
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 08 August 2022
Sec. Cardiovascular Epidemiology and Prevention
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.969259
Background: Statins are frequently prescribed with direct oral anticoagulants (DOACs), and previous studies have raised concerns about the increased risk of intracerebral hemorrhage or other major bleeding in concurrent statins and DOACs use. The objective of this study is to evaluate the risk of major bleeding in non-valvular atrial fibrillation patients taking DOACs with or without statins.
Methods: This nationwide, retrospective cohort study used data from the Taiwan National Health Insurance Research Database, enrolled a total of 90,731 non-valvular atrial fibrillation patients receiving rivaroxaban, dabigatran, apixaban or edoxaban from January 1st, 2012 to December 31st, 2017. Major bleeding was defined as a hospitalization or emergency department visit with a primary diagnosis of intracerebral hemorrhage, gastrointestinal tract bleeding, urogenital tract bleeding, or other sites of bleeding. Adjusted incidence rate ratios (IRR) and differences of major bleeding between person-quarters of DOACs with or without statins were estimated using a Poisson regression and inverse probability of treatment weighting using the propensity score.
Results: 50,854 (56.0%) of them were male with a mean age of 74.9 (SD, 10.4) years. Using DOACs without statins as a reference, the adjusted IRR for all major bleedings in concurrent use of DOACs and statins was 0.8 (95% CI 0.72–0.81). Lower major bleeding risk was seen in both low-to-moderate-intensity statins (IRR: 0.8, 95% CI 0.74–0.84) and high-intensity statins (IRR: 0.8, 95% CI 0.74–0.88). Concurrent use of DOACs and statins decreased the risk for intracerebral hemorrhage with an IRR of 0.8 (95% CI 0.66–0.93), and gastrointestinal tract bleeding with an IRR of 0.7 (95% CI 0.69–0.79). The protective effect of statins on intracerebral hemorrhage was observed only in female patients (IRR 0.67, 95% CI 0.51–0.89), but not in male patients (IRR 0.87, 95% CI 0.70–1.08).
Conclusions: Among non-valvular atrial fibrillation patients who were taking DOACs, concurrent use of statins decreased major bleeding risk, including intracerebral hemorrhage and gastrointestinal tract bleeding. Considering this and other cardioprotective effects, statins should be considered in all eligible patients prescribed with DOACs.
Atrial fibrillation (AF) is the most common arrhythmia; patients having AF and other comorbidities, especially those with a high CHA2DS2-VASc score, are at an increased risk of embolic stroke (1). Oral anticoagulation is a widely-used management strategy for AF, and direct oral anticoagulants (DOACs) are often prescribed due to their favorable efficacy and safety profiles compared to vitamin K antagonists (VKA) (2–5). However, their bleeding risks cannot be neglected (6), especially in patients with multiple medications and potential drug-drug interactions. Hyperlipidemia is another important risk factor for stroke and cardiovascular disease. Intensive lipid control with statins is widely recommended for patients at high risks (7, 8). The relationship between statins and bleeding risk, especially intracerebral hemorrhage (ICH), has been debated for years with inconsistent findings. In the SPARCL trial, high-dose statins were associated with a slightly increased ICH incidence (9). A meta-analysis of several randomized controlled trials also showed that statins might increase the risk of ICH in patients with a history of stroke or cardiovascular disease (10). However, in other meta-analyses and cohort studies, statins were not associated with a higher ICH risk (11–13). Even in patients with a history of ICH, statins may not increase the risk of recurrent ICH (14). Expert consensus currently supports the notion that the benefits of statins in preventing cardiovascular events outweighs the possible increased risk of ICH (15).
AF and dyslipidemia are common co-morbidities and thus DOACs and statins are frequently prescribed together. An earlier study reported reduced ICH risk in patients taking atorvastatin and DOACs (16), while other research found that dabigatran users had a higher risk of major hemorrhage when prescribed with simvastatin or lovastatin than with other statins (17). Whether concurrent use of different intensities of statins and DOACs increases the risk of ICH or other major bleeding remains unclear. To clarify this, we used a nationwide cohort of non-valvular AF patients to estimate the bleeding risk of simultaneous DOACs and different intensities of statins use.
This study was approved by the institutional review board of the Chang Gung Memorial Hospital, Taoyuan, Taiwan (IRB No.: 201701397B1). Informed consent was waived as no identifiable information was used in this study.
This retrospective cohort study investigated all patients that were registered in the Taiwan National Health Insurance (NHI) program. The Taiwan NHI program was founded in 1995 as a single-payer insurance system and is mandatory for all citizens and foreigners living in Taiwan for more than 6 months. By the end of 2017, ~23 million beneficiaries were registered in the NHI with a coverage rate of more than 99.5%. The NHI maintains an anonymous database, National Health Insurance Research Database (NHIRD), since 1995 that records comprehensive characteristics of all beneficiaries, including medical diagnoses, detailed prescriptions, examinations, surgical procedures, and incurred fees. From 1997 to 2015, the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes were used for diagnosis and procedure coding, and the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes have been used since 2016.
The data repository of NHIRD is Health and Welfare Data Science Center, Taiwan. Researchers can obtain the data through formal application to the Health and Welfare Data Science Center, Department of Statistics, Ministry of Health and Welfare, Taiwan (https://dep.mohw.gov.tw/DOS/np-2500-113.html).
All patients with 2 consecutive records of non-valvular AF diagnosis (ICD-9-CM code 427.31 or ICD-10-CM code I48) (16) and at least one DOAC prescription (rivaroxaban, dabigatran, apixaban or edoxaban) from January 1st 2012 to December 31st 2017 were enrolled. Patients who had valvular heart disease, joint surgery within 6 months prior to the first DOAC prescription, end-stage renal disease, previous ICH history, or cerebral vascular anomaly were excluded (Figure 1).
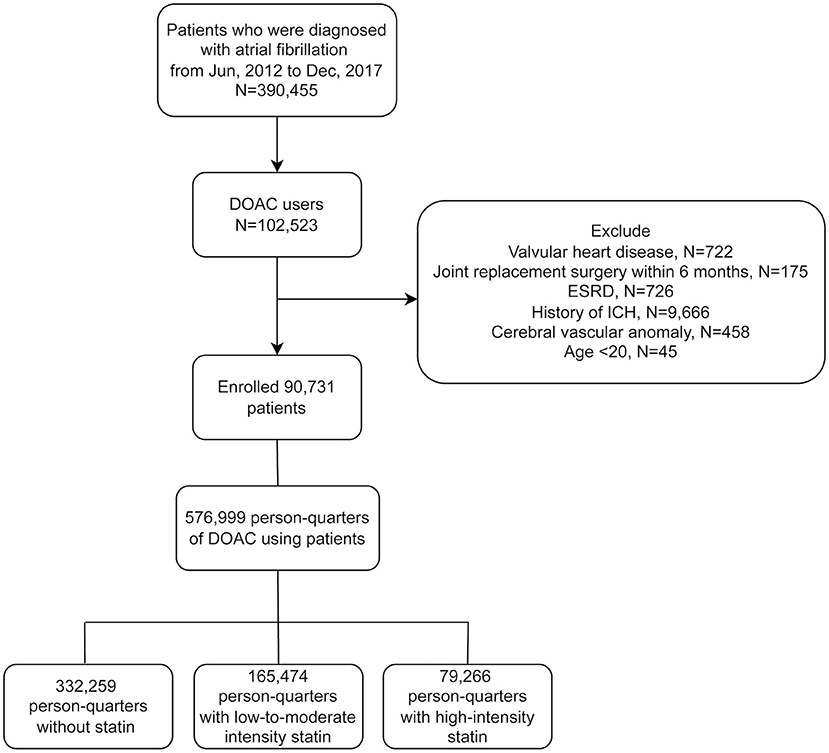
Figure 1. Flowchart of the study protocol. DOAC, direct oral anticoagulant; ESRD, end-stage renal disease; ICH, intracerebral hemorrhage.
We partitioned each calendar year into four quarters for each patient and each year after the first prescription of DOAC. One person-quarter (PQ) was used as an analytical unit. We used PQs because medications for chronic illnesses were refilled with a maximum duration of 3 months per the Taiwan NHI reimbursement policy (16, 18). Medications and covariates were assessed for each PQ, and PQs exposed to DOACs with or without statins were identified. The major bleeding risks of PQs exposed to statins and DOACs were compared with PQs exposed to DOACs alone. PQ with statin using were further subdivided into low-to-moderate- and high-intensity statin groups. High-intensity statins were defined as atorvastatin 40–80 mg or rosuvastatin 20 mg per day (Supplementary Table 1) (19). This design may help to overcome the complexity of prescription adjustments (changing the intensities of statins or stopping statins due to side effects) in real-world practice and to identify the events under specific medications.
The primary outcome was major bleeding, defined as a hospitalization or an emergency department visit with a primary diagnosis of ICH, gastrointestinal tract (GI) bleeding, urogenital bleeding, or other sites of bleeding (listed in Supplementary Table 1). Within the PQ, the time-points of DOACs prescription, statin prescription, and bleedings were all recorded, as to ensure that only bleedings after DOACs prescription were counted, and only bleedings after statin prescription were recorded as bleeding events with statin using. Examples are shown in Supplementary Figure.
Patient demographics, including sex, age, socioeconomic status, occupation, comorbidities, and relevant medications were identified as covariates. Comorbidities were the components of the Charlson comorbidity index (myocardial infarction, congestive failure, peripheral vascular disease, stroke, transient ischemic attack, dementia, chronic obstructive lung disease, connective tissue disease, peptic ulcer disease, liver disease, diabetes mellitus, moderate to severe chronic kidney disease, cancer, leukemia, lymphoma, acquired immune-deficient syndrome). We also included medications of proton pump inhibitors, warfarin, non-steroidal anti-inflammatory drugs, glucocorticoids, aspirin, clopidogrel, ticlopidine and HAS-BLED score as covariates. In our study, HAS-BLED score was ranged from 0 to 8 and calculated by assigning one point each for hypertension, chronic kidney disease, mild to severe liver disease, stroke history, age > 65 years, antiplatelet use, and non-steroidal anti-inflammation drug use (Supplementary Table 2). Since the international normalized ratio (INR) was not available in the NHIRD, we did not include labile INR for HAS-BLED scoring, consistent with other registry database studies (20–22). These covariates were assessed for each PQ on the first date of the pertinent PQ and are listed in Supplementary Table 3.
The selection of one medication over another is confounded by the indication and may result in a non-random treatment allocation. We used the inverse probability of treatment weighting by the propensity score to account for this bias (23). The propensity score was the probability that a patient was prescribed the concurrent medication during a PQ. For each PQ, a specific propensity score was calculated according to the aforementioned covariates that were pertinent to the first date of the PQ.
We used generalized estimating equations (24) for a Poisson regression model to account for intra-individual correlation across PQs to calculate the incidence rate, incidence rate ratios (IRR), incidence rate difference (IRD) and 95% confidence intervals (CIs) that considered the inverse probability of treatment weighting using the propensity score.
Data from patients without a valid insurance status were considered missing data and were excluded from this study (estimated at <0.1%). The analysis was performed using SAS (SAS Institute) version 9.4.
From 2012 to 2017, a total of 390,455 AF patients were identified. Of these, 102,523 were DOACs users. Only adult patients (age ≥ 20) were enrolled. Patients receiving joint replacement surgery or prosthetic valve surgery within 6 months before the first DOACs prescription (N = 175) were excluded, as well as those with valvular heart disease (N = 722), because the focus of this study was non-valvular AF. Patients with end-stage renal disease (ESRD) (N = 726) were excluded due to excessive bleeding risks. Finally, we excluded patients who had previous intracerebral hemorrhage (ICH) (N = 9,666) or cerebral vascular anomaly (N = 458), because these patients may carry excessive intracranial bleeding risks (25). A total of 90,731 patients were enrolled, and a total of 576,999 PQs were identified. There was a total of 165,474 PQs for low-to-moderate-intensity statin group and 79,266 PQs for high-intensity statin group (Figure 1).
Table 1 shows the demographic data of our study population. The mean age was 74.94 years (SD, 10.39) and 56.05% of them were male. The HAS-BLED score was 3.00 (SD, 1.13). The average CHA2DS2-VASc score [congestive heart failure, hypertension, age ≥75 years (two points), age 65–74 years, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism (two points), vascular disease history, female] was 4.59 (SD, 1.73). Half of our patients had congestive heart failure (51.71%), and more than one third of them had cerebral vascular disease (46.07%), or diabetes mellitus (41.28%).
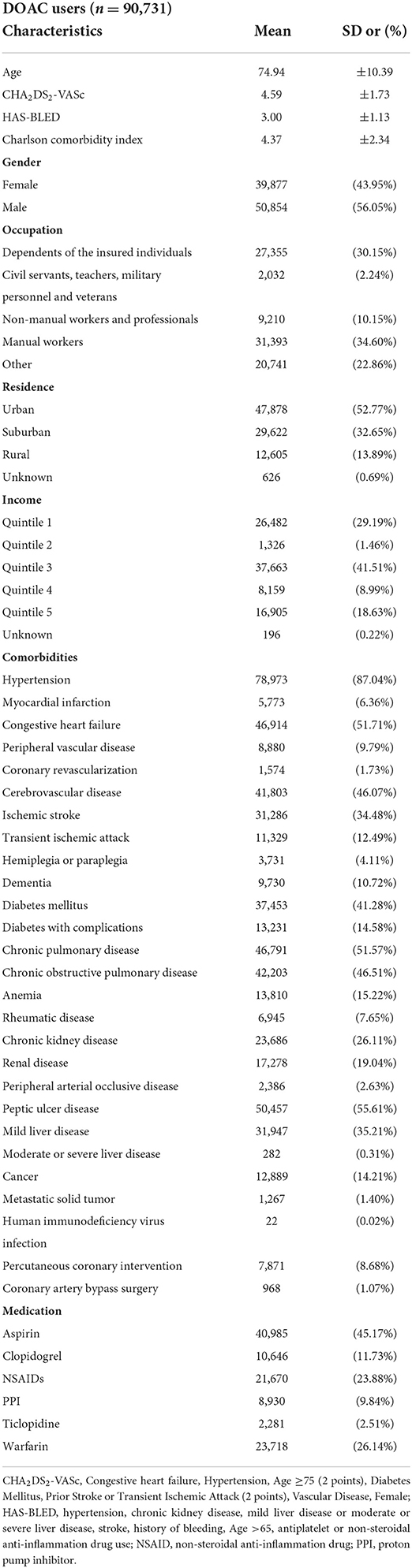
Table 1. Characteristics, comorbidities and medications among patients with non-valvular atrial fibrillation using DOACs.
Major bleeding was defined as bleeding requiring hospitalization or an emergency department visit. During follow-up, 4,580 major bleeding events occurred among 332,259 no-statin PQs, while 2,289 major bleeding events were recorded among 238,923 statin-using PQs. The adjusted incidence rate for all major bleeding events of no-statin PQs was 50.86 (95% CI 48.73–53.09) per 1,000 person-year and 38.71 (95% CI 37.02–40.48) per 1,000 person-year for statin-using. Lower bleeding incidence was observed in both high-intensity and low-to-moderate-intensity statin-using PQs (incidence rate of 40.62 and 38.30 per 1,000 person-year, respectively) than in no-statin PQs. Compared with PQs of DOACs alone, the adjusted IRR of major bleeding for a DOACs with statins, high-intensity statins, and low-to-moderate-intensity statins were 0.76 (95% CI 0.72–0.81), 0.81 (95% CI 0.74–0.88), and 0.75 (95% CI 0.70–0.80), respectively. The adjusted IRD per 1,000 person-year of major bleeding among concurrent statin and DOACs use was −12.15 (95% CI −14.94 to −9.37) (Table 2).
Examining sex differences, the IRR for all major bleeding in statin-using males was 0.79 (95% CI 0.73–0.86), and 0.70 (95% CI 0.64–0.77) in statin-using females. The IRD for all major bleeding in statin-using males was −9.81 (95% CI −13.40 to −6.22) per 1,000 person-year, and −17.24 (95% CI −21.68 to −12.79) per 1,000 person-year in statin-using females. Figure 2 demonstrated the forest plots of major bleeding risks among different sites.
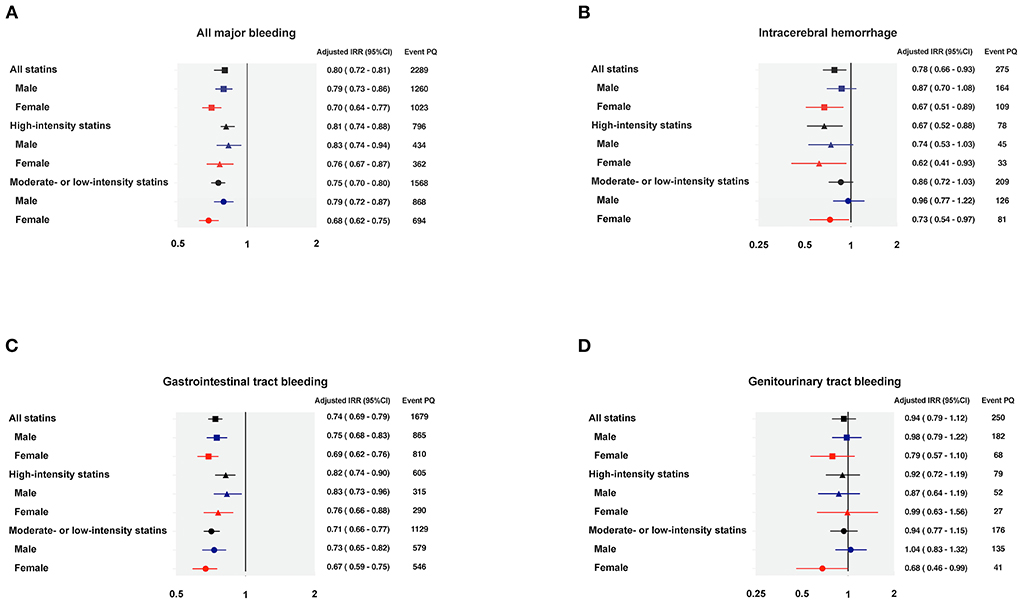
Figure 2. Forest plots of the adjusted incidence rate ratio regarding major bleedings. (A) All major bleeding; (B) Intracerebral hemorrhage; (C) Gastrointestinal tract bleeding; (D) Genitourinary tract bleeding. IRR, incidence rate ratio; PQ, person-quarter.
The adjusted incidence rate for ICH was 5.95 (95% CI 5.26–6.73) per 1,000 person-year among no-statin PQs, and 4.65 (95% CI 4.12–5.25) per 1,000 person-year among statin-using PQs (Table 3). A lower ICH risk was observed in concurrent statin and DOACs use, especially in high-intensity statin group. The IRR of concurrent high-intensity statin use was 0.67 (95% CI 0.52–0.88) and the IRD was−1.93 (95% CI −3.13 to −0.73). The IRR of concurrent low-to-moderate-intensity statin use was 0.86 (95% CI 0.72–1.03) and the IRD was −0.84 (95% CI −1.84 to 0.17) per 1,000 person-year. While statin-using did not show significant difference on ICH risk among male DOAC users [IRR 0.87 (95% CI 0.70–1.08); IRD −0.72 (95% CI −1.86 to 0.41)] per 1,000 person-year, combined prescription of statin decreased the risk of ICH in female DOAC users with an IRR of 0.67 (95% CI 0.51–0.89) and IRD of −2.07 (95% CI −3.56 to −0.59) per 1,000 person-year. It is of note that high-intensity statins seemed to provide better protection among female patients, with an IRR of 0.62 (95% CI 0.41–0.93) and IRD of −2.44 (95% CI −4.35 to −0.54) per 1,000 person-year (Table 3).
Concurrent statin use reduced the risk of gastrointestinal (GI) tract bleeding in patients taking DOACs, regardless of statin intensities or sex differences. Compared to no-statin PQs, the IRR during statin-using PQs was 0.74 (95% CI 0.69–0.79) and the IRD was −10.12 (95% CI −12.51 to −7.73) per 1,000 person-year (Table 4). The IRRs were 0.82 (95% CI 0.74–0.90) and 0.71 (95% CI 0.66–0.77) under the prescription of high-intensity statin and low-to-moderate-intensity statin, respectively. There were no sex-differences regarding the protective effect of concurrent statin use in GI tract bleeding (Table 4).
DOACs use alone and co-medication with statins had similar incidence rates of urogenital tract bleeding events. Compared with PQs of no-statin use, the IRR for urogenital tract bleeding during statin-using PQs was 0.94 (95% CI 0.79–1.12). No significant sex difference was observed in the incidence of urogenital tract bleeding. For statin-using males, the IRR was 0.98 (95% CI 0.79–1.22) for urogenital tract bleeding, while for statin-using females, the IRR was 0.79 (95% CI 0.57–1.10) (Table 5).
Concurrent statin use did not increase the risk of bleeding at other sites with an IRR of 0.76 (95% CI 0.52–1.11) (Table 6). No significant differences between statin intensities or sex were found.
This nationwide population-based cohort study showed that concurrent use of statins and DOACs did not increase the risk of major bleeding but decreased ICH and GI bleeding. This is the first large cohort study investigating the risk of major bleedings in patients taking different intensities statins with DOACs.
Reviewing the literature, only a few studies evaluated the risk of major bleeding in AF patients receiving statins and long-term anticoagulant therapy. Evidence shows that concurrent use of statins and VKA (warfarin) did not increase the risk of bleeding, but data with simultaneous DOACs and statins use was controversial (26, 27). An earlier study reported a higher ICH risk in patients taking dabigatran and either simvastatin or lovastatin (17). One observational study also suggested an increased bleeding risk with simultaneous use of DOACs and statins (27). On the other hand, a nationwide study from Taiwan found reduced major bleeding events with concurrent use of atorvastatin and DOACs (16). Another prospective study also observed lower bleeding risk with dabigatran and statin use compared to dabigatran alone (28). Like the above two studies, our data further confirmed that decreased major bleeding risks were observed among DOAC users taking statins, regardless of the intensity. This study may provide a more comprehensive insight with several advantages. First, we included four DOACs (rivaroxaban, dabigatran, apixaban or edoxaban); second, we evaluated the bleeding risk of statins with different intensities, though individual statins were not evaluated due to insufficient users and events of certain statins.
Statins have several pleiotropic effects include improvement of endothelial function, increased nitric oxide bioavailability, and anti-inflammation property (29). The pleiotropic effect of statins is considered a class effect. However, different statins have different metabolism, and this may result in having different drug-drug interaction profiles. Atorvastatin and simvastatin are CYP3A4 substrates, but rosuvastatin, fluvastatin, and pitavastatin are less susceptible to cytochrome P450. DOACs are also CYP3A4 substrates and previous studies have showed atorvastatin did not impact the trough activity of DOACs (30, 31). On the other hand, the absorption of DOACs is dependent on intestinal P-glycoproteins (P-gp), as DOACs are P-gp substrates (32–34). P-gp is an ATP-binding cassette drug efflux transporter and can limit the cellular uptake of drugs from the intestinal lumen into epithelial cells, limit substrate uptake from the blood circulation into the brain, and enhance the excretion of substrates from hepatocytes and renal tubules (35, 36). The serum or tissue concentration of a substrate, such as DOACs, may increase in the presence of P-gp inhibitors. Previous in vitro models have shown lovastatin, simvastatin and atorvastatin are P-gp inhibitors while fluvastatin and rosuvastatin only interact with P-gp in vitro at high concentrations (37, 38). Despite the possibility of increased serum or tissue DOAC concentrations with concurrent statin use, this study did not find an increased risk of major bleeding but found a protective effect of statins in our DOAC-using population. Hence, there might be other mechanisms that reduced the risk of bleeding during co-prescription of DOACs and statins.
A lower ICH risk was observed in concurrent use of statins and DOACs. ICH is one of the most devastating complications during anticoagulation. Most primary ICH is a manifestation of small vessel disease, with long-standing hypertension and cerebral amyloid angiopathy (CAA) being the primary causes. Hypertensive vasculopathy may cause cerebral small vessel lipohyalinosis (also known as fibrinoid necrosis) and endothelium injury, while CAA is characterized by the deposition of amyloid-β peptide in the walls of small cerebral cortical and leptomeningeal vessels (39). Recently, endothelial cell dysfunction and loss of endothelial nitric oxide have been reported to be associated with cerebrovascular amyloid formation (40). Statins have cholesterol-independent vascular pleiotropic effects and may improve and stabilize endothelial function by increasing the bioavailability of nitric oxide, thereby reducing oxidative stress and inhibiting inflammation (41). This might partly explain why statins reduced ICH incidence via their pleiotropic effects in our patients. Another important finding is the different ICH risks with different statin intensities and among different sex. Our study showed a reduction in the risk of ICH was mainly observed in high-intensity statin using. Aggressive management of hyperlipidemia with high-intensity statins could be suggested for AF patients on DOAC therapy and should not be withheld due to concerns of ICH. It is worthy of note that statin might have stronger protective effect on the risk of ICH among female patients. Estrogen level was found to correlate with endothelial function and endothelial nitric oxide synthase expression (42). But this still cannot paint the whole picture why statin use reduced female ICH incidence. Further studies are needed to understand the underlying pathophysiology.
We also found concurrent statin use reduced GI bleeding, regardless of intensity. This result supports an earlier study, which suggested statins might have protective effects against GI bleeding in patients with acute coronary syndrome (43). However, other previous studies showed mixed results. In one claims database study, statin users had a higher risk of GI bleeding than other chronic medication users (44). Another retrospective cohort study reported a higher risk of GI bleeding during rosuvastatin combined with warfarin use compared to other statins (45). More studies are needed to clarify the risk of GI bleeding among simultaneous statins and DOACs use.
Statins did not increase major genitourinary tract bleeding in non-valvular AF patients taking DOACs. An earlier study reported that rosuvastatin had a rate of transient microscopic hematuria <1.5% (46), and another study showed the use of statins was associated with a lower risk of uterine myoma and menorrhagia (47). Considering this, statin use would not be expected to further increase genitourinary tract bleeding risk among DOAC users.
Our study has several limitations. First, this was a claims database cohort study and coding inaccuracy might exist, though the coding accuracy of AF and other comorbidities in NHIRD has been validated before (48–50). Second, this study was conducted in Taiwan and more than 95% of the general population was Han-Chinese during the study period. Asians have a high incidence of ICH (51, 52), and thus the ability to generalize these results to other ethnicities is limited. Third, we did not have the detailed medical records and laboratory data for each patient. We could not evaluate the correlation of cholesterol level and major bleeding risk. Fourth, the indication of each medication might result in non-random treatment allocation. Although the inverse probability of treatment weighting was used in our study to account for this bias, confounding could still exist. Last but not least, DOAC dosage was beyond the scope of this study and was not considered in the calculations.
Compared with DOACs use alone, concurrent statins and DOACs use decreased the risk of major bleeding, including ICH and GI bleeding. High-intensity statins tended to be more beneficial than low-to-moderate-intensity statins in decreasing the incidence of ICH, especially among females. Statins should be considered in all eligible patients with non-valvular AF who are taking DOACs.
The data analyzed in this study is subject to the following licenses/restrictions: The data repository of NHIRD is Health and Welfare Data Science Center, Taiwan. Researchers can obtain the data through formal application to the Health and Welfare Data Science Center, Department of Statistics, Ministry of Health and Welfare, Taiwan (https://dep.mohw.gov.tw/DOS/np-2500-113.html). Requests to access these datasets should be directed to https://dep.mohw.gov.tw/DOS/np-2500-113.html.
The studies involving human participants were reviewed and approved by Institutional review board of the Chang Gung Memorial Hospital, Taoyuan, Taiwan. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
H-HW and T-YC: study design and manuscript writing. S-HC, C-HL, and H-TT: data acquisition. H-HW and H-TT: data analysis. T-HL: research mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
This work was supported through a grant, CMRPG5D0091, from the Chang Gung Medical Research Council. The funders had no role in study design or interpretation of the findings.
The authors would like to thank Dr. Yu-Tung Huang for the statistical assistance. and acknowledge the support of the Maintenance Project of the Center for Big Data Analytics and Statistics (Grant CLRPG3D0046) at Chang Gung Memorial Hospital for assisting with the study design, monitoring, data analysis and interpretation of results. We would also like to thank the Research Services Center for Health Information, Chang Gung University, for administrative, technical, and funding support. This study is based in part on data from the National Health Insurance research database provided by the National Health Insurance Administration and managed by Health and Welfare Data Science Center, Ministry of Health and Welfare. However, the interpretation and conclusions contained herein do not represent the position of Chang Gung Memorial Hospital, the National Health Insurance Administration, or the Ministry of Health and Welfare.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.969259/full#supplementary-material
1. Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY. The value of the Cha2ds2-vasc score for refining stroke risk stratification in patients with atrial fibrillation with a chads2 score 0-1: a nationwide cohort study. Thromb Haemost. (2012) 107:1172–9. doi: 10.1160/TH12-03-0175
2. Ng SS, Lai NM, Nathisuwan S, Jahan NK, Dilokthornsakul P, Kongpakwattana K, et al. Comparative efficacy and safety of warfarin care bundles and novel oral anticoagulants in patients with atrial fibrillation: a systematic review and network meta-analysis. Sci Rep. (2020) 10:662. doi: 10.1038/s41598-019-57370-2
3. Ntaios G, Papavasileiou V, Makaritsis K, Vemmos K, Michel P, Lip GYH. Real-World setting comparison of nonvitamin-k antagonist oral anticoagulants versus vitamin-k antagonists for stroke prevention in atrial fibrillation: a systematic review and meta-analysis. Stroke. (2017) 48:2494–503. doi: 10.1161/STROKEAHA.117.017549
4. Lip GY, Keshishian A, Kamble S, Pan X, Mardekian J, Horblyuk R, et al. Real-world comparison of major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or Warfarin. A propensity score matched analysis. Thromb Haemost. (2016) 116:975–86. doi: 10.1160/TH16-05-0403
5. Chan YH, See LC, Tu HT, Yeh YH, Chang SH, Wu LS, et al. Efficacy and safety of apixaban, dabigatran, rivaroxaban, and warfarin in asians with nonvalvular atrial fibrillation. J Am Heart Assoc. (2018) 7:e008150. doi: 10.1161/JAHA.117.008150
6. Lip Gregory YH, Lane Deirdre A. Matching the noac to the patient. J Am Coll Cardiol. (2015) 66:2282–4. doi: 10.1016/j.jacc.2015.07.086
7. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 Aha/Acc/Aacvpr/Aapa/Abc/Acpm/Ada/Ags/Apha/Aspc/Nla/Pcna guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2019) 139:e1082–43. doi: 10.1161/CIR.0000000000000624
8. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 Esc/Eas guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the European society of cardiology (Esc) and European atherosclerosis society (Eas). Eur Heart J. (2019) 41:111–88. doi: 10.1093/eurheartj/ehz455
9. Amarenco P, Bogousslavsky J, Callahan A 3rd, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. (2006) 355:549–59. doi: 10.1056/NEJMoa061894
10. Pandit AK, Kumar P, Kumar A, Chakravarty K, Misra S, Prasad K. High-dose statin therapy and risk of intracerebral hemorrhage: a meta-analysis. Acta Neurol Scand. (2016) 134:22–8. doi: 10.1111/ane.12540
11. Ziff OJ, Banerjee G, Ambler G, Werring DJ. Statins and the risk of intracerebral haemorrhage in patients with stroke: systematic review and meta-analysis. J Neurol Neuros Psychiatry. (2019) 90:75–83. doi: 10.1136/jnnp-2018-318483
12. Saliba W, Rennert HS, Barnett-Griness O, Gronich N, Molad J, Rennert G, et al. Association of statin use with spontaneous intracerebral hemorrhage. Cohort Study. (2018) 91:e400–9. doi: 10.1212/WNL.0000000000005907
13. Ribe AR, Vestergaard CH, Vestergaard M, Pedersen HS, Prior A, Lietzen LW, et al. Statins and risk of intracerebral hemorrhage in individuals with a history of stroke. Stroke. (2020) 51:1111–9. doi: 10.1161/STROKEAHA.119.027301
14. Åsberg S, Farahmand B, Henriksson KM, Appelros P. Statins as secondary preventives in patients with intracerebral hemorrhage. Int J Stroke. (2020) 15:61–8. doi: 10.1177/1747493018816476
15. Newman CB, Preiss D, Tobert JA, Jacobson TA, Page RL 2nd, Goldstein LB, et al. Statin safety and associated adverse events: a scientific statement from the American heart association. Arterioscler Thromb Vasc Biol. (2019) 39:e38–81. doi: 10.1161/ATV.0000000000000073
16. Chang S-H, Chou I-J, Yeh Y-H, Chiou M-J, Wen M-S, Kuo C-T, et al. Association between use of non–vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA. (2017) 318:1250–9. doi: 10.1001/jama.2017.13883
17. Antoniou T, Macdonald EM, Yao Z, Hollands S, Gomes T, Tadrous M, et al. Association between statin use and ischemic stroke or major hemorrhage in patients taking dabigatran for atrial fibrillation. CMAJ. (2017) 189:E4–10. doi: 10.1503/cmaj.160303
18. Fan PY, Lee CC, Liu SH, Li IJ, Weng CH, Tu KH, et al. Preventing arteriovenous shunt failure in hemodialysis patients: a population-based cohort study. J Thromb Haemost. (2019) 17:77–87. doi: 10.1111/jth.14347
19. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 Acc/Aha guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. (2014) 63:2889–934. doi: 10.1016/j.jacc.2013.11.002
20. Tsai CT, Liao JN, Chiang CE, Lin YJ, Chang SL, Lo LW, et al. Association of ischemic stroke, major bleeding, and other adverse events with warfarin use vs non-vitamin k antagonist oral anticoagulant use in patients with atrial fibrillation with a history of intracranial hemorrhage. JAMA Netw Open. (2020) 3:e206424. doi: 10.1001/jamanetworkopen.2020.6424
21. Blin P, Fauchier L, Dureau-Pournin C, Sacher F, Dallongeville J, Bernard MA, et al. Effectiveness and safety of rivaroxaban 15 or 20 mg versus vitamin k antagonists in nonvalvular atrial fibrillation. Stroke. (2019) 50:2469–76. doi: 10.1161/STROKEAHA.119.025824
22. Nielsen PB, Skjøth F, Søgaard M, Kjældgaard JN, Lip GY, Larsen TB. Effectiveness and safety of reduced dose non-vitamin k antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. (2017) 356:j510. doi: 10.1136/bmj.j510
23. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. (2014) 33:1242–58. doi: 10.1002/sim.5984
24. Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. (1986) 73:13–22. doi: 10.1093/biomet/73.1.13
25. Hill MD, Silver FL, Austin PC, Tu JV. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. (2000) 31:123–7. doi: 10.1161/01.STR.31.1.123
26. Korhonen MJ, Tiittanen P, Kastarinen H, Helin-Salmivaara A, Hauta-Aho M, Rikala M, et al. Statins do not increase the rate of bleeding among warfarin users. Basic Clin Pharmacol Toxicol. (2018) 123:195–201. doi: 10.1111/bcpt.12998
27. Jimenez-Serrania MI, Treceno-Lobato C. Influence of concomitant treatments under anticoagulants and statins in detecting signals of adverse drug reactions. Semin Thromb Hemost. (2019) 45:837–45. doi: 10.1055/s-0039-1695734
28. Ho BL, Lin YJ, Lin SF, Chou PS, Chen CF, Lin RT, et al. Statins and the risk of bleeding in patients taking dabigatran. Acta Neurol Scand. (2019) 139:455–61. doi: 10.1111/ane.13077
29. Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. (2004) 109(23_suppl_1):III-39–III-43. doi: 10.1161/01.CIR.0000131517.20177.5a
30. Škornová I, Samoš M, Bolek T, Stančiaková L, Vádelová L, Galajda P, et al. Does atorvastatin therapy change the anti-xa activity in xabans-treated patients with atrial fibrillation? Pharmacol Res Perspect. (2021) 9:e00730. doi: 10.1002/prp2.730
31. Stangier J, Rathgen K, Stähle H, Reseski K, Körnicke T, Roth W. Coadministration of dabigatran etexilate and atorvastatin. Am J Cardiov Drugs. (2009) 9:59–68. doi: 10.1007/BF03256595
32. Wang L, He K, Maxwell B, Grossman SJ, Tremaine LM, Humphreys WG, et al. Tissue distribution and elimination of [14c]Apixaban in rats. Drug Metab Dispos. (2011) 39:256–64. doi: 10.1124/dmd.110.036442
33. Gnoth MJ, Buetehorn U, Muenster U, Schwarz T, Sandmann S. In Vitro and in Vivo P-glycoprotein transport characteristics of rivaroxaban. J Pharmacol Exp Ther. (2011) 338:372–80.
34. Härtter S, Sennewald R, Nehmiz G, Reilly P. Oral bioavailability of dabigatran etexilate (Pradaxa(®)) after co-medication with verapamil in healthy subjects. Br J Clin Pharmacol. (2013) 75:1053–62. doi: 10.1111/j.1365-2125.2012.04453.x
35. Lin JH, Yamazaki M. Role of p-glycoprotein in pharmacokinetics: clinical implications. Clin Pharmacokinet. (2003) 42:59–98. doi: 10.2165/00003088-200342010-00003
36. Staud F, Ceckova M, Micuda S, Pavek P. Expression and function of p-glycoprotein in normal tissues: effect on pharmacokinetics. Methods Mol Biol. (2010) 596:199–222. doi: 10.1007/978-1-60761-416-6_10
37. Holtzman CW, Wiggins BS, Spinler SA. Role of p-glycoprotein in statin drug interactions. Pharmacotherapy. (2006) 26:1601–7. doi: 10.1592/phco.26.11.1601
38. Goard CA, Mather RG, Vinepal B, Clendening JW, Martirosyan A, Boutros PC, et al. Differential interactions between statins and p-glycoprotein: implications for exploiting statins as anticancer agents. Int J Cancer. (2010) 127:2936–48. doi: 10.1002/ijc.25295
39. Caceres JA, Goldstein JN. Intracranial hemorrhage. Emerg Med Clin North Am. (2012) 30:771–94. doi: 10.1016/j.emc.2012.06.003
40. Austin SA, Katusic ZS. Partial loss of endothelial nitric oxide leads to increased cerebrovascular beta amyloid. J Cerebral Blood Flow Metabol. (2020) 40:392–403. doi: 10.1177/0271678X18822474
41. Wolfrum S, Jensen KS, Liao JK. Endothelium-dependent effects of statins. Arterioscl Thromb Vasc Biol. (2003) 23:729–36. doi: 10.1161/01.ATV.0000063385.12476.A7
42. Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor α is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab. (2009) 94:3513–20. doi: 10.1210/jc.2009-0278
43. Atar S, Cannon CP, Murphy SA, Rosanio S, Uretsky BF, Birnbaum Y. Statins are associated with lower risk of gastrointestinal bleeding in patients with unstable coronary syndromes: analysis of the orbofiban in patients with unstable coronary syndromes-thrombolysis in myocardial infarction 16 (Opus-Timi 16) trial. Am Heart J. (2006) 151:976.e1–6. doi: 10.1016/j.ahj.2006.02.013
44. Martinez AI, Freeman PR, Moga DC. Statin use and gastrointestinal hemorrhage: a large retrospective cohort study. Am J Cardiovasc Drugs. (2019) 19:65–74. doi: 10.1007/s40256-018-0301-4
45. Shin D, Yoon D, Lim SG, Hong JM, Park RW, Lee JS. Comparison of the risk of gastrointestinal bleeding among different statin exposures with concomitant administration of warfarin: electronic health record-based retrospective cohort study. PLoS ONE. (2016) 11:e0158130. doi: 10.1371/journal.pone.0158130
46. Scott LJ, Curran MP, Figgitt DP. Rosuvastatin: a review of its use in the management of dyslipidemia. Am J Cardiovasc Drugs. (2004) 4:117–38. doi: 10.2165/00129784-200404020-00005
47. Borahay MA, Fang X, Baillargeon JG, Kilic GS, Boehning DF, Kuo Y-F. Statin use and uterine fibroid risk in hyperlipidemia patients: a nested case-control study. Am J Obstet Gynecol. (2016) 215:750.e1–8. doi: 10.1016/j.ajog.2016.06.036
48. Hsieh C-Y, Su C-C, Shao S-C, Sung S-F, Lin S-J, Kao Yang Y-H, et al. Taiwan's national health insurance research database: past and future. Clin Epidemiol. (2019) 11:349–58. doi: 10.2147/CLEP.S196293
49. Hsieh CY, Chen CH, Li CY, Lai ML. Validating the diagnosis of acute ischemic stroke in a national health insurance claims database. J Formos Med Assoc. (2015) 114:254–9. doi: 10.1016/j.jfma.2013.09.009
50. Sung SF, Hsieh CY, Lin HJ, Chen YW, Yang YH, Li CY. Validation of algorithms to identify stroke risk factors in patients with acute ischemic stroke, transient ischemic attack, or intracerebral hemorrhage in an administrative claims database. Int J Cardiol. (2016) 215:277–82. doi: 10.1016/j.ijcard.2016.04.069
51. Tsai C-F, Anderson N, Thomas B, Sudlow CLM. Comparing risk factor profiles between intracerebral hemorrhage and ischemic stroke in chinese and white populations: systematic review and meta-analysis. PLoS ONE. (2016) 11:e0151743. doi: 10.1371/journal.pone.0151743
52. van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. (2010) 9:167–76. doi: 10.1016/S1474-4422(09)70340-0
Keywords: atrial fibrillation, DOACs, direct-acting oral anticoagulant, major bleeding, intracerebral hemorrhage, statin
Citation: Wu H-H, Chang S-H, Lee T-H, Tu H-T, Liu C-H and Chang T-Y (2022) Concurrent use of statins decreases major bleeding and intracerebral hemorrhage in non-valvular atrial fibrillation patients taking direct oral anticoagulants—A nationwide cohort study. Front. Cardiovasc. Med. 9:969259. doi: 10.3389/fcvm.2022.969259
Received: 14 June 2022; Accepted: 19 July 2022;
Published: 08 August 2022.
Edited by:
Rajeev Gupta, Medicilinic, United Arab EmiratesReviewed by:
Vito Maurizio Parato, Marche Polytechnic University, ItalyCopyright © 2022 Wu, Chang, Lee, Tu, Liu and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting-Yu Chang, dF95X2NoYW5nQGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.