- 1Cardiovascular Surgery Unit, Maria Cecilia Hospital GVM Care and Research, Cotignola, Italy
- 2Interventional Cardiology Unit, Maria Cecilia Hospital GVM Care and Research, Cotignola, Italy
- 3Cardiology Unit, Maria Cecilia Hospital GVM Care and Research, Cotignola, Italy
Clinically significant tricuspid regurgitation (TR) is common and associated with excess mortality. At the same time right ventricular (RV) failure is a complex clinical syndrome that results from many causes, but is often associated with long-term prognosis. Whilst results of isolated tricuspid valve (TV) surgery are often unsatisfactory and limited by the prohibitive risk of most patients, the recent development of percutaneous recovery techniques has opened new scenarios. In consideration of the complexity of the mechanisms that lead to right heart failure and RV dysfunction it is important to understand the real advantages that percutaneous TV treatment can offer, more specifically the effect of TR reduction on RV remodeling in the setting of functional tricuspid regurgitation (fTR).
Introduction
Functional tricuspid regurgitation (fTR) is the most common cause of TR as it usually comes as a result of annular dilation and leaflets tethering caused by right ventricle (RV) enlargement and dysfunction due to pressure/volume overload related to left-sided heart disease (ventricular fTR) or atrial fibrillation (atrial fTR) (1, 2). It has become clear from surgical data of isolated tricuspid treatment that right ventricle (RV) end-systolic area, degree of RV dilatation, and presence of advanced heart failure can be predictors of poor outcomes (3, 4). On the other side, it has been recently demonstrated that patients showing early reverse remodeling of RV after tricuspid surgery were associated with a better long-term survival (5). Among the predictors of effective RV improvement in patients with pre-existing RV dysfunction, only right atrial pressure has been previously identified as associated with worse outcomes (5, 6).
As surgical repair tipically addresses annular dilatation, it is still unclear whether the same assumptions can be made for percutaneous treatments, in particular for those not directly acting on annulus size. In addition, scarce data is available about the effect of tricuspid treatment on RV function as assessed by advanced imaging techniques.
Aim of the present review is to evaluate the effect of percutaneous leaflet repair on RV function and size in the subgroup of patients with fTR.
Percutaneous treatment of functional TR
FTR usually develops due to structural alterations in right atrial and/or ventricular myocardial geometry, leading to tricuspid annulus dilation and/or leaflet tethering, both associated with impaired leaflet coaptation. This is by far the most common cause of TR in adults, as shown by echocardiographic studies in which over than 90% patients with severe TR had a functional etiology (7, 8). Multiple etiologies of fTR have been described. Most commonly, fTR is associated with LV dysfunction and/or left-sided heart disease, leading to left atrial dilation, increased pulmonary wedge pressure and RV afterload (9, 10). Pulmonary hypertension arising from other causes than left heart disease, such as primary pulmonary hypertension, pulmonary embolism and chronic pulmonary disease, can also cause TR due to increased RV afterload, RV dilation and dysfunction (11). On the other hand, primary RV diseases, including isolated RV infarction or arrhythmogenic RV dysplasia, occur less frequently (12, 13). In addition, it should be acknowledged that RV dysfunction associated with significant TR is often difficult to assess (14). In all cases, anyway, when severe TR occurs, the increase in volume overload contributes to further RV dilation and dysfunction, with further malcoaptation of the TV leaflets perpetuating a vicious circle that impacts on prognosis. It is noteworthy, however, that RV reshaping process associated with fTR varies tremendously between patients (15). The different patterns of RV remodeling may be related to the underlying pathophysiology and to the timing in natural history of fTR when these patterns are assessed. We could consider two different pathophysiological groups:
1) patients without left-sided heart disease and without pulmonary hypertension, in whom significant fTR may appear attributable to right atrial dilation and atrial fibrillation, whereas the RV dimensions and function are usually within the normal values;
2) patients with left-sided heart disease and pulmonary hypertension, in whom TR is associated with various grades of RV dilation and dysfunction, usually related to myocyte loss and fibrosis that would finally affect prognosis.
While the benefit of tricuspid repair looks high in the former group, it is still not clear to what degree of RV dysfunction this process is reversible. In a study by Topilsky et al. (11), idiopathic TR was associated with basal RV enlargement (conical deformation) and tricuspid annulus dilation, whereas pulmonary hypertension–related TR was associated with increased RV length (elliptical deformation), causing tenting of the tricuspid leaflets and depicting a technically more challenging scenario to be solved with a percutaneous repair.
Tricuspid transcatheter edge-to-edge repair using the TriClip™ (Abbott Vascular, Santa Clara, CA, USA) or leaflet approximation with the PASCAL systems (Edwards Lifesciences, Irvine, CA, USA) are the currently available systems for percutaneous tricuspid repair. In the core lab-adjudicated TRILUMINATE study, percutaneous tricuspid repair in 85 prospectively enrolled patients (STR 84%; severe, massive and torrential in 29, 26 and 37%, respectively) was associated with a durable reduction to moderate TR or less in 71% at 1 year (16, 17). Interestingly, a significant reduction of RV and RA dimensions and improvement was reported in patients showing clinical and functional improvement. Of note, patients with severe RV dysfunction and severe pulmonary hypertension were excluded from the study and are currently not treated with percutaneous tricuspid repair. Favorable results have been obtained with the PASCAL system in a US-based early feasibility study (18); however, less experience has been accumulated and follow-up is shorter.
Evaluation of right ventricular reverse remodeling
Evaluation of RV size and function is universally considered more complex than the one of left system. The biomechanics of the RV are significantly different from those of the LV and are optimized to deal with changes in volume load and to work at low pressure. This complex biomechanical construct and the change in relative components of radial function and longitudinal function with loading adds to the complexity in overall assessment of RV function (19).
Among all possible imaging modalities, echocardiography and cardiac magnetic resonance (CMR) should be considered as first-line tools to assess RV function changes over time (20, 21).
Echocardiography
Echocardiography is by far the most common way to evaluate RV dimensions and function but technical issues are well known. Quantitative assessment of the RV by 2D transthoracic echocardiography is challenging due to its complex asymmetric geometry that leads to incomplete visualization in a single projection and lack of precise anatomic landmarks. Differently from LV, there are no geometrical assumptions for end-diastolic volume (EDV) and end-systolic volume (ESV) measurements. In addition, 2D echocardiography diameters of the RV may significantly vary just with minor rotation of the probe due to the lack of a precise and specific landmark, leading to a significant under- or over- estimation of RV size.
Evaluation of RV size and function is usually multiparametric and different echocardiographic techniques can be used. However, this usually leads to data fragmentation, thus preventing inter-study correlations. 3D echo can overcome these limitations but requires specific reconstruction software, it's time consuming and is limited by acoustic impedance.
3D echocardiography has demonstrated excellent correlation with CMR for the calculation of RV volumes and ejection fraction with a slight systematic underestimation of EDV and ESV but no difference in terms of RV ejection fraction (22).
Cardiac magnetic resonance
CMR, even if limited by availability and costs, is considered the gold standard for evaluating RV volume, mass and function due to the volumetric acquisition providing excellent myocardial definition and tissue characterization. CMR permits direct EDV, ESV and EF measurement without geometrical assumption. Atrial arrhythmias might interfere with image acquisition and the presence of intrathoracic prosthetic material (prosthetic valves, pacemakers, etc.) in certain cases can lead to significant artifact making CMR images uninterpretable.
CMR not only allows precise evaluation of RV ventricular volumes and function but is able to calculate both left and right actual forward stroke and regurgitation volumes.
Right ventricle remodeling after percutaneous tricuspid leaflet repair
Atrial fTR and ventricular fTR usually show two different remodeling patterns. The atrial fTR is linked with aging, atrial fibrillation, diastolic dysfunction, associated with a predominant atrial and annular dilatation with the latter as the predominant mechanism of TR. RV dilation in these cases is usually mild and limited to the basal segment.
Ventricular fTR has a different remodeling pattern with significant RV enlargement, less prominent annular dilatation and marked leaflets tethering as the leading mechanism of regurgitation. It is often due to left-sided ventricular or valve diseases, primary RV dysfunction as a consequence of RV infarction or cardiomyopathy, or pulmonary hypertension.
Residual TR and RV reverse remodeling after cardiac surgery are sensitive predictors of long-term prognosis (23) after surgery. In fact, successful reduction of TR after surgery can lead to a significant RV reverse remodeling by RV volume reduction and systolic function preservation, which translates into LV increase in preload and cardiac output.
Although available data is scarce, it seems that different degrees of reverse remodeling are linked to different etiologies of TR and degree of RV remodeling and dysfunction at the time of surgery. Several different papers showed a positive reverse remodeling after tricuspid surgical repair during mitral valve surgery (24), pulmonary endarterectomy or balloon pulmonary angioplasty (25, 26). On the contrary, there is some evidence that RV reverse remodeling is frequently absent in ischemic cardiomyopathy after CABG and mitral and tricuspid surgery (27).
Surgical series should be considered as a reference guide to predict the effect of percutaneous repair on RV remodeling. Data derived from the Triluminate study (16) shows significant global and early RV reverse remodeling after successful procedure in terms of EDV, annular diameter, and atrial volumes at 30 days. These positive remodeling lasts till 1 year leading to an increase of RV contractility in terms of FAC and TAPSE. The study did not show if this positive reverse remodeling happens in all patients or in selected cases and it did not question whether it depends on the amount of TR reduction. RV reverse remodeling seems to happen early after the procedure, within 1 month (28) or even before discharge (29), with no further changes at 6 months or 1 year as a consequence of changed loading condition, as described by reduction in volumes without changes in contractility, as assessed by longitudinal strain (28). Reasons for the lack of RV reverse remodeling after 6 months are unclear and maybe due to incomplete TR reduction or irreversible structural changes in the RV myocardium at the time of Triclip intervention. Successful percutaneous tricuspid repair tends to reduce RV EDV with no significant changes in RVESV, which may be related to a significant reduction in RV volume overload with no significant reduction in RV afterload (30) (Figure 1). As a result, despite total RV stroke volume is reduced, forward RV stroke volume is significantly increased as documented by CMR studies (Figure 2) (28). The amount of reduction of RV end diastolic area, immediately after the procedure, is directly correlated with event-free survival at follow-up, confirming that RV reverse remodeling is an independent prognostic predictor (31).
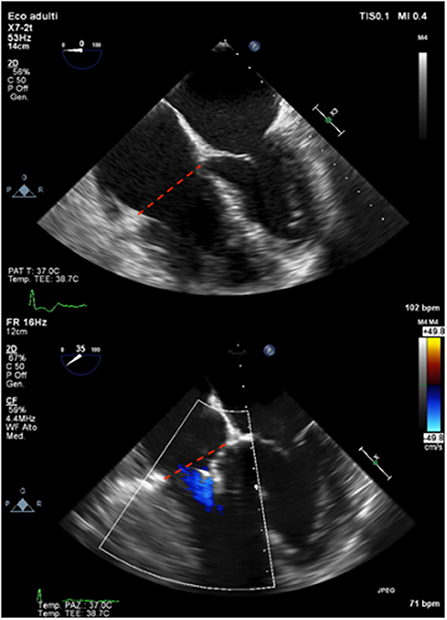
Figure 1. A case of RV reverse remodeling after percutaneous tricuspid leaflet repair. Baseline TEE (upper panel) and final result immediately after Triclip (bottom panel) showing early and significant reduction of TV annulus in end-diastole (red dashed lines).
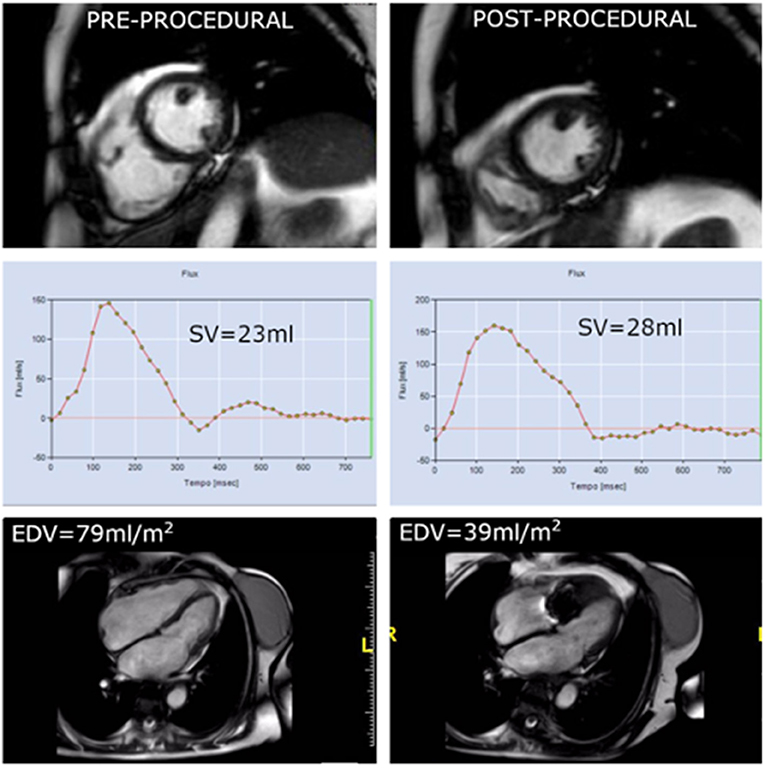
Figure 2. In the same patient showing early annulus size reduction, the comparison between pre-procedural CMR (left column) and immediately after procedure (right column) depicts a significant reduction in EDV as consequence of acute remodeling the day after the procedure. Phase contrast imaging shows a slight increase in absolute forward stroke due to reduction in regurgitant volume.
Changes in RV loading condition and forward stroke volume lead to an improvement in left ventricular (LV) hemodynamic performance through an increase in LV preload documented by an increase of EDV and EF in the context of the so called “chronic latent LV under filling” (32). In this condition, a reduction of LV preload caused by TR can lead to an underestimation of mitral regurgitation. Moderate mitral regurgitation can become severe after tricuspid percutaneous repair as a consequence of an increase in RV forward stroke volume.
Finally, volume overload determined by severe TR is a major determinant of right atrium remodeling resulting in structural, electrical, or metabolic changes often leading to chronic atrial fibrillation (33). Irrespective of cardiac rhythm and RV loading conditions, right atrium volume is a major determinant of tricuspid annulus area in patients with FTR, and RA enlargement is an important mechanism for tricuspid annulus dilatation (34). Being able to reduce tricuspid annulus dimensions in the earlier phases of right atrium remodeling may be a promising target in preventing or delaying atrial fibrillation occurrence.
In conclusion, available preliminary data indicate an initial efficacy of percutaneous tricuspid repair on RV remodeling, with possible benefits to both ventricles. However, these results need confirmation from further and longer studies able to identify the subgroups of patients who may benefit more from the procedure.
Author contributions
AA contributed to conception and design of the study. RN, AS, and FC wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kim MJ, Chung CH., Choo SJ, Song MG, Song JM, et al. Factors associated with development of late significant tricuspid regurgitation after successful left-sided valve surgery. Heart. (2009) 95:931–6. doi: 10.1136/hrt.2008.152793
2. Kwak JJ, Kim YJ., Kim MK, Kim HK, Park JS, Kim KH, et al. Development of tricuspid regurgitation late after left-sided valve surgery: a single-center experience with long-term echocardiographic examinations. Am Heart J. (2008) 155:732–7. doi: 10.1016/j.ahj.2007.11.010
3. Khanna AD, Oh JK., Nishimura RA, Enriquez-Sarano M, Jeon YB, et al. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation. (2011) 123:1929–39. doi: 10.1161/CIRCULATIONAHA.110.991018
4. Kim Y-J, Kwon D-A, Kim H-K, Park JS, Hahn S, Kim KH, et al. Determinants of surgical outcome in patients isolated tricuspid regurgitation. Circulation. (2009) 120:1672–78. doi: 10.1161/CIRCULATIONAHA.109.849448
5. Patlolla SH, Schaff HV., Greason KL, Pochettino A, Daly RC, Frye RL, et al. Early right ventricular reverse remodeling predicts survival after isolated tricuspid valve surgery. Ann Thorac Surg. (2021) 112:1402–9. doi: 10.1016/j.athoracsur.2021.02.051
6. Fender EA, Petrescu I, Ionescu F, Zack CJ, Pislaru SV, Nkomo VT, et al. Prognostic importance and predictors of survival in isolated tricuspid regurgitation: a growing problem. Mayo Clin Proc. (2019) 94:2032–9. doi: 10.1016/j.mayocp.2019.04.036
7. Mutlak D, Lessick J, Reisner SA, Aronson D, Dabbah S. Agmon Y. Echocardiography-based spectrum of severe tricuspid regurgitation: the frequency of apparently idiopathic tricuspid regurgitation. J Am Soc Echocardiography. (2007) 20:405–8. doi: 10.1016/j.echo.2006.09.013
8. Taramasso M, Vanermen H, Maisano F, Guidotti A, La Canna G, Alfieri O. The growing clinical importance of secondary tricuspid regurgitation. J Am Coll Cardiol. (2012) 59:703–10. doi: 10.1016/j.jacc.2011.09.069
9. Prihadi EA, Delgado V, Leon MB, Enriquez-Sarano M, Topilsky Y, Bax JJ. Morphologic types of tricuspid regurgitation: characteristics and prognostic implications. JACC: Cardiovascular Imaging. (2019) 12:491–9. doi: 10.1016/j.jcmg.2018.09.027
10. Dreyfus GD, Martin RP, Chan KMJ, Dulguerov F, Alexandrescu C. Functional tricuspid regurgitation: a need to revise our understanding. J Am Coll Cardiol. (2015) 65:2331–6. doi: 10.1016/j.jacc.2015.04.011
11. Topilsky Y, Khanna A, Le Tourneau T, Park S, Michelena H, Suri R, et al. Clinical context and mechanism of functional tricuspid regurgitation in patients with and without pulmonary hypertension. Circulation. (2012) 5:314–23. doi: 10.1161/CIRCIMAGING.111.967919
12. Taramasso M, Gavazzoni M, Pozzoli A, Dreyfus GD, Bolling SF, George I, et al. Tricuspid regurgitation: predicting the need for intervention, procedural success, and recurrence of disease. JACC. (2019) 12:605–21. doi: 10.1016/j.jcmg.2018.11.034
13. Pinamonti B, Dragos AM, Pyxaras SA, Merlo M, Pivetta A, Barbati G, et al. Prognostic predictors in arrhythmogenic right ventricular cardiomyopathy: results from a 10-year registry. Eur Heart J. (2011) 32:1105–13. doi: 10.1093/eurheartj/ehr040
14. Kammerlander AA, Marzluf BA, Graf A, Bachmann A, Kocher A, Bonderman D, Mascherbauer J.right ventricular dysfunction, but not tricuspid regurgitation, is associated with outcome late after left heart valve procedure. J Am Coll Cardiol. (2014) 64: 2633–42. doi: 10.1016/j.jacc.2014.09.062
15. Dietz MF, Prihadi EA., van der Bijl P, Goedemans L, Mertens BJA, Gursoy E, et al. Prognostic implications of right ventricular remodeling and function in patients with significant secondary tricuspid regurgitation. Circulation. (2019) 140:836–45. doi: 10.1161/CIRCULATIONAHA.119.039630
16. Lurz P, Stephan von Bardeleben R, Weber M, itges M, Sorajja P, Hausleiter J, et al. TRILUMINATE investigators. transcatheter edge-to-edge repair for treatment of tricuspid regurgitation. J Am Coll Cardiol. (2021) 77: 229–39. doi: 10.1016/j.jacc.2020.11.038
17. Nickenig G, Weber M, Lurz P, von Bardeleben RS, Sitges M, Sorajja P, et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet. (2019) 394: 2002–11. doi: 10.1016/S0140-6736(19)32600-5
18. Hahn RT, Eleid MF., Kipperman R, Smith R, Lim DS, et al. CLASP TR EFS Investigators. Feasibility study of the transcatheter valve repair system for severe tricuspid regurgitation. J Am Coll Cardiol. (2021) 77:345–56. doi: 10.1016/j.jacc.2020.11.047
19. Hashimoto I, Watanabe K. Geometry-Related right ventricular systolic function assessed by longitudinal and radial right ventricular contractions. Echocardiography. (2016) 33:299–306. doi: 10.1111/echo.13039
20. Lang RM, Badano LP., Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:233–70. doi: 10.1093/ehjci/jev014
21. Surkova E, Cosyns B, Gerber B, Gimelli A, La Gerche A, Ajmone Marsan N. The dysfunctional right ventricle: the importance of multi-modality imaging. Eur Heart J Cardiovasc Imaging. (2022) 23:885–97. doi: 10.1093/ehjci/jeac037
22. Park JB, Lee SP., Lee JH, Yoon YE, Park EA, Kim HK, et al. Quantification of right ventricular volume and function using single- beat three-dimensional echocardiography: a vali- dation study with cardiac magnetic resonance. J Am Soc Echocardiogr. (2016) 29:392–401. doi: 10.1016/j.echo.2016.01.010
23. Ji-Hyun K, Hyung-Kwan K, Seung-Pyo L, Kim YJ, Cho GY, Kim KH, et al. Right ventricular reverse remodeling, but not subjective clinical amelioration, predicts long-term outcome after surgery for isolated severe tricuspid regurgitation. Circ J. (2014) 78:385–92. doi: 10.1253/circj.CJ-13-0790
24. Zhong Y, Bai W, Wang H, Qian H. Rao L. Impact of concomitant tricuspid annuloplasty on right ventricular remodeling in patients with rheumatic mitral valve disease. Cardiovascular Ultrasound. (2021) 9:16. doi: 10.1186/s12947-021-00245-2
25. Fukui S, Ogo T, Morita Y, Tsuji A, Tateishi E. Ozaki K. Right ventricular reverse remodelling after balloon pulmonary angioplasty. Eur Respir J. (2014) 43:1394–402. doi: 10.1183/09031936.00012914
26. Berman M, Gopalan D, Sharples L, Screaton N, Maccan C, Sheares K, et al. Right ventricular reverse remodeling after pulmonary endarterectomy: magnetic resonance imaging and clinical and right heart catheterization assessment. Pulm Circ. (2014) 4:36–44. doi: 10.1086/674884
27. Elgharably H, Javadikasgari H, Koprivanac M, Lowry AM, Sato K, Blackstone EH, et al. Right versus left heart reverse remodelling after treating ischaemic mitral and tricuspid regurgitation. Eur J Cardiothorac Surg. (2020) 14:ezaa326. doi: 10.1093/ejcts/ezaa326
28. Rommel KP, Besler C, Noack T, Blazek S, von Roeder M, Fengler K, et al. Physiological and clinical consequences of right ventricular volume overload reduction after transcatheter treatment for tricuspid regurgitation. J Am Coll Cardiol Intv. (2019) 12:1423–34. doi: 10.1016/j.jcin.2019.02.042
29. Sticchi A, Toselli M, Garhwal K, Squeri A, Colombo A, Giannini F. Acute right ventricular reshaping after triclip in ebstein's-like anomaly assessed by multimodality imaging. J Invasive Cardiol. (2021) 33: E1005-E1007.
30. Lurz P, Giardini A, Taylor AM, Nordmeyer J, Muthurangu V, Odendaal D, et al. Effect of altering pathologic right ventricular loading con- ditions by percutaneous pulmonary valve implantation on exercise capacity. Am J Cardiol. (2010) 105:721–6. doi: 10.1016/j.amjcard.2009.10.054
31. Orban M, Braun D, Deseive S, Stolz L, Stocker TJ, Stark K, et al. Transcatheter edge-to-edge repair for tricuspid regur- gitation is associated with right ventricular reverse remodeling in patients with right-sided heart failure. J Am Coll Cardiol Img. (2019) 12:559–60. doi: 10.1016/j.jcmg.2018.10.029
32. Andersen MJ, Nishimura RA., Borlaug BA. The hemodynamic basis of exercise intolerance in tricuspid regurgitation. Circ Heart Fail. (2014) 7:911–7. doi: 10.1161/CIRCHEARTFAILURE.114.001575
33. Lang RM, Cameli M, Sade LE, Faletra FF, Fortuni F, Rossi A, et al. Imaging assessment of the right atrium: anatomy and function. Eur Heart J Cardiovasc Imaging. (2022) 23:867–84. doi: 10.1093/ehjci/jeac011
34. Muraru D, Addetia K, Guta AC, Ochoa-Jimenez RC, Genovese D, Veronesi F, et al. Right atrial volume is a major determinant of tricuspid annulus area in functional tricuspid regurgitation: a three- dimensional echocardiographic study. Eur Heart J Cardiovasc Imaging. (2021) 22:660–9. doi: 10.1093/ehjci/jeaa286
Keywords: tricuspid regurgitation, transcatheter tricuspid valve repair, right ventricular remodeling, right ventricular failure, cardiac magnetic resonance imaging
Citation: Albertini A, Nerla R, Castriota F and Squeri A (2022) Right ventricle remodeling after transcatheter tricuspid leaflet repair in patients with functional tricuspid regurgitation: Lessons from the surgical experience. Front. Cardiovasc. Med. 9:977142. doi: 10.3389/fcvm.2022.977142
Received: 24 June 2022; Accepted: 29 August 2022;
Published: 27 September 2022.
Edited by:
Guido Tavazzi, University of Pavia, ItalyReviewed by:
Francesco Ancona, Ospedale San Raffaele (IRCCS), ItalyCopyright © 2022 Albertini, Nerla, Castriota and Squeri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto Albertini, YWxiZXJ0by5hbGJlcnRpbmlAYmx1Lml0
 Alberto Albertini
Alberto Albertini Roberto Nerla
Roberto Nerla Fausto Castriota2
Fausto Castriota2 Angelo Squeri
Angelo Squeri