- 1Department of Cardiovascular Medicine, State Key Laboratory of Medical Genomics, Shanghai Key Laboratory of Hypertension, Shanghai Institute of Hypertension, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Renal Division, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of Endocrine and Metabolic Diseases, State Key Laboratory of Medical Genomics, Ruijin Hospital, Clinical Trial Center, Shanghai Institute of Endocrine and Metabolic Diseases, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 4Renal Division, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 5Division of Endocrinology, Diabetes, and Bone Disease, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Introduction: The risks associated with non-albuminuric chronic kidney disease (CKD) have been investigated in diabetes mellitus but not in hypertensive patients. The objective of this study was to investigate the risks associated with non-albuminuric CKD in treated hypertensive patients in the Systolic Blood Pressure Intervention Trial (SPRINT) population.
Methods: Based on baseline albuminuria status (urine albumin/creatinine ratio [UACR], ≥30 or <30 mg/g) and the levels of estimated glomerular filtration rate ([eGFR], ≥60, 45–59, or <45 mL/min/1.73 m2), participants were classified into six subgroups to assess the risks associated with the primary outcome and mortality. The primary composite outcome was myocardial infarction, other acute coronary syndromes, stroke, heart failure, or mortality from cardiovascular causes.
Results: During a median follow-up of 3.26 years in 8,866 hypertensive patients, there were 352 deaths and 547 participants with the primary outcome. In adjusted Cox regression analysis using non-CKD and non-albuminuria (eGFR ≥60 mL/min/1.73 m2 combined with UACR <30 mg/g) as reference, albuminuria whether combined with CKD or not, showed significantly higher risk of both primary outcome and all-cause mortality in the total population. Whereas, non-albuminuria only combined with eGFR <45 mL/min/1.73 m2 showed significantly higher risk of both primary outcome and all-cause mortality in the intensive-therapy group.
Discussion: Non-albuminuric CKD did have higher risk of all-cause and CVD mortality only if the eGFR <45 mL/min/1.73 m2. Increased albuminuria conferred higher risk of primary outcome and all-cause mortality irrespective the levels of eGFR.
Clinical trial registration: ClinicalTrials.gov, number: NCT01206062.
Introduction
Diabetes mellitus and hypertension are the two leading risk factors of chronic kidney disease (CKD), in which increased albuminuria and decreased estimated glomerular filtration rate (eGFR) are the major determinants (1, 2). Previous studies on diabetic kidney disease showed a decreased prevalence of albuminuria and increased prevalence of decreased eGFR compared to those without diabetes (3, 4). At present, non-albuminuric renal impairment (i.e., non-albuminuric diabetic kidney disease [DKD]) has become prevalent in diabetes mellitus, and might play an important role of CKD progression and carry higher risk of outcomes (5–9). Indeed, non-albuminuric CKD did have higher risks of death or major cardiovascular events, but was not comparable to those with albuminuria (albuminuric CKD or albuminuric non-CKD) in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial (10). However, a recent observational study in Japan failed to find non-albuminuric CKD had higher risks of mortality, cardiovascular disease (CVD) events, or renal function decline than those in no-CKD (11). It seems that albuminuria, rather than reduced eGFR, is the main driver for CKD progression and outcomes in diabetes mellitus. In fact, some studies have indicated that any degree of albuminuria is a risk factor for cardiovascular events in individuals with or without diabetes mellitus (12).
After diabetes mellitus, hypertension is the second important cause of CKD, and a major risk factor for the development and progression of CKD (1, 2, 13, 14). In patients with CKD, masked hypertension and elevated nighttime BP are common, and are associated with a lower eGFR and higher levels of albuminuria (15). A recent large meta-analysis has shown a significant reduction in all-cause mortality following BP reduction in patients with CKD (16). Reduction of albuminuria has also been considered as a therapeutic target in hypertensive patients (17). However, the risks associated with non-albuminuric CKD in non-diabetic hypertensive patients have rarely been investigated.
The Systolic Blood Pressure Intervention Trial (SPRINT) was the largest randomized clinical trial to date, to test the efficacy of a target systolic blood pressure goal of <120 mmHg compared to a standard blood pressure goal of <140 mmHg on lowering the risk of clinical outcomes in hypertensive patients free of diabetes mellitus (18), which enabled us to investigate the risks associated with various eGFR subgroups and albuminuria categories, as well as the non-albuminuric CKD. In the present study, we employed the data from the SPRINT trial to investigate the risks associated with non-albuminuric CKD in hypertensive patients.
Research design and methods
Study population
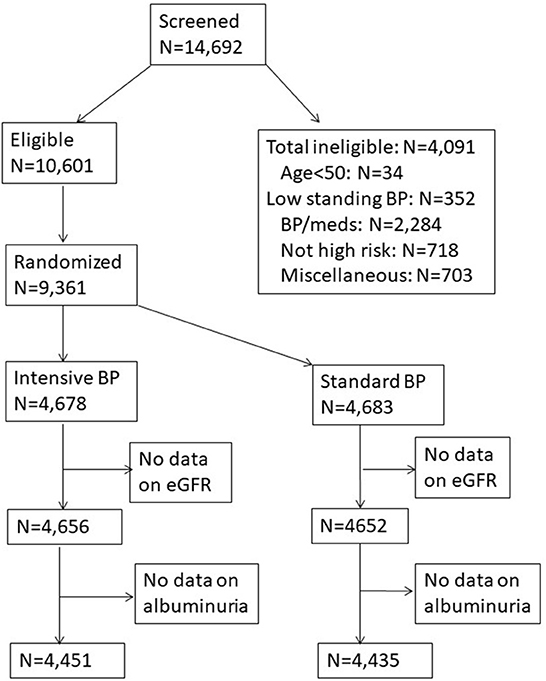
SPRINT was a multicenter randomized clinical trial sponsored by the National Heart, Lung, and Blood Institute (NHLBI), the National Institute for Diabetes and Digestive and Kidney Diseases, National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS) (online study protocols: https://biolincc.nhlbi.nih.gov/studies/sprint/). A thorough description of the rationale and design of SPRINT has been published (18). SPRINT was the largest randomized clinical trial to date, to test the efficacy of a target systolic blood pressure goal of <120 mmHg compared to a standard blood pressure goal of <140 mmHg on lowering the risk of clinical outcomes. SPRINT was approved by Institutional Review Boards at participating sites and all participants provided written informed consent. Figure 1 presents the flow chart of the study population selected for our analysis.
Measurements
Urine albumin/creatinine ratio (UACR) was performed using a spot urine sample obtained at the baseline visit and measured in milligrams of albumin/gram of creatinine (mg/g). Estimated GFR (eGFR) was calculated in mL/min/1.73 m2 using the serum creatinine level obtained at the baseline visit. The 2021 Chronic Kidney Disease Epidemiology Collaboration equation (2021 CKD-EPI creatinine-based equations to estimate GFR without race) was used for eGFR calculating (18). Detailed inclusion and exclusion criteria have been described previously (19). Both UACR and serum creatinine were measured at the SPRINT central laboratories.
Albuminuria was defined as a UACR ≥30 mg/g. CKD was defined as eGFR <60 mL/min/1.73 m2. Based on baseline albuminuria status and the levels of eGFR (≥60, 45–59, or <45 mL/min/1.73 m2), participants were classified into six subgroups: non-albuminuric non-CKD (UACR <30 mg/g and eGFR ≥60 mL/min/1.73 m2), non-albuminuric CKD 3a (UACR <30 mg/g and eGFR of 45–59 mL/min/1.73 m2), non-albuminuric CKD 3b/4/5 (UACR <30 mg/g and eGFR of <45 mL/min/1.73 m2), albuminuric non-CKD (UACR ≥30 mg/g and eGFR ≥60 mL/min/1.73 m2), albuminuric CKD 3a (UACR ≥30 mg/g and eGFR of 45–59 mL/min/1.73 m2), and albuminuric CKD 3b/4/5 (UACR ≥30 mg/g and eGFR of <45 mL/min/1.73 m2). A 10-year risk of cardiovascular disease was calculated on the basis of the Framingham risk score.
Study outcomes
The primary composite outcome was considered to have occurred with myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, or death from cardiovascular causes. Total mortality and cardiovascular mortality refer to death from any cause and to death from cardiovascular cause.
Pre-specified subgroups of interest for primary outcome were defined according to status with respect to cardiovascular disease at baseline (yes vs. no), age (<75 vs. ≥75 years), sex, race (black vs. non-black), and baseline systolic blood pressure in three levels (≤ 132 mm Hg, >132 to <145 mm Hg, and ≥145 mm Hg).
Statistics
For database management and statistical analysis, we used SAS software version 9.4 (SAS Institute Inc, Cary, NC). Significance was a 2-tailed α-level of ≤ 0.05. Means and proportions were compared using the large-sample z-test and the χ2 statistic, respectively. Comparisons of continuous variables among the six eGFR and albuminuria phenotypes were performed by one-way ANOVA.
Kaplan-Meier survival probabilities were estimated according to CKD and albuminuria phenotypes, and differences were analyzed by the log-rank test in four subgroups. Cox proportional hazards analysis was used to compute the hazard ratio (HR) with 95% CI and cumulative survival rate according to CKD phenotypes in the total population as well as in the standard-therapy group analyzed separately, using the UACR <30 mg/g combined with eGFR ≥60 mL/min/1.73 m2 as reference group, adjusted for baseline age, sex, race, smoking, history of CVD, BMI, systolic and diastolic blood pressures, and glucose and serum lipid levels.
Results
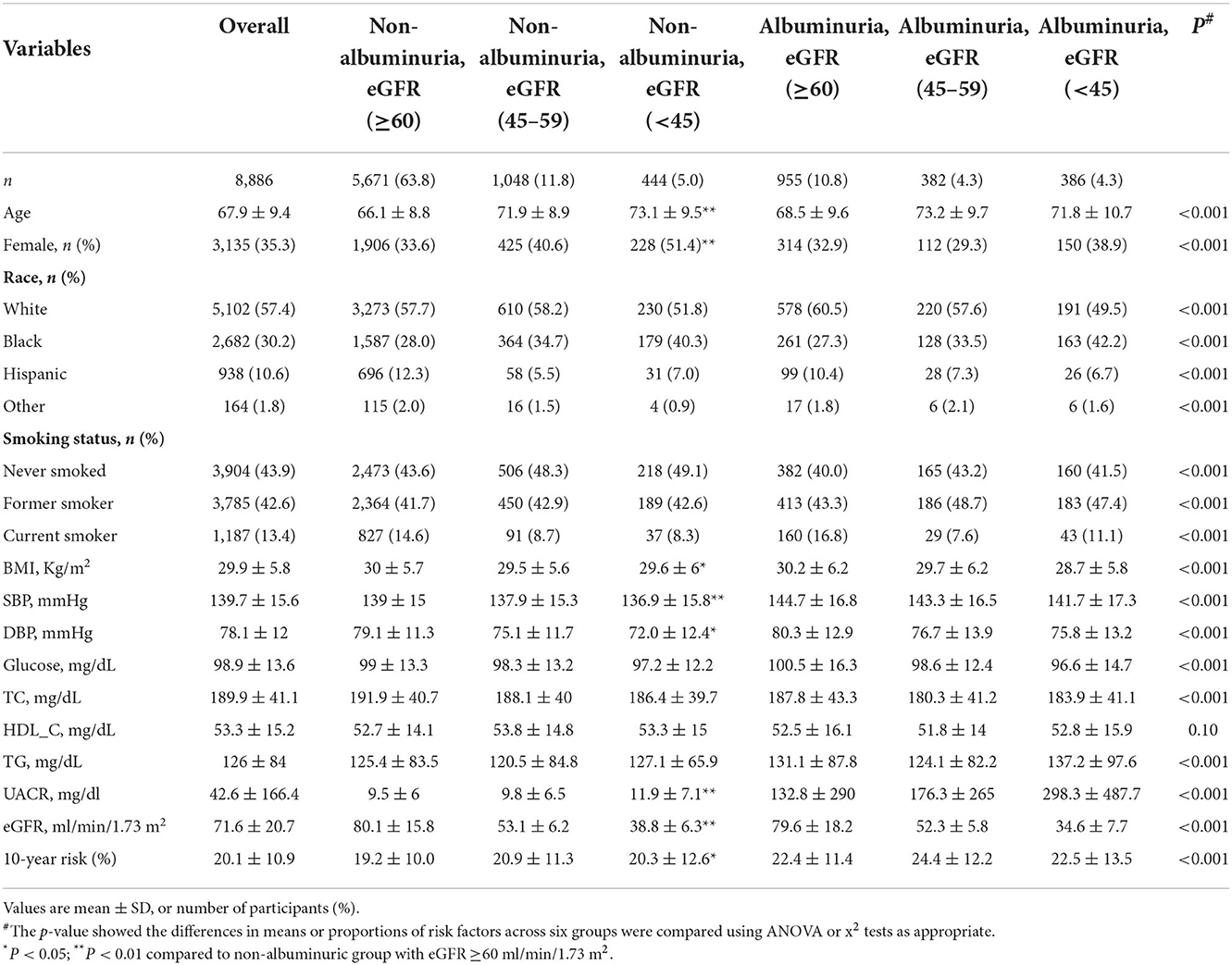
This study included 8,886 people with hypertensive patients who were enrolled into the SPRINT clinical trial. The median follow-up duration was 3.3 (25th−75th percentile, 2.8–3.8) years. Characteristics of the study participants at baseline were summarized in Table 1. The prevalence of chronic kidney disease phenotypes was 10.8% for albuminuric non-CKD, 16.8% for non-albuminuric CKD, and 8.6% for albuminuric CKD. Compared with non-albuminuric non-CKD group, individuals with non-albuminuric CKD 3b/4/5 (eGFR <45 mL/min/1.73 m2) were older (73.1 vs. 66.1 years), more frequently women (51.4 vs. 33.6%), and nonsmokers (49.1 vs. 43.6%), had lower BMI (29.6 vs. 30.0 Kg/m2), and systolic (136.9 vs. 139.0 mmHg) and diastolic blood pressure (72.0 vs. 79.1 mmHg), had decreased eGFR (38.8 vs. 80.1 ml/min/1.73 m2), but had higher levels of UACR (11.9 vs. 9.5 mg/dl) and higher 10-year risk (20.3 vs.19.2%).
During a median follow-up of 3.3 years, 547 (6.2%) people developed the primary outcome, and 352 (3.9%) and 99 (1.1%) people died of any cause and of cardiovascular causes, respectively. The cumulative survival rates for these outcomes differed among the six CKD phenotypes, non-albuminuric non-CKD, non-albuminuric CKD 3a, non-albuminuric CKD 3b/4/5, albuminuric non-CKD, albuminuric CKD 3a, and albuminuric CKD 3b/4/5. Compared with non-albuminuric non-CKD, patients with eGFR < 45 ml/min/1.73 m2 or UACR≥30 mg/g had significantly lower survival rate of primary outcome and all-cause and cardiovascular mortality, and patients with albuminuric CKD 3b/4/5 had the lowest survival rate of all outcomes (Figure 2).
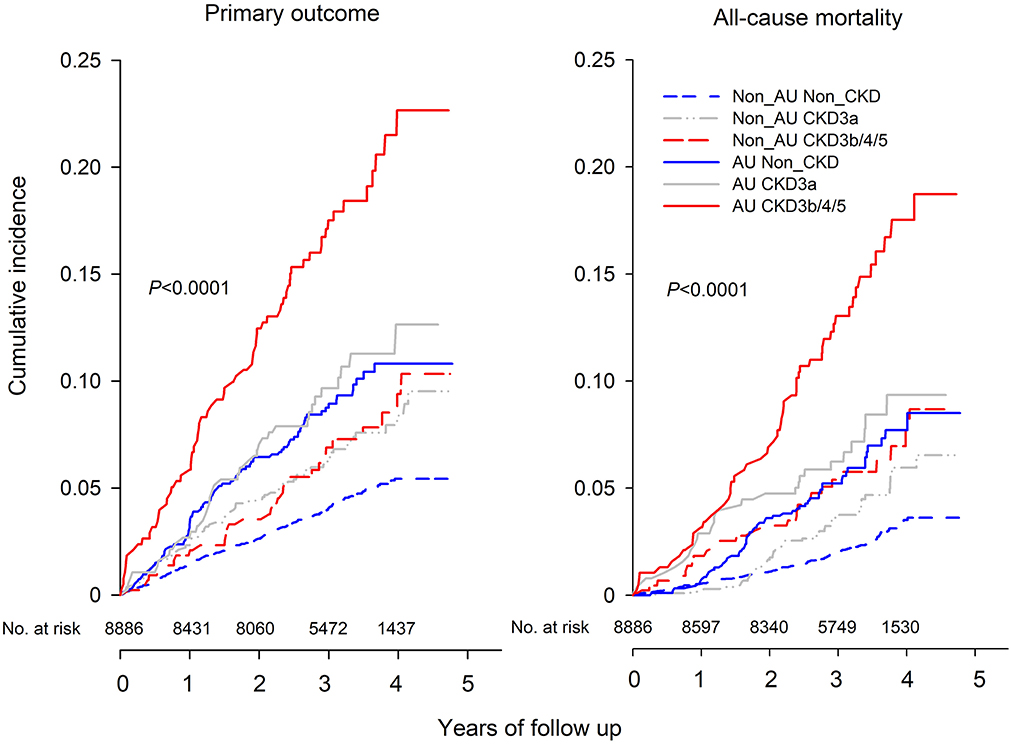
Figure 2. Kaplan-Meier curve of cumulative incidence for primary outcome (left) and all-cause mortality (right) according to the GFR and Albuminuria categories at baseline. The six subgroups included Non_AU Non_CKD (UACR < 30 mg/g and eGFR ≥60 mL/min/1.73 m2), Non_AU CKD3a (UACR < 30 mg/g and eGFR of 45–59 mL/min/1.73 m2), Non_AU CKD3b/4/5 (UACR < 30 mg/g and eGFR of < 45 mL/min/1.73 m2), AU Non_CKD (UACR ≥30 mg/g and eGFR ≥60 mL/min/1.73 m2), AU CKD 3a (UACR ≥30 mg/g and eGFR of 45–59 mL/min/1.73 m2), and AU CKD 3b/4/5 (UACR ≥30 mg/g and eGFR of < 45 mL/min/1.73 m2).
In the adjusted Cox regression models, we investigated the risks associated with other CKD phenotypes compared with non-albuminuric non-CKD. Patients with albuminuric CKD 3b/4/5 had increased likelihood of the primary outcome, of total mortality, and of CVD mortality in the total population as well as in the intensive- and standard-therapy group analyzed separately (HR from 2.94 to 6.47). Albuminuric non-CKD was associated with an increase in the primary outcome and in all-cause mortality in the total population as well as in the intensive- and standard-therapy group analyzed separately (HR from 1.77 to 2.16).
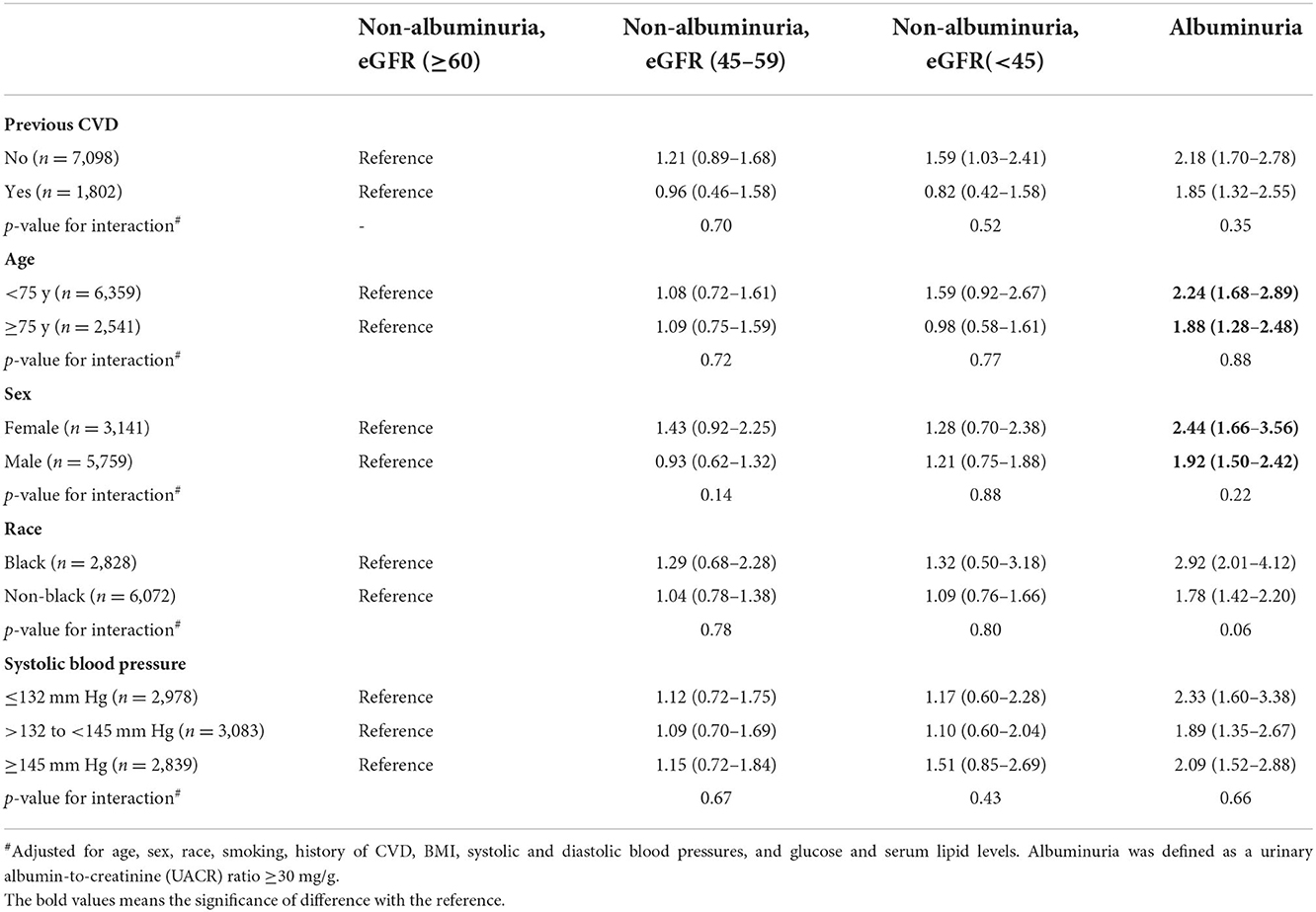
Non-albuminuric CKD, generally, was neither associated with a significant increase in adverse outcomes in the total population nor in the standard- and intensive-therapy groups analyzed separately (Table 2). Furthermore, no prognostic significance was seen with non-albuminuric CKD on primary outcome across the pre-specified subgroups (Table 3). However, in the non-albuminuric CKD 3b/4/5 group (n = 444), there was a suggestion of increased both all-cause and CVD mortality which was statistically significant in the intensive-treatment subset (HR [95% CI], 2.55 [1.43–4.56] and 6.46 [2.57–16.3], respectively), but not in the standard-treatment subset (Table 2). When a sub analysis of the components of the composite CVD end-points was further conducted, only heart failure significantly increased in the non-albuminuric CKD 3b/4/5 group in intensive-treatment subset (HR [95% CI], 5.79 (2.34–14.4) but not in standard-treatment subset (Supplementary Table S1).
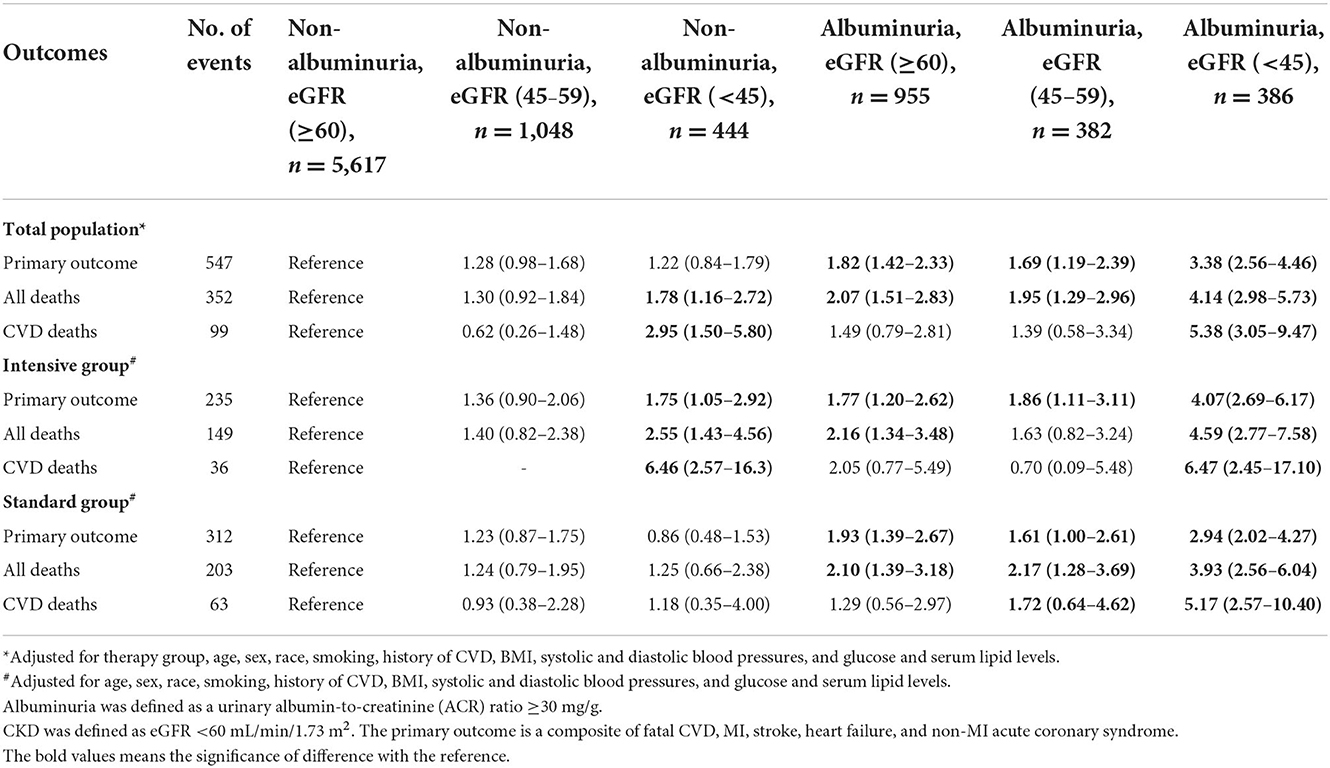
Table 2. Risk of Primary outcomes and total and cardiovascular mortality by baseline CKD and albuminuria status.
Comparisons of primary outcome and mortality in the subgroups defined by the presence or absence of albuminuria and by eGFR ≥60, ≥45 to < 60, or < 45 showed reduction in both outcomes in participants randomized intensive vs. standard BP goals in trial participants with normoalbuminuria and eGFR ≥60, with normoalbuminuria and eGFR ≥45 to < 60, and with albuminuria in all the eGFR subgroups (Supplementary Table S1). However, among trial participants with eGFR < 45 and normoalbuminuria the likelihood of mortality was 1.39-fold (95% CI 0.65–2.99) greater with the intensive BP goal than that with the standard BP goal (Supplementary Table S2).
Discussion
This post-hoc analysis of the SPRINT trial found that non-albuminuric CKD did not have higher risk of primary outcome and mortality in hypertensive patients alone. However, increased mortality was shown in the intensive treatment subset if the eGFR < 45 mL/min/1.73 m2. Generally, increased albuminuria, rather than decreased eGFR, might be the main driver for adverse outcomes in hypertensive patients.
Our study exhibited similar results to those conducted in persons with diabetes mellitus, and found that non-albuminuric CKD did not have higher risk of primary outcome and mortality. One study of 2,953 Japanese patients with type 2 diabetes and estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2 found that the risks of death or CVD were not higher in those with non-albuminuric CKD than those with non-CKD (adjusted hazard ratio, 1.02 [95% CI 0.66–1.60]) (11). Another study conducted in 1,908 participants with diabetes and reduced eGFR found that the absence of albuminuria carried a much lower risk for ESKD, CKD progression, or rapid decline in eGFR compared to that in patients with albuminuria (20). However, among persons with type 2 diabetes enrolled in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study (10), patients with non-albuminuric CKD had higher risk of death or major cardiovascular events than those in non-CKD patients. Nevertheless, the risk of those with non-albuminuric CKD was much lower than that of those with albumuniuric CKD and albuminuric non-CKD. Our study of non-diabetic hypertensive patients found similar results:persons with nonalbuminuric reduced eGFR did not have higher risk of adverse outcomes than those in hypertensive patients with normal glomerular filtration. However, our study firstly found that non-albuminuric CKD 3b/4/5 did have higher risk of all-cause and cardiovascular mortality. The risk was only seen in heart failure when the composite CVD components was further analyzed, similar to the original SPRINT study (19). However, intensive BP-lowering was associated with greater rather than lower risk of adverse outcome.
There are several explanations for these results. Firstly, non-albuminuria with reduced eGFR could happen in histopathologically diagnosed tubulointerstitial disease which is known by no significant proteinuria, no clinically evident edema, and no known cause of chronic renal failure (21). Secondly, it is possible that non-albuminuric reduced GFR mainly reflects an age-related decline in renal function, rather than a primary renal disease. In this study, non-albuminuric CKD showed about 8 years older than the reference group, though slightly younger than albuminuric CKD.
Population-based studies consistently showed that prevalence of CKD depends strongly on age, with prevalence increased approximately 10-fold between young adulthood and middle age in the US Kidney Early Evaluation Program (22). Thirdly, another possibility is that the non-albuminuric reduced GFR group is enriched with people with who respond particularly favorably to RAAS blockade, leading to normalization of urinary albumin-to-creatinine ratio and increased serum creatinine (23).
Although risk for end-stage kidney disease was very low in people with non-albuminuric CKD, reduced eGFR remains an important marker for all-cause mortality and CVD (24). Studies have showed that reduced eGFR is an important prognostic factor in total mortality and cardiovascular events, inspective of albuminuria, in general population or uncomplicated hypertensive patients (24) or resistant hypertensive patients (25).
Our study should be interpreted within the context of its strengths and limitations. The strengths of our study include a large sample size and long follow up, which enable us to classify the study population into six subgroups for detailed analysis. Some limitations of this analysis should be discussed. Our study was a post-hoc analysis of a highly selected group of individuals with prior CVD, and the results should be validated in unselected hypertensive populations. The study population was classified into six subgroups, which might be underpowered to conduct subgroup analysis, such as greater HRs and wide range of CIs for NACKD predicting CVD mortality. Our study did not take frequent serum creatinine sampling, and we could not compare the rate of CKD progression between non-albuminuric CKD and other groups. The study did not use the KDIGO definition of CKD, as only one measure of serum creatinine and albumninuria at baseline was conducted.
Conclusion
Non-albuminuric CKD did have higher risk of all-cause and CVD mortality only if the eGFR <45 mL/min/1.73 m2. Increased albuminuria conferred higher risk of primary outcome and all-cause mortality irrespective the levels of eGFR.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
C-SS, JT, and ZB had full access to all the data in the study, take responsibility for the accuracy of the data analysis, and participated in the study design and paper revision. DW, JY, and YC performed the studies and drafted the manuscript. SS and YY helped with the statistical analysis. YM and WW checked the accuracy of the analysis and involved in review the English language and grammar. All the authors read and approved the final manuscript.
Funding
This work was supported by the Shanghai Talent Development Fund (2021087), the Chinese National Natural Science Foundation (82270452, 81770418, 81400346, and 81270935), Ministry of Health (2016YFC1300103 and 2016YFC0905001), and Shanghai Pujiang Talents Plan (18PJ1407200).
Acknowledgments
The investigators acknowledge and thank the SPRINT investigators and the National Heart, Lung, and Blood Institute for conducting the trials and making datasets publicly available.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.977938/full#supplementary-material
References
1. Gaitonde DY, Cook DL, Rivera IM. Chronic kidney disease: detection and evaluation. Am Fam Physician. (2017) 96:776–83.
2. National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kid Dis Nat Kid Found. (2002) 39(2 Suppl 1):S1–266.
3. Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. (2012) 380:1662–73. doi: 10.1016/S0140-6736(12)61350-6
4. Berhane AM, Weil EJ, Knowler WC, Nelson RG, Hanson RL. Albuminuria and estimated glomerular filtration rate as predictors of diabetic end-stage renal disease and death. Clin J Am Soc Nephrol. (2011) 6:2444–51. doi: 10.2215/CJN.00580111
5. Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, et al. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens. (2011) 29:1802–9. doi: 10.1097/HJH.0b013e3283495cd6
6. Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. Jama. (2016) 316:602–10. doi: 10.1001/jama.2016.10924
7. Thorn LM, Gordin D, Harjutsalo V, Hagg S, Masar R, Saraheimo M, et al. The presence and consequence of nonalbuminuric chronic kidney disease in patients with type 1 diabetes. Diabetes Care. (2015) 38:2128–33. doi: 10.2337/dc15-0641
8. Kramer H, Boucher RE, Leehey D, Fried L, Wei G, Greene T, et al. Increasing mortality in adults with diabetes and low estimated glomerular filtration rate in the absence of albuminuria. Diabetes Care. (2018) 41:775–81. doi: 10.2337/dc17-1954
9. Penno G, Solini A, Orsi E, Bonora E, Fondelli C, Trevisan R, et al. Non-albuminuric renal impairment is a strong predictor of mortality in individuals with type 2 diabetes: the renal insufficiency and cardiovascular events (RIACE) Italian multicentre study. Diabetologia. (2018 N) 61:2277–89. doi: 10.1007/s00125-018-4691-2
10. Buyadaa O, Magliano DJ, Salim A, Koye DN, Shaw JE. Risk of rapid kidney function decline, all-cause mortality, and major cardiovascular events in nonalbuminuric chronic kidney disease in type 2 diabetes. Diabetes Care. (2020) 43:122–9. doi: 10.2337/dc19-1438
11. Yokoyama H, Araki SI, Kawai K, Yamazaki K, Shirabe SI, Sugimoto H, et al. The prognosis of patients with type 2 diabetes and nonalbuminuric diabetic kidney disease is not always poor: implication of the effects of coexisting macrovascular complications (JDDM 54). Diabetes Care. (2020) 43:1102–10. doi: 10.2337/dc19-2049
12. Gerstein HC, Mann JF Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. Jama. (2001) 286:421–6. doi: 10.1001/jama.286.4.421
13. Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, et al. Hypertension awareness, treatment, and control in adults with CKD: results from the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis J Nat Kid Found. (2010) 55:441–51. doi: 10.1053/j.ajkd.2009.09.014
14. Rao MV, Qiu Y, Wang C, Bakris G. Hypertension and CKD: kidney early evaluation program (KEEP) and national health and nutrition examination survey (NHANES), 1999–2004. Am J Kid Dis J Nat Kidney Found. (2008) 51(4 Suppl 2):S30–7. doi: 10.1053/j.ajkd.2007.12.012
15. Drawz PE, Alper AB, Anderson AH, Brecklin CS, Charleston J, Chen J, et al. Masked hypertension and elevated nighttime blood pressure in CKD: prevalence and association with target organ damage. Clin J Am Soc Nephrol. (2016) 11:642–52. doi: 10.2215/CJN.08530815
16. Tsai WC, Wu HY, Peng YS, Yang JY, Chen HY, Chiu YL, et al. Association of intensive blood pressure control and kidney disease progression in non-diabetic patients with chronic kidney disease: a systematic review and meta-analysis. JAMA Intern Med. (2017) 177:792–9. doi: 10.1001/jamainternmed.2017.0197
17. Ibsen H, Olsen MH, Wachtell K, Borch-Johnsen K, Lindholm LH, Mogensen CE, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension. (2005) 45:198–202. doi: 10.1161/01.HYP.0000154082.72286.2a
18. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. Chronic kidney disease epidemiology collaboration. New creatinine- and cystatin C-based equations to estimate GFR without Race. N Engl J Med. (2021) 385:1737–49. doi: 10.1056/NEJMoa2102953
19. Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Eng J Med. (2015) 373:2103–16. doi: 10.1056/NEJMoa1511939
20. Koye DN, Magliano DJ, Reid CM, Jepson C, Feldman HI, Herman WH, et al. Risk of progression of non-albuminuric CKD to end-stage kidney disease in people with diabetes: the CRIC (chronic renal insufficiency cohort) study. Am J Kidney Dis J Nat Kidney Found. (2018) 72:653–61. doi: 10.1053/j.ajkd.2018.02.364
21. Mani MK. Experience with a program for prevention of chronic renal failure in India. Kidney Int. (2005) 67:S75–8. doi: 10.1111/j.1523-1755.2005.09419.x
22. Brown WW, Peters RM, Ohmit SE, Keane WF, Collins A, Chen SC, et al. Early detection of kidney disease in community settings: the kidney early evaluation program (KEEP). Am J Kidney Dis. (2003) 42:22–35. doi: 10.1016/S0272-6386(03)00405-0
23. Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. (2001) 345:870–8. doi: 10.1056/NEJMoa011489
24. Viazzi F, Leoncini G, Conti N, Tomolillo C, Giachero G, Vercelli M, et al. Combined effect of albuminuria and estimated glomerular filtration rate on cardiovascular events and all-cause mortality in uncomplicated hypertensive patients. J Hypertens. (2010) 28:848–55. doi: 10.1097/HJH.0b013e328336ed09
Keywords: albuminuria, hypertension, SPRINT trial, chronic kidney disease, cardiovascular risk factors
Citation: Sheng C-S, Wang D, Yuan J, Cheng Y, Sun S, Yang Y, Miao Y, Wang W, Tian J and Bloomgarden ZT (2022) CVD risk in non-albuminuric chronic kidney disease in hypertensive, non-diabetic subjects: A post-hoc analysis from SPRINT. Front. Cardiovasc. Med. 9:977938. doi: 10.3389/fcvm.2022.977938
Received: 25 June 2022; Accepted: 18 November 2022;
Published: 07 December 2022.
Edited by:
Belen Ponte, Hôpitaux Universitaires de Genève (HUG), SwitzerlandReviewed by:
(EE) Marius Miglinas, Vilnius University, LithuaniaRichard James Glassock, University of California, Los Angeles, United States
Giuseppe Pugliese, Sapienza University of Rome, Italy
Copyright © 2022 Sheng, Wang, Yuan, Cheng, Sun, Yang, Miao, Wang, Tian and Bloomgarden. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chang-Sheng Sheng, c2NzaGVuZzIwMDRAMTYzLmNvbQ==; Jingyan Tian, dGlhbmp5cGFwZXJAMTYzLmNvbQ==; Zachary T. Bloomgarden, emJsb29tQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Chang-Sheng Sheng
Chang-Sheng Sheng Dan Wang1†
Dan Wang1† Ya Miao
Ya Miao Zachary T. Bloomgarden
Zachary T. Bloomgarden