
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 09 September 2022
Sec. Cardiovascular Epidemiology and Prevention
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.976817
This article is part of the Research Topic Frontiers in Cardiovascular Medicine: Rising Stars 2022 View all 75 articles
 Nian Huang1†
Nian Huang1† Chengyao Tang2†
Chengyao Tang2† Shiyang Li1
Shiyang Li1 Wenzhi Ma1
Wenzhi Ma1 Xiaobing Zhai3
Xiaobing Zhai3 Keyang Liu4
Keyang Liu4 Haytham A. Sheerah5,6
Haytham A. Sheerah5,6 Jinhong Cao1*
Jinhong Cao1*Objective: The potential effects of pulmonary dysfunction on cardiovascular diseases (CVD) and all-cause mortality are receiving attention. The current study aimed to explore whether reduced lung function predicts CVD and all-cause mortality in people with diabetes.
Methods: A total of 1,723 adults with diabetes (mean age 60.2 years) were included in the National Health and Nutrition Examination Survey (NHANES III). Death outcomes were ascertained by linkage to the database records through 31 December 2015. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for coronary heart disease (CHD), CVD, and all-cause mortalities. We conducted stratified analyses based on age, body mass index (BMI), history of hypertension, and dyslipidemia.
Results: During a mean follow-up of 14.62 years (25,184 person-year), a total of 1,221 deaths were documented, of which 327 were CHD, 406 were CVD, and 197 were cancer. After multi-factor adjustment, participants with lower FEV1 and FVC had a higher risk of CHD, CVD, and all-cause mortality. This association was also found in lower FVC and a higher risk of cancer mortality [HR: 3.85 (1.31–11.32); P for trend = 0.040], but the association of FEV1 was attenuated after adjustment for covariates [HR:2.23 (0.54–9.17); P for trend = 0.247]. In subgroup analysis, we found that the adverse associations of FEV1 and FVC with CVD mortality were observed in subgroups of age, BMI, and history of hypertension and dyslipidemia.
Conclusion: Declined lung function was associated with a higher risk of CVD and all-cause mortality in people with diabetes. Lung function tests, especially FEV1 and FVC, should be encouraged to provide prognostic and predictive information for the management of CVD and all-cause mortality in patients with diabetes.
Several studies have extensively recognized that lung function was associated with the risk of all-cause and cardiovascular risk (1–6). In the general population, lung function, as indicated by a low forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC), demonstrated an inverse association with coronary heart disease (CHD), stroke, and other cardiovascular diseases mortality (CVD) (7, 8). Several mechanisms have been established for the association between poor lung function and the increased risk of CVD. Previous studies showed that lung function was associated with mortality among smokers and non-smokers (4, 9). Inflammation, which leads to the degradation of lung function, might be the likely mechanism. Another potential mechanism that vascular injury and atherosclerosis owing to airflow limitation and mediating effects of chronic diseases on the association between lung function and mortality (10).
The incidence rate of type 2 diabetes mellitus has increased and became a global public health issue. Adults with diabetes are expected to surpass 700 million by 2025 (11). Diabetes is one of the most critical risk factors for CVD (12) and elevates morbidity and mortality of CVD mainly attributsed to vascular inflammation and endothelial dysfunction (13). Common complications of diabetes include microvascular and macrovascular conditions, such as retinopathy, nephropathy, neuropathy, cardiovascular, and peripheral vascular diseases (14). There is an increasing evidence that the lung is one of the target organs of diabetic damage (15). A meta-analysis reported that diabetes was associated with a decreased predicted percentage of forced expiratory volume in 1 s (FEV1%) and percentage of forced vital capacity (FVC%) (16). Reduced lung function is also associated with the risk of diabetes (8) and is often considered one of the complications of diabetes. Throughout the mechanisms of the association between lung function and CVD, systemic inflammation seems a common one, which contributes to the association of lung dysfunction with both CVD and diabetes. Besides, highly related characteristics between diabetes and CVD may also contaminate or exert effect modification on the association of lung dysfunction with CVD (8, 17). However, as an essential part of secondary prevention from cardiovascular mortality, the association between lung function and cardiovascular mortality among patients with diabetes has been neglected. The current study aimed to investigate whether reduced lung function is associated with the risk of all-cause and cardiovascular mortality among patients with diabetes by using participants in the Health Examination Survey (NHANES 1988–1994), which is a nationwide prospective cohort study.
The participants were from the National Health and Nutrition Examination Survey (NHANES 1988–1994). The NHANES used a multi-stage, stratified, closeted, probability sampling design to identify a nationally representative sample of non-institutionalized civilians in the United States. The participants completed a household interview, laboratory measurements, and physical examinations. A detailed cohort profile was published previously (18). Data on the baseline lifestyle and participants' characteristics, including demographic data, medical history of related diseases, alcohol and smoking status, and other items, were compiled via a self-administered questionnaire. From 1988 to 1994, a total of 33,994 participants were enrolled in this study. In the current study, 18,390 participants were excluded owing to insufficient or missing spirometry data or being underage (age < 20 years), 13,869 were excluded without a history of diabetes, and 12 were excluded without mortality data A total of 1,723 individuals were included (760 men and 963 women) (Figure 1). The criteria of diabetic medical history are as follows: fasting plasma glucose >7.8 mmol/L; glycohemoglobin ≥ 6.5%; taking insulin or diabetic pills; and being told to have diabetes by a doctor. Hypertension was defined as being told by a doctor to have a high blood pressure. The definition of dyslipidemia was high-density lipoprotein cholesterol (HDL-C) < 40 mg/dl, as well as total cholesterol, low-density lipoprotein cholesterol (LDL-C), and TG levels of ≥200, ≥130, and ≥130 mg/dl, respectively.
NHANES is a publicly released dataset, so informed consent is not required.
Lung function measurement was performed by following the standards (19) of the American Thoracic Society. Lung function parameters in the present study included FEV1, FVC, and FEV1/FVC. The FEV1 and FVC measurements were acquired from the spirometry data as part of the NHANES. Detailed information on the spirometry equipment, examination protocol, calibration procedures, and quality control for the NHANES was available and reported previously (20). FEV1 and FVC measurements were performed by trained technicians using a dry-rolling seal spirometry and involved the performance of at least five FVC maneuvers. Until it is accepted, it has to ensure a maneuver that is free of hesitation, leak, cough, mouthpiece obstruction, additional effort, and early termination. Only valid and reproducible spirometry measurements were chosen according to the reference values (21). The designated FEV1 and FVC values for each subject were obtained from the largest values of FEV1 and FVC, respectively, from the spirometry performed by each participant. We also calculated FEV1 and FVC as the percentage of predicted values for each participant according to Hankinson's predicted value equation (21).
From its baseline (1988–1994) to the end of follow-up on 31 December 2015, participants' vital status and cause-of-death information were confirmed by the National Center for Health Statistics. Vital status was determined by the probabilistic matching of participants to the National Death Index based on identifying information, including social security number, name, sex, and date of birth (22). The identical matching methodology applied to the NHANES I Epidemiological Follow-up Study found that 96.1% of deceased participants and 99.4% of living participants were correctly classified (23). Details of the linkage methods have been reported previously (22). The International Classification of Disease, 10th revision (ICD10) codes, were applied to determine the underlying causes of death (24). In the present study, our primary outcome was the total CVD mortality (ICD I00-I09, I11, I13, I20-I51, I60-I69). Other cause-specific outcomes included mortalities from CHD (ICD I00-I09, I11, I13, I20-I51), cancer (C00-C97) and all-cause death. This death certificate ascertainment was applied to all deaths within our cohort, except for deaths with insufficient information on these matching criteria, which were considered as a lost follow-up.
FEV1 and FVC were categorized by quartile. The significance of differences in means or proportions of participants' characteristics and risk factors of CVD and diabetes or covariates related to lung function was tested by covariance or χ2 test. Person-years of follow-up were calculated from the baseline (1988-1994) to their first endpoint in this follow-up as follows: death, moving out, or the end of follow-up, whichever came first. Adult participants in NHANES III were followed for mortality up to 31 December 2015. The Cox proportional hazard model was used to calculate crude and multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (95% CIs) for estimating the risk of mortality from CVD or all-cause mortality during the follow-up period across quartiles of FEV1 and FVC, respectively. Multiplicative interactions of FEV1 and FVC with sex were tested in deciding whether to present the data sex-specifically or to combine the results of men and women. We hypothesized known risk factors of CVD and covariates related to lung function as confounders, including sex, age, race, education level, BMI, drinking status, smoking status, high-density lipoprotein-cholesterol (HDL-c) level, serum C-reactive protein, serum albumin, the ratio of FEV1 and FVC, percentage of predicted values of FEV1 and FVC, history of hypertension and dyslipidemia, history of whistling and/or wheezing, persist phlegm status, asthma status, history of chronic bronchitis, and cold or flu. To avoid multicollinearity caused by multiple covariates, we conducted a multivariable Cox regression, including all covariates, and calculated the variance inflation factors (VIFs) as a diagnostic tool of multicollinearity. We assigned the median values to each quartile of FEV1 and FVC and examined their significance to calculate the trends across quartiles of FEV1 and FVC. Besides, we investigated the trends of FEV1 and FVC with increasing age and height by calculating the mean value and 95% CIs of FEV1 and FVC in different age or height groups.
Additionally, we conducted a stratified analysis according to BMI, age, history of hypertension, and dyslipidemia to examine the potential effect modification. In sensitivity analyses, we excluded those who died within 2 years to avoid potential as-yet-undiagnosed diseases at baseline, and who had a medical history of respiratory diseases (asthma, chronic bronchitis, and emphysema). All probability values for the statistical test were two-tailed, and P < 0.05 was considered statistically significant. Statistical analyses of the present study were conducted on the SAS statistical package (Version 9.4; SAS Inc., Cary, NC). NHANES recommends using sample weights to calculate estimates that represent the U.S. civilian non-institutionalized population or any subpopulation of interest. “PROC SURVEYREG” were used in computing descriptive and regression analyses as these protocols account for both the weighted data and the complexity of sample design.
Since no interaction with sex was observed in the association of FEV1 and FVC with CVD and specific endpoints, the presented results of men and women were combined in the main analyses. During the follow-up of 25,184 person-year of 1,723 included participants, 1,221 deaths were documented; 406 deaths due to CVD (327 of which were due to coronary heart disease) and 197 due to cancer.
In the multilinearity diagnosis, the highest VIF (1.56) occurred in the medical history of asthma, and no strong multilinearity was observed in the covariates (data not shown). In Table 1, participants in the lowest quartiles of FEV1 and FVC were older age, less likely to be current drinkers or smokers, had lower education levels, lower percentage of predicted values of FEV1 and FVC, lower serum albumin levels, higher HDL-c, and serum C-reactive protein level. They were also less likely to have a medical history of whistling or wheezing, and more likely to have a history of asthma, chronic bronchitis, emphysema, dyslipidemia, and hypertension.
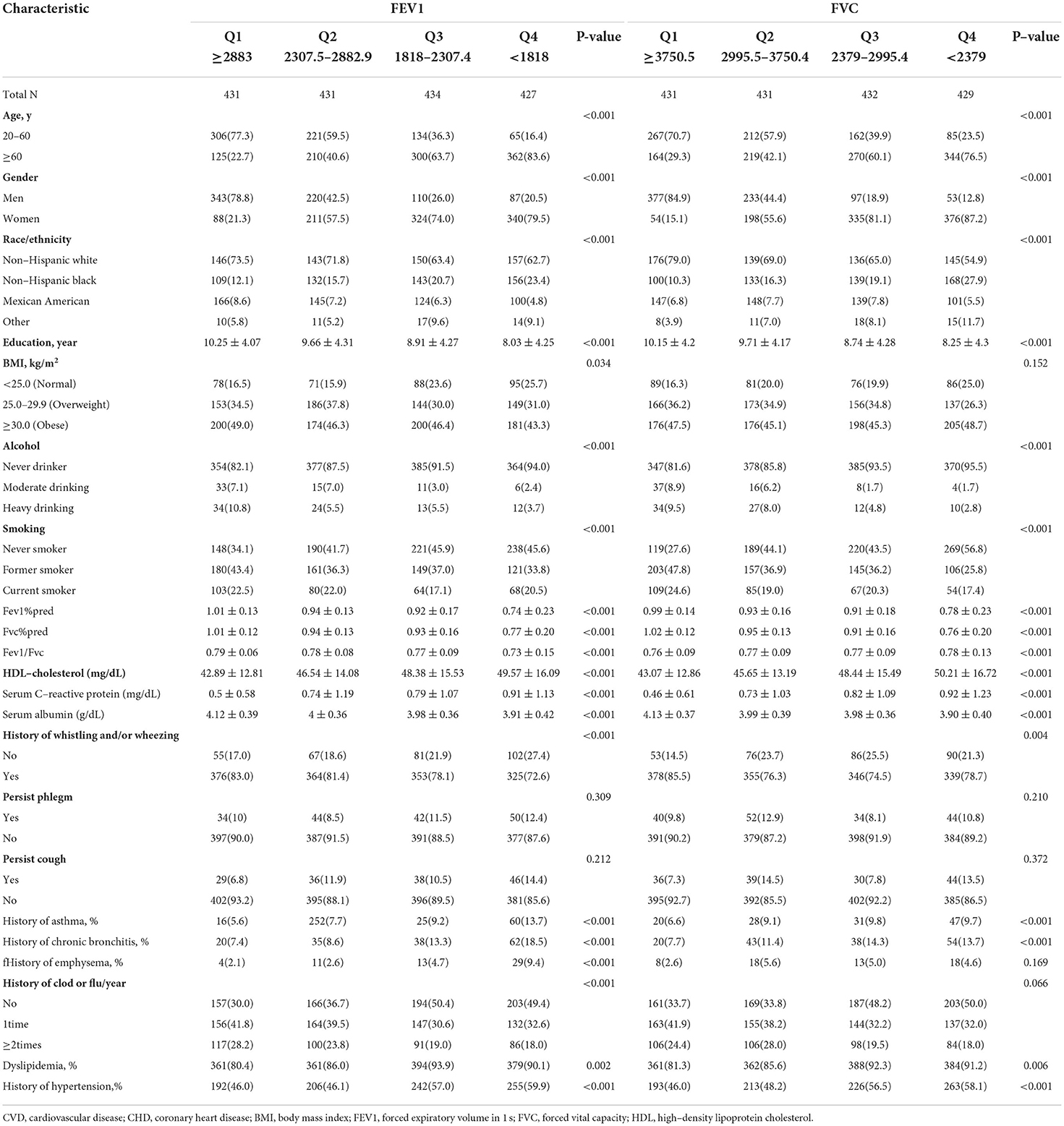
Table 1. Baseline demographic characteristics of the study population, according to quartiles of FEV1, FVC.
In Table 2, both lower FEV1 and FVC were associated with a higher risk of CHD and CVD death. Compared with the highest group, HRs (95%CIs) of the lowest FEV1 were 2.71 (1.15–6.38; P for trend = 0.009) for CHD and 2.89 (1.19–3.87; P for trend = 0.001) for CVD mortality. HRs (95%CIs) of the lowest FVC were 5.30 (2.68-10.47; P for trend <0.001) for CHD and 6.32 (3.19–12.53; P for trend <0.001) for CVD. Although the lowest FVC was associated with a higher risk of cancer mortality (HR: 3.85 (1.31-11.32); P for trend = 0.040), the association of FEV1 was not related after adjustment for covariates (HR:2.23 (0.54–9.17); P for trend = 0.247). In addition, the lowest FEV1 and FVC were observed to be associated with all-cause deaths; HRs (95%CIs) of FEV1 and FVC were 2.96 (1.92–4.56; P for trend <0.001) and 4.15 (2.58–6.65; P for trend <0.001), respectively.
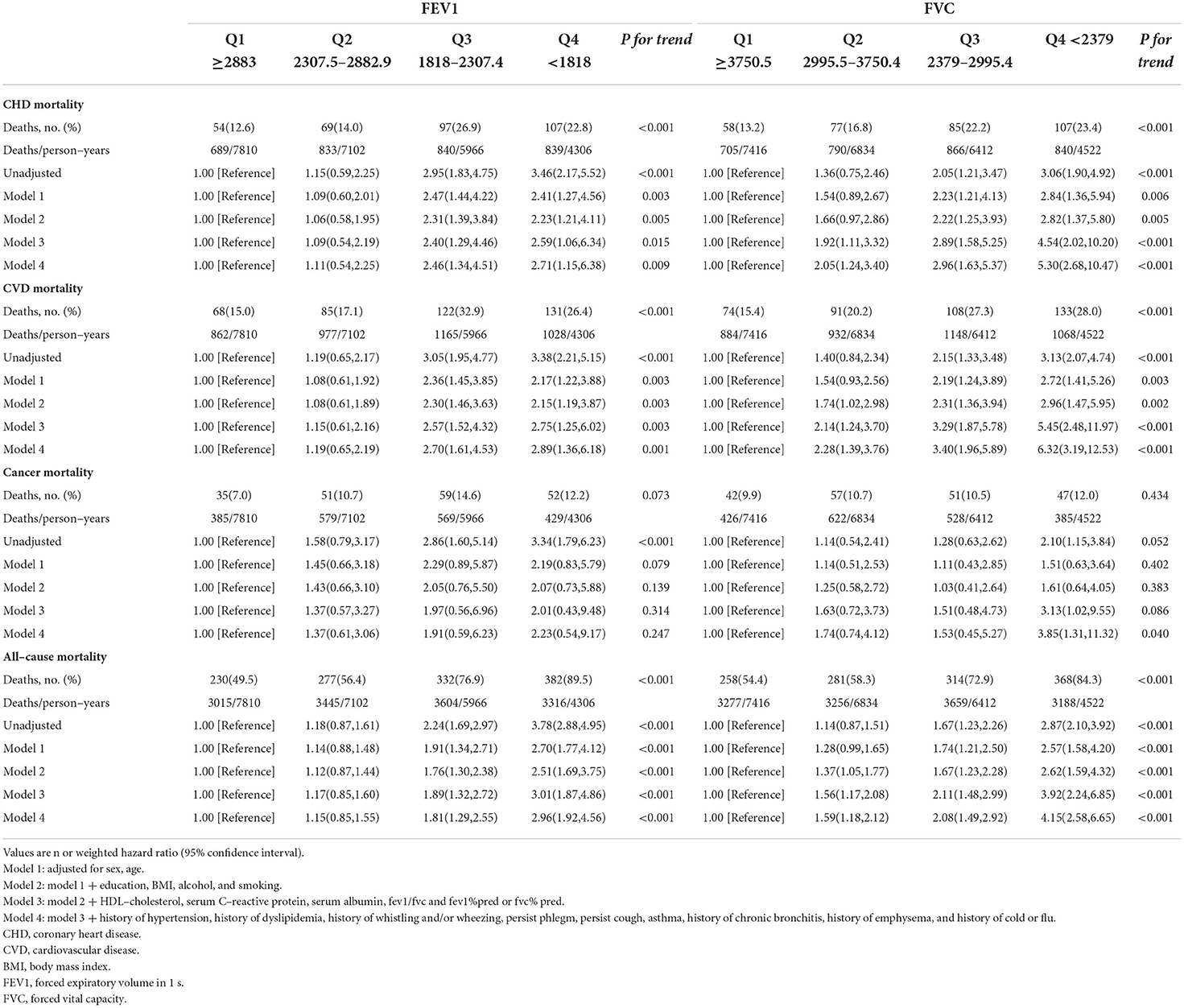
Table 2. Associations of FEV1 and FVC with coronary heart disease, cardiovascular, cancer and all–cause mortality in U.S. adults aged at least 20 years.
As shown in Tables 3.1–3.4, we conducted stratified analyses based on BMI, age, history of hypertension, and dyslipidemia. Among participants with BMI of ≥25, lower FEV1 was associated with a higher risk of CHD, CVD, and all-cause mortality; HRs (95% CIs) were 2.91(1.19–7.10), 3.19 (1.40–7.24), and 3.86 (2.12–7.03), respectively, but only associated with CHD and CVD among the participants whose BMI was <25. For FVC, the associations with CHD, CVD, and all-cause death were observed in both subgroups of BMI. The association of FEV1 was observed with all-cause mortality in both subgroups stratified by age. On the other hand, the association of FEV1 with CVD was only observed in the subgroup aged ≥60 (HR:3.61; 95%CI: 1.23–10.58). For FVC, the associations were significant in both subgroups with CHD, CVD, and all-cause mortality.
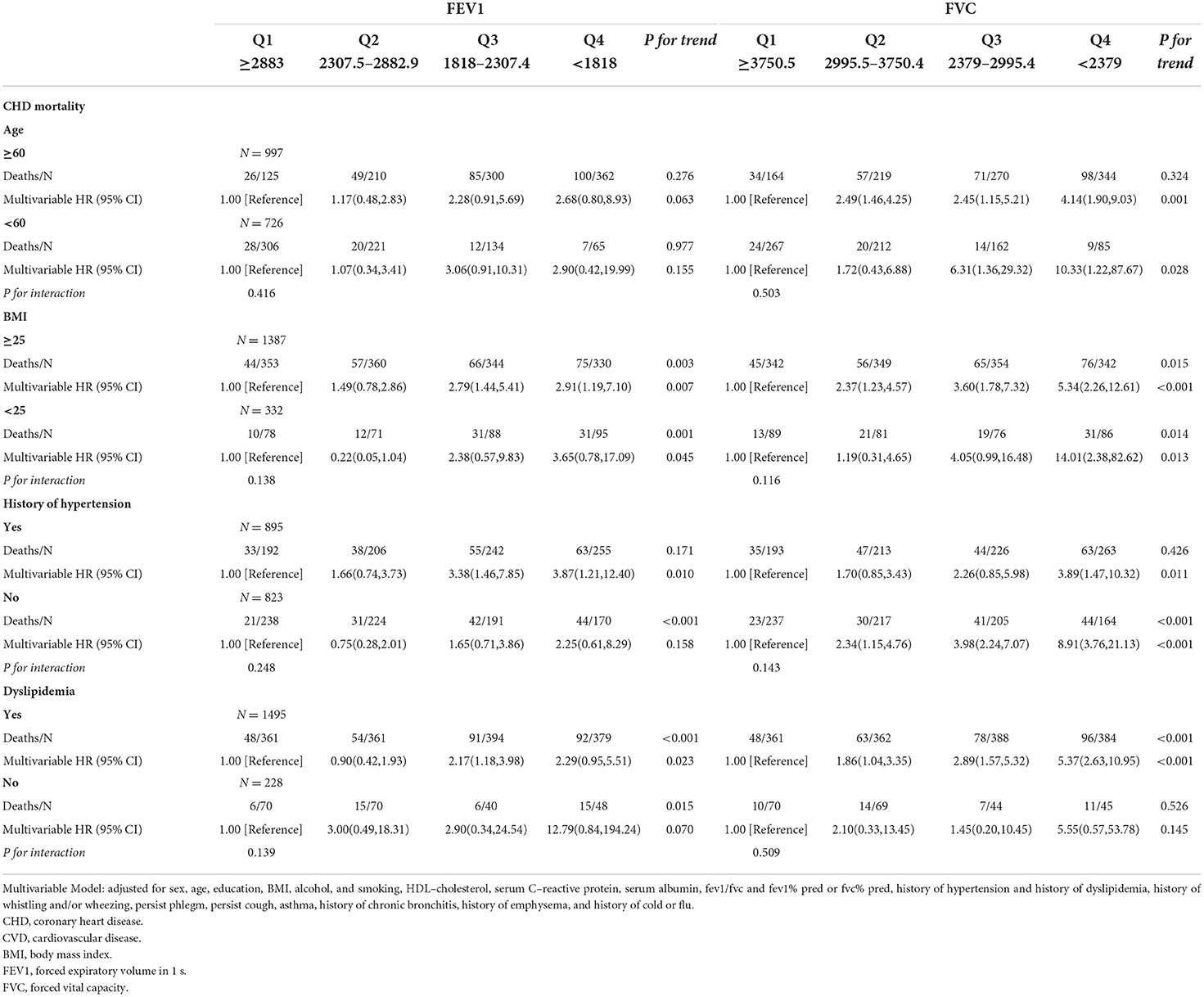
Table 3.1. Associations of FEV1 and FVC with coronary heart disease mortality in U.S. adults aged at least 20 years among different groups.
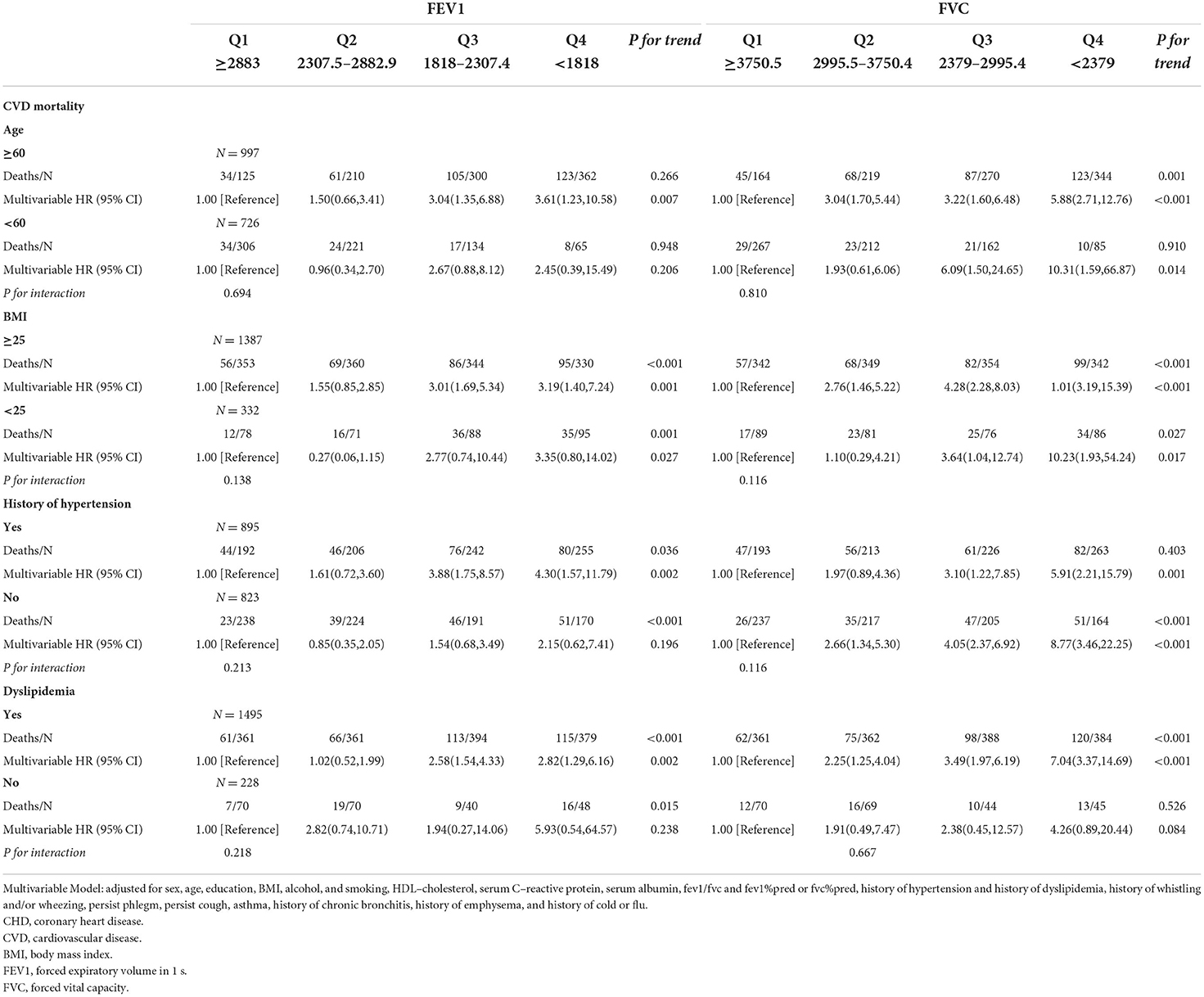
Table 3.2. Associations of FEV1 and FVC with cardiovascular mortality in U.S. adults aged at least 20 years among different groups.
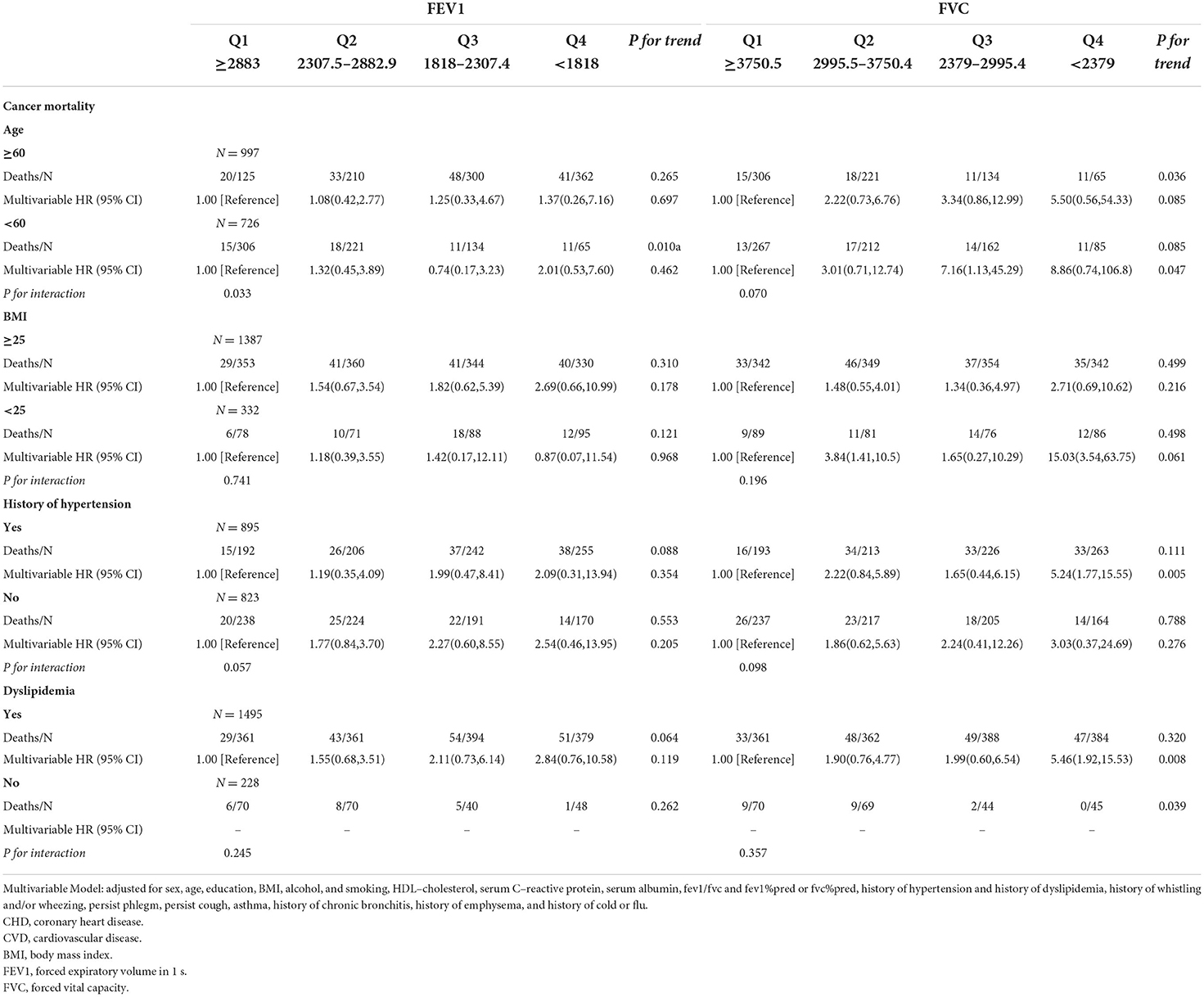
Table 3.3. Associations of FEV1 and FVC with cancer mortality in U.S. adults aged at least 20 years among different groups.
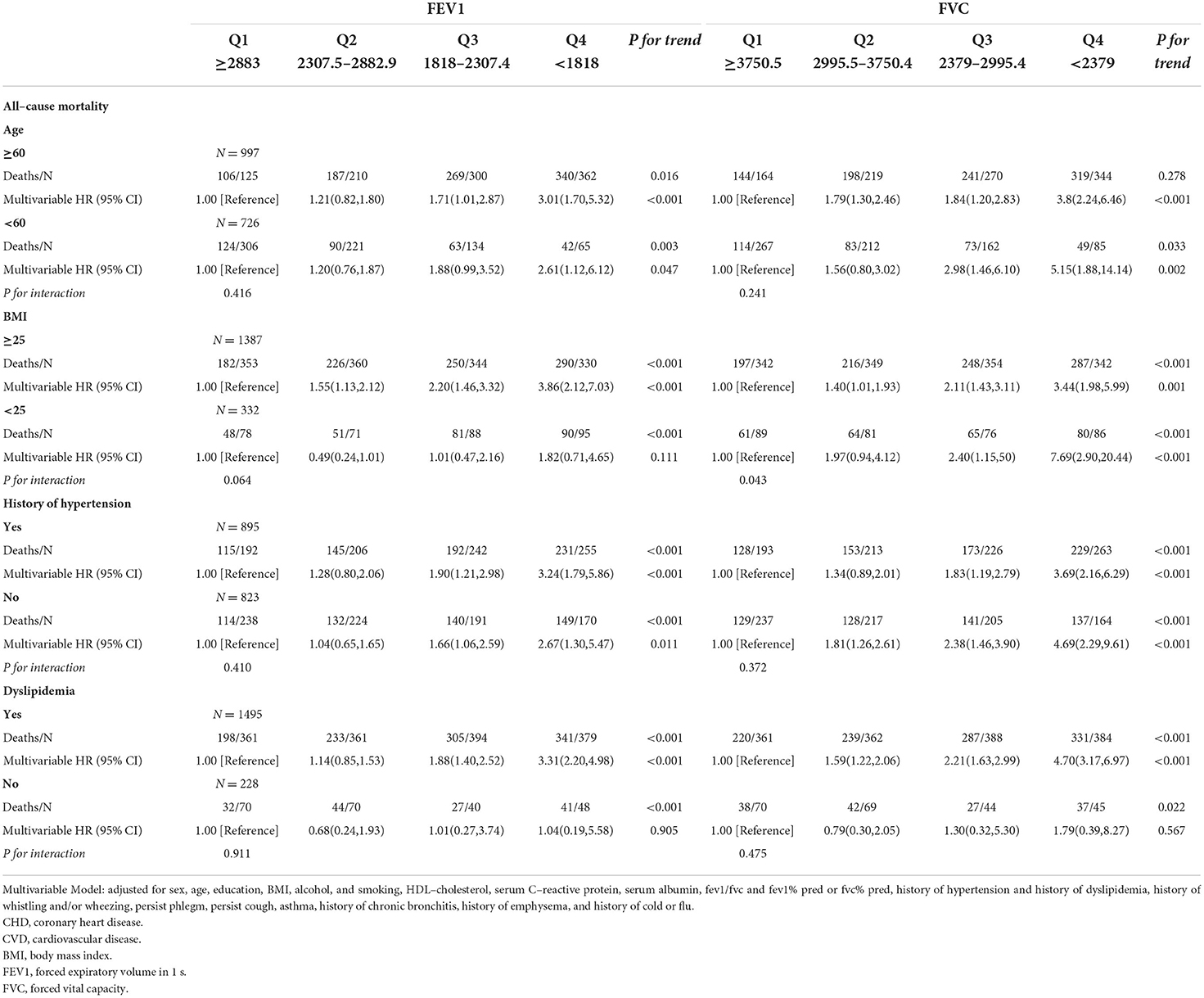
Table 3.4. Associations of FEV1 and FVC with all–cause mortality in U.S. adults aged at least 20 years among different groups.
In the subgroup analysis of hypertension, lower FEV1 was associated with a higher risk of all-cause mortality in with and without hypertension subgroups. The associations of CHD and CVD were only observed in the hypertension subgroup; HRs were 3.87 (95%CI: 1.21–12.40) and 4.30 (95%CI: 1.57–11.79), respectively. The associations of FVC were significant in subgroups with and without hypertension. Stratified by the history of dyslipidemia, the association of FEV1 and CHD became significant in both subgroups. The associations of FEV1 and FVC with CHD, CVD, and all-cause mortality were observed in the dyslipidemia subgroup (all P for trend <0.050), but not associated in the without dyslipidemia subgroup. A total of 936 participants died within 2 years of follow-up and 951 participants had a medical history of diseases related to lung function and excluding them resulted in no substantial changes in the associations of FEV1 and FVC with CVD and all-cause mortality (Supplementary Tables 1.1,1.2). FEV1 and FVC increased with height, but the opposite trend appeared with age (Figures 2A,B).
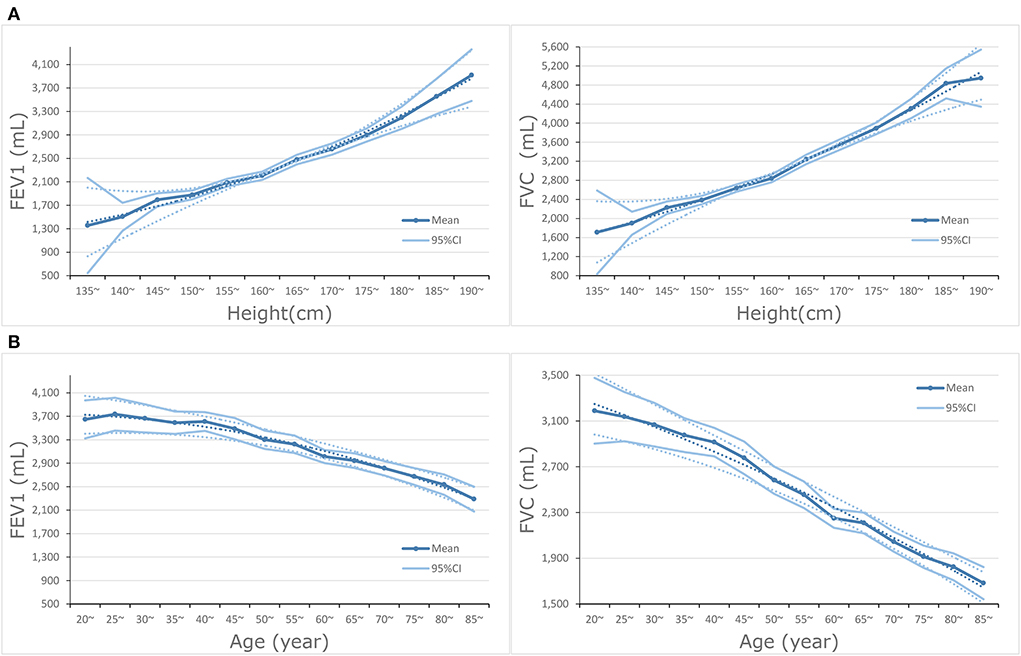
Figure 2. (A) Dose-response association between height and FEV1 and FVC. (B) Dose-response association between age and FEV1 and FVC.
In this prospective cohort study, lower FEV1 and FVC were associated with a higher risk of CHD, CVD, and all-cause mortality among USA adults with diabetes. After adjustment for cardiovascular risk factors and other covariates related to lung function, the HRs were not attenuated. In addition, we also observed the association between lower FVC and cancer mortality, whereas lower FEV1 was not associated with cancer mortality after adjusting the confounders.
In previous studies, decreased lung function was associated with a higher cardiovascular risk among the general population (2, 5, 7, 25, 26). A cohort study on the general Chinese people found that each 5% decrease in FEV1/FVC was associated with a 0.47% increase in 10-year CVD risk (P < 0.001) (25). Similarly, in a population-based prospective cohort study with a follow-up of over 18 years, there is a reported decrease of 28%-35% mortality risk from CVD for every 70 L/s increase in FEV1 (26). In a cohort study of 14,503 adults from the Moli-sani study, the HRs of FEV1% pred and FVC% pred in the lowest quartile for CVD mortality were 1.59 (95%CI: 1.02–2.47) and 1.97 (95%CI: 1.97–3.08), respectively (2). In the same population (NHANS III) of the present study, the reduced lung function was associated with CVD mortality (HR of FEV1% pred: 1.7, 95%CI: 1.4–2.1; HR of FVC% pred: 2.1, 95%CI: 1.7–2.6) among the general population (5). The present study reinforced the association of lung function with CVD mortality. Moreover, previous studies reported an inverse association of reduced lung function with CHD (4, 5, 8, 26, 27). A prospective study of 4,434 men with no history of CVD (CHD or stroke) and diabetes demonstrated that lower FEV1 levels were associated with a higher risk of fatal CHD (Relative risk: 1.63; 95%CI: 1.03–2.67), but not associated with higher risks of major CHD and non-fatal MI (8). In a prospective study with a 29-year follow-up of the Buffalo Health Study cohort, the authors found that lung function was inversely associated with the risk of CHD (27). The HRs of FEV1% pred in the 1st quintile for CHD mortality were 2.11 (95%CI: 1.20–3.71) among men and 1.96 (95%CI:0.99–3.88) among women. In a cohort study of 15,411 adults, a lower relative FEV1 was shown to be associated with a higher risk of CHD (HRs: 1.56 (1.26–1.92) for men and 1.88 (1.44–2.47) for women) (4). The previous study investigating the general population of the same cohort as the present study reported a similar risk estimate, an approximately 2-fold increased risk of CHD mortality, compared with other previous studies (5).
Our findings indicated a potentially stronger association of lung function with CVD or CHD mortality in the population with diabetes than that in the general population. They were in line with some previous studies. In a cohort study of 1,743 adults, compared with clinically normal participants (as reference), the adjusted odds ratio of participants with diabetes depicted a stronger association between FEV1 (HR: 1.67; P < 0.01) and FVC (HR:1.51; P = 0.02) with overall mortality, which was nearly a 1.5-fold risk with comparison to the general population (1). In addition, logistic regression analysis elaborated that the adjusted risk of CVD mortality was reinforced for all participants with any lung function impairment, current or former smoker, and patients with type 2 diabetes (1), which was in accordance with our findings. Hedblad et al. examined the association of FVC with insulin resistance and CVD incidence and found that subjects who had developed insulin resistance had the highest risk of CVD events (28). Taking the high FVC group without insulin resistance as a reference, the adjusted relative risk of low FVC among subjects with insulin resistance was 1.7 (95%CI: 1.02–2.70).
Additionally, after excluding those who had developed diabetes at the follow-up examination, the result did not change substantially. However, in some studies, whether the association is more robust in a population with diabetes is inconclusive. For instance, Wannamethe et al. found that exclusion of men with diabetes resulted in little difference in the association of lung function with fatal or non-fatal CHD events (8).
Although lung function has been extensively recognized and considered as an effective predictor of CVD, the mechanism underlying the association still requires evidence. Smoking has been considered responsible for the association ever (29). However, the association was additionally observed in non-smokers (4, 9, 30). Furthermore, in a study examining the extent to which risk factors explain the association, smoking history was only reduced by 4.9%, suggesting that smoking history was not the predominant explanation for this association (10). Some other potential mechanisms have been nominated. For example, few studies proposed that poor lung function may result from long-term exposure to air pollution or diesel exhaust fumes, finally causing diseases or death (27). Recently, inflammation has seized considerable attention regarding the association between lung function and CVD mortality. Sabia et al. highlighted the prominence of inflammatory markers, which account for the association of lung function with mortality more than any other risk factors (10). C-reactive protein (CRP) has been consistently regarded as a reliable indicator of underlying low-grade systemic inflammation and as a critical biomarker for the onset and mortality of CVD (31–34). There is an inverse association between FVC and CRP in a cross-sectional study (35). Association has also been identified between FVC and plasma levels of inflammation-sensitive plasma proteins, another inflammatory marker (3). Engström et al. also suggested that this association contributes to the risk of CVD mortality (relative risk: 3.7; 95%CI: 2.2–6.3) in men with low FVC levels. Notably, previous studies reported that the subjects with elevated CRP had an approximately 2-fold increased risk of cardiac injury (5, 6).
In the study including all subjects of NHANES III, high CRP significantly increased HR of CVD mortality among subjects with the lowest FVC% pred or FEV1% pred (5). Our findings are consistent with their results (data not shown), implying that systemic inflammation greatly affects the connection between declined lung function and CVD mortality. Besides, low-grade systemic inflammation was associated with diabetes (13, 36), and previous studies indicated that CRP was an independent predictor of cardiovascular risk in the population with diabetes (37–40). Since the association of lung function with CVD mortality lies in systemic inflammation, especially CRP, and CRP could convey independent prediction information of cardiovascular risk in subjects with diabetes, a stronger association of lung function with CVD mortality in subjects with diabetes may be established. In a previous study, poor lung function was associated with the increased risk of fatal events and case fatality of CHD (HR: 1.63; 95% CI: 1.03–2.67), and inflammatory pathway adjustment further attenuated FEV1 with both diabetes and the association of both diabetes and fatal CHD, suggesting that reduced lung function may be a potential factor linking diabetes to increased risk of CHD and increased susceptibility to a fatal episode in the event of a cardiac event (8). However, few studies suggested that the magnitude of association between CRP and CVD mortality is comparable in people with and without diabetes (39, 41). Therefore, further studies will need to provide the magnitude of this association in people with and without diabetes.
Notably, in the present study, the association of FEV1 with CVD and CHD mortality was only observed in the subgroup, in which subjects with over 25 BMI were included. BMI is a known and well-established risk factor for both CVD, CHD, and diabetes. A previous study reported a similar result (42). Each 1 kg/m2 increase in BMI resulted in a 5% increase in the risk of CVD mortality for a reduction in relative FEV1 of 10% (42). Nevertheless, few studies suggested no substantial difference in HRs in the subgroups stratified by BMI (5, 7, 26). The inconclusive results between previous and present studies may be attributed to an averagely greater BMI in people with diabetes than that in the general population. Similar findings were observed in the subgroups stratified by medical history of dyslipidemia. The associations of both FVC and FEV1 with CVD mortality were only identified in the subjects with dyslipidemia. Dyslipidemia, as an important cardiovascular risk factor, was related to decreased FVC% pred and FEV1% pred (43). The diagnosis criterion is usually the reduced high-density lipoprotein (HDL), which is <40 mg/dl. A previous study indicated the association of low HDL with reduced lung function, owing to its roles in reverse cholesterol transport and anti-inflammation (44). Therefore, dyslipidemia may affect the association of lung function with CVD mortality. Further studies, however, are required to identify the effect modification of dyslipidemia. In addition, we only observed a significant association of FEV1 with CVD and CHD in the stratum with a history of hypertension. The result is consistent with the previous study. Taking clinically normal participants as a reference, the ORs of FEV1 and FVC with risk of CVD in participants with hypertension were 2.15 (1.63–2.83) and 2.19 (1.66–2.88), respectively (1). For CHD, it was shown that hypertension was associated with insulin resistance and glucose intolerance, with evidence of endothelial dysfunction, which is mainly responsible for the increased risk of CHD mortality (45). In our stratified analysis, the results of FEV1 and FVC are not consistent. For instance, there was a significant association of FEV1 with CHD mortality in the stratum with over 25 BMI but not in another subgroup, while we observed significant associations of FVC in both subgroups. This may be attributed to the different prediction effects of these two spirometric parameters. Previous studies also reported that FEV 1 was a stronger predictor for CVD mortality in a population with chronic obstructive pulmonary disease than FVC (46) or FVC was superior to FEV1 in the general population (47, 48). However, the evidence was not conclusive.
To our knowledge, the present study is the first to investigate the association of lung function with CVD mortality in people with diabetes and hypothesize a stronger association in people with diabetes than in the general population. FEV1 and FVC are spirometric parameters extensively used in lung function tests, which can be a non-invasive approach to provide additional prognostic and predictive information on CVD and the risk of further cardiovascular events. Our findings imply that FEV1 and FVC can be utilized in spirometric tests and for the prevention and management of CVD, especially for those with diabetes or metabolic syndrome. Meanwhile, exploring emerging data for the association in different population can provide better health outcomes for patients and sufficient evidence for the management of the chronic disease.
The present study has some limitations. First, the spirometric measurement in the present study may be inadequate, owing to only one testing at the baseline. Extreme or inaccurate values by the single lung function test may affect the accuracy of results. Second, the period from the baseline to the follow-up outcome was excessively long. We recognize that lung function was not unchangeable, and the influence of lung function changes on the risk of CVD mortality was not addressed. Third, CVD mortality may be overestimated, especially for the elderly (49), because the NHANES III Linked Mortality File overly attributed the cause of death to CVD mortality. Lastly, we cannot determine all confounding effects although we have already adjusted for some known cardiovascular risk factors and lung function-related covariates.
Declined lung function was associated with a higher risk of CVD and all-cause mortality in people with diabetes. Lung function tests, especially FEV1 and FVC, should be encouraged to provide prognostic and predictive information for the management of CVD and all-cause mortality in patients with diabetes.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
JC supervised the study. JC, NH, and CT designed the study. NH, SL, WM, XZ, KL, and HS collected and organized the data. NH analyzed the data. JC, NH, CT, and SL interpreted the results. CT wrote the first draft. All authors read and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (grant number 82173626), the Fundamental Research Funds for the Central Universities (grant number 2020YJ066), the Fundamental Research Funds for the Health Commission of Hubei Province (grant number SWSZFY2021), and the Fundamental Research Funds for the Health Commission of Wuhan (grant number WHWSZFY2021).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.976817/full#supplementary-material
1. Collaro AJ, Chang AB, Marchant JM, Chatfield MD, Dent A, Blake T, et al. Associations between lung function and future cardiovascular morbidity and overall mortality in a predominantly First Nations population: a cohort study. Lancet Reg Health West Pac. (2021) 13:100188. doi: 10.1016/j.lanwpc.2021.100188
2. Costanzo S, Magnacca S, Bonaccio M, Di Castelnuovo A, Piraino A, Cerletti C, et al. Reduced pulmonary function, low-grade inflammation and increased risk of total and cardiovascular mortality in a general adult population: prospective results from the Moli-sani study. Respir Med. (2021) 184:106441. doi: 10.1016/j.rmed.2021.106441
3. Engström G, Lind P, Hedblad B, Wollmer P, Stavenow L, Janzon L, et al. Lung function and cardiovascular risk: relationship with inflammation-sensitive plasma proteins. Circulation. (2002) 106:2555–60. doi: 10.1161/01.CIR.0000037220.00065.0D
4. Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. (1996) 313:711–5. doi: 10.1136/bmj.313.7059.711
5. Min KB, Min JY. Reduced lung function, C-reactive protein, and increased risk of cardiovascular mortality. Circulation J. (2014) 78:2309–U423. doi: 10.1253/circj.CJ-14-0308
6. Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? the potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. (2003) 107:1514–9. doi: 10.1161/01.CIR.0000056767.69054.B3
7. Lee HM, Liu MA, Barrett-Connor E, Wong ND. Association of lung function with coronary heart disease and cardiovascular disease outcomes in elderly: the Rancho Bernardo study. Respir Med. (2014) 108:1779–85. doi: 10.1016/j.rmed.2014.09.016
8. Wannamethee SG, Shaper AG, Rumley A, Sattar N, Whincup PH, Thomas MC, et al. Lung function and risk of type 2 diabetes and fatal and nonfatal major coronary heart disease events: possible associations with inflammation. Diabetes Care. (2010) 33:1990–6. doi: 10.2337/dc10-0324
9. Chinn S, Gislason T, Aspelund T, Gudnason V. Optimum expression of adult lung function based on all-cause mortality: results from the Reykjavik study. Respir Med. (2007) 101:601–9. doi: 10.1016/j.rmed.2006.06.009
10. Sabia S, Shipley M, Elbaz A, Marmot M, Kivimaki M, Kauffmann F, et al. Why does lung function predict mortality? results from the Whitehall II. Cohort Study. (2010) 172:1415–23. doi: 10.1093/aje/kwq294
11. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. (2016) 387:1513–30. doi: 10.1016/S0140-6736(16)00618-8
12. Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U S adults. Diabetes Care. (2012) 35:1835–44. doi: 10.2337/dc12-0002
13. Akinboboye O, Williams JS, Garacci E, Egede LE. The relationship between C-Reactive protein and mortality in adults with diabetes: Influences of demographic characteristics, lifestyle behaviors, and medications. Nutr Metab Cardiovasc Dis. (2022) 32:176–85. doi: 10.1016/j.numecd.2021.09.022
14. Nathan DM. Diabetes: advances in diagnosis and treatment. JAMA. (2015) 314:1052–62. doi: 10.1001/jama.2015.9536
15. Lecube A, Simó R, Pallayova M, Punjabi NM, López-Cano C, Turino C, et al. Pulmonary function and sleep breathing: two new targets for type 2 diabetes care. Endocr Rev. (2017) 38:550–73. doi: 10.1210/er.2017-00173
16. van den Borst B, Gosker HR, Zeegers MP, Schols AM. Pulmonary function in diabetes: a metaanalysis. Chest. (2010) 138:393–406. doi: 10.1378/chest.09-2622
17. Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. (2008) 32:962–9. doi: 10.1183/09031936.00012408
18. Plan Plan and operation of the Third National Health and Nutrition Examination Survey 1988-94. Series 1: programs and collection procedures. Vital Health Stat 1. (1994) 32:1–407.
19. Standardization Standardization of Spirometry, 1994 Update. American thoracic society. Am J Respir Crit Care Med. (1995) 152:1107–36. doi: 10.1164/ajrccm.152.3.7663792
20. Manual SP. Third National Health and Nutrition Examination Survey III. (1988). Available online at: https://wwwn.cdc.gov/nchs/data/nhanes3/manuals/spiro.pdf (accessed April 4,2022).
21. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U S population. Am J Respir Crit Care Med. (1999) 159:179–87. doi: 10.1164/ajrccm.159.1.9712108
22. Epidemiology OoAa. NCHS 2011 Linked Mortality Files Matching Methodology National Center for Health Statistics: National Center for Health Statistics. (2013). Available online at: http://www.cdc.gov/nchs/data_access/data_linkage/mortality/linkage_methods_analytical_support/2011_linked_mortality_file_matching_methodology.pdf (accessed April 4,2022).
23. Loria CM, Sempos CT, Vuong C. Plan and operation of the NHANES II Mortality Study, 1992. Vital Health Stat 1. (1999) 38:1–16.
24. Organization WH. International classification of diseases and related health problems, 10th revision. (2007). Available online at: http://www.whoint/classifications/apps/icd/icd10online (accessed April 4,2022).
25. Wang B, Zhou Y, Xiao LL, Guo YJ, Ma JX, Zhou M, et al. Association of lung function with cardiovascular risk: a cohort study. Respiratory Res. (2018) 19:920. doi: 10.1186/s12931-018-0920-y
26. Ching SM, Chia YC, Lentjes MAH, Luben R, Wareham N, Khaw KT. FEV1 and total Cardiovascular mortality and morbidity over an 18 years follow-up Population-Based Prospective EPIC-NORFOLK Study. BMC Public Health. (2019) 19:501. doi: 10.1186/s12889-019-6818-x
27. Schünemann HJ, Dorn J, Grant BJ, Winkelstein W. Jr., Trevisan M. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. (2000) 118:656–64. doi: 10.1378/chest.118.3.656
28. Engström G, Hedblad B, Nilsson P, Wollmer P, Berglund G, Janzon L. Lung function, insulin resistance and incidence of cardiovascular disease: a longitudinal cohort study. J Intern Med. (2003) 253:574–81. doi: 10.1046/j.1365-2796.2003.01138.x
29. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. (2004) 43:1731–7. doi: 10.1016/j.jacc.2003.12.047
30. Batty GD, Gunnell D, Langenberg C, Smith GD, Marmot MG, Shipley MJ. Adult height and lung function as markers of life course exposures: associations with risk factors and cause-specific mortality. Eur J Epidemiol. (2006) 21:795–801. doi: 10.1007/s10654-006-9057-2
31. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. (2010) 375:132–40. doi: 10.1016/S0140-6736(09)61717-7
32. Gan WQ, Man SFP, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. (2004) 59:574–80. doi: 10.1136/thx.2003.019588
33. Man SFP, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. (2006) 61:849–53. doi: 10.1136/thx.2006.059808
34. Rasmussen F, Mikkelsen D, Hancox RJ, Lambrechtsen J, Nybo M, Hansen HS, et al. High-sensitive C-reactive protein is associated with reduced lung function in young adults. Eur Res J. (2009) 33:382–8. doi: 10.1183/09031936.00040708
35. Hancox RJ, Poulton R, Greene JM, Filsell S, McLachlan CR, Rasmussen F, et al. Systemic inflammation and lung function in young adults. Thorax. (2007) 62:1064–8. doi: 10.1136/thx.2006.076877
36. Ford ES. Body mass index, diabetes, and C-reactive protein among US adults. Diabetes Care. (1999) 22:1971–7. doi: 10.2337/diacare.22.12.1971
37. Bruno G, Fornengo P, Novelli G, Panero F, Perotto M, Segre O, et al. C-Reactive protein and 5-year survival in type 2 diabetes the casale monferrato study. Diabetes. (2009) 58:926–33. doi: 10.2337/db08-0900
38. Cox AJ, Agarwal S, Herrington DM, Carr JJ, Freedman BI, Bowden DW. C-reactive protein concentration predicts mortality in type 2 diabetes: the diabetes heart study. Diabetic Med. (2012) 29:767–70. doi: 10.1111/j.1464-5491.2011.03560.x
39. Kengne AP, Batty GD, Hamer M, Stamatakis E, Czernichow S. Association of C-reactive protein with cardiovascular disease mortality according to diabetes status pooled analyses of 25,979 participants from four UK prospective cohort studies. Diabetes Care. (2012) 35:396–403. doi: 10.2337/dc11-1588
40. Soinio M, Marniemi J, Laakso M, Lehto S, Rönnemaa T. High-sensitivity C-reactive protein and coronary heart disease mortality in patients with type 2 diabetes: a 7-year follow-up study. Diabetes Care. (2006) 29:329–33. doi: 10.2337/diacare.29.02.06.dc05-1700
41. Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Yudkin JS, Nijpels G, et al. von Willebrand factor, C-reactive protein, and 5-year mortality in diabetic and non-diabetic subjects: the Hoorn Study. Arterioscler Thromb Vasc Biol. (1999) 19:3071–8. doi: 10.1161/01.ATV.19.12.3071
42. Stavem K, Aaser E, Sandvik L, Bjørnholt JV, Erikssen G, Thaulow E, et al. Lung function, smoking and mortality in a 26-year follow-up of healthy middle-aged males. Eur Respir J. (2005) 25:618–25. doi: 10.1183/09031936.05.00008504
43. Yang K, Wu Y, Chen D, Liu S, Chen R. The impact of lung function on extra-pulmonary diseases and all-cause mortality in US Adult population with and without COPD. Clin Epidemiol. (2020) 12:997–1005. doi: 10.2147/CLEP.S270599
44. Rogliani P, Curradi G, Mura M, Lauro D, Federici M, Galli A, et al. Metabolic syndrome and risk of pulmonary involvement. Respir Med. (2010) 104:47–51. doi: 10.1016/j.rmed.2009.08.009
45. Reaven G. Insulin resistance, hypertension, and coronary heart disease. J Clin Hypertens (Greenwich). (2003) 5:269–74. doi: 10.1111/j.1524-6175.2003.01764.x
46. Bikov A, Lange P, Anderson JA, Brook RD, Calverley PMA, Celli BR, et al. FEV(1) is a stronger mortality predictor than FVC in patients with moderate COPD and with an increased risk for cardiovascular disease. Int J Chron Obstruct Pulmon Dis. (2020) 15:1135–42. doi: 10.2147/COPD.S242809
47. Burney PG, Hooper R. Forced vital capacity, airway obstruction and survival in a general population sample from the USA. Thorax. (2011) 66:49–54. doi: 10.1136/thx.2010.147041
48. Friedman GD, Klatsky AL, Siegelaub AB. Lung function and risk of myocardial infarction and sudden cardiac death. N Engl J Med. (1976) 294:1071–5. doi: 10.1056/NEJM197605132942001
Keywords: forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), cardiovascular disease (CVD), coronary heart disease (CHD), diabetes, National Health and Nutrition Examination Survey (NHANES)
Citation: Huang N, Tang C, Li S, Ma W, Zhai X, Liu K, Sheerah HA and Cao J (2022) Association of lung function with the risk of cardiovascular diseases and all-cause mortality in patients with diabetes: Results from NHANES III 1988-1994. Front. Cardiovasc. Med. 9:976817. doi: 10.3389/fcvm.2022.976817
Received: 23 June 2022; Accepted: 12 August 2022;
Published: 09 September 2022.
Edited by:
Yong-Jae Kim, Catholic University of Korea, South KoreaReviewed by:
Suvasini Lakshmanan, University of Iowa Hospitals and Clinics, United StatesCopyright © 2022 Huang, Tang, Li, Ma, Zhai, Liu, Sheerah and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinhong Cao, Y2FvamhreTIwMDdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.