- 1Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Cardiovascular Diseases Center, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 3Beijing University of Traditional Chinese Medicine, Beijing, China
- 4Shanxi University of Traditional Chinese Medicine, Taiyuan, China
Purpose: There is increasing evidence that left atrial appendage flow velocity (LAAFV) is linked to the recurrence of atrial fibrillation (AF) after catheter ablation (CA), suggesting the potential predictable significance of LAAFV in this setting. We performed a systematic review and meta-analysis to assess whether LAAFV is association with AF recurrence after CA.
Methods: Up to May 1, 2022, six databases (PubMed, EMBASE, Web of Science, Cochrane Library, Scopus, and CINAHL) were searched for literature reporting the association between LAAFV and AF recurrence after CA. All statistical analyses were carried out using STATA version 16 software. Heterogeneity was determined by the Cochrane’s Q test and I2 statistics. The Newcastle-Ottawa Scale (NOS) was used to assess the methodological quality of each included study, and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method was adopted to evaluate the quality of evidence.
Result: Sixteen studies with 5,006 AF patients after CA (1,479 patients with AF recurrence, 3,527 without AF recurrence) were included in the meta-analysis. The meta-analysis of 15 studies (16 data sets) showed that patients with recurrence exhibited lower LAAFV values than those without recurrence [standardized mean difference (SMD): −0.65, 95% CI: −0.88 to −0.42, P < 0.01]. Moreover, we evaluated the association of LAAFV and the risk of AF recurrence after CA. Nine studies (11 data sets) defined LAAFV as continuous variables, and the pooled analysis suggested that for every 1 cm/s rise in LAAFV values, the risk of AF recurrence after CA decreased by 3% [Odds Ratio (OR): 0.97, 95% CI: 0.95 to 0.99, P < 0.01]. Seven studies defined LAAFV as categorical variables, and the pooled analysis showed that lower LAAFV were associated with an increased risk of AF recurrence after CA [OR: 2.28, 95% CI: 1.46 to 3.57, P < 0.01]. The subgroup analyses showed that the association between LAAFV and AF recurrence after CA was not significantly affected by the AF type and ablation procedure. The NOS indicated that included studies were moderate to high quality, while the GRADE assessment suggested a low certainty of the evidence.
Conclusion: Lower LAAFV may be associated with an increased risk of AF recurrence after CA. Further studies with well designed and randomized studies for LAAFV should be conducted.
Systematic review registration: [https://www.crd.york.ac.uk/PROSPERO/], identifier [CRD42022333627].
Introduction
Atrial fibrillation (AF), affecting more than 46.3 million individuals worldwide, is the most common sustained arrhythmia (1). As one of the main risk factors for heart failure, myocardial infarction, and thromboembolic strokes, AF is associated with high mortality and hospitalization rates and ultimately imposes a considerable burden on individuals and society (2). Catheter ablation (CA), an effective therapeutic option for drug-refractory symptomatic AF (3), was recommended as first-line therapy for patients with paroxysmal AF in a recent meta-analysis (4). However, AF recurrence remains a challenging issue with a recurrence rate of up to 30% (5), which make it more essential to use screening factors to predict patients at high risk of AF recurrence and post-operative complications. The risk factors for AF recurrence include age, type of AF, duration of AF, left atrial (LA) enlargement, left ventricular ejection fraction, structure and function of left atrial appendage (LAA), atrial natriuretic peptide level, sleep apnea, obesity, and hypertension (6–8). The investigations of the association between LAAFV and AF recurrence following CA have increased exponentially in recent years. Nevertheless, these studies were small and contradictory. Therefore, we performed a systematic review and meta-analysis to evaluate whether LAAFV is association with AF recurrence after CA.
Methods
This systematic review and meta-analysis was reported followed the criteria outlined in the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) and the PRISMA 2020 (9). The systematic evaluation program for this study was registered in PROSPERO (number: CRD42022333627).
Search strategy
A systematic literature search was conducted independently by two investigators (Peng-fei Chen and Yu-jiao Shi) in six databases (PubMed, EMBASE, Web of Science, Cochrane Library, Scopus, and CINAHL). We searched for English-language literature published up to May 1, 2022. The following search MESH terms and keywords were used: “left atrial appendage flow velocity,” “atrial fibrillation,” “catheter ablation,” “radiofrequency ablation,” “cryoablation,” “recurrence.” Detailed search strategies were shown in the Supplementary material. The disagreements were resolved by consulting a third investigator (Jian-peng Du).
Eligible studies
Two investigators (Peng-fei Chen and Yu-jiao Shi) independently screened titles, abstracts, and full-text material to select eligible studies. The criteria for inclusion in this meta-analysis were as follows: (1) observational studies with at least 6 months and completeness of follow-up period; (2) the target population was patients with AF (including paroxysmal AF and persistent AF) after CA (including radiofrequency and cryoballoon ablation); (3) comparing means and standard deviations (SDs) of LAAFV values in individuals with AF recurrence after CA to those without recurrence; and (4) reporting odds ratios (ORs) or hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs) for LAAFV as a predictor of AF recurrence after CA. The abstracts, editorial, animal experiment, or review were excluded.
Data extraction
Pre-specified data variables were extracted independently by two investigators (Peng-fei Chen and Yu-jiao Shi) using a standard form. The following information was extracted from the eligible literature: the first author’s name, publication year, study location, study design, baseline characteristics of patients (gender, age, and sample size), numbers of patients (paroxysmal AF patients, persistent AF patients, and recurrent post-operative patients), LAAFV measurement method, blanking period in months, and follow-up duration in months.
Quality evaluation
The quality of the included research was assessed according to the Newcastle-Ottawa Scale (NOS), an assessment tool focused on three aspects: participant selection, comparability, and exposure. The NOS score ranged from 0 to 9, with more than or equal to 8 stars defined as high quality, 6–8 stars as moderate quality, and less than 6 stars as low quality (10). To assess the certainty of the evidence, we adopted the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (11), which classifies evidence as high, moderate, low, or very low certainty based on the following factors: risk of bias, inconsistency, indirectness, imprecision, and publication bias. Since all included studies were observational studies, the preliminary definition of the quality evidence was low, and other factors may then upgrade or downgrade the quality level. Any disagreements were resolved by consulting a third investigator (Jian-peng Du).
Statistical analysis
In the analysis of LAAFV values in AF patients with and without recurrence after CA, means and SDs of LAAFV values were extracted, and standardized mean difference (SMD) and 95% CI were calculated for each study. In analyzing the association between LAAFV and the risk of AF recurrence after CA, univariate or multivariate ORs for AF recurrence reported by logistic regression analysis were extracted. For the study that only reported HRs, HRs was adopted as the best estimate of ORs. Since the studies included in this meta-analysis used LAAFV values as either a categorical or continuous variable to evaluate ORs, two separate meta-analyses were conducted for both variables. Heterogeneity was assessed by the Cochrane’s Q test (P < 0.1 was considered statistical heterogeneity) and I2 Statistics (25, 50, and 75% were considered to represent low, medium, and high heterogeneity, respectively). We adopted a random-effect model for the meta-analysis because it incorporates the potential effects of heterogeneity and therefore allows for the retrieval of more generalizable results. Subgroup analyses were stratified by study location (Europe or Asia), study design (prospective or retrospective), sample size (≤100 or >100), AF type (persistent AF or paroxysmal AF), ablation procedure (circumferential pulmonary vein isolation or additional linear ablations), follow-up time (≤12 or >12 months), and ablation type (cryoballoon ablation or radiofrequency ablation). The inverted funnel plot and Egger’s test were performed to assess publication bias. Sensitivity analyses by removing one individual study at a time to confirm the robustness of the results. All statistical analyses were carried out using STATA version 16 software.
Results
Study search
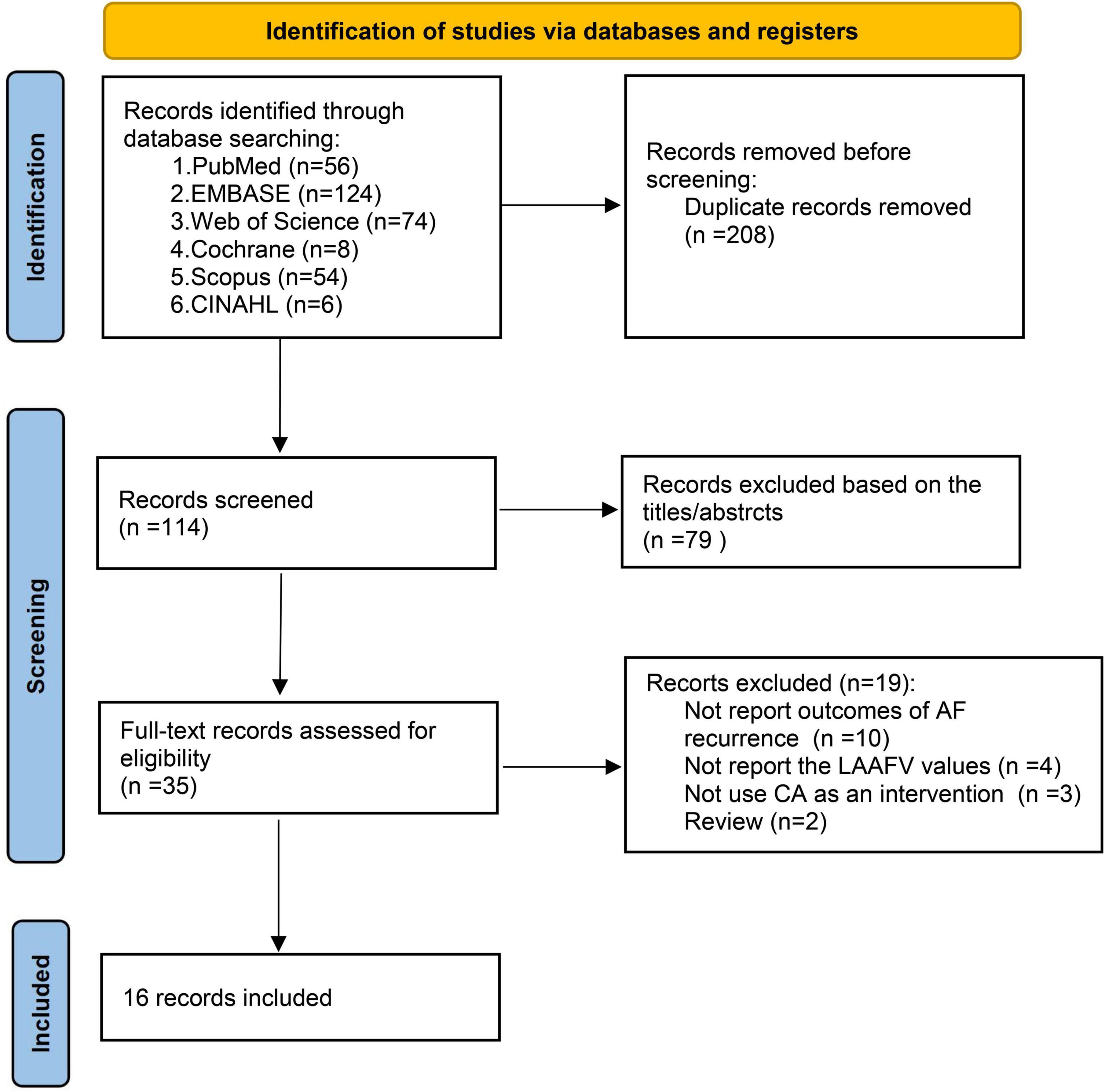
The database search and study identification procedure was presented in Figure 1. A total of 322 records were retrieved, 208 of which were duplicates, and 79 studies were excluded after reading the title and abstract, primarily because they were irrelevant to the study purpose. The remaining 35 articles were evaluated for eligibility by full-text screening. Of these, 19 studies were further excluded because 10 did not report the AF recurrence outcomes, 4 did not report the LAAFV values, 3 did not use CA as an intervention, and the other 2 were reviews. Finally, 16 studies (12–27) were included in our systematic review and meta-analysis.
Study characteristics
Table 1 displayed the study characteristics of 16 included studies, comprising 9 (12, 15, 18, 20, 21, 23–25, 27) prospective studies and 7 (13, 14, 16, 17, 19, 22, 26) retrospective studies. Six studies (19, 22–25, 27) were conducted in China, 4 studies (15, 16, 20, 21) in Japan, 2 studies (14, 17) in Hungary, 1 study (26) in South Korea, 1 study (13) in Poland, 1 study (12) in Türkiye, and 1 study (18) in Romania. Sixteen studies with 5,006 AF patients after CA (1,479 patients with AF recurrence, 3,527 without AF recurrence) were included, and the proportion of AF patients with recurrence ranged from 24.1 to 41.5%. The proportion of males was higher than that of females, varying from 59.3 to 92.7%. The mean age ranged from 54.6 ± 10.4 to 67.5 ± 7.5 years, and the median follow-up time varied from 6 to 48 months. Nine (12–15, 17, 18, 22, 23, 25) studies performed circumferential pulmonary vein isolation (CPVI) alone, whereas 7 (16, 19–21, 24, 26, 27) studies conducted additional linear ablations. The LAAFV, which refers to the peak flow emptying velocity of the left atrial appendage at late diastole, was measured by transthoracic echocardiography (TEE) in all selected research. All studies used 24-h Holter and/or surface electrocardiogram recording to diagnose asymptomatic AF recurrence. All studies blanking period of post-CA procedure were 3 months, except for 1 study (15) was 2 months.
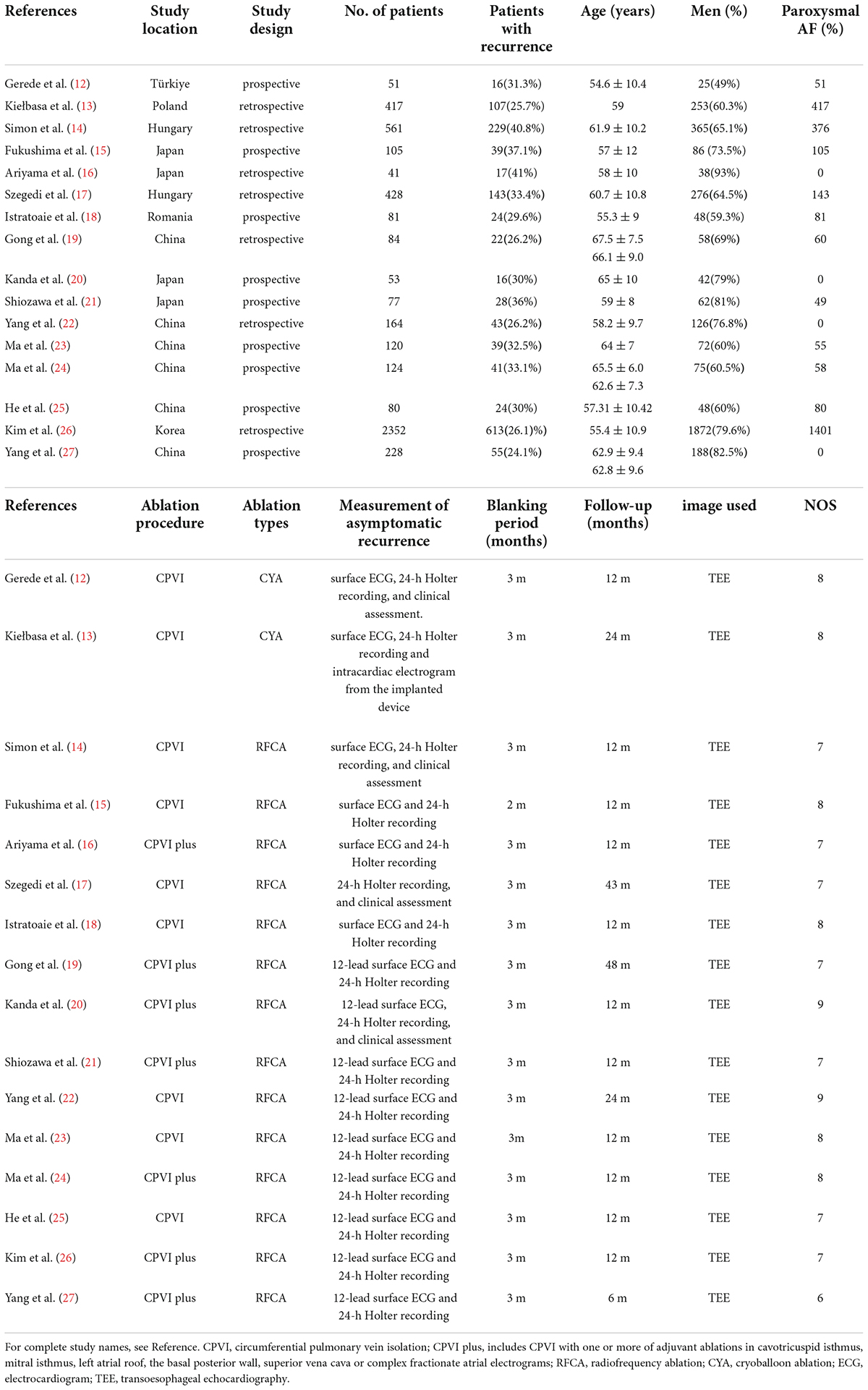
Table 1. Characteristics of 16 studies included in the meta-analysis of difference in LAAFV between patients with and without post-CA AF recurrence.
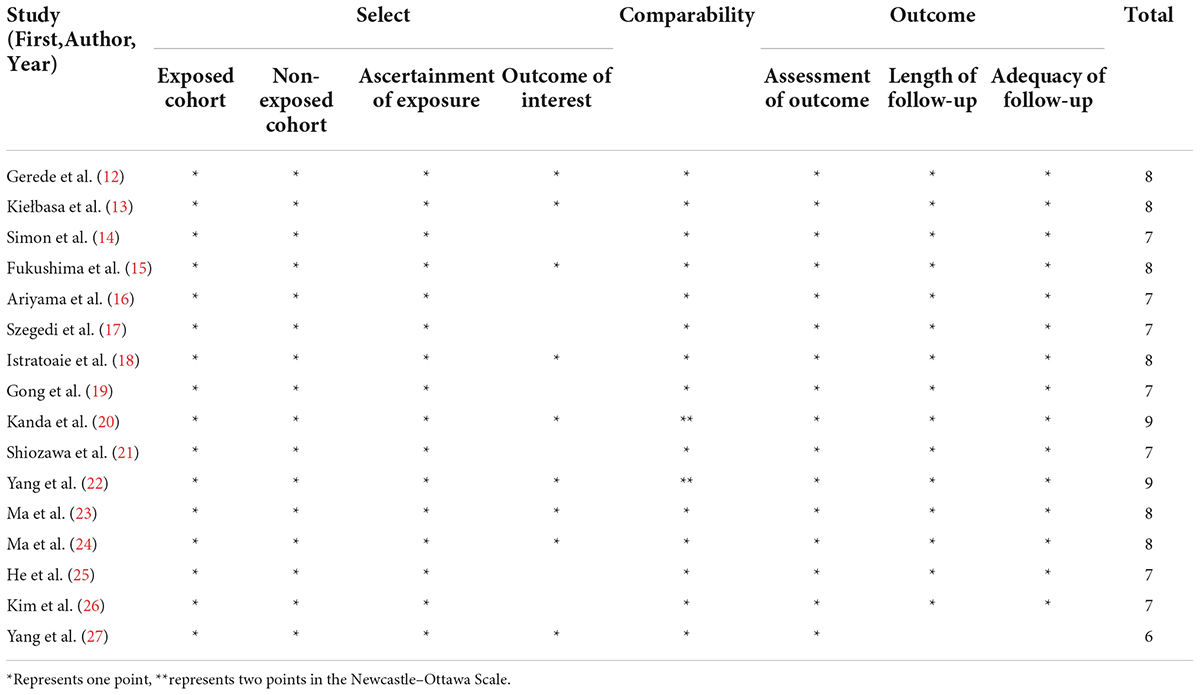
Study quality
Based on NOS for observational studies, all examined studies had quality ratings ranging from 6 to 9 (mean score: 7.6), indicating moderate to high quality. Detailed quality assessment is presented in Table 2.
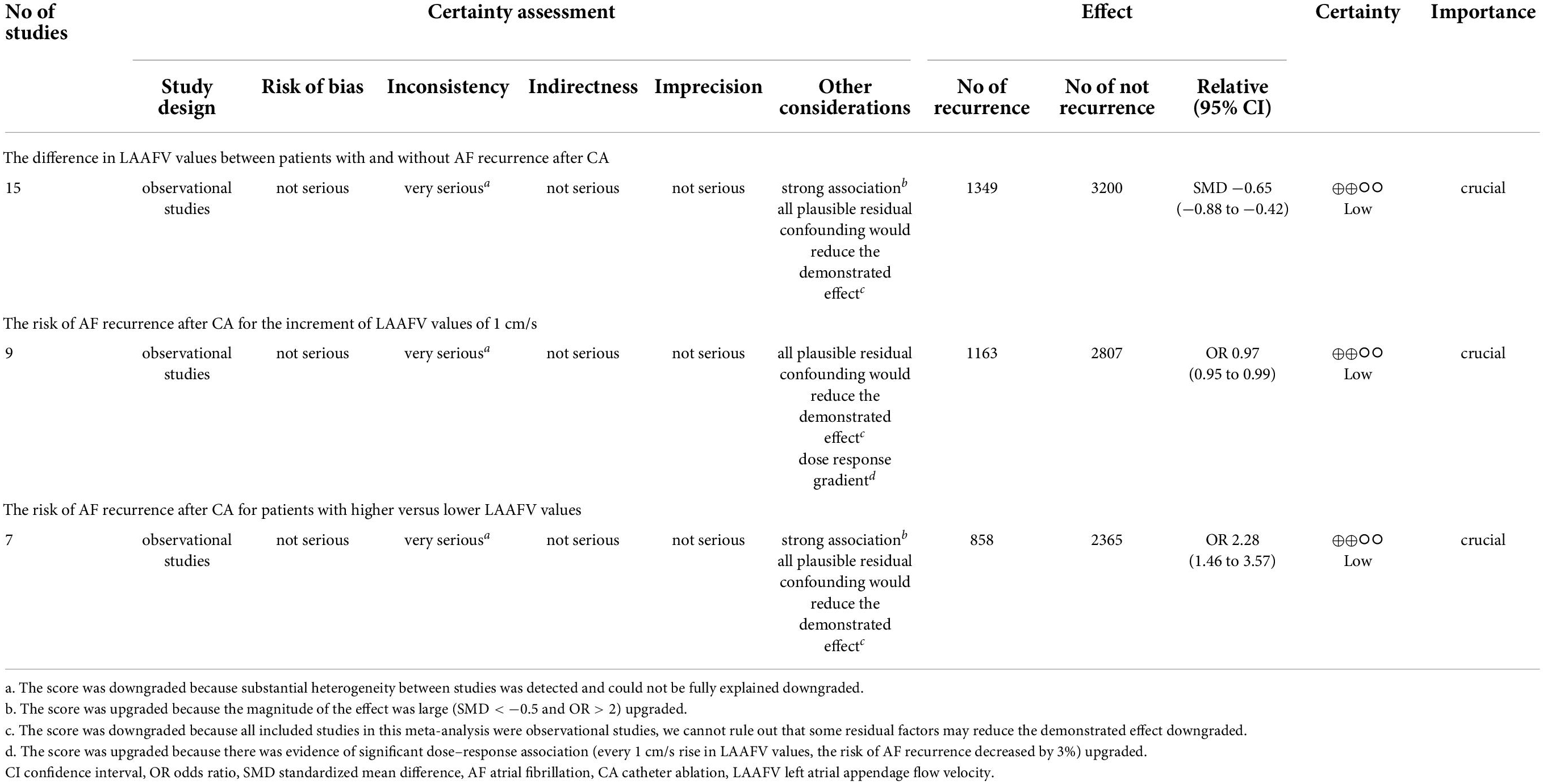
According to the GRADE grade system, the evidence supporting the link between LAAFV and AF recurrence following CA was of low certainty. Table 3 displays the certainty assessment ratings and a description of the results.
Results of meta-analysis
The difference in left atrial appendage flow velocity values between patients with and without atrial fibrillation recurrence after catheter ablation
Fifteen studies (12, 14–27) (16 data sets) reported the difference in LAAFV values between patients with (n = 1,349) and without (n = 3,200) AF recurrence after CA. Patients with recurrence exhibited lower LAAFV values than those without recurrence [SMD:−0.65, 95% CI: −0.88 to −0.42, P < 0.01; I2 = 87.9% Figure 2]. The Funnel plot was symmetrical upon visual inspection (Supplementary Figure 1), and the P-value of Egger’s test was 0.12 (Supplementary Figure 2), indicating no significant publication bias. The sensitivity analysis results were consistent (SMD: −0.71 to −0.59, p all < 0.05, Supplementary Figure 3). The subgroup analyses were summarized in Table 4. When we performed subgroup analyses stratified by study design and follow-up time, the results were not statistically significant in the subgroup of retrospective study [SMD: −0.23, 95% CI: −0.52 to 0.06, P > 0.05] and follow-up times > 12m study [SMD: −0.16, 95% CI: −0.60 to 0.28, P > 0.05].
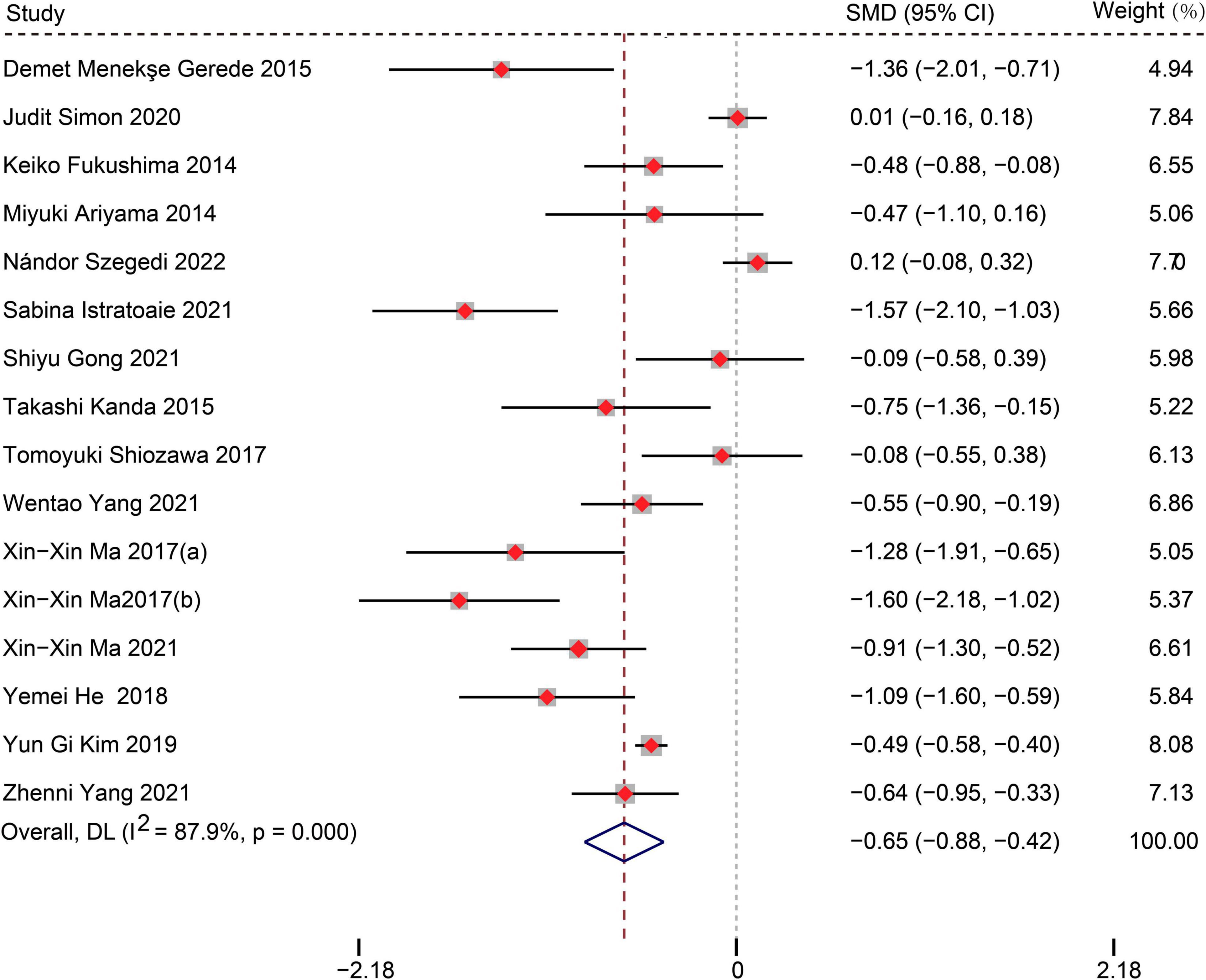
Figure 2. Forest plots show the difference in LAAFV values between patients with and without AF recurrence after CA.
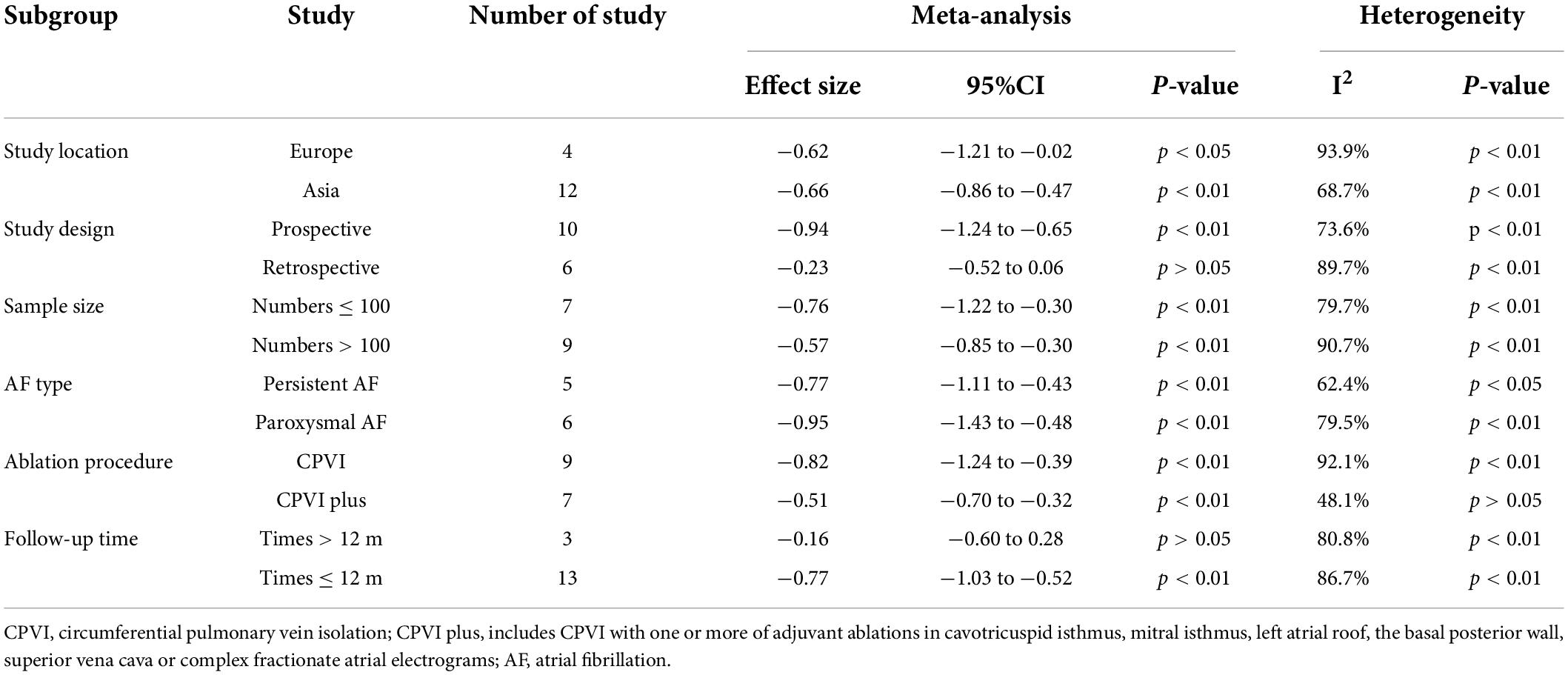
Table 4. Subgroup analyses of difference in LAAFV values between patients with and without AF recurrence after CA.
The risk of atrial fibrillation recurrence after catheter ablation for the increment of left atrial appendage flow velocity values of 1 cm/s
Fourteen studies (12–15, 17–27) reported the relationship between LAAFV values and the risk of AF recurrence after CA. Nine studies (14, 17–19, 21, 23–26) (11 data sets) defined LAAFV as continuous variables, and the pooled analysis showed that for every 1cm/s rise in LAAFV values, the risk of AF recurrence after CA decreased by 3% [OR:0.97, 95% CI: 0.95 to 0.99, P < 0.01; I2 = 91.4% Figure 3]. The sensitivity analysis results were consistent (OR: 0.96 to 0.98, p all < 0.05, Supplementary Figure 4). The subgroup analyses were summarized in Table 5, and the effect sizes were consistent regardless of AF types and ablation procedure. When we stratified the studies by study location, study design, sample size, and follow-up time, the results were not statistically significant in the subgroup of Europe study [OR: 0.99, 95% CI: 0.96 to 1.01, P > 0.05], retrospective study [OR: 1, 95% CI: 0.98 to 1.02, P > 0.05], numbers < 100 study [OR: 0.94, 95% CI: 0.88 to 1.01, P > 0.05], numbers > 100 study [OR: 0.98, 95% CI: 0.96 to 1.01, P > 0.05], and follow-up times > 12 m [OR: 1.01, 95% CI: 1.00 to 1.02, P > 0.05].
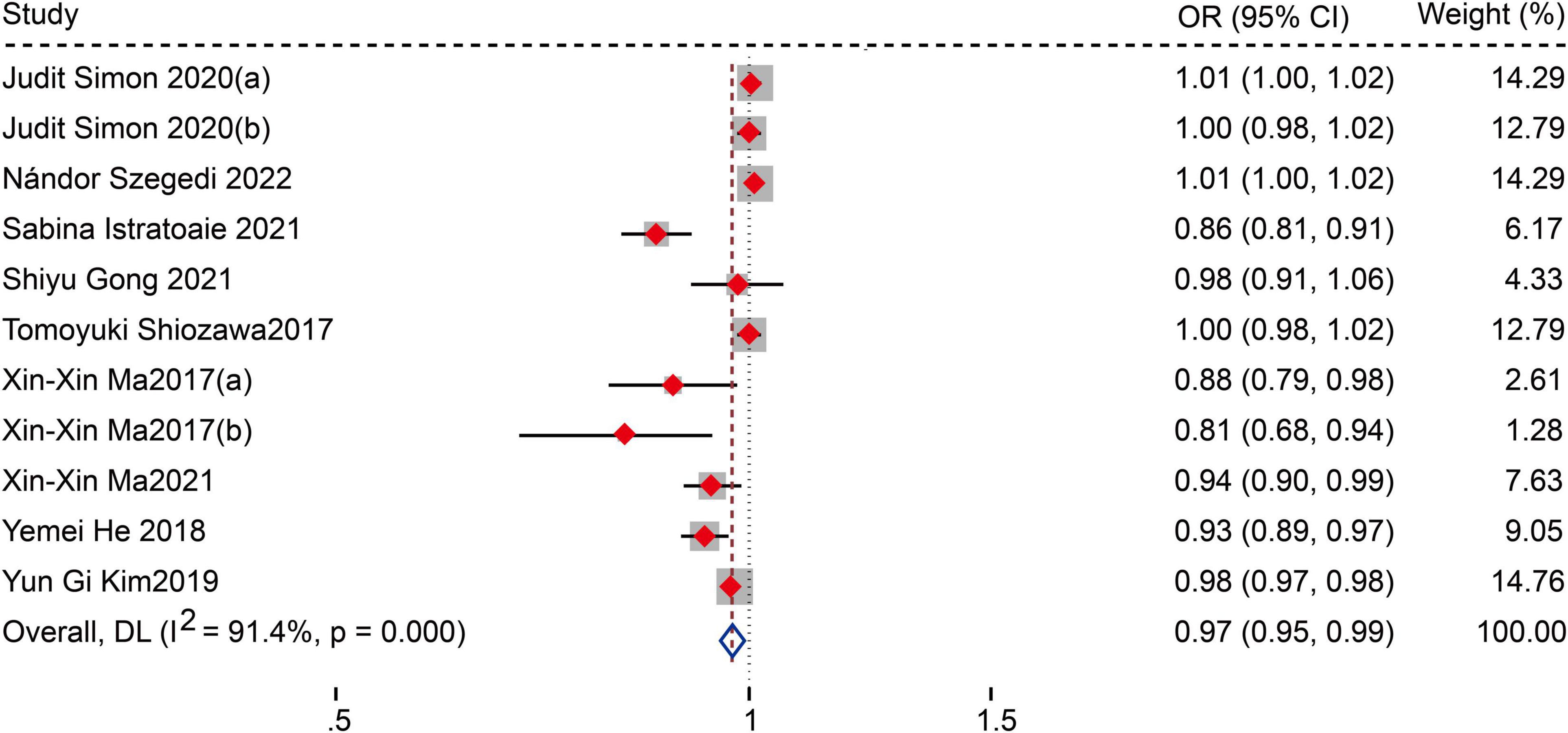
Figure 3. Forest plots show the relationship between LAAFV (continuous variables) and the risk of AF recurrence after CA.
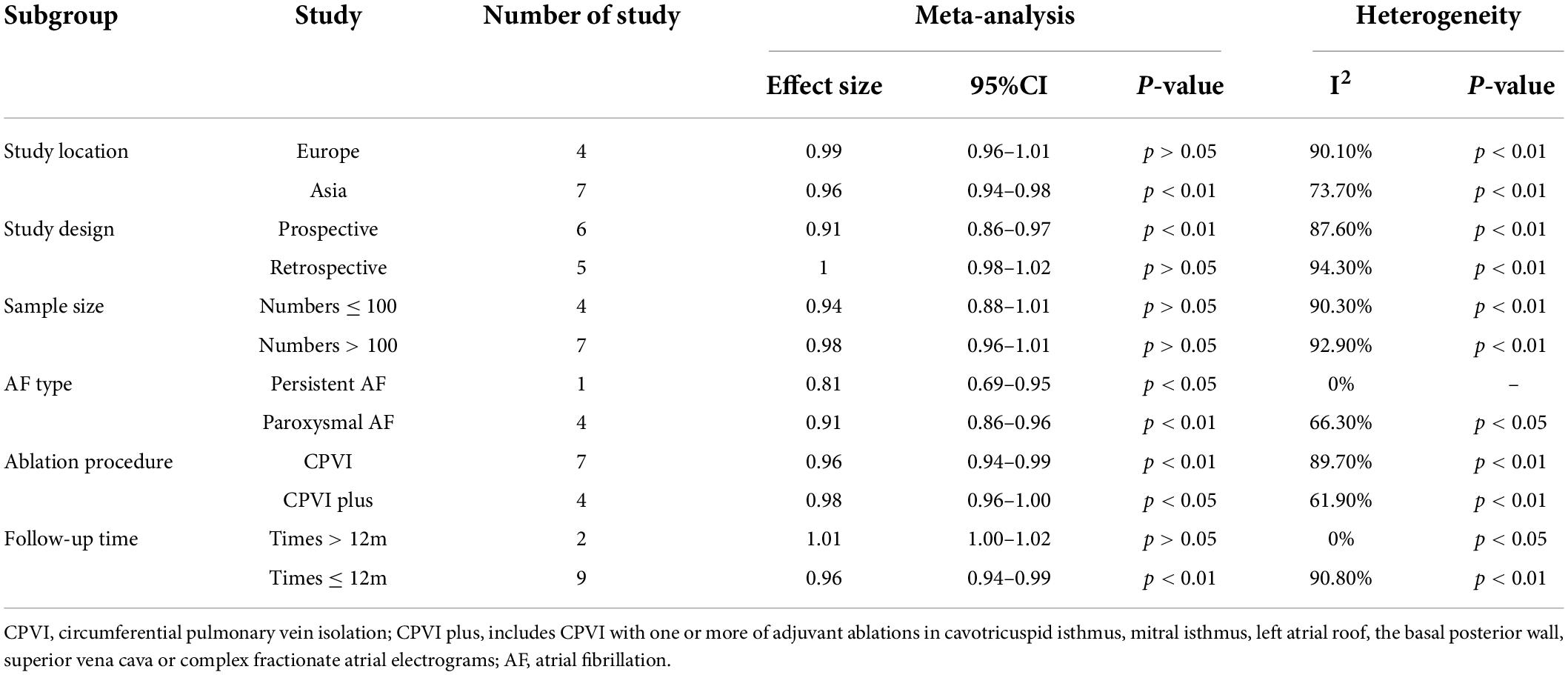
Table 5. Subgroup analyses of the risk of AF recurrence after CA based on LAAFV (continuous variable).
The risk of atrial fibrillation recurrence after catheter ablation for patients with higher versus lower left atrial appendage flow velocity values
Seven studies (12, 13, 15, 18, 20, 22, 26) defined LAAFV as categorical variables. The pooled analysis showed that lower LAAFV was associated with an increased risk of AF recurrence after CA [OR:2.28, 95% CI: 1.46 to 3.57, P < 0.01; I2 = 93.4% Figure 4]. The sensitivity analysis results were consistent (OR: 1.96 to 2.48, p all < 0.05, Supplementary Figure 5). The subgroup analyses were summarized in Table 6. When we stratified the studies by study location, sample size, and ablation type, the results were not statistically significant in the subgroup of Europe study [OR: 2.05, 95% CI: 0.99 to 4.22, P > 0.05], numbers < 100 study [OR: 2.93, 95% CI: 0.81 to 10.65, P > 0.05], and cryoballoon ablation study [OR: 1.27, 95% CI: 0.97 to 1.78, P > 0.05].
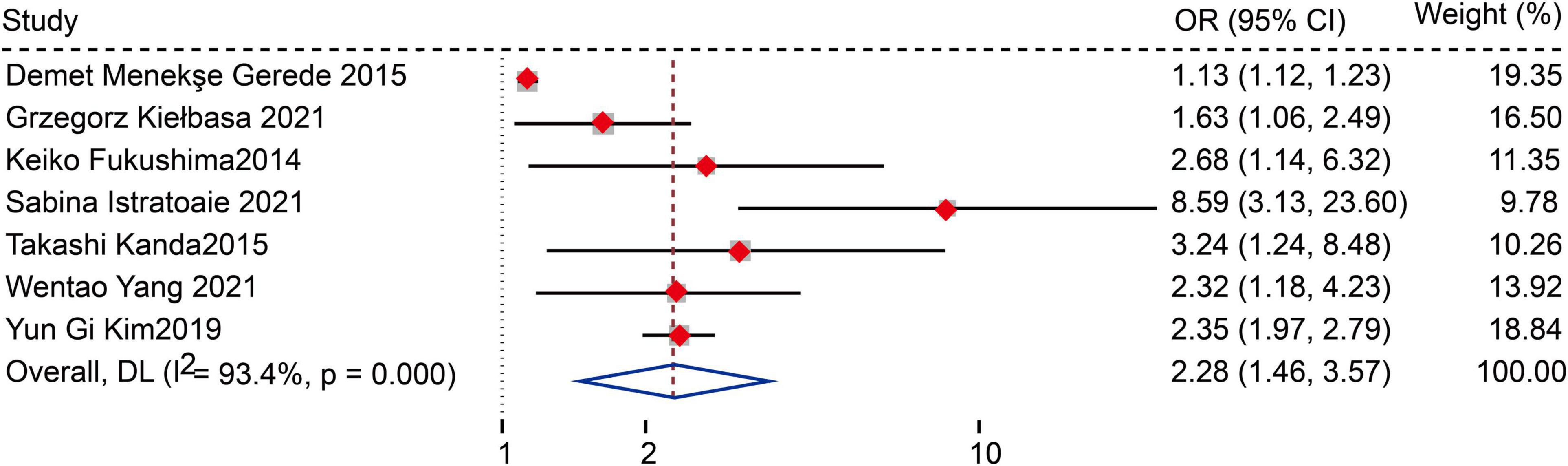
Figure 4. Forest plots show the relationship between LAAFV (categorical variables) and the risk of AF recurrence after CA.
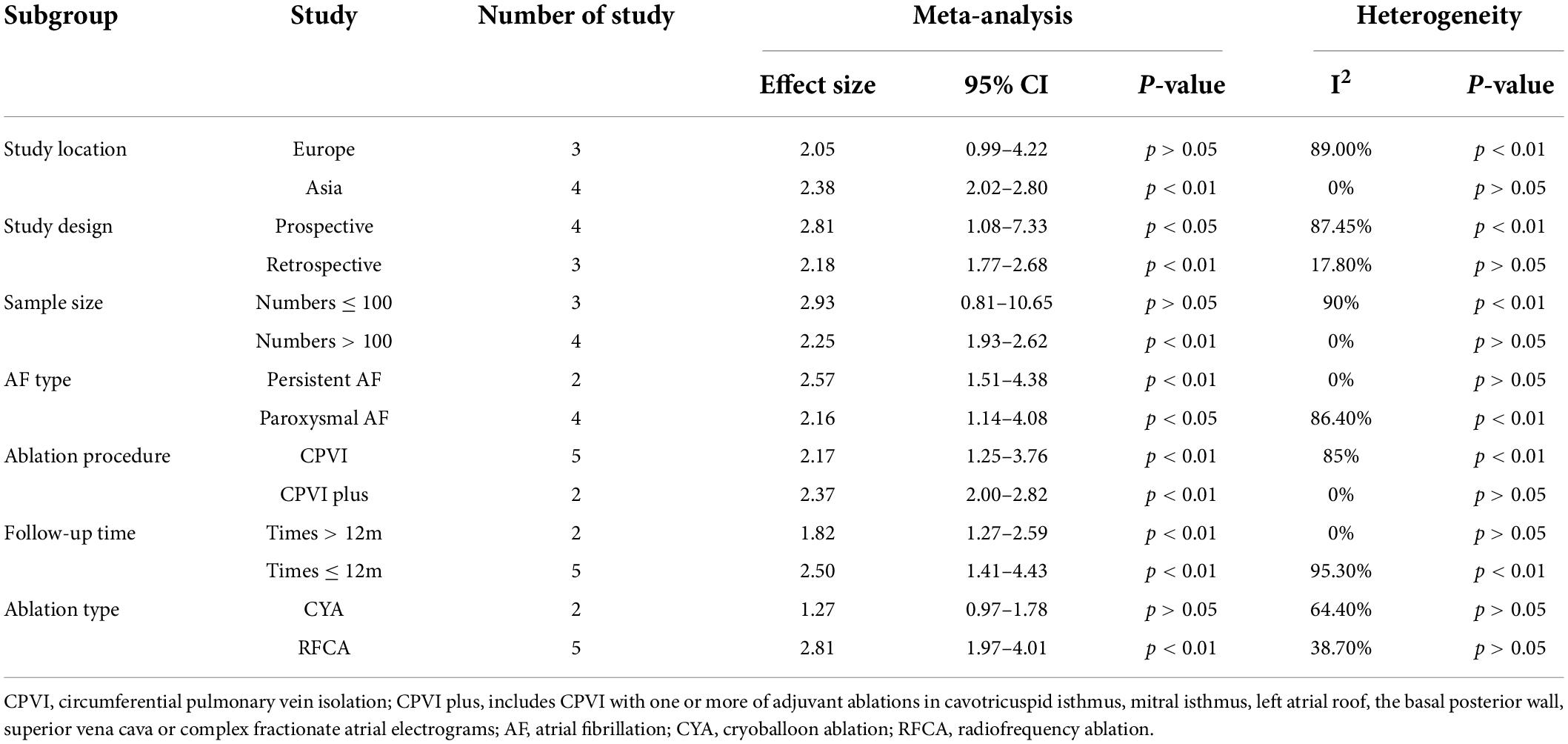
Table 6. Subgroup analyses of the risk of AF recurrence after CA based on LAAFV (categorical variable).
Discussion
The aim of this meta-analysis and systematic review was to examine whether LAAFV is a reliable predictor of AF recurrence after CA. Our meta-analysis showed that patients with AF recurrence had lower mean LAAFV values than those without recurrence. Moreover, we evaluated the association between LAAFV and the risk of AF recurrence after CA. Every 1cm/s rise in LAAFV, the risk of AF recurrence after CA decreased by 3% in the pooled analysis of continuous factors, whereas lower LAAFV was associated with an increased risk of AF recurrence in the pooled analysis of categorical variables. The subgroup analyses showed that the association between LAAFV and AF recurrence after CA was not significantly affected by the AF type and ablation procedure.
Catheter ablation is the most frequently performed interventional electrophysiological therapy for AF. Ectopic pacing sites in patients with AF usually originate from pulmonary veins (PV), and pulmonary vein isolation (PVI) is the cornerstone of CA. Although the surgical effect is satisfactory, the long-term recurrence rate post-operative remains high, because non-PV areas other than PVI may be the source of initiation and maintenance of AF (28–30). The most common areas include the superior vena cava, the coronary sinus, the ligament of marshall, the crista terminalis, the LA posterior wall and the LAA (31–33). LAA is a finger-like projection extending from the main body of the LA and is primarily formed by the adsorption of the primordial PV and their branches (34). It is demonstrated that LAA is a significant source of AF and atrial tachycardia (35). A study (29) found that nearly 30 percent of AF triggers originate from non-pulmonary veins, especially the LAA. Di Biase et al. (36) analyzed 987 patients undergoing AF cablation, demonstrating that 27% of AF patients were triggered by LAA, and LAA electrical isolation can improve the success rate of AF cablation. The function of the LAA is most commonly determined by measuring emptying velocity with pulsed-wave Doppler (15). Previous studies have reported that lower LAAFV in AF patients was associated with a higher risk of thromboembolism (37) and a lower success rate of long-term cardioversion (38, 39). LAAFV, representing a hemodynamic feature of LA and the LAA (40), is widely acknowledged as a marker of LAA function (including contractility, stunning, and fibrosis) (41, 42). AF is associated with pathological changes such as remodeling, electrical intolerance changes, and atrial mass loss, which lead to enlargement and dysfunction of LAA, thus causing a decrease in LAA blood flow and ultimately a reduction in LAAFV values (43).
Left atrial remodeling, including LA enlargement, hypertrophy, and/or fibrosis is the basis of AF recurrence (44). Moreover, LA size increases and LA voltages decrease due to LA remodeling, which are considered the surrogate for LA fibrosis and the significant predictor of AF recurrence after CA (45, 46). According to recent research, LAAFV is positively correlated with LA voltage and negatively correlated with LA volume (47, 48). As an indicator reflecting LA contractile and reserve function, LAAFV demonstrates the severity of LA functional remodeling, which may occur in the early stage of LA remodeling (49). Paroxysmal AF patients are generally in the early stage of LA remodeling. Chronic pressure overload causes LA enlargement, while Impaired LA function precedes LA expansion. Therefore, LAAFV may be a more sensitive predictor for AF recurrence than LA size and volume, particularly in patients with paroxysmal AF. LAA has a stronger contraction and extension function than the LA, the distensible at a greater degree than the LA, as buffering effect on reducing LA pressure (50). It provides a theoretical basis that flow velocity of LAA may be a more dependable parameter for AF recurrence than LA. LAAFV, which reflects the more comprehensive LAA dysfunction and atrial remodeling as mentioned above, mainly depends on the contraction of LAA, and therefore, would be a more reliable predictor of AF recurrence. The primary imaging method for assessing LAAFV is TEE (51), which provides a more accurate risk assessment because it allows the characterization of the AF substrates (52).
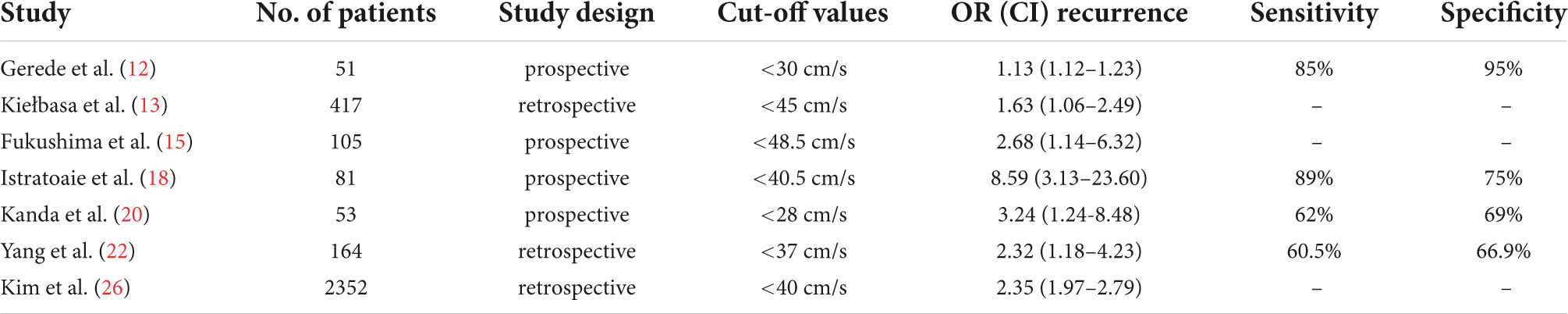
Although there have been many studies on the relationship between LAAFV and AF recurrence after CA, how to determine the cut-off value of LAAFV is a crucial question to be answered. In our meta-analysis, the cut-off values of the seven studies that defined LAAFV as categorical variables were displayed in Table 7. The cut-off values of most studies are similar, and the differences across studies were probably caused by different research populations or methods, and in the largest related studies so far, the cut-off value of 40 cm/s has been proposed.
To our knowledge, this is the first meta-analysis to summarize the association between the LAAFV and AF recurrence after CA. The advantages of the meta-analysis may include the following. First, the results of this study were relatively stable and reliable because the meta-analysis covered studies from different countries and had a large sample size. Second, the finding that LAAFV is associated with the risk of AF recurrence after CA was based on most adequately adjusted ORs, suggesting that the finding may not be affected by potential confounding factors. Third, studies with LAAFV analyzed as categorized and continuous data were summarized separately and derived consistent results, which further verified the stability of the results. Fourth, the sensitivity analyses by removing one individual study at a time had no significant impact on the results, suggesting the outcomes were credible. Fifth, multiple subgroup analyses were conducted to assess the potential study characteristics of the relationship between LAAFV and AF recurrence after CA.
However, this meta-analysis also had some limitations. First, as a meta-analysis of observational studies, it carries inherent limitations of the study design. Second, the heterogeneity of our study was significant. Even if sensitivity and subgroup analyses were adopted, the origin of heterogeneity could not be explored. Third, we cannot rule out that some residual factors may confuse the link between LAAFV and AF recurrence. Forth, studies that defined LAAFV as categorical variables have different cut-off values, which would impact our study result.
Conclusion
Meta-analyses of observational studies show that patients with AF recurrence after CA have lower mean LAAFV values than patients without recurrence. Lower LAAFV was associated with an increased risk of AF recurrence after CA, and the assessment of LAAFV before CA could be used as a potential and feasible screening method to predict the risk of AF recurrence. Further studies with larger, well designed, and randomized studies with longer follow up periods for LAAFV should be conducted. In addition, the mechanism of LAAFV and AF recurrences remains to be further explored.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JD and ZC were involved in conceptualization and supervision. PC, LM, and XG collected, analyzed, and interpreted the data. PC, YS, and DP drew pictures and wrote the original draft. JJ and JD edited and modified the final version. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the project of Major New Drug Creation (No. 2018ZX09301-011-001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.971848/full#supplementary-material
SUPPLEMENTARY FIGURE 1 | Funnel plot for the difference in LAAFV values between patients with and without AF recurrence after CA.
SUPPLEMENTARY FIGURE 2 | Egger’s test for the difference in LAAFV values between patients with and without AF recurrence after CA.
SUPPLEMENTARY FIGURE 3 | Sensitivity analysis for the difference in LAAFV values between patients with and without AF recurrence after CA.
SUPPLEMENTARY FIGURE 4 | Sensitivity analysis for the relationship between LAAFV (continuous variables) and the risk of AF recurrence after CA.
SUPPLEMENTARY FIGURE 5 | Sensitivity analysis for the relationship between LAAFV (categorical variables) and the risk of AF recurrence after CA.
References
1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. American heart association council on epidemiology and prevention statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. (2019) 139:e56–528. doi: 10.1161/CIR.0000000000000659
2. Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. (2021) 16:217–21. doi: 10.1177/1747493019897870
3. Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. (2019) 321:1275–85. doi: 10.1001/jama.2019.0692
4. Turagam MK, Musikantow D, Whang W, Koruth JS, Miller MA, Langan MN, et al. Assessment of catheter ablation or antiarrhythmic drugs for first-line therapy of atrial fibrillation: a meta-analysis of randomized clinical trials. JAMA Cardiol. (2021) 6:697–705. doi: 10.1001/jamacardio.2021.0852
5. Erhard N, Metzner A, Fink T. Late arrhythmia recurrence after atrial fibrillation ablation: incidence, mechanisms and clinical implications. Herzschrittmacherther Elektrophysiol. (2022) 33:71–6. doi: 10.1007/s00399-021-00836-6
6. Liżewska-Springer A, Dąbrowska-Kugacka A, Lewicka E, Drelich Ł, Królak T, Raczak G. Echocardiographic predictors of atrial fibrillation recurrence after catheter ablation: a literature review. Cardiol J. (2020) 27:848–56. doi: 10.5603/CJ.a2018.0067
7. Knight BP, Novak PG, Sangrigoli R, Champagne J, Dubuc M, Adler SW, et al. Long-term outcomes after ablation for paroxysmal atrial fibrillation using the second-generation cryoballoon: final results from STOP AF post-approval study. JACC Clin Electrophysiol. (2019) 5:306–14. doi: 10.1016/j.jacep.2018.11.006
8. Sultan A, Lüker J, Andresen D, Kuck KH, Hoffmann E, Brachmann J, et al. Predictors of atrial fibrillation recurrence after catheter ablation: data from the German ablation registry. Sci Rep. (2017) 7:16678. doi: 10.1038/s41598-017-16938-6
9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
10. Margulis AV, Pladevall M, Riera-Guardia N, Varas-Lorenzo C, Hazell L, Berkman ND, et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol. (2014) 6:359–68. doi: 10.2147/CLEP.S66677
11. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
12. Gerede DM, Candemir B, Vurgun VK, Aghdam SM, Acıbuca A, Özcan ÖU, et al. Prediction of recurrence after cryoballoon ablation therapy in patients with paroxysmal atrial fibrillation. Anatol J Cardiol. (2015) 16:482–8. doi: 10.5152/AnatolJCardiol.2015.6309
13. Kiełbasa G, Bednarek A, Bednarski A, Olszanecka A, Sondej T, Kusiak A, et al. Patent foramen ovale and left atrial appendage flow velocity predict atrial fibrillation recurrence post cryoballoon ablation. Kardiol Pol. (2021) 79:756–64. doi: 10.33963/KP.a2021.0004
14. Simon J, El Mahdiui M, Smit JM, Száraz L, van Rosendael AR, Herczeg S, et al. Left atrial appendage size is a marker of atrial fibrillation recurrence after radiofrequency catheter ablation in patients with persistent atrial fibrillation. Clin Cardiol. (2022) 45:273–81. doi: 10.1002/clc.23748
15. Fukushima K, Fukushima N, Ejima K, Kato K, Sato Y, Uematsu S, et al. Left atrial appendage flow velocity and time from P-wave onset to tissue Doppler-derived A’ predict atrial fibrillation recurrence after radiofrequency catheter ablation. Echocardiography. (2015) 32:1101–8. doi: 10.1111/echo.12823
16. Ariyama M, Kato R, Matsumura M, Yoshimoto H, Nakajima Y, Nakano S, et al. Left atrial appendage wall-motion velocity associates with recurrence of nonparoxysmal atrial fibrillation after catheter ablation. Echocardiography. (2015) 32:272–80. doi: 10.1111/echo.12647
17. Szegedi N, Simon J, Szilveszter B, Salló Z, Herczeg S, Száraz L, et al. Abutting left atrial appendage and left superior pulmonary vein predicts recurrence of atrial fibrillation after point-by-point pulmonary vein isolation. Front Cardiovasc Med. (2022) 9:708298. doi: 10.3389/fcvm.2022.708298
18. Istratoaie S, Vesa ŞC, Cismaru G, Pop D, Roşu R, Puiu M, et al. Value of left atrial appendage function measured by transesophageal echocardiography for prediction of atrial fibrillation recurrence after radiofrequency catheter ablation. Diagnostics (Basel). (2021) 11:1465. doi: 10.3390/diagnostics11081465
19. Gong S, Zhou J, Li B, Kang S, Ma X, Cai Y, et al. The association of left atrial appendage morphology to atrial fibrillation recurrence after radiofrequency ablation. Front Cardiovasc Med. (2021) 8:677885. doi: 10.3389/fcvm.2021.677885
20. Kanda T, Masuda M, Sunaga A, Fujita M, Iida O, Okamoto S, et al. Low left atrial appendage flow velocity predicts recurrence of atrial fibrillation after catheter ablation of persistent atrial fibrillation. J Cardiol. (2015) 66:377–81. doi: 10.1016/j.jjcc.2015.04.009
21. Shiozawa T, Shimada K, Sekita G, Hayashi H, Tabuchi H, Miura S, et al. Left atrial appendage volume and plasma docosahexaenoic acid levels are associated with atrial fibrillation recurrence after catheter ablation. Cardiol Res. (2017) 8:96–104. doi: 10.14740/cr542w
22. Yang W, Zhao Q, Yao M, Li X, Zhang Y, Liu C, et al. The prognostic significance of left atrial appendage peak flow velocity in the recurrence of persistent atrial fibrillation following first radiofrequency catheter ablation. J Thorac Dis. (2021) 13:5954–63. doi: 10.21037/jtd-21-1363
23. Ma XX, Zhang YL, Hu B, Jiang WJ, Wang M, Zheng DY, et al. Association between left atrial appendage emptying velocity, N-terminal plasma brain natriuretic peptide levels, and recurrence of atrial fibrillation after catheter ablation. J Interv Card Electrophysiol. (2017) 48:343–50. doi: 10.1007/s10840-016-0216-4
24. Ma XX, Wang A, Lin K. Incremental predictive value of left atrial strain and left atrial appendage function in rhythm outcome of non-valvular atrial fibrillation patients after catheter ablation. Open Heart. (2021) 8:e001635. doi: 10.1136/openhrt-2021-001635
25. He Y, Zhang B, Zhu F, Hu Z, Zhong J, Zhu W. Transesophageal echocardiography measures left atrial appendage volume and function and predicts recurrence of paroxysmal atrial fibrillation after radiofrequency catheter ablation. Echocardiography. (2018) 35:985–90. doi: 10.1111/echo.13856
26. Kim YG, Choi JI, Boo KY, Kim DY, Oh SK, Park HS, et al. Clinical and echocardiographic risk factors predict late recurrence after radiofrequency catheter ablation of atrial fibrillation. Sci Rep. (2019) 9:6890. doi: 10.1038/s41598-019-43283-7
27. Yang Z, Xu M, Zhang C, Liu H, Shao X, Wang Y, et al. A predictive model using left atrial function and B-type natriuretic peptide level in predicting the recurrence of early persistent atrial fibrillation after radiofrequency ablation. Clin Cardiol. (2021) 44:407–14. doi: 10.1002/clc.23557
28. Arbelo E, Brugada J, Hindricks G, Maggioni AP, Tavazzi L, Vardas P, et al. The atrial fibrillation ablation pilot study: a European survey on methodology and results of catheter ablation for atrial fibrillation conducted by the European heart rhythm association. Eur Heart J. (2014) 35:1466–78. doi: 10.1093/eurheartj/ehu001
29. Di Biase L, Santangeli P, Natale A. How to ablate long-standing persistent atrial fibrillation? Curr Opin Cardiol. (2013) 28:26–35. doi: 10.1097/HCO.0b013e32835b59bb
30. Gianni C, Atoui M, Mohanty S, Trivedi C, Bai R, Al-Ahmad A, et al. Difference in thermodynamics between two types of esophageal temperature probes: insights from an experimental study. Heart Rhythm. (2016) 13:2195–200. doi: 10.1016/j.hrthm.2016.07.021
31. Della Rocca DG, Mohanty S, Trivedi C, Di Biase L, Natale A. Percutaneous treatment of non-paroxysmal atrial fibrillation: a paradigm shift from pulmonary vein to non-pulmonary vein trigger ablation? Arrhythm Electrophysiol Rev. (2018) 7:256–60. doi: 10.15420/aer.2018.56.2
32. Nishimura M, Lupercio-Lopez F, Hsu JC. Left atrial appendage electrical isolation as a target in atrial fibrillation. JACC Clin Electrophysiol. (2019) 5:407–16. doi: 10.1016/j.jacep.2019.02.009
33. Goldenberg GR, Ono M, Aryana A, d’Avila A, Saad EB, Singh SK, et al. The incremental benefit of non-pulmonary vein left atrial ablation in patients undergoing a repeat persistent atrial fibrillation ablation procedure. J Interv Card Electrophysiol. (2017) 48:185–91. doi: 10.1007/s10840-016-0204-8
34. Beigel R, Wunderlich NC, Ho SY, Arsanjani R, Siegel RJ. The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging. (2014) 7:1251–65. doi: 10.1016/j.jcmg.2014.08.009
35. Yamada T, McElderry HT, Allison JS, Kay GN. Focal atrial tachycardia originating from the epicardial left atrial appendage. Heart Rhythm. (2008) 5:766–7. doi: 10.1016/j.hrthm.2007.12.025
36. Di Biase L, Burkhardt JD, Mohanty P, Sanchez J, Mohanty S, Horton R, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. (2010) 122:109–18. doi: 10.1161/CIRCULATIONAHA.109.928903
37. Yaghi S, Song C, Gray WA, Furie KL, Elkind MS, Kamel H. Left atrial appendage function and stroke risk. Stroke. (2015) 46:3554–9. doi: 10.1161/STROKEAHA.115.011273
38. Pálinkás A, Varga A, Nyúzó B, Gruber N, Forster T, Nemes A, et al. A bal pitvari fülcse áramlás szerepe a cardioversio rövid és hosszú távú sikerességének elórejelzésében nem valvularis eredetú pitvarfibrilláció fennállásakor [Clinical role of flow velocity of left atrial auricle for prediction of short and long term success of cardioversion in patients with non-valvular atrial fibrillation]. Orv Hetil. (2002) 143:2035–41.
39. Antonielli E, Pizzuti A, Pálinkás A, Tanga M, Gruber N, Michelassi C, et al. Clinical value of left atrial appendage flow for prediction of long-term sinus rhythm maintenance in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol. (2002) 39:1443–9. doi: 10.1016/s0735-1097(02)01800-4
40. Wang YC, Lin LC, Lin MS, Lai LP, Hwang JJ, Tseng YZ, et al. Identification of good responders to rhythm control of paroxysmal and persistent atrial fibrillation by transthoracic and transesophageal echocardiography. Cardiology. (2005) 104:202–9. doi: 10.1159/000088174
41. Combes S, Jacob S, Combes N, Karam N, Chaumeil A, Guy-Moyat B, et al. Predicting favourable outcomes in the setting of radiofrequency catheter ablation of long-standing persistent atrial fibrillation: a pilot study assessing the value of left atrial appendage peak flow velocity. Arch Cardiovasc Dis. (2013) 106:36–43. doi: 10.1016/j.acvd.2012.09.002
42. Donal E, Grimm RA, Yamada H, Kim YJ, Marrouche N, Natale A, et al. Usefulness of doppler assessment of pulmonary vein and left atrial appendage flow following pulmonary vein isolation of chronic atrial fibrillation in predicting recovery of left atrial function. Am J Cardiol. (2005) 95:941–7. doi: 10.1016/j.amjcard.2004.12.031
43. Ezzeddine FM, DeSimone CV. Left atrial appendage emptying velocity as a predictor of recurrence of atrial fibrillation post-ablation. J Cardiovasc Electrophysiol. (2022) doi: 10.1111/jce.15583. [Epub ahead of print].
44. Jalife J, Kaur K. Atrial remodeling, fibrosis, and atrial fibrillation. Trends Cardiovasc Med. (2015) 25:475–84. doi: 10.1016/j.tcm.2014.12.015
45. Nedios S, Lindemann F, Heijman J, Crijns HJGM, Bollmann A, Hindricks G. Atrial remodeling and atrial fibrillation recurrence after catheter ablation : past, present, and future developments. Herz. (2021) 46:312–7. doi: 10.1007/s00059-021-05050-1
46. Lo LW, Chen SA. Cardiac remodeling after atrial fibrillation ablation. J Atr Fibrillation. (2013) 6:877. doi: 10.4022/jafib.877
47. Kiedrowicz RM, Wielusinski M, Wojtarowicz A, Kazmierczak J. Left and right atrial appendage functional features as predictors for voltage-defined left atrial remodelling in patients with long-standing persistent atrial fibrillation. Heart Vessels. (2021) 36:853–62. doi: 10.1007/s00380-020-01752-4
48. Thotamgari SR, Sheth AR, Ahmad J, Bawa D, Thevuthasan S, Babbili A, et al. Low left atrial appendage emptying velocity is a predictor of atrial fibrillation recurrence after catheter ablation. J Cardiovasc Electrophysiol. (2022) doi: 10.1111/jce.15580. [Epub ahead of print].
49. Kim MN, Lee JJ, Kim SA, Kim YH, Choi JI, Park SM, et al. The difference of predictors for recurrence after catheter ablation of non-paroxysmal atrial fibrillation according to follow-up period. Int Heart J. (2014) 55:312–8. doi: 10.1536/ihj.13-370
50. Delgado V, Di Biase L, Leung M, Romero J, Tops LF, Casadei B, et al. Structure and function of the left atrium and left atrial appendage: AF and stroke implications. J Am Coll Cardiol. (2017) 70:3157–72. doi: 10.1016/j.jacc.2017.10.063
51. Zhu F, Zhang B, Zhu W. Evaluation of the volume and function of left atrial appendage and left atrium in patients with atrial fibrillation by three-dimensional transesophageal echocardiography and transthoracic echocardiography. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2018) 43:1309–14. doi: 10.11817/j.issn.1672-7347.2018.12.005
Keywords: left atrial appendage flow velocity, atrial fibrillation, catheter ablation, recurrence, systematic review, meta-analysis
Citation: Chen P, Shi Y, Ju J, Pan D, Miao L, Guo X, Chen Z and Du J (2022) Left atrial appendage flow velocity predicts recurrence of atrial fibrillation after catheter ablation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 9:971848. doi: 10.3389/fcvm.2022.971848
Received: 17 June 2022; Accepted: 08 August 2022;
Published: 06 September 2022.
Edited by:
Young Keun On, Sungkyunkwan University, South KoreaReviewed by:
Eue-Keun Choi, Seoul National University Hospital, South KoreaMyung-Jin Cha, University of Ulsan, South Korea
Copyright © 2022 Chen, Shi, Ju, Pan, Miao, Guo, Chen and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuhong Chen, MTMyNjQyMTQ5MTlAMTYzLmNvbQ==; Jianpeng Du, MTM4MTE1MTgwNjJAMTYzLmNvbQ==
†These authors share first authorship
 Pengfei Chen
Pengfei Chen Yujiao Shi
Yujiao Shi Jianqing Ju
Jianqing Ju Deng Pan3
Deng Pan3 Lina Miao
Lina Miao Zhuhong Chen
Zhuhong Chen Jianpeng Du
Jianpeng Du