
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 19 October 2022
Sec. Atherosclerosis and Vascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.971141
This article is part of the Research Topic Aortic Stiffness Assessment in Daily Clinical Practice View all 5 articles
 Nonhlanhla Mthembu1†
Nonhlanhla Mthembu1† Vernice R. Peterson1†
Vernice R. Peterson1† Gavin R. Norton1†
Gavin R. Norton1† Eitzaz Sadiq2
Eitzaz Sadiq2 Andrea Kolkenbeck-Ruh1
Andrea Kolkenbeck-Ruh1 Ravi Naran1
Ravi Naran1 Suraj M. Yusuf1
Suraj M. Yusuf1 Grace Tade1
Grace Tade1 Hamza Bello1
Hamza Bello1 Adamu Bamaiyi1
Adamu Bamaiyi1 Carlos D. Libhaber1
Carlos D. Libhaber1 Patrick Dessein1
Patrick Dessein1 Ferande Peters1
Ferande Peters1 Taalib Monareng2
Taalib Monareng2 Talib Abdool-Carrim2
Talib Abdool-Carrim2 Ismail Cassimjee2
Ismail Cassimjee2 Pinhas Sareli1
Pinhas Sareli1 Girish Modi2
Girish Modi2 Angela J. Woodiwiss1*†
Angela J. Woodiwiss1*†Aims: A lower heart rate (HR) increases central blood pressure through enhanced backward wave pressures (Pb). We aimed to determine whether these relationships are modified by increases in aortic stiffness.
Methods: Using non-invasive central pressure, aortic velocity and diameter measurements in the outflow tract (echocardiography), we assessed the impact of aortic stiffness on relationships between HR and arterial wave morphology in 603 community participants < 60 years of age, 221 ≥ 60 years, and in 287 participants with arterial events [stroke and critical limb ischemia (CLI)].
Results: As compared to community participants < 60 years, those ≥ 60 years or with events had increased multivariate adjusted proximal aortic characteristic impedance (Zc) and carotid femoral pulse wave velocity (PWV) (p < 0.05 to < 0.0001). Community participants ≥ 60 years and those with events also had a greater slope of the inverse relationship between HR and Pb (p < 0.001 for comparison). While in community participants < 60 years, no interaction between indexes of aortic stiffness and HR occurred, in those ≥ 60 years (p < 0.02) and in those with arterial events (p = 0.001), beyond aortic root diameter, an interaction between Zc and HR, but not between PWV and HR independently associated with Pb. This translated into stepwise increases in the slope of HR-Pb relationships at incremental tertiles of Zc. Although HR was inversely associated with the systemic reflection coefficient in community participants ≥ 60 years (p < 0.0001), adjustments for the reflection coefficient failed to modify HR-Pb relations.
Conclusion: Beyond the impact on systemic wave reflection, increases in proximal aortic stiffness enhance the adverse effects of HR on Pb and hence central BP.
A lower heart rate (HR) is a well-established determinant of an increased central arterial (PPc), but not peripheral pulse pressure (PP) (1–3). A reduction in HR with β1 selective adrenergic receptor blockers increases PPc without modifying peripheral BP (4, 5). This effect is thought to in-part account for the limited ability of these agents to reduce the risk of cardiovascular events in hypertension when employed as first line-agents (6, 7). Although guidelines consequently recommend that β-blockers should not be used as first-line therapy for uncomplicated hypertension (8), little attention has been given to developing approaches to limit the adverse effects on PPc of any HR reducing agent, when required for use in cardiac conditions (9). In this regard, only more recent studies have identified the detailed mechanisms that explain HR relationships with central arterial pressure wave morphology (10). However, the factors that modify the impact of HR on PPc have not been determined. In this regard, detecting those most at risk for the adverse effects of HR on PPc may assist in planning therapeutic strategies that may minimize the impact of these effects. One possible factor that may modify the impact of HR on PPc is an increased aortic stiffness, a change that frequently accompanies the major risk factors for cardiac pathology.
Conventional thought is that a lower HR is associated with an increased PPc primarily through a prolonged filling period and hence ejection duration and stroke volume (SV) (11). The increased SV is thought to enhance forward traveling pressure waves (Pf) both through increases in peak aortic flow and ejection volume (reservoir pressure effect) (11). As increases in systemic flow may be advantageous in cardiac conditions, the potential deleterious impact of HR reduction on PPc in these conditions has been given little consideration. However, contemporary evidence shows that the relationship between HR and Pf is explained not by an increased SV or aortic flow, but because of an enhanced magnitude of re-reflected pressure waves generated by increases in reflected (backward traveling) pressure waves (Pb) (10). Importantly, in contrast to increases in SV or flow, which are potentially beneficial in cardiac conditions, increases in Pb create an impedance (resistance in a pulsatile system) to flow, which are likely to have adverse effects. The mechanisms that explain HR relationships with Pb include the inverse frequency dependency of the reflection coefficient and the harmonics of the pulse wave (which moves to a lower frequency at decreasing HR) (12). Thus, increases in aortic stiffness could produce two potential effects on the relationships between HR and Pb and hence PPc. Aortic stiffness increases aortic characteristic impedance to flow (Zc) and hence reduces the impedance mismatch between the aorta and more distal arterial vessels (1). As wave reflection occurs at points of impedance mismatch, an increased aortic stiffness could decrease the impact of HR on wave reflection and hence reduce the inverse relationship noted between HR and Pb. Alternatively, increases in aortic stiffness may also reduce the harmonic frequencies of the pulse wave (13) and increase the impact of a lower HR on Pb. Importantly, the effect of aortic stiffness on relationships between HR and central aortic pulse wave characteristics have not been determined in vivo. In the present study, we therefore aimed to compare relationships between HR and central arterial pulse wave morphology in those with either age-related increases in aortic stiffness (community sample ≥ 60 years of age) or arteriosclerotic-related arterial events that occur across the full adult age range in developing countries (14, 15) with a community sample < 60 years of age. We selected 60 years of age as the threshold (inflection point) at which age-related increases in aortic stiffness begin to markedly increase at a population level. This is indeed the age at which HR effects on PPc begin to produce clinically significant effects (10).
The present study was conducted according to the principles outlined in the Helsinki declaration. The Committee for Research on Human Subjects of the University of the Witwatersrand approved the protocols (approval numbers: M11-08-29, M14-04-29, M19-06-88, M16-04-11, M21-111-55, M02-04-72, M07-04-69, M12-04-108, M17-04-01, and M22-03-93). Participants gave informed, written consent. The present study design has previously been described (14–19). To obtain community participants either < or ≥ 60 years of age, nuclear families of black African descent (Nguni and Sotho chiefdoms) with siblings older than 16 years of age were randomly recruited (population census figures of 2001) from the South West Township (SOWETO) of Johannesburg, South Africa. In the present sub-study 824 participants had high quality aortic velocity measurements in the outflow tract. To obtain participants with arterial events, 287 consecutive black South Africans with stroke (n = 109) who did not have atrial fibrillation at the time of assessment or critical limb ischemia (CLI) (n = 178) with high quality aortic velocity assessments in the outflow tract were recruited from the Charlotte Maxeke Johannesburg Academic Hospital, South Africa. The presence of CLI or stroke was identified as described (14, 15). In this regard, black African patients attending the Charlotte Maxeke-Johannesburg Academic Hospital are of the same socioeconomic class as those living in the SOWETO community.
A questionnaire was administered to obtain demographic and clinical data as described (19). Clinical information was also extracted from the hospital records and confirmed by the attending physician (14, 15). Clinical data included the presence of risk factors and the therapy thereof. Height and weight were measured using standard approaches and participants were considered to be obese if their body mass index (BMI) was ≥ 30 kg/m2. Laboratory blood tests of renal function, liver function, blood glucose, hematological parameters, and percentage glycated hemoglobin (HbA1c) were performed. Diabetes mellitus (DM) was defined as the use of insulin or oral glucose lowering agents, a fasting plasma glucose concentration ≥ 7 mmol/l or an HbA1c value greater than 6.5%. High quality office brachial blood pressure (BP) measurements were obtained in the seated position and after 5 min of rest, by a trained nurse-technician using a standard mercury sphygmomanometer as previously described (19) and according to guidelines. The mean of 5 measurements obtained at least 30 s apart was taken as office BP. Hypertension was defined as a mean office BP ≥ 140 mm Hg systolic or ≥ 90 mm Hg diastolic BP or the use of antihypertensive medication.
Central arterial hemodynamics were determined from central arterial pressure recordings using pulse wave analysis and aortic velocity and diameter assessments obtained in the outflow tract as previously described (14–18). After participants had rested for 15 min in the supine position, arterial waveforms at the radial (dominant arm) pulse were recorded by applanation tonometry and SphygmoCor software. Central arterial waveforms were generated from peripheral waveforms using a validated generalized transfer function in SphygmoCor software. Immediately after peripheral and central arterial pressure waveforms were acquired, aortic velocity and diameter measurements were obtained by an experienced observer (AJW) in the left lateral decubitus position using an Acuson SC2000 Diagnostic ultrasound system (Siemens Medical Solutions, USA, Inc.). Velocity waveforms were obtained in the 5-chamber view. High quality velocity assessments were identified as those with a smooth velocity waveform with a dense leading (outer) edge and a clear maximum velocity. Aortic diameter measurements were obtained just proximal to the aortic leaflets in the long axis parasternal view. The largest diameter recorded in early systole was used to construct an aortic flow waveform.
Central arterial waveforms were generated as previously described (14–18) based on prior studies (20–22). Taking care to avoid any overshoot of the image, the leading (outer) edge or the most dense, or brightest, portion of the spectral image of the velocity waveform was outlined using graphics software. Aortic velocity and cross-sectional area were employed to construct a flow (Q) waveform. Characteristic impedance (Zc) was determined in the time domain using approaches previously described (20, 21) and validated against invasive pressure measurements (22). Using Zc values and flow and pressure waveforms, wave separation analysis was performed and Pb determined from (aortic PP – QxZc)/2 and Pf from (aortic PP + QxZc)/2 (14–18). The impact of HR on Pb independent of Pf was identified from reflection magnitude (Pb/Pf).
Heart rate (HR) was determined from the length (period, PD) of an averaged peripheral waveform captured over a 10 s period, using the formula: HR = 1,000/PDx60. The systemic (global) reflection coefficient was determined as (1 − Zc/SVR)/(1 + Zc/SVR) (12, 23), where SVR is systemic vascular resistance (or resistance vessel impedance, Zr) calculated from (mean arterial pressure-right atrial pressure)/cardiac output assuming right atrial pressure = 0 mm Hg. Carotid-femoral pulse wave velocity (PWV) was determined using standard approaches from SphygmoCor software (14, 15).
For database management and statistical analysis, SAS software, version 9.4 (SAS Institute Inc., Cary, NC) was employed. Continuous variables are expressed as mean (SD or SEM). Dichotomous variables are expressed as percentages. For graphical representation of variables at different HR values, multiple variable adjusted data are shown across septiles of HR. Multiple linear regression analysis was performed to determine the independent relations between HR and hemodynamic variables. In regression analysis, adjustments were for age, sex, regular alcohol intake, regular tobacco intake, BMI, DM, mean arterial pressure (MAP) and the use of antihypertensive treatment. Probability values < 0.05 were considered to be significant. As age may affect the impact of aortic stiffness on HR-Pb relationships, sensitivity analysis was performed in those with arterial events < 60 years of age.
The participant characteristics are given in Table 1. Arterial events occurred over an age range from 18 to 92 years. Participants with arterial events and community participants ≥ 60 years of age had a greater prevalence of several major risk factors including diabetes mellitus and hypertension, and regular smoking. Moreover, HDL cholesterol concentrations were lower in patients with vascular events. Although LDL cholesterol concentrations were also lower in cases, 86.6% of these patients were receiving lipid-lowering therapy at the time of obtaining fasting blood samples.
With adjustments for age, sex, and associated risk factors, in both community participants ≥ 60 years of age and in those with arterial events, an increase in carotid-femoral PWV and proximal aortic characteristic impedance (Zc) was noted as compared to community participants < 60 years of age (Table 2). Differences in Zc were attributed to variations in aortic stiffness as these differences were retained with further adjustments for aortic root diameter (Table 2). In sensitivity analysis conducted in participants with arterial events < 60 years of age, both PWV (p < 0.0001) and Zc (p < 0.02) were also increased as compared with age-matched community participants. As compared to community participants < 60 years of age, community participants ≥ 60 years of age and those with arterial events had increases in multivariate adjusted Pf and peak pressures generated by the product of Zc and Q (PQxZc) (Table 2). With adjustments, community participants ≥ 60 years of age, but not those with arterial events showed a trend for increases in Pb and RM (Table 2). In sensitivity analysis conducted in participants with arterial events < 60 years of age, Pf and PQxZc were also increased compared with age-matched community participants (p < 0.0001), while neither Pb nor RM showed differences (p = 0.14 and p = 0.73, respectively).
Independent of confounders, HR was strongly and inversely associated with both Pb and PPc in both community participants and in those with arterial events (Figure 1 and Table 3). However, the slope of the regression relationship (β-coefficient) between HR and both Pb and PPc was increased in community participants ≥ 60 years of age and in those with events as compared to community participants < 60 years of age (Figure 1). In sensitivity analysis conducted in those with events < 60 years of age, the slope of the relationship between HR and Pb or PPc was similarly greater than in community participants < 60 years of age (β-coefficients ± SEM, HR vs. Pb; community sample = −0.097 ± 0.013, arterial events = −0.169 ± 0.022, p < 0.01 for comparison, HR vs. PPc; community sample = −0.198 ± 0.030, arterial events = −0.326 ± 0.040, p < 0.05 for comparison).
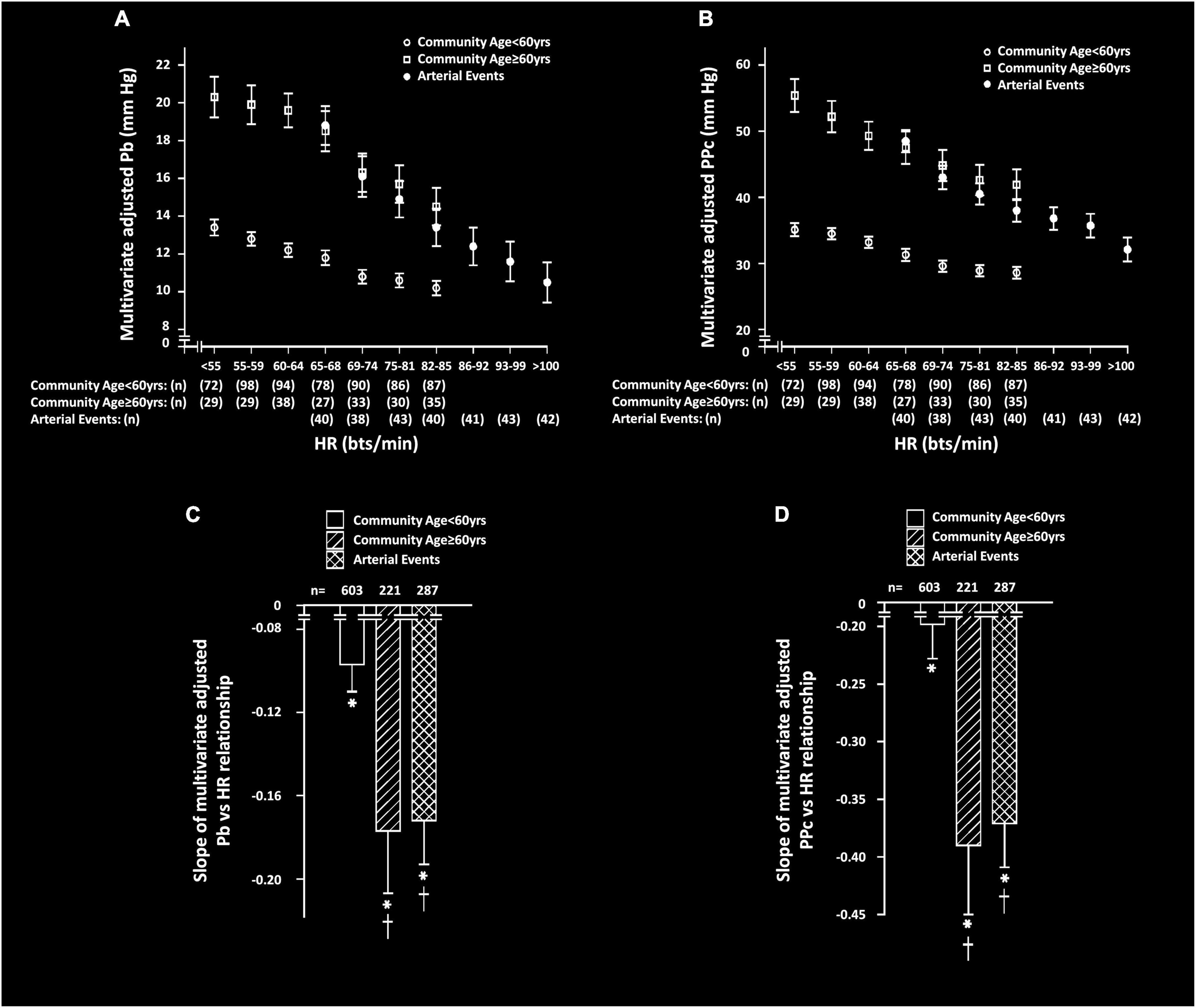
Figure 1. Multivariate adjusted backward wave pressures (Pb) or central arterial pulse pressure (PPc) across septiles of heart rate (HR) (A,B) and slopes of HR-Pb relationships (C,D) in participants from a community sample either younger (community < 60 years) or older (community ≥ 60 years) than 60 years or in those with arterial events (stroke or critical limb ischemia). Panels C and D show comparison of the multivariate adjusted slope (β-coefficient) of the relationships. All data are adjusted for age, sex, MAP, BMI, regular smoking, regular alcohol, DM, and antihypertensive therapy. *p < 0.0001 for relationships. †p < 0.01 vs. slopes of relationships in community participants < 60 years.
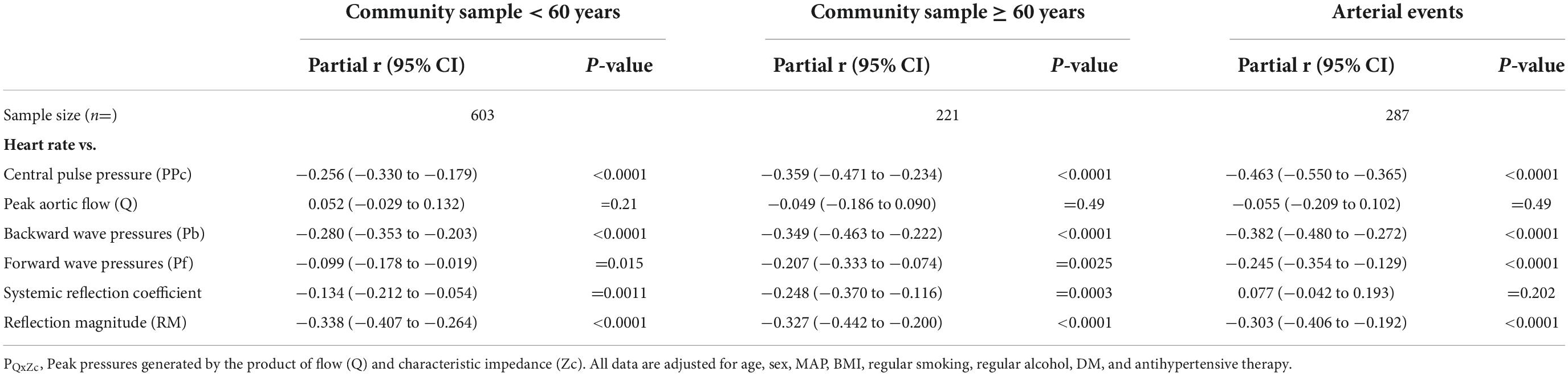
Table 3. Multivariate adjusted relationships (partial r) between heart rate (HR) and central arterial function.
Beyond the individual terms and additional confounders, an interaction between Zc and HR was independently associated with Pb in both community participants ≥ 60 years of age (p < 0.02) and in participants with arterial events (p = 0.001). However, no independent interaction was noted between Zc and HR as a determinant of Pb in community participants < 60 years of age (p = 0.77). These interactions translated into a stepwise increase in the slope of the relationships between HR and Pb in community participants ≥ 60 years of age and in those with arterial events, while no increase in the relationship was noted in community participants < 60 years of age (Figure 2). In sensitivity analysis conducted in participants with arterial events < 60 years of age, an independent interaction between HR and Zc was similarly independently associated with Pb (p = 0.02). In contrast to the interactions between Zc and HR, in neither community participants ≥ 60 years of age (p = 0.12), nor in participants with arterial events (p = 0.24) was an interaction between HR and carotid-femoral PWV independently associated with Pb.
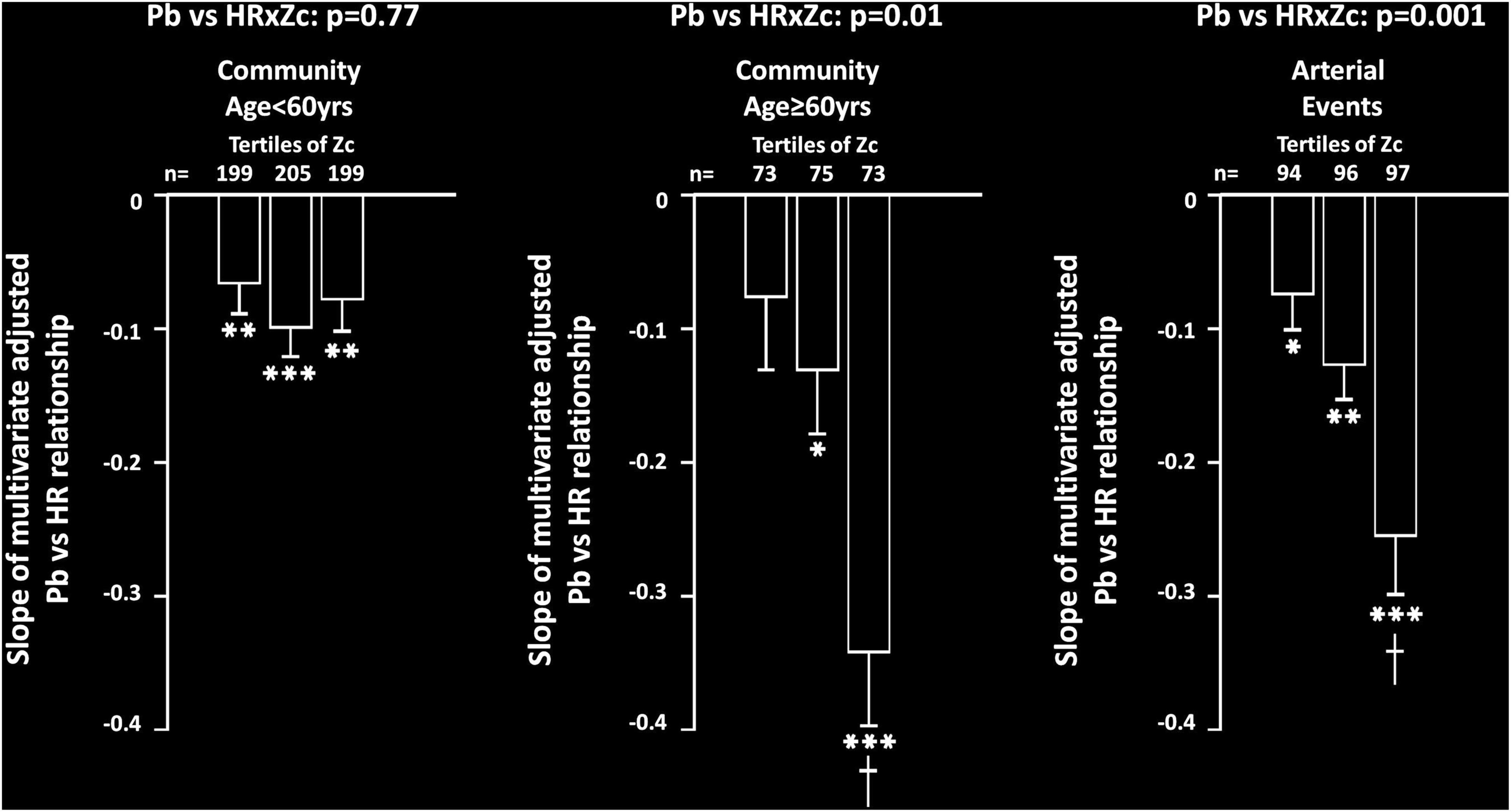
Figure 2. Impact of proximal aortic characteristic impedance (Zc) on multivariate adjusted slopes (β-coefficient) of relationships between heart rate (HR) and backward wave pressures (Pb) in participants from a community sample either younger (community < 60 years) or older (community ≥ 60 years) than 60 years or in those with arterial events (stroke or critical limb ischemia). Relationships are shown across tertiles of Zc in each group. All data are adjusted for age, sex, MAP, BMI, regular smoking, regular alcohol, DM, and antihypertensive therapy. *p < 0.02, **p < 0.005, ***p < 0.0001 for relationships. †p < 0.01 vs. slopes of relationships in first and second tertiles of Zc.
HR was strongly and independently associated with a decrease in the systemic reflection coefficient in community participants, but not in participants with arterial events (Table 3). Importantly, relationships between HR and Pb were unaffected by adjustments for the systemic reflection coefficient in either community participants < or ≥ 60 years of age or in participants with arterial events (Figure 3).
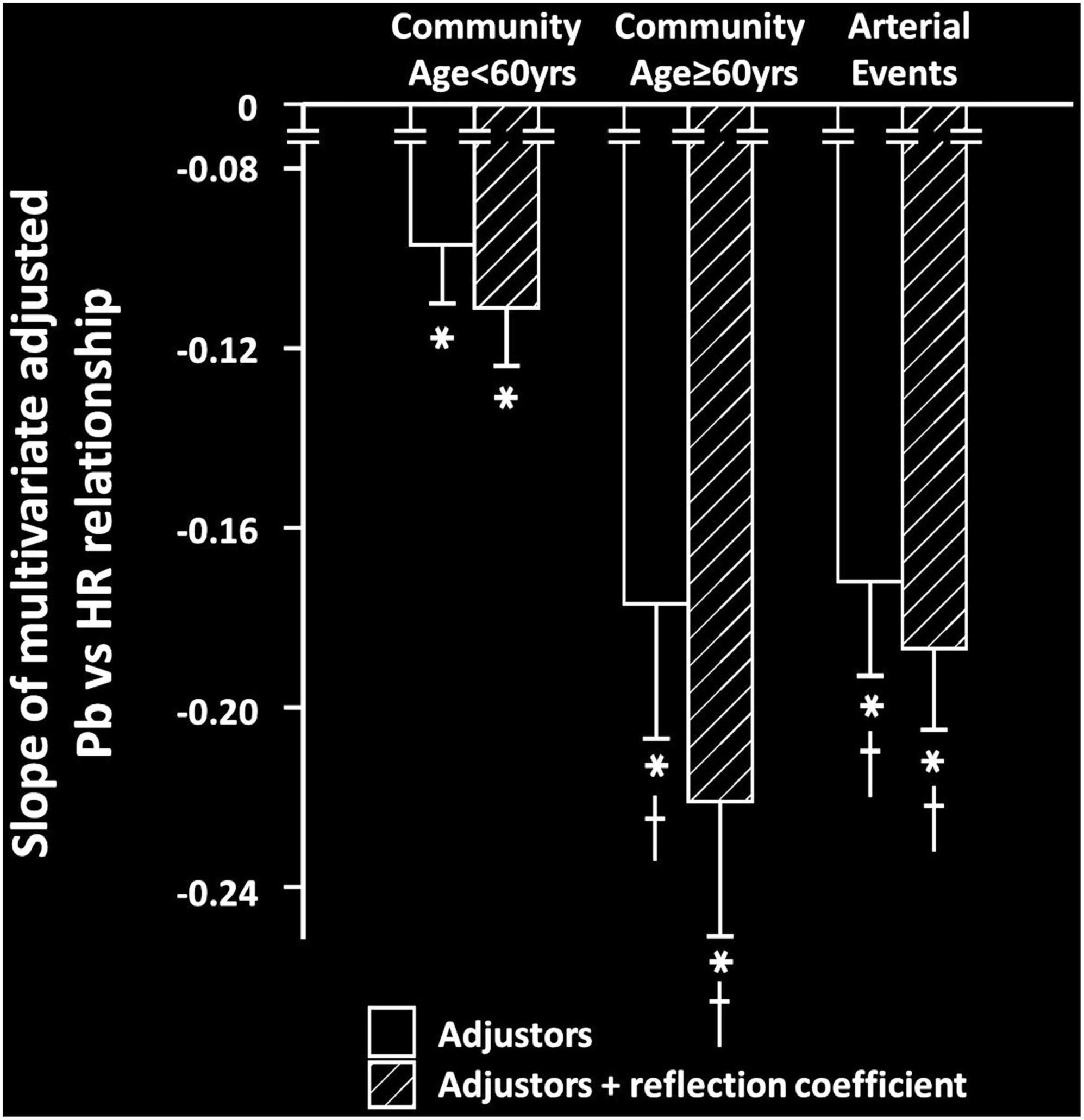
Figure 3. Impact of adjustments for the systemic reflection coefficient on the multivariate adjusted slopes (β-coefficient) of relationships between heart rate (HR) and backward wave pressures (Pb) in participants from a community sample either younger (community < 60 years) or older (community ≥ 60 years) than 60 years or in those with arterial events (stroke or critical limb ischemia). All data are adjusted for age, sex, MAP, BMI, regular smoking, regular alcohol, DM, and antihypertensive therapy. *p < 0.0001 for relationships. †p < 0.01 vs. slopes of relationships in community participants < 60 years. No differences between relationships were noted before and after adjustments for the systemic reflection coefficient.
In the present study we assessed the impact of aortic stiffness on the relationships between a lower HR and increased central arterial backward wave pressures (Pb) and hence pulse pressure (PPc). As compared to community participants < 60 years of age, we noted an increased slope of these relationships in both community participants ≥ 60 years of age with an increased aortic stiffness and in those with arterial events (stroke and CLI) also with an increased aortic stiffness. The increased slope of the HR-Pb relationships in the groups with an increased aortic stiffness was explained by an interaction between proximal aortic characteristic impedance (Zc) and HR, independent of aortic root diameter. In this regard, in these groups, but not in community participants < 60 years of age, HR-Pb relationships showed stepwise increases across increasing tertiles of Zc. In contrast, no interaction between HR and carotid-femoral PWV was noted. Thus, increases in stiffness in the proximal, but not distal portion of the aorta accounted for the enhanced HR-Pb relations in groups with an increased aortic stiffness. Although HR was independently associated with the systemic reflection coefficient in community participants, adjustments for the systemic reflection coefficient failed to modify HR-Pb relationships in any group.
Through their limited benefits on outcomes (6, 7) β1 selective adrenergic receptor blockers are not recommended for use as first line agents in uncomplicated hypertension. However, underlying cardiac disease is a compelling indication for their use and they may be used as second, or third line agents even without cardiac disease (8). A well-recognized mechanism that may explain the adverse effects of β1 selective adrenergic receptor blockers is the increases in central, but not peripheral PP that occur with decreases in HR (2–5). Little attention has nevertheless been given to these possible adverse effects (2–5) as traditional thought is that the primary mechanisms responsible for these changes is an increased systemic flow, which may have possible beneficial effects in cardiac disease. However, more recent evidence indicates that decreases in HR increase central PP through an impact on Pb and not flow (10). As Pb creates an impedance to flow, the benefits produced by decreases in HR in cardiac disease may be offset by adverse effects on the LV. Therefore, identifying those most at risk of the adverse effects of HR reducing agents may improve approaches to the use of these agents in cardiac disease. In this regard, the present study is the first to show that those with an increased aortic stiffness are most likely to develop deleterious effects of HR reducing agents on central arterial PP and that strategies to manage the effects of HR reduction on central PP are therefore required in these patients.
The present study suggests that increases in aortic stiffness primarily reduce the harmonic frequencies of the pulse wave and in so doing enhance the magnitude of the pulse wave at lower HR values (13). In this regard, in silico studies demonstrate that the mechanisms that explain HR relationships with Pb include the inverse frequency dependency of the reflection coefficient and the harmonics of the pulse wave (which moves to a lower frequency at a decreasing HR) (12). In this regard, the reflection factor is well described as having primarily an inverse relationship with the frequency of the pulse in the ascending aorta (24). A lower HR is associated with lower frequency pulses in the aorta (12). As aortic stiffness increases aortic impedance, it decreases the impedance mismatch between the aorta and more distal vessels, an effect that will decrease the reflection coefficient. Thus, aortic stiffness may decrease wave reflection and reduce the impact of HR on Pb. However, in the present study, although HR was inversely associated with the reflection coefficient in community participants, adjustments for the reflection coefficient failed to modify HR-Pb relations in any of the groups. Thus, the dominant effect of HR on Pb is likely to be determined by harmonic effects on the pulse wave.
An older age is strongly associated with increases in Pb (18). As the average age of older community participants was greater than community participants < 60 years of age, it may be argued that the greater HR-Pb relations in those with an increased aortic stiffness can be attributed to an age-related increase in Pb. However, multivariate adjusted Pb values in those with arterial events was no greater than community participants < 60 years of age and HR-Pb relations were markedly increased in those with events. Moreover, in sensitivity analysis conducted in those with arterial events < 60 years of age, a markedly greater slope of the HR-Pb relationship was similarly noted as compared to community participants < 60 years of age. Hence, it is unlikely that an age-related increase in Pb is the explanation for the greater HR-Pb relations in those groups with an increased aortic stiffness.
There are several clinical implications of the present study. First, the present study explains the markedly greater relations between HR and central PP in those over 60 years of age (10). In this regard, older individuals have a greater aortic stiffness. Importantly, previous work suggests that the use of HR reducing agents in those younger than 60 years of age produces little clinical impact on BP (10). However, the present study indicates that even in those younger than 60 years of age with a high aortic stiffness, HR reduction will produce important adverse effects on BP that will not be detected at the peripheral pulse. Thus, PWV should be determined in younger individuals with risk factors and if HR reducing agents are required, approaches to limit these effects should be employed. Although speculative, the possible approaches to limiting the adverse effects of HR on Pb should therefore be considered. In this regard, although there is no proven ability to attenuate aortic stiffness, Zc may be reduced by decreasing aortic distending pressures (MAP). This may be achieved through the use of a variety of antihypertensives and intense brachial BP reduction may be required to decrease Zc and hence HR effects on Pb in those with increases in aortic stiffness.
The present study has several limitations. First, the present study was cross-sectional in design and hence causality may not be inferred. Further studies with HR reducing agents or with artificial pacing are required to identify whether aortic stiffness enhances the impact of HR on central PP. Second, the present study was not conducted in those with cardiac disease requiring HR reducing agents. Further work is therefore also required in patients with coronary artery disease or heart failure with a reduced ejection fraction who require HR reduction.
In conclusion, in the present study we show that increases in aortic stiffness noted to occur in either the elderly or in high risk patients at any age, enhance the adverse effect of a reduced HR on central arterial PP. This occurs as increments in proximal aortic stiffness (as indexed by characteristic impedance independent of aortic root diameter) augment the increase in backward wave pressures that occur at lower HR values. As HR relationships with backward wave pressures occurred largely independent of the systemic reflection coefficient, these adverse effects of aortic stiffness are explained by the impact of stiffness on the harmonics of the pulse wave. These data suggest that aortic stiffness should be determined before HR reducing agents are initiated and that approaches to oppose the adverse effects of HR on backward wave pressures require identification in future studies.
The raw data supporting the conclusions of this article will be made available by the authors, upon reasonable request.
The studies involving human participants were reviewed and approved by the Human Research Ethics Committee of the University of the Witwatersrand. The patients/participants provided their written informed consent to participate in this study.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This study was supported by the Medical Research Council of South Africa, the University Research Council of the University of the Witwatersrand, the South African National Research Foundation, and the Circulatory Disorders Research Trust.
This study would not have been possible without the voluntary collaboration of the participants and the excellent technical assistance of Mthuthuzeli Kiviet, Nomonde Molebatsi, Nkele Maseko, and Delene Nciweni.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BMI, body mass index; BP, blood pressure; CLI, critical limb ischemia; DBP, diastolic blood pressure; DM, diabetes mellitus; HbA1c, glycated hemoglobin; HDL, cholesterol high density lipoprotein cholesterol; HR, heart rate; LDL, cholesterol low density lipoprotein cholesterol; LV, left ventricle; MAP, mean arterial pressure; Pb, backward traveling (reflected) pressure wave; Pf, forward traveling pressure wave; PP, pulse pressure; PPc, central arterial pulse pressure; PQxZc, peak pressure generated by product of flow and characteristic impedance; PWV, carotid femoral pulse wave velocity; Q, peak aortic flow; RM, reflection magnitude (Pb/Pf); SBP, systolic blood pressure; SV, stroke volume; SVR, systemic vascular resistance; Zc, aortic characteristic impedance; Zr, resistance vessel impedance.
1. Nichols WW, O’Rourke MF, Vlachopoulos C. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. 6th Edn. Boca Raton, FL: CRC-Taylor & Francis Group (2011).
2. Tan I, Kiat H, Barin E, Butlin M, Avolio AP. Effects of pacing modality on noninvasive assessment of heart rate dependency of indices of large artery function. J Appl Physiol. (2016) 121:771–80. doi: 10.1152/japplphysiol.00445.2016
3. Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. (2000) 525:263–70.
4. Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. (2006) 113:1213–25.
5. Williams B, Lacy PS. CAFE and the ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) investigators. impact of heart rate on central aortic pressures and hemodynamics: analysis from the CAFE (Conduit Artery Function Evaluation) study: CAFE-heart rate. J Am Coll Cardiol. (2009) 54:705–13. doi: 10.1016/j.jacc.2009.02.088
6. Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH. Beta-blockers for hypertension. Cochrane Database Syst Rev. (2017) 11:CD002003. doi: 10.1002/14651858.CD002003.pub5
7. Wright JM, Musini VM, Gill R. First-line drugs for hypertension. Cochrane Database Syst Rev. (2018) 4:CD001841. doi: 10.1002/14651858.CD001841.pub3
8. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. (2018) 71:e127–248. doi: 10.1016/j.jacc.2017.11.006
9. András Dézsi C, Szentes V. The real role of β-blockers in daily cardiovascular therapy. Am J Cardiovasc Drugs. (2017) 17:361–73.
10. Mthembu N, Norton GR, Peterson VR, Naran R, Yusuf SM, Tade G, et al. Increased backward wave pressures rather than flow explain age-dependent heart rate effects on central, but not peripheral arterial pressure. Hypertension. (2022) 79:435–46. doi: 10.1161/HYPERTENSIONAHA.121.18271
11. Sluyter JD, Hughes AD, Lowe A, Parker KM, Camargo CA Jr, Hametner B, et al. Different associations between beta-blockers and other antihypertensive medication combinations with brachial blood pressure and aortic waveform parameters. Int J Cardiol. (2016) 219:257–63. doi: 10.1016/j.ijcard.2016.06.051
12. Xiao H, Tan I, Butlin M, Li D, Avolio AP. Mechanism underlying the heart rate dependency of wave reflection in the aorta: a numerical simulation. Am J Physiol Heart Circ Physiol. (2018) 314:H443–51. doi: 10.1152/ajpheart.00559.2017
13. Wang SH, Hsu TL, Jan MY, Wang YYL, Wang WK. Age-related changes in specific harmonic indices of pressure pulse waveform. Proceedings of the 13th International Conference on Biomedical Engineering. IFMBE Proceedings. Berlin (2009).
14. Motau TH, Norton GR, Sadiq E, Manyatsi N, Kolkenbeck-Ruh A, Robinson C, et al. Marked arterial functional changes in patients with arterial vascular events across the early adult lifespan. Arterioscler Thromb Vasc Biol. (2020) 40:1574–86. doi: 10.1161/ATVBAHA.119.313734
15. Motau TH, Norton GR, Mmopi KN, Bello H, Peterson VR, Libhaber C, et al. Relations of aortic stiffness with arterial damage beyond brachial pressure are both dependent and independent of central arterial pulsatile load. J Hypertens. (2021) 39:718–28. doi: 10.1097/HJH.0000000000002695
16. Woodiwiss AJ, Mmopi KN, Peterson V, Libhaber C, Bello H, Masiu M, et al. Distinct contribution of systemic blood flow to hypertension in an African population across the adult lifespan. Hypertension. (2020) 76:410–9. doi: 10.1161/HYPERTENSIONAHA.120.14925
17. Mmopi KN, Norton GR, Bello H, Libhaber C, Masiu M, Da Silva Fernandes D. Increased aortic characteristic impedance explains relations between urinary Na+/K+ and pulse or systolic blood pressure. Hypertension. (2020) 75:1260–70.
18. Bello H, Norton GR, Peterson VR, Mmopi KN, Mthembu N, Libhaber CD, et al. Hemodynamic determinants of age versus left ventricular diastolic function relations across the full adult age range. Hypertension. (2020) 75:1574–83. doi: 10.1161/HYPERTENSIONAHA.119.14622
19. Woodiwiss AJ, Molebatsi N, Maseko MJ, Libhaber E, Libhaber C, Majane OH, et al. Nurse-recorded auscultatory blood pressure at a single visit predicts target organ changes as well as ambulatory blood pressure. J Hypertens. (2009) 27:287–97. doi: 10.1097/HJH.0b013e328317a78f
20. Segers P, Rietzschel ER, De Buyzere ML, Vermeersch SJ, De Bacquer D, Van Bortel LM, et al. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension. (2007) 49:1248–55. doi: 10.1161/HYPERTENSIONAHA.106.085480
21. Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, et al. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. (2010) 122:1379–86. doi: 10.1161/CIRCULATIONAHA.109.914507
22. Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of new technique. J Am Coll Cardiol. (1997) 20:952–63. doi: 10.1016/0735-1097(92)90198-v
23. London GM, Pannier B, Safar ME. Arterial stiffness gradient, systemic reflection coefficient, and pulsatile pressure wave transmission in essential hypertension. Hypertension. (2019) 74:1366–72. doi: 10.1161/HYPERTENSIONAHA.119.13387
Keywords: heart rate, aortic pressure, flow, forward waves, backward waves, age
Citation: Mthembu N, Peterson VR, Norton GR, Sadiq E, Kolkenbeck-Ruh A, Naran R, Yusuf SM, Tade G, Bello H, Bamaiyi A, Libhaber CD, Dessein P, Peters F, Monareng T, Abdool-Carrim T, Cassimjee I, Sareli P, Modi G and Woodiwiss AJ (2022) Proximal aortic stiffness modifies the relationship between heart rate and backward wave and hence central arterial pulse pressure. Front. Cardiovasc. Med. 9:971141. doi: 10.3389/fcvm.2022.971141
Received: 16 June 2022; Accepted: 21 September 2022;
Published: 19 October 2022.
Edited by:
Paolo Salvi, Istituto Auxologico Italiano (IRCCS), ItalyReviewed by:
Daniele Bianchi, Campus Bio-Medico University, ItalyCopyright © 2022 Mthembu, Peterson, Norton, Sadiq, Kolkenbeck-Ruh, Naran, Yusuf, Tade, Bello, Bamaiyi, Libhaber, Dessein, Peters, Monareng, Abdool-Carrim, Cassimjee, Sareli, Modi and Woodiwiss. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angela J. Woodiwiss, YW5nZWxhLndvb2Rpd2lzc0B3aXRzLmFjLnph
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.