
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 20 January 2023
Sec. Hypertension
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.969148
This article is part of the Research Topic Women in Hypertension View all 10 articles
 Federica Canfora1
Federica Canfora1 Elena Calabria1*
Elena Calabria1* Giuseppe Pecoraro1
Giuseppe Pecoraro1 Stefania Leuci1
Stefania Leuci1 Noemi Coppola1
Noemi Coppola1 Cristina Mazzaccara2,3
Cristina Mazzaccara2,3 Francesca Spirito4
Francesca Spirito4 Massimo Aria5
Massimo Aria5 Luca D'Aniello6
Luca D'Aniello6 Michele Davide Mignogna1†
Michele Davide Mignogna1† Daniela Adamo1†
Daniela Adamo1†Background: The relationship between hypertension (HTN) and chronic pain is still a matter of debate, and its prevalence in patients with burning mouth syndrome (BMS) has never been evaluated. This study aimed to assess the prevalence of HTN in women with BMS and to evaluate its relationship with potential predictors such as risk factors for cardiovascular diseases, pain, and mental health status analyzing differences with healthy women.
Methods: In total, 250 women with BMS (WBMS) were prospectively recruited and compared with an equal number of healthy women (HW) matched for age. Education, body mass index, smoke and alcohol consumption, intensity and quality of pain, and psychological profile were further investigated to identify the potential predictors of HTN. Specifically, pain assessment [the Numeric Rating Scale (NRS) and Short-Form McGill Pain Questionnaire (SF-MPQ)] and psychological assessment [Hamilton Rating Scale for Depression and Anxiety (HAM-D and HAM-A), Pittsburgh Sleep Quality Index (PSQI), and Epworth Sleepiness Scale (ESS)] was carried out for the participants.
Results: HTN was found in 128 (51.2%) WBMS and 76 (30.4%) HW (p < 0.001**). The scores of the NRS, SF-MPQ, HAM-D, HAM-A, and PSQI were statistically significantly higher in the WBMS than in the HW (p < 0.001**). A strongly linear correlation between HTN and employment status, systemic diseases, and education level (p < 0.001**) was found in WBMS, while a strong correlation between HTN and employment status, hypercholesterolemia, systemic diseases, and drug consumption was found in HW (p < 0.001**). No statistically significant correlation was found between HTN and pain, anxiety, depression, and sleep disturbances.
Conclusion: These results suggest that WBMS showed a higher prevalence of HTN compared with controls. Unemployed WBMS with lower education and other systemic comorbidities are at an increased risk of developing HTN. HTN is associated with alteration in the vascular structure and function of the brain, and these processes accelerate brain aging, which contributes to a reduction in intracortical connectivity, thus affecting the modulatory system of control of pain in patients with BMS, independently of their mental health assessment. Predictors that may underlie this association remain unclear, taking into account the differences found in HW, and should be further elucidated.
Hypertension (HTN) is the leading cause of cardiovascular disease, and it is responsible for 8.5 million premature deaths from stroke, ischemic heart disease, and kidney disease worldwide (1–3). In addition, HTN is an evidence-based risk factor for brain aging and dementia (4, 5).
The number of people affected by HTN aged 30–79 years has doubled from 1990 to 2021, reaching 626 and 652 millions of women and men, respectively, in the world (6). Gender disparity in the HTN epidemiology reveals differences in age stratification; indeed, the prevalence of HTN in patients aged between 18 and 29 years is 3% in women vs. 8.5% in men, while in patients aged between 30 and 44 years, it is 7.3% in women vs. 15.8% in men (7). In contrast, this prevalence increases strongly after menopause, and it is more common in women than in men in the elderly population above the age of 75 years, reaching 78% (8).
The mechanisms in which sex interacts with vascular aging and subsequently with an increase in blood pressure are complex, including a multitude of hormonal, chromosomal, or even psychosocial factors (9). Indeed, sex steroids and the receptors through which they act are emerging as important mediators in the promotion and maintenance of sexual divergence in blood pressure regulation across the lifespan (10).
Obesity, dyslipidemia, impaired fasting glucose, and chronic pain are the most frequent comorbidity associated with HTN (11–14).
Burning mouth syndrome (BMS) is a chronic orofacial pain disorder with a strong female predilection; it is characterized by a generalized or localized intraoral burning or dysesthetic sensation or pain of the oral mucosa without any evidence of any specific mucosal lesions and/or laboratory findings (15). The overall prevalence of BMS was 1.73% in the general population and 7.72% in the clinical settings of dental practice with an average of 4%, reaching a prevalence of 18% in postmenopausal women (16). Several studies reported a consistent gender difference associated with BMS (16). Nasri-Heir et al. (17) reported the highest prevalence in women of middle age (>50 years) with a female/male ratio of 7:1, whereas in the recent meta-analysis by Wu et al. (16), the female/male ratio reported was 3:1. Possible factors behind these gender differences could include genetic factors affecting pain vulnerability as well as hormonal and psychosocial factors (18).
The pathophysiology of BMS includes central nervous system dysfunctions, which increase the central pain sensitization processes and reduce the functioning of descending pain inhibitory mechanisms (17). The higher pain sensitivity in women is probably due to biological sex differences in the ascending and descending modulation pathways and also for psychological phenomena that predominantly affect women (19).
While functional interactions between the pain inhibitory mechanism and the cardiovascular system exist (20), blood pressure is consistently and inversely associated with pain perception in chronic pain-free subjects. Indeed, elevated blood pressure may determine the attenuation of acute pain sensitivity (blood pressure-related hypoalgesia), and presumably, a similar phenomenon should be attended also in chronic pain status (20). However, recent studies found that, in patients with chronic pain, the relationship between blood pressure and pain sensitivity is completely reversed, and consequently, higher blood pressure has been associated with increased or higher sensitivity in the perception of chronic pain intensity (14, 20, 21).
Based on the above studies (14, 20, 21) and taking into account the positive relationship between elevated blood pressure and impaired pain perception, we assumed a possible association between HTN and a condition of chronic orofacial pain such as BMS. In addition, several studies underline that changes in hormonal profile and psychological factors during menopause could have a role in the development of both conditions in women (9, 22–24).
No published studies have examined the prevalence of HTN in the BMS population, specifically in women who are the most frequently affected population.
Therefore, this study aimed to investigate the prevalence of HTN in a wide sample of women with BMS (WBMS) compared with a control group of healthy women (HW) matched for age and to identify the potential predictors of HTN in WBMS and HW, analyzing the differences between the two groups and taking in account sociodemographic profile (age, employment, marital status), body mass index (BMI), risk factors (smoking and alcohol use), other systemic comorbidities and drug consumption, pain evaluation, and psychological factors.
This was an observational case-control study that was conducted between April 2020 and January 2022 at the Oral Medicine Department of the University of Naples “Federico II” in accordance with the ethical principles of the World Medical Association Declaration of Helsinki. It was approved by the Ethical Committee of the University (Approval Number: 251/19—the date of approval was February 20, 2019). The adopted methods conformed with the Strengthening of the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for observational studies (25).
At the baseline appointment (time 0), 270 patients in the study group and 265 individuals in the control group were considered eligible for this study. However, only 250 individuals in each group met the inclusion and exclusion criteria (Figure 1). All the participants prospectively recruited were women aged at least 18 years. The case group included patients suffering from BMS at the first consultation, which referred to the BMS symptom onset antecedent to any new drugs introduced in their treatment to exclude any causative effect, as described in previous studies (26–28). The control group included healthy subjects presenting at the hospital during the study period for dental treatments. Every subject considered eligible has been included in this study after having provided written informed consent. No payment was provided for participation. The patients and controls were matched by age. First, we recruited the patients and calculated their average age; then, we recruited the controls to obtain a matched sample.
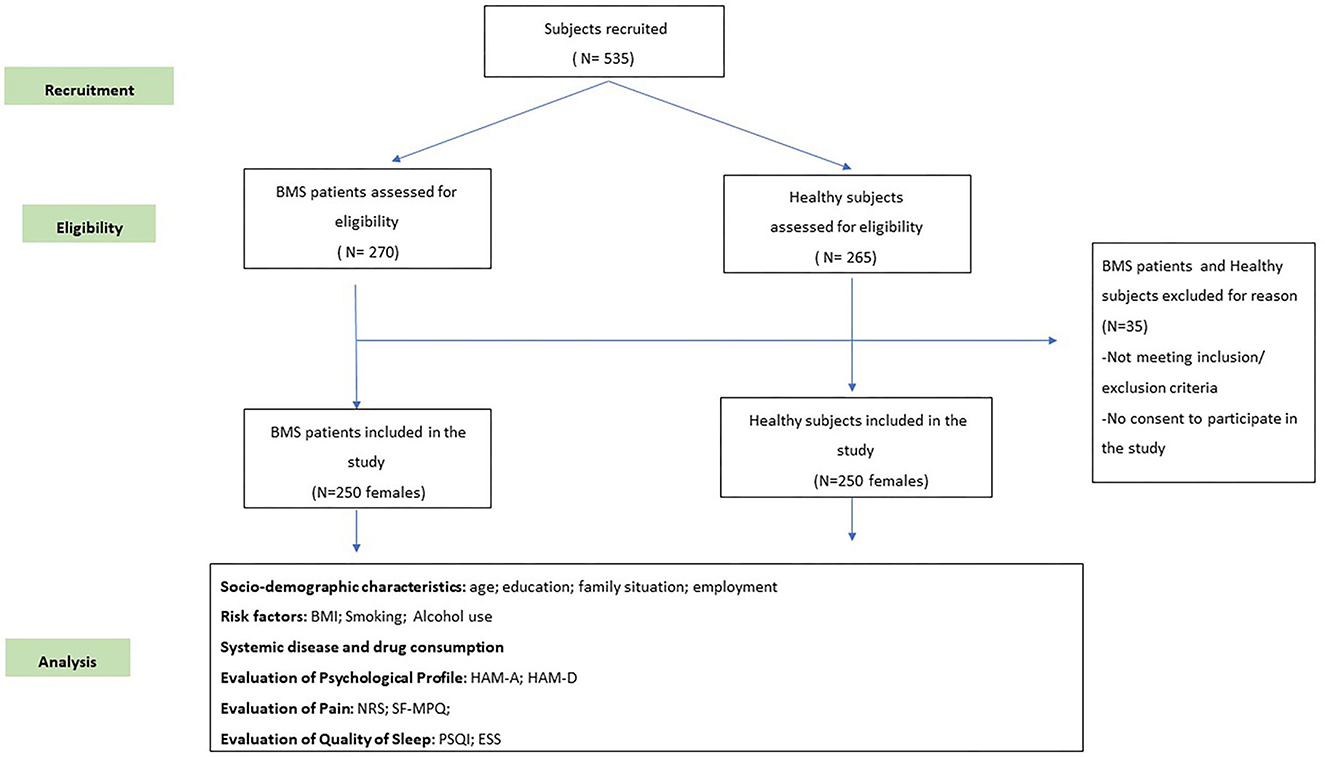
Figure 1. A flow chart of the study. BMS, burning mouth syndrome; BMI, Body Mass Index; ESS, Epworth Sleepiness Scale; HAM-A, Hamilton Rating Scale for Anxiety; HAM-D, Hamilton Rating Scale for Depression; NRS, Numeric Rating Scale; PSQI, Pittsburgh Sleep Quality Index; SF-MPQ, Short-Form McGill Pain Questionnaire.
In accordance with the International Classification of Orofacial Pain (ICOP 2020) 1st edition (15), the inclusion criteria of the WBMS group were as follows:
- Female patients aged at least 18 years;
- Patients experiencing an intraoral burning or dysesthetic sensation, recurring daily for more than 2 h per day for more than 3 months, without evident causative lesions on clinical examination and investigation; the pain has the characteristics of burning quality and is experienced superficially in the oral mucosa;
- Patients with normal blood test findings (including blood count, blood glucose levels, glycated hemoglobin, serum iron, ferritin, and transferrin); and
- Patients who are not currently in treatment with psychotropic drugs.
The WBMS group exclusion criteria were as follows:
- Patients suffering from diseases that could be recognized as a causative factor of BMS,
- Patients unable to understand or complete the questionnaires,
- Patients having a history of a psychiatric disorder or a neurological or organic brain disorder,
- Patients undergoing treatment with psychotropic drugs or systemic drugs possibly associated with oral symptoms,
- Patients having a history of alcohol or substance abuse, and
- Patients suffering from obstructive sleep apnoea syndrome (OSAS).
The inclusion criteria of the HW were as follows:
- Female subjects aged at least 18 years,
- Subjects without any lesion of the oral mucosa,
- Subjects without psychiatric disorder or a neurological or organic brain disorder,
- Subjects without a history of BMS,
- Subjects with normal blood test findings (including blood count, blood glucose levels, glycated hemoglobin, serum iron, ferritin, and transferrin), and
- Subjects who had not undergone treatment with psychotropic drugs.
The exclusion criteria of the HW were as follows:
- Subjects unable to understand or complete the questionnaires,
- Subjects having a history of alcohol or substance abuse,
- Subjects suffering from OSAS.
In the course of routine initial clinical evaluation, all the patients underwent a careful medical analysis, specifically an intra- and extra-oral examination by a board-certified expert clinician in oral medicine (DA). The patients had subsequently been assessed with regard to oral symptoms and the sites involved, age, years of education, family situation, job status, risk factors (current smoking status and alcohol consumption), medical comorbidities, and systemic drugs taken. The blood pressure (BP) has been recorded after the patient was seated for a minimum of 5 min in a standardized fashion during each examination cycle (29). The BP was calculated as the mean of two measurements recorded by a physician. We defined hypertension as having systolic blood pressure of 140 mmHg or greater, diastolic blood pressure of 90 mmHg or greater, or taking medication for hypertension (30). Moreover, we used measured weight and height to calculate the body mass index (BMI) as weight (kilograms) divided by the square of height (meters) (31). According to the WHO classification, the cutoff values considered were 18.5–24.9 kg/m2 for normal, 25.0–29.9 kg/m2 for overweight, and > 30 kg/m2 for obesity. In particular, obesity class I: BMI of 30–34.9 kg/m2, obesity class II: BMI of 35–39.9 kg/m2, obesity class III: BMI of ≥40 kg/m2 (also referred to as severe, extreme, or massive obesity) (32, 33).
A set of predefined questionnaires were administered to patients and controls to comprehensively analyze the intensity and quality of pain experienced, psychological profile, and sleep quality.
The Numerical Rating Scale (NRS) (34) and the Short Form of the McGill Pain Questionnaire (SF-MPQ) (35) were administered to evaluate the intensity and quality of pain of the sample group.
The NRS is an 11-point scale where the two endpoints are, respectively, the extremes of no pain and worst pain (34). This could be graphically administered, through a linear 11-box scale, or verbally administered. The SF-MPQ (35) is a multidimensional pain questionnaire that measures the quality of pain. This scale has 15 items considering the sensory, affective, and evaluative aspects of the perceived pain (34). Each item scored from 0 (none) to 3 (severe). There are no established critical cutoff points for the interpretation of the scores, and a higher score indicates worse pain.
The Hamilton Depression Rating Scale (HAM-A) (36) and the Hamilton Rating Scale for Anxiety (HAM-D) (37) were administered to evaluate anxiety and depression symptoms, respectively.
The HAM-A is a clinician-administered anxiety assessment scale containing 14 items, scored from 0 to 4, which evaluate both somatic anxiety and psychic anxiety. A total score of <17 indicates mild severity, a score between 18 and 24 indicates mild to moderate, and a score between 25 and 30 indicates moderate to severe (37).
The HAM-D is a hetero-administered scale containing 21 items that explore the affective field scoring from 0 to 54. The cutoff score considered are as follows: a score between 7 and 17 indicates mild depression, a score between 18 and 24 indicates moderate depression, and a score of >24 indicate severe depression. The HAM-A is a clinician-administered anxiety assessment scale containing 14 items, scored from 0 to 4, which evaluates both somatic anxiety and psychic anxiety. A total score of <17 indicates mild severity, a score between 18 and 24 indicates mild to moderate severity, and a score of 25–30 indicates moderate to severe severity (38).
The daytime sleepiness and the subjective sleep quality were evaluated using the Epworth Sleepiness Scale (ESS) (39) and the pittsburgh sleep quality index (PSQI) (40), respectively. First, the ESS evaluates the sleep propensity in daily life through 8 items, each scored from 0 to 3. On this scale, a higher score corresponds to higher daytime sleepiness (41, 42). Second, the PSQI considers a period of 1 month to evaluate sleep quality, evaluating seven components each scored from 0 to 3: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction (43). PSQI total score ranges between 0 and 21, and a higher score corresponds to the worst sleep quality (44).
The statistical analysis was performed using the R software (v. 4.2.0 – R Core Team, 2016) (45). Descriptive statistics, including means, standard deviations (SDs), medians, and interquartile ranges (IQRs), were measured to summarize the sociodemographic and clinical characteristics of the WBMS and HW.
Fisher's exact test was used to assess the significant differences between frequencies for systematic diseases, drug consumption, antihypertensive drugs, oral symptoms, sites involved, and clinical parameters (psychological profile, and sleep and pain assessment) between WBMS and HW and between WBMS with and without hypertension, while the Mann–Whitney U test was computed for comparing median values.
Dependence analysis among WBMS and HW with HTN and qualitative predictors was performed. A significant difference between frequencies was measured by Fisher's exact test.
Dependence analysis among WBMS and HW with HTN and quantitative predictors was performed. Differences between groups were tested with the Mann–Whitney U test comparing median values. In all analyses, the Bonferroni correction was used to counteract the multiple comparisons problem.
The demographic variables and the risk factors are shown in Table 1. WBMS reported a statistically significantly lower education level (in years) and a higher level of unemployment compared with the HW (p < 0.001**). With respect to the family and marital status, a statistically significantly higher proportion of HW was divorced (p = 0.013*) in comparison to the WBMS. Additionally, WBMS presented a statistically significant higher percentage of heavy smokers (>15 cigarettes, 25 subjects-10%; p = 0.003**), while, overall, WBMS consumed less alcohol as there were statistically significantly more non-habitual alcohol users (p = 0.016*) compared to the control group. The frequency distributions of participants depending on the BMI categories revealed that overall, WBMS showed a considerably higher BMI than HW (p = 0.001**), especially with regard to the overweight and class I obesity categories.
The prevalence of systemic disease and drug consumption are summarized in Table 2. A statistically significantly higher proportion of WBMS presented HTN and hypercholesterolemia compared to HW (p < 0.001*), while no significant differences were found with respect to all the other comorbidities. In detail, 128 (51.2%) WBMS and 76 (30.4%) HW showed HTN (p < 0.001**); similar results were present for hypercholesterolemia affecting 90 (36%) WBMS and 53 HW (21.2%; p < 0.001**).
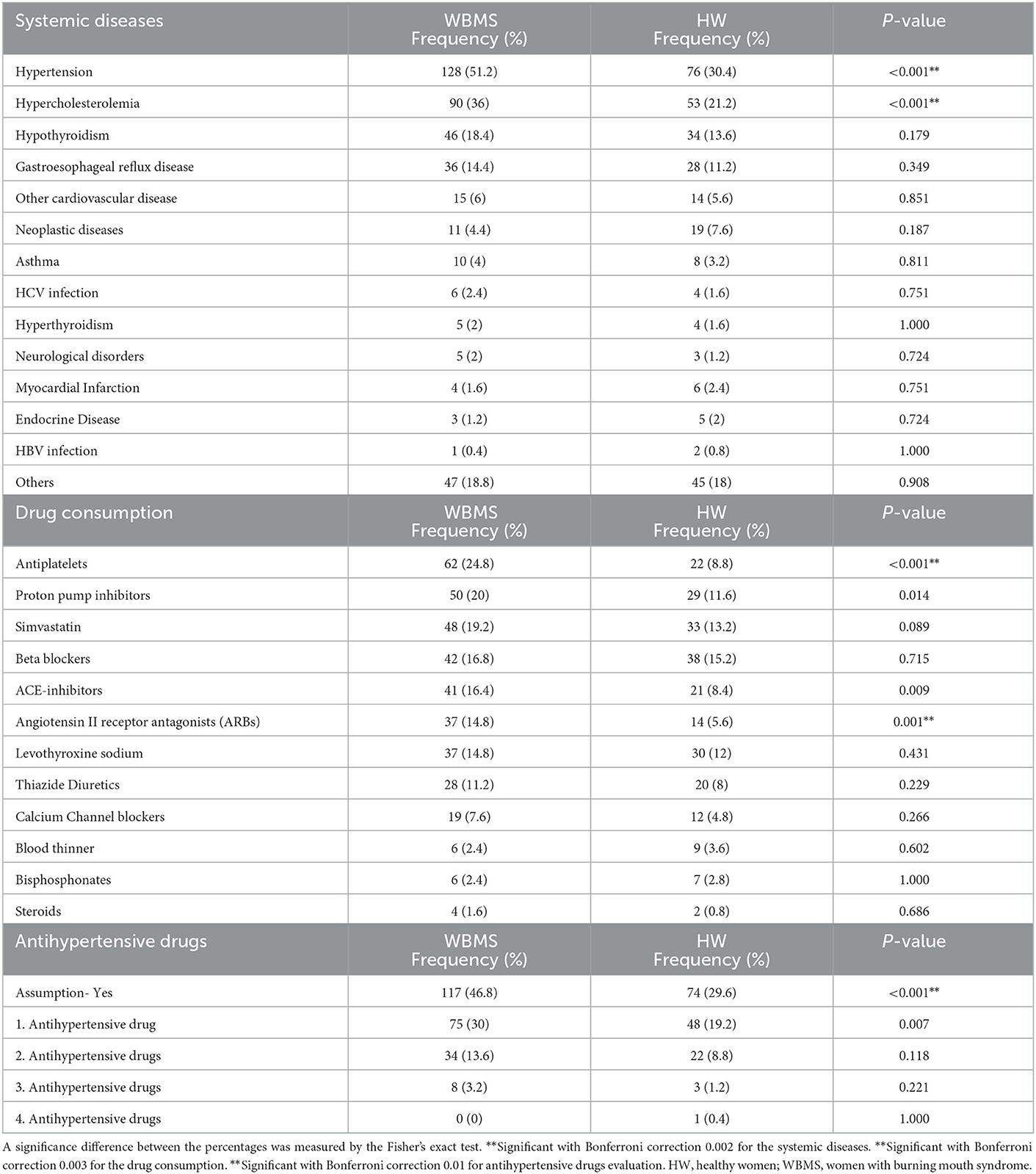
Table 2. Prevalence of systemic diseases, drug consumption and antihypertensive drugs evaluation in 250 WBMS patients and 250 HW.
As shown in Figure 2, the bar plot underlines the distribution of HTN considering the age stratification in which the highest percentage of WBMS with HTN (42.9%) is classified between the ages of 65 and 75 years, while for the HW, the highest percentage (38.2%) is between the ages of 55 and 65 years. Moreover, HTN was found in 29 WBMS (22.6%) and 10 HW (13.2%), aged >75 years.
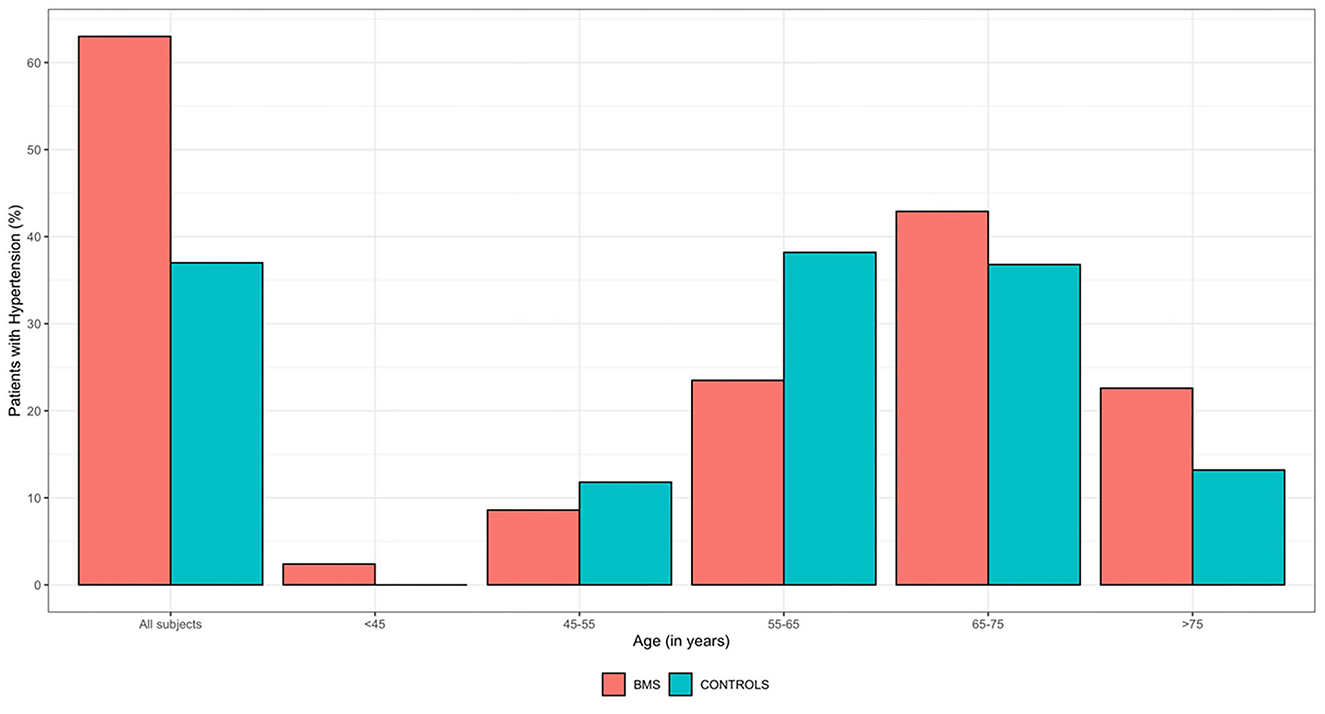
Figure 2. Frequency distribution of HTN by age ranges of 250 WBMS and 250 HW. HW, healthy women; WBMS, women with BMS.
Moreover, considering all the antihypertensive drugs, a statistically significantly higher proportion of WBMS (117; 46.8%) was on antihypertensive therapy compared to the HW (74; 29.6%) (p < 0.001**). The frequency distribution of the antihypertensive drugs among the WBMS is shown in Table 2 and Figure 3. In addition, the majority of WBMS (75; 30%) and HW (48; 19.2%) assumed only one antihypertensive drug, 35 WBMS (13.6%) assumed two antihypertensive drugs, 8 WBMS (3.2) assumed three antihypertensive drugs, and no WBMS assumed four antihypertensive drugs. On the contrary, only 11 out of 128 WBMS and 2 out of 76 HW were found to have HTN without assuming antihypertensive medications. A statistically significant difference was found in a higher percentage of WBMS (37; 14.8%) treated with the angiotensin II receptor antagonist (ARB) molecule compared to the HW (14; 5.6%) (p = 0.001**).

Figure 3. Frequency distribution of the antihypertensive drugs among 250 WBMS and 250 HW. HW, healthy women; WBMS, women with BMS.
When comparing sociodemographic variables and risk factors between the subgroup of 128 WBMS with HTN and the subgroup of 122 WBMS without HTN, some differences were also detected (Supplementary Table 1). As expected, WBMS suffering from HTN were statistically older than those without HTN (67.2 ± 9.58 years vs. 51.1 ± 10.9 years) and had a lower education (8.03 ± 4.3 years vs. 10.4 ± 4.49 years) (p < 0.001**). In relation to the family status, there was a statistically higher prevalence of widowed (18; 14.1%) among the WBMS with HTN and a lower number of employed (18; 14.1%) (p < 0.001**). Differences were also detected with respect to the BMI as a statistically significantly higher proportion of WBMS with HTN (77; 602%) in comparison to the WBMS with no HTN (70; 57.4%) (p < 0.001). On the contrary, a higher percentage of WBMS without HTN showed a normal BMI (40; 32.8%) compared to the WBMS with HTN (29; 22.7%) (p < 0.001). In addition, as displayed in Supplementary Table 2, a comparison of the prevalence of systemic diseases and drug consumption between WBMS with and without HTN revealed no statistically significant difference, except from the antiplatelets, as a higher number of WBMS with HTN were under this medication (48; 37.5%) (p < 0.001*). Further information on the frequency distribution by age ranges of the total WBMS with and without HTN is displayed in Supplementary Figure 1 and Supplementary Table 3.
The type and location of the oral symptoms are shown in Table 3. Statistically significant differences were found between the cases and controls in relation to most of the symptoms. All the WBMS reported a burning sensation (250; 100%), which was the worst symptom reported, followed by xerostomia (151; 60.4%), dysgeusia (115; 46.2%), globus pharyngeus (103; 41.2%), intraoral foreign body sensation (60; 24%), sialorrhea (57; 22.8%), change in the tongue morphology (46; 18.4%), and itching (41; 16.4%). In contrast, halitophobia was the only symptom reported more in HW (19; 7.6%) than in WBMS (16; 6.4%) without any statistically significant difference. The most frequent sites involved were, in order, the tongue (225; 90%), followed by the anterior palate (163; 65.2%), the lips (162; 64.8%), the gums (154; 61.6%), and the cheeks (139; 55.6%).
Comparisons of the clinical parameters between the WBMS and HW are summarized in Table 4. A statistically significant difference was found in the NRS and SF-MPQ score between the two groups (p < 0.001**). The majority of WBMS (229; 91.6%) reported severe pain (NRS > 8) and the median and IQR of the SF-MPQ total score were 10 (7–12).
Statistically significant higher percentages of WBMS suffered from anxiety, depression, and sleep disturbances in comparison to the HW (p < 0.001**). Precisely, 246 WBMS (98.4%) and 89 HW (35.6%) showed anxiety (HAM-A>7), while 247 WBMS (98.8%) and 81 HW (32.4%) showed depression (HAM-D>7). In particular, the majority of WBMS (124; 49.6%) showed mild anxiety (HAM-A: 8–17), while 104 WBMS (41.6%) suffered from mild to moderate anxiety (HAM-A: 18–25). Regarding depression, 116 WBMS (46.4%) had mild depression (HAM-D: 8–18) and 102 WBMS (40.8%) had moderate depression (HAM-D: 17–23). Severe anxiety (HAM-A: 25–30) and severe depression (HAM-D>24) were found in 18 WBMS (7.2%) and in 29 WBMS (11.6%), respectively.
With respect to sleep evaluation, the WBMS showed a strongly statistically significant difference in the PSQI total score (p < 0.001**), as poor sleep (PSQI > 5) was found in 226 WBMS (90.4%) and in only 133 HW (53.2%), while no statistically significant difference was found in the ESS total score between the two groups (p = 0.101).
When comparing oral symptoms, the sites involved and the scores of pain (NRS, T-PRI), anxiety and depression (HAM-A, HAM-D), and sleep quality (PSQI, ESS), no differences were detected between WBMS with and without HTN (Supplementary Tables 4, 5).
A dependence analysis between HTN and qualitative and quantitative predictors was performed separately for WBMS and HW to analyze differences in the predictors of HTN between cases and controls. The results of the dependence analysis between HTN and qualitative and quantitative predictors in WBMS are summarized in Table 5. In detail, employment status was found to correlate with HTN (p < 0.001**); in particular, unemployed WBMS in this group were 110 (85.9%), while the employed WBMS suffering from HTN were only 18 (14.1%). Also, the systemic diseases were positively correlated with HTN (p < 0.001**), and specifically, WBMS suffering from diseases other than HTN were 124 (96.9%). Among the quantitative predictors, only education level, expressed in years, was found to correlate with HTN (p < 0.001**).
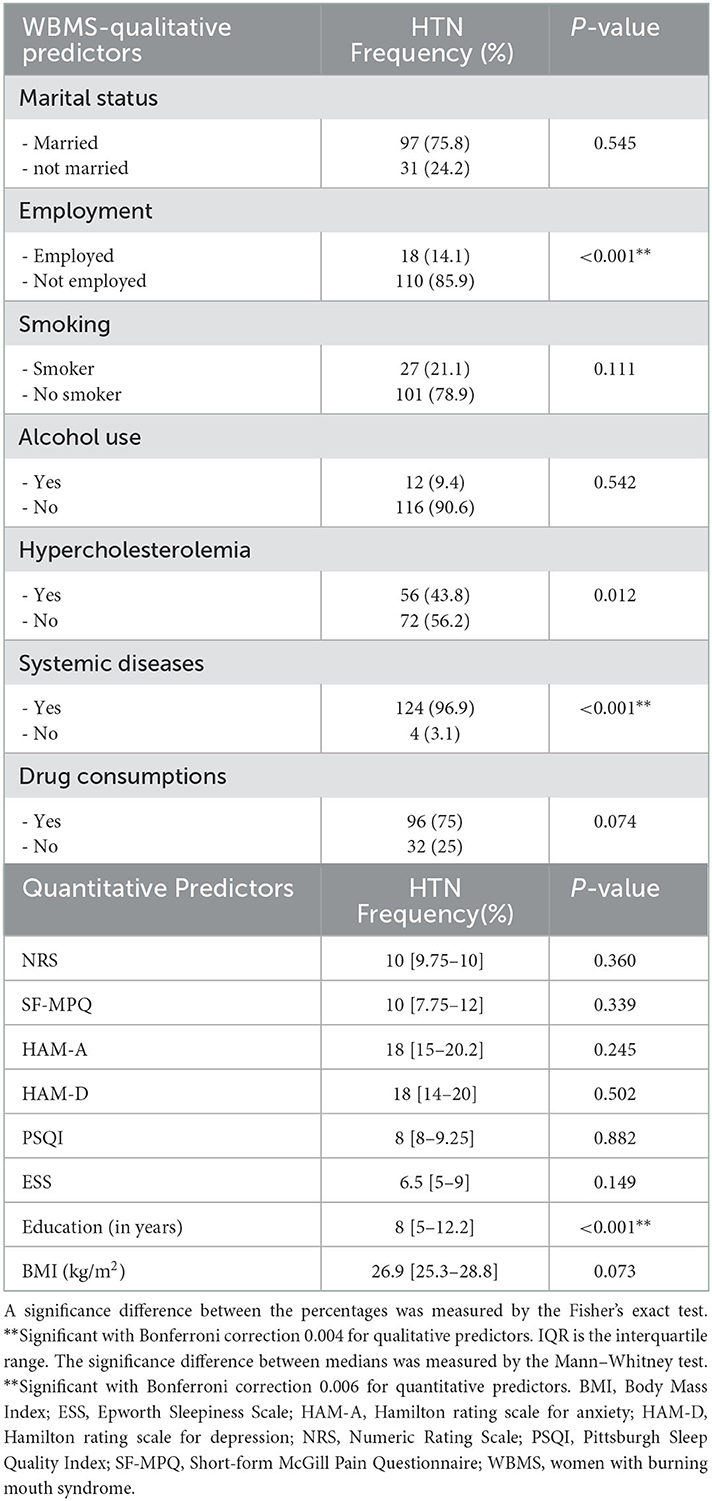
Table 5. Dependence analysis among 128 WBMS patients with HTN and qualitative and quantitative predictors.
The results of the dependence analysis between HTN and qualitative and quantitative predictors in HW are summarized in Table 6. The evaluation of qualitative predictors showed a correlation not only with employment status and systemic diseases (p < 0.001**) as in WBMS but also with hypercholesterolemia and drug consumption as in HW (p < 0.001**). No correlation was found between HTN and quantitative predictors in HW.
Blood pressure and its regulatory systems have been proven to be deeply interconnected with pain modulation (20). For instance, both essential HTN and secondary HTN are effective in reducing acute pain perception throughout a process known as blood pressure-related hypoalgesia (46). On the contrary, in chronic pain sufferers, this mechanism seems to be under-regulated, and as a consequence, elevated blood pressure is associated with greater chronic pain intensity (14).
Nevertheless, the prevalence and role of HTN in chronic pain conditions are poorly understood, especially in women. The female's prevalence of HTN increases after menopause (>40 years), suggesting the pivotal role of sexual hormone imbalance in the pathophysiology of the disease (1, 10, 22). In detail, menopause promotes a derangement of the cardiocirculatory system with a marked decline in endothelium-dependent vasodilation and also in the emergence of other atherogenic factors (47, 48); in addition, the menopause transition broadly affects health and wellbeing in the midlife woman being associated with decreased physical activity and weight gain, impaired sleep, and negative mood (47, 49). All these aspects are also involved in the chronic pain experience, further causing an increase in blood pressure. Indeed, the fluctuations of estrogen hormones and, subsequently, their reduction influence the development and exacerbation of both HTN and pain (50, 51). This theory is supported by epidemiological studies in which the perimenopausal and postmenopausal women showed a higher prevalence of chronic pain and HTN compared to men (9, 52). Moreover, women showed lower pain threshold and tolerance, resulting in increased pain intensity (53). Additionally, it has been proven that women experiencing pain are less prone to use coping strategies, are predisposed to pain chronicization, and are more likely to seek the help of a pain specialist for treatment (50, 54).
BMS epidemiology highlights that it affects more female subjects, especially after menopause (16). Structural and functional alterations in the peripheral and central nervous system are considered in the etiopathogenesis of the disease, thus affecting the pain perception and predisposition for mood disorders, sleep disturbance, and cognitive impairment (17, 19, 55, 56), as suggested also in a recent study from Canfora et al. (57).
This is the first study that evaluated the prevalence of HTN in a wide sample of WBMS in comparison with an age-matched control group and explored the possible predictors of HTN in this disease. Finally, this study analyzed the potential role of HTN in the disease progression and the mutual interaction between HTN and pain, mood disorders, sleep, and other comorbidities.
The results of the study suggested a statistically significant difference in the prevalence of HTN in the sample with a higher prevalence of HTN in WBMS (51.2%) compared with HW (30.4%). Considering age stratification, the prevalence of HTN was higher in HW until 65 years but it increased in WBMS aged higher than 65 years. Precisely, the prevalence of HTN in HW with age < 65 years was in line with the epidemiological studies that investigate women's HTN in the general population (38.2%), and this percentage was found to be consistently lower in WBMS (23.5%).
Instead, this prevalence increased to 42.9% in WBMS aged between 65 and 75 years, resulting in a higher prevalence compared with the general prevalence of HTN in women and in patients suffering from chronic pain. Indeed, even if this prevalence is slightly higher compared with the study of Bruehl et al. (14) on 300 chronic pain patients (39%), these results support the possibility of the functional and overlapping links of the anti-nociceptive and the cardiovascular systems in which impairment in the mechanism of modulating both pain and blood pressure may increase HTN prevalence in WBMS (58–60).
Indeed, blood pressure is modulated by functional circuitry linking the hypothalamus, the nucleus tractus solitarius, the nucleus raphe magnus, and the rostral ventrolateral medulla in which the activity of central adrenergic fibers and alpha-2 receptors may prolong the activation of anti-nociceptive pathways in patients with BMS (61, 62).
Moreover, the bidirectional relationship between pain and blood pressure may involve the levels of cerebral catecholamine, as a result of changed catechol-O-methyltransferase (COMT)-dependent metabolism (63–65). Indeed, the gene which codes for this protein activity is highly polymorphic, and some variants contribute to a lower metabolism of norepinephrine to normetanephrine; the increase of these neurotransmitters not only contributes to higher blood pressure but also modifies pain response (66).
The impact of long-term elevated blood pressure on cerebral health involves structural pathological changes of the brain such as WMH, cortical thinning, enlarged Virchow-Robin spaces, and brain atrophy (67), suggesting that HTN can accelerate brain aging in the same areas involved in chronic pain. However, it is difficult to determine if these brain alterations are a direct consequence of HTN but it could explain the previous study results in which a high prevalence of WMH was found in patients with BMS (68, 69).
In this study, a statistically significant difference in years of education was found between WBMS and HW with lower educational attainment found in WBMS compared with HW, and it may be implicated in the impairment of blood pressure control and considered a predictor of HTN. These results are in line with previous studies' results, in which an increase in the year of education leads to an increase in the individual's knowledge and skills about the disease; on the contrary, unschooled patients were at greater risk of developing uncontrolled HTN (70, 71).
In addition, less educational attainment has a significant role also on the limited knowledge about healthcare and disease (72), such as BMS. For this reason, the patient's ability to manage both diseases is strictly dependent on their education (73).
Moreover, in this study, a higher prevalence of unemployment was found in WBMS (72.8%) compared with HW (57.6%), and this condition was a predictor of HTN as suggested from the results of the dependence analysis. This finding was in line with previous reports in which employment status was inversely associated with HTN in women (22, 74), maybe due to the employment's influence on a woman's socioeconomic status (75).
In line with previous studies, the coexistence of other systemic comorbidities represents a predictor of HTN in patients and in controls, and unfortunately, this is a non-modifiable risk factor of HTN (76, 77).
Therefore, unemployed WBMS with few years of education and other systemic comorbidities are at increased risk to develop HTN. Instead, the predictors of HTN are slightly different in HW group, showing that unemployment, systemic comorbidities, hypercholesterolemia, and drug consumption increase the risk of HTN.
In our sample, the common modifiable health risk behaviors of HTN such as tobacco use (78, 79) and alcohol consumption (80, 81) were not predictors of HTN in patients and controls, but probably these results have to be considered in light of the prevalence of non-smokers (74.4 and 79.9%, respectively) and no alcohol consumers (89.2 and 81.2%, respectively) among WBMS and HW.
In addition, in our sample, BMI was not a predictor of HTN but probably because the majority of WBMS and HW were not obese. In addition, disability related to diseases, such as BMS, makes patients more sedentary and prone to develop obesity. Therefore, body weight reduction in overweight women and the promotion of healthy lifestyle behaviors (50) represent an essential part of both HTN and BMS treatment.
From the analysis of the psychological profile, WBMS showed a higher prevalence of anxiety, depression, and sleep disturbance compared with HW, but no differences were found between WBMS with or without HTN.
Moreover, pain, anxiety depression, and sleep disturbances were not predictors of HTN both in WBMS and in HW. Therefore, the association between depression and anxiety and increased HTN risk remains inconsistent in this study in contrast with other studies that found depression associated with an increased (82, 83) or decreased (84) risk of HTN.
Patients receiving the HTN diagnosis could increase their psychological distress, which may further aggravate the adjunctive diagnosis of BMS (85, 86). Indeed, the coexistence of several medical comorbidities may have a labeling effect causing mental distress and a decrease in the quality of life, resulting in increased healthcare utilization (77).
Sleep disturbance (PSQI > 5) was found in 90.4% of WBMS, confirming the results of previous studies in which a high prevalence of poor sleep was found in patients with BMS (55, 87). Despite sleep disturbance not being considered a predictor of HTN in our sample, it is known that sleep disturbance is associated with an independent HTN risk (88, 89). Particularly, a 2016 American Heart Association (AHA) scientific statement concluded that there is strong epidemiological evidence that self-reported short sleep duration (<6 h) is a risk factor for HTN where women may be more prone to the effects of short sleep duration on HTN risk (89). This statement was confirmed by a review of 2012 in which higher HTN risk among short sleepers has been reported (90).
Short sleep may increase HTN risk through several physiological mechanisms, including disturbed autonomic balance, hormonal imbalances, inflammation and oxidative stress, greater predisposition to obesity, metabolic syndrome, and unhealthy lifestyle behaviors (88–90). Thus, when present simultaneously, BMS, HTN, mood disorder, and sleep disturbance represent a toxic combination that affects the quality of life of individuals, worsening the outcome of the disease (88, 91).
The results of this study highlight that BMS is a complex disease, with several intertwined comorbidities that may aggravate and prevent the healing of patients if a complete medical and psychological assessment and treatment of all conditions is not carried out. Therefore, despite dental professionals playing a central role in the diagnosis and the management of disease, it is crucial to improve the knowledge about BMS also among medical professionals and promote multidisciplinary collaboration to identify and treat the possible associated comorbidities and reduce the societal burden caused by BMS, further worsening the association with HTN and mood disorder.
WBMS showed a higher prevalence of HTN compared with HW. Unemployed WBMS with lower education and other systemic comorbidities are at an increased risk to develop HTN. The mechanism whereby these phenomena are associated is not completely clear by the results of this study although it is reasonable to consider the interaction of the genetic, environmental, and biological factors that could contribute to both HTN and BMS development. Indeed, the effects of the cardiovascular sympathetic stimulation in response to the failure of pain-regulatory mechanisms may contribute to broadening pain perception and HTN. The association of BMS and HTN may, in turn, accelerate brain aging contributing to the occurrence of WMH, resulting in intracortical connectivity reduction, which further affects pain processing and produces a vicious circle.
Moreover, a higher prevalence of anxiety, depression, and sleep disturbance was found in WBMS compared with HW. Considering the deleterious effects of concomitant HTN and mood disorders, early recognition and proper treatment of both conditions in patients affected by BMS are important.
Healthy lifestyle behaviors, in addition to treatments, are essential in WBMS to promote psychological wellbeing, improve the quality of life, and prevent early brain aging. Further studies will be needed to confirm the association between HTN and BMS.
This study has some limitations. First, an important limitation of the study is related to the hypertension diagnosis because it was not possible to verify if hypertension preceded the chronic pain onset or, on the contrary, the chronic pain came first. This could be important in the etiopathogenesis interpretation. Second, the duration of antihypertensive drug assumption and eventual switching in the therapy could not be reported by the patients; as a consequence, we do not know if there were resistant hypertension among these patients; and consequently, although no correlation has been found with HTN and pain scores, it is not possible to address the question of whether controlling the high pressure may have a role in modulating pain perception.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethical Committee of the University of Naples Federico II (Approval Number: 251/19—the date of approval was February 20, 2019). The patients/participants provided their written informed consent to participate in this study.
DA, FC, and MM: conceptualization. DA, FC, EC, GP, and MM: methodology. LD'A and MA: software and formal analysis. MM and DA: validation and supervision. FC, EC, SL, NC, CM, GP, FS, LD'A, MA, and MM: investigation. FC, EC, SL, NC, and MM: resources. FC, EC, CM, SL, NC, FS, LD'A, MA, and MM: data curation. FC, DA, and EC: writing—original draft preparation. FC, EC, LD'A, MA, GP, DA, and MM: writing—review and editing. DA, FC, EC, and MM: visualization. All authors have contributed to the work and are familiar with the primary data, each has read the final version of the manuscript, approved its content, and have agreed to have their name added to the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.969148/full#supplementary-material
1. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. (2020) 16:223–37. doi: 10.1038/s41581-019-0244-2
2. Messerli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heart failure: contemporary update. JACC Heart Fail. (2017) 5:543–51. doi: 10.1016/j.jchf.2017.04.012
3. Barri YM. Hypertension and kidney disease: a deadly connection. Curr Hypertens Rep. (2008) 10:39–45. doi: 10.1007/s11906-008-0009-y
4. Wang J, Sun W, Wells GA Li Z, Li T, Wu J, et al. Differences in prevalence of hypertension and associated risk factors in urban and rural residents of the northeastern region of the People's Republic of China: a cross-sectional study. PLoS ONE. (2018) 13:e0195340. doi: 10.1371/journal.pone.0195340
5. Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep. (2017) 19:24. doi: 10.1007/s11906-017-0724-3
6. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. (2021) 398:957–80. doi: 10.1016/S0140-6736(21)01330-1
7. Reckelhoff JF. Gender differences in hypertension. Curr Opin Nephrol Hypertens. (2018) 27:176–81. doi: 10.1097/MNH.0000000000000404
8. Scheidt-Nave C, Kamtsiuris P, Gößwald A, Hölling H, Lange M, Busch MA, et al. German health interview and examination survey for adults (DEGS) - design, objectives and implementation of the first data collection wave. BMC Public Health. (2012) 12:730. doi: 10.1186/1471-2458-12-730
9. Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. (2002) 53:688–708. doi: 10.1016/S0008-6363(01)00527-2
10. Connelly PJ, Casey H, Montezano AC, Touyz RM, Delles C. Sex steroids receptors, hypertension, and vascular ageing. J Hum Hypertens. (2022) 36:120–5. doi: 10.1038/s41371-021-00576-7
11. Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res. (2017) 122:1–7. doi: 10.1016/j.phrs.2017.05.013
12. Robbins CL, Dietz PM, Bombard J, Tregear M, Schmidt SM, Tregear SJ. Lifestyle interventions for hypertension and dyslipidemia among women of reproductive age. Prev Chronic Dis. (2011) 8:A123.
13. Ferdinand KC, Kleinpeter MA. Management of hypertension and dyslipidemia. Curr Hypertens Rep. (2006) 8:489–96. doi: 10.1007/s11906-006-0028-5
14. Bruehl S, Chung OY, Jirjis JN, Biridepalli S. Prevalence of clinical hypertension in patients with chronic pain compared to nonpain general medical patients. Clin J Pain. (2005) 21:147–53. doi: 10.1097/00002508-200503000-00006
15. Orofacial Pain Classification Committee. International classification of orofacial pain (ICOP). Cephalalgia. (2020) 40:129–221. doi: 10.1177/0333102419893823
16. Wu S, Zhang W, Yan J, Noma N, Young A, Yan Z. Worldwide prevalence estimates of burning mouth syndrome: a systematic review and meta-analysis. Oral Dis. (2021) 28:1431–40. doi: 10.1111/odi.13868
17. Nasri-Heir C, Zagury JG, Thomas D, Ananthan S. Burning mouth syndrome: current concepts. J Indian Prosthodont Soc. (2015) 15:300–7. doi: 10.4103/0972-4052.171823
18. Suzuki N, Mashu S, Toyoda M, Nishibori M. Oral burning sensation: prevalence and gender differences in a Japanese population. Pain Pract. (2010) 10:306–11. doi: 10.1111/j.1533-2500.2010.00361.x
19. Adamo D, Celentano A, Ruoppo E, Cucciniello C, Pecoraro G, Aria M, et al. The relationship between sociodemographic characteristics and clinical features in burning mouth syndrome. Pain Med. (2015) 16:2171–9. doi: 10.1111/pme.12808
20. Saccò M, Meschi M, Regolisti G, Detrenis S, Bianchi L, Bertorelli M, et al. The relationship between blood pressure and pain. J Clin Hypertens. (2013) 15:600–5. doi: 10.1111/jch.12145
21. Bruehl S, Harden RN, Galer BS, Saltz S, Backonja M, Stanton-Hicks M. Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain. (2002) 95:119–24. doi: 10.1016/S0304-3959(01)00387-6
22. Rose KM, Newman B, Tyroler HA, Szklo M, Arnett D, Srivastava N. Women, employment status, and hypertension: cross-sectional and prospective findings from the atherosclerosis risk in communities (ARIC) Study. Ann Epidemiol. (1999) 9:374–82. doi: 10.1016/S1047-2797(99)00015-0
23. Dahiya P, Kamal R, Kumar M. Niti, Gupta R, Chaudhary K. Burning mouth syndrome and menopause. Int J Prev Med. (2013) 4:15–20.
24. Yao H, Zhang Q, Song Q, Liu M, Tang G. Characteristics of oral mucosal lesions and their association with socioeconomic status and systemic health: a cross-sectional study of consecutively collected oral medicine clinic data in a remote rural area of China. Front Public Health. (2022) 10:897814. doi: 10.3389/fpubh.2022.897814
25. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. doi: 10.1016/j.ijsu.2014.07.013
26. Obara T, Naito H, Nojima T, Koga H, Nakao A. Burning mouth syndrome induced by angiotensin-converting enzyme inhibitors. Cureus. (2020) 12:e11376. doi: 10.7759/cureus.11376
27. Azzi L, Veronesi G, Tagliabue A, Croveri F, Maurino V, Reguzzoni M, et al. Is there an association between drugs and burning mouth syndrome? A case-control study. Oral Dis. (2019) 25:1634–44. doi: 10.1111/odi.13116
28. Jin JQ, Cui HM, Han Y, Su S, Liu HW. Multifactor analysis of patients with oral sensory complaints in a case-control study. Chin Med J. (2020) 133:2822–8. doi: 10.1097/CM9.0000000000001190
29. Drawz PE, Beddhu S, Kramer HJ, Rakotz M, Rocco MV, Whelton PK. Blood pressure measurement: a KDOQI perspective. Am J Kidney Dis. (2020) 75:426–34. doi: 10.1053/j.ajkd.2019.08.030
30. Brouwers S, Sudano I, Kokubo Y, Sulaica EM. Arterial hypertension. Lancet. (2021) 398:249–61. doi: 10.1016/S0140-6736(21)00221-X
31. Landi F, Calvani R, Picca A, Tosato M, Martone AM, Ortolani E, et al. Body mass index is strongly associated with hypertension: results from the longevity check-up 7+ study. Nutrients. (2018) 10:E1976. doi: 10.3390/nu10121976
32. Weir CB, Jan A. BMI Classification Percentile Cut Off Points. StatPearls. StatPearls Publishing. (2021). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK541070/ (accessed May 28, 2022).
33. Consultation WH. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. (2000) 894:1–253.
34. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), Mcgill pain questionnaire (MPQ), short-form Mcgill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res. (2011) 63:S240–252. doi: 10.1002/acr.20543
35. Melzack R. The short-form McGill pain questionnaire. Pain. (1987) 30:191–7. doi: 10.1016/0304-3959(87)91074-8
36. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
37. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
38. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. (1967) 6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x
39. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. (1991) 14:540–5. doi: 10.1093/sleep/14.6.540
40. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
41. Trimmel K, Zebrowska M, Böck M, Stefanic A, Mayer D, Klösch G, et al. Wanted: a better cut-off value for the epworth sleepiness scale. Wien Klin Wochenschr. (2018) 130:349–55. doi: 10.1007/s00508-017-1308-6
42. Vignatelli L, Plazzi G, Barbato A, Ferini-Strambi L, Manni R, Pompei F, et al. Italian version of the Epworth sleepiness scale: external validity. Neurol Sci. (2003) 23:295–300. doi: 10.1007/s100720300004
43. Curcio G, Tempesta D, Scarlata S, Marzano C, Moroni F, Rossini PM, et al. Validity of the Italian version of the pittsburgh sleep quality index (PSQI). Neurol Sci. (2013) 34:511–9. doi: 10.1007/s10072-012-1085-y
44. Carpenter JS, Andrykowski MA. Psychometric evaluation of the pittsburgh sleep quality index. J Psychosom Res. (1998) 45:5–13. doi: 10.1016/S0022-3999(97)00298-5
46. Olsen RB, Bruehl S, Nielsen CS, Rosseland LA, Eggen AE, Stubhaug A. Hypertension prevalence and diminished blood pressure-related hypoalgesia in individuals reporting chronic pain in a general population: the Tromsø study. Pain. (2013) 154:257–62. doi: 10.1016/j.pain.2012.10.020
47. Moreau KL, Hildreth KL. Vascular aging across the menopause transition in healthy women. Adv Vasc Med. (2014) 2014:204390. doi: 10.1155/2014/204390
48. Meadows JL, Vaughan DE. Endothelial biology in the post-menopausal obese woman. Maturitas. (2011) 69:120–5. doi: 10.1016/j.maturitas.2011.03.012
49. Zhu D, Chung HF, Pandeya N, Dobson AJ, Kuh D, Crawford SL, et al. Body mass index and age at natural menopause: an international pooled analysis of 11 prospective studies. Eur J Epidemiol. (2018) 33:699–710. doi: 10.1007/s10654-018-0367-y
50. Tan MN, Kartal M, Guldal D. The effect of physical activity and body mass index on menopausal symptoms in Turkish women: a cross-sectional study in primary care. BMC Women's Health. (2014) 14:38. doi: 10.1186/1472-6874-14-38
51. Maas AHEM, Franke HR. Women's health in menopause with a focus on hypertension. Neth Heart J. (2009) 17:68–72. doi: 10.1007/BF03086220
52. Gibson CJ Li Y, Bertenthal D, Huang AJ, Seal KH. Menopause symptoms and chronic pain in a national sample of midlife women veterans. Menopause. (2019) 26:708–13. doi: 10.1097/GME.0000000000001312
53. Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. (2013) 111:52. doi: 10.1093/bja/aet127
54. Arman M, Gebhardt A, Hök Nordberg J, Andermo S. Women's lived experiences of chronic pain: faces of gendered suffering. Qual Health Res. (2020) 30:772–82. doi: 10.1177/1049732319888478
55. Adamo D, Sardella A, Varoni E, Lajolo C, Biasotto M, Ottaviani G, et al. The association between burning mouth syndrome and sleep disturbance: a case-control multicentre study. Oral Dis. (2018) 24:638–49. doi: 10.1111/odi.12807
56. Adamo D, Pecoraro G, Fortuna G, Amato M, Marenzi G, Aria M, et al. Assessment of oral health-related quality of life, measured by OHIP-14 and GOHAI, and psychological profiling in burning mouth syndrome: a case-control clinical study. J Oral Rehabil. (2020) 47:42–52. doi: 10.1111/joor.12864
57. Canfora F, Calabria E, Cuocolo R, Ugga L, Buono G, Marenzi G, et al. Burning fog: cognitive impairment in burning mouth syndrome. Front Aging Neurosci. (2021) 13:727417. doi: 10.3389/fnagi.2021.727417
58. Pinto E. Blood pressure and ageing. Postgrad Med J. (2007) 83:109–14. doi: 10.1136/pgmj.2006.048371
59. Hackett J, Naugle KE, Naugle KM. The decline of endogenous pain modulation with aging: a meta-analysis of temporal summation and conditioned pain modulation. J Pain. (2020) 21:514–28. doi: 10.1016/j.jpain.2019.09.005
60. González-Roldán AM, Terrasa JL, Sitges C, van der Meulen M, Anton F, Montoya P. Age-related changes in pain perception are associated with altered functional connectivity during resting state. Front Aging Neurosci. (2020) 12:116. doi: 10.3389/fnagi.2020.00116
61. Sapru HN. Role of the hypothalamic arcuate nucleus in cardiovascular regulation. Auton Neurosci. (2013) 175:38–50. doi: 10.1016/j.autneu.2012.10.016
62. Matsushita Y, Manabe M, Kitamura N, Shibuya I. Adrenergic receptors inhibit TRPV1 activity in the dorsal root ganglion neurons of rats. PLoS One. (2018) 13:e0191032. doi: 10.1371/journal.pone.0191032
63. Nackley-Neely AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both β2 and β3 adrenergic receptors. Pain. (2007) 128:199–208. doi: 10.1016/j.pain.2006.09.022
64. Xu J, Boström AE, Saeed M, Dubey RK, Waeber G, Vollenweider P, et al. A genetic variant in the catechol-O-methyl transferase (COMT) gene is related to age-dependent differences in the therapeutic effect of calcium-channel blockers. Medicine. (2017) 96:e7029. doi: 10.1097/MD.0000000000007029
65. Diatchenko L, Nackley AG, Slade GD, Bhalang K, Belfer I, Max MB, et al. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain. (2006) 125:216–24. doi: 10.1016/j.pain.2006.05.024
66. Gonzalez-Lopez E, Vrana KE. Dopamine beta-hydroxylase and its genetic variants in human health and disease. J Neurochem. (2020) 152:157–81. doi: 10.1111/jnc.14893
67. Alateeq K, Walsh EI, Cherbuin N. Higher blood pressure is associated with greater white matter lesions and brain atrophy: a systematic review with meta-analysis. J Clin Med. (2021) 10:637. doi: 10.3390/jcm10040637
68. Hay M, Barnes C, Huentelman M, Brinton R, Ryan L. Hypertension and age-related cognitive impairment: common risk factors and a role for precision aging. Curr Hypertens Rep. (2020) 22:80. doi: 10.1007/s11906-020-01090-w
69. Adamo D, Canfora F, Calabria E, Coppola N, Leuci S, Pecoraro G, et al. White matter hyperintensities in burning mouth syndrome assessed according to the age-related white matter changes scale. Front Aging Neurosci. (2022) 14:923720. doi: 10.3389/fnagi.2022.923720
70. Di Chiara T, Scaglione A, Corrao S, Argano C, Pinto A, Scaglione R. Education and hypertension: impact on global cardiovascular risk. Acta Cardiol. (2017) 72:507–13. doi: 10.1080/00015385.2017.1297626
71. Babaee Beigi MA, Zibaeenezhad MJ, Aghasadeghi K, Jokar A, Shekarforoush S, Khazraei H. The effect of educational programs on hypertension management. Int Cardiovasc Res J. (2014) 8:94–8.
72. Tedesco MA, Di Salvo G, Caputo S, Natale F, Ratti G, Iarussi D, et al. Educational level and hypertension: how socioeconomic differences condition health care. J Hum Hypertens. (2001) 15:727–31. doi: 10.1038/sj.jhh.1001249
73. Sorel JE, Ragland DR, Syme SL, Davis WB. Educational status and blood pressure: the second national health and nutrition examination survey, 1976-1980, and the hispanic health and nutrition examination survey, 1982-1984. Am J Epidemiol. (1992) 135:1339–48. doi: 10.1093/oxfordjournals.aje.a116245
74. Rumball-Smith J, Nandi A, Kaufman JS. Working and hypertension: gaps in employment not associated with increased risk in 13 European countries, a retrospective cohort study. BMC Public Health. (2014) 14:536. doi: 10.1186/1471-2458-14-536
75. Rose KM, Newman B, Bennett T, Tyroler HA. Employment status and high blood pressure in women: variations by time and by sociodemographic characteristics. Ann Epidemiol. (1997) 7:107–14. doi: 10.1016/S1047-2797(96)00127-5
76. Noh J, Kim HC, Shin A, Yeom H, Jang SY, Lee JH, et al. Prevalence of Comorbidity among people with hypertension: the Korea national health and nutrition examination survey 2007-2013. Korean Circ J. (2016) 46:672–80. doi: 10.4070/kcj.2016.46.5.672
77. Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T, et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American heart association. Circulation. (2016) 134:e535–78. doi: 10.1161/CIR.0000000000000450
78. Primatesta P, Falaschetti E, Gupta S, Marmot MG, Poulter NR. Association between smoking and blood pressure: evidence from the health survey for England. Hypertension. (2001) 37:187–93. doi: 10.1161/01.HYP.37.2.187
79. Virdis A, Giannarelli C, Neves MF, Taddei S, Ghiadoni L. Cigarette smoking and hypertension. Curr Pharm Des. (2010) 16:2518–25. doi: 10.2174/138161210792062920
80. Husain K, Ansari RA, Ferder L. Alcohol-induced hypertension: Mechanism and prevention. World J Cardiol. (2014) 6:245–52. doi: 10.4330/wjc.v6.i5.245
81. Tasnim S, Tang C, Musini VM, Wright JM. Effect of alcohol on blood pressure. The Cochrane Database of Systematic Reviews. (2020). Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8130994/ (accessed June 5, 2020). doi: 10.1002/14651858.CD012787.pub2
82. Rubio-Guerra AF, Rodriguez-Lopez L, Vargas-Ayala G, Huerta-Ramirez S, Serna DC, Lozano-Nuevo JJ. Depression increases the risk for uncontrolled hypertension. Exp Clin Cardiol. (2013) 18:10–2.
83. DeMoss DS, Teigen KJ, Claassen CA, Fisk MJ, Blair SE, Bakre SA, et al. Association between depression and hypertension using classic and revised blood pressure thresholds. Fam Pract. (2020) 37:616–22. doi: 10.1093/fampra/cmaa010
84. Licht CMM, de Geus EJC, Seldenrijk A, van Hout HPJ, Zitman FG, van Dyck R, et al. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension. (2009) 53:631–8. doi: 10.1161/HYPERTENSIONAHA.108.126698
85. Hamer M, Batty GD, Stamatakis E, Kivimaki M. Hypertension awareness and psychological distress. Hypertension. (2010) 56:547–50. doi: 10.1161/HYPERTENSIONAHA.110.153775
86. Mucci N, Giorgi G, De Pasquale Ceratti S, Fiz-Pérez J, Mucci F, Arcangeli G. Anxiety, stress-related factors, and blood pressure in young adults. Front Psychol. (2016) 7:1682. doi: 10.3389/fpsyg.2016.01682
87. Adamo D, Schiavone V, Aria M, Leuci S, Ruoppo E. Dell'Aversana G, et al. Sleep disturbance in patients with burning mouth syndrome: a case-control study. J Orofac Pain. (2013) 27:304–13. doi: 10.11607/jop.1109
88. Makarem N, Alcántara C, Williams N, Bello NA, Abdalla M. Effect of sleep disturbances on blood pressure. Hypertension. (2021) 77:1036–46. doi: 10.1161/HYPERTENSIONAHA.120.14479
89. Gangwisch JE, Feskanich D, Malaspina D, Shen S, Forman JP. Sleep duration and risk for hypertension in women: results from the nurses' health study. Am J Hypertens. (2013) 26:903–11. doi: 10.1093/ajh/hpt044
90. Wang Q, Xi B, Liu M, Zhang Y, Fu M. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertens Res. (2012) 35:1012–8. doi: 10.1038/hr.2012.91
Keywords: burning mouth syndrome (BMS), hypertension, women, chronic orofacial pain, cardiovascular risk factor
Citation: Canfora F, Calabria E, Pecoraro G, Leuci S, Coppola N, Mazzaccara C, Spirito F, Aria M, D'Aniello L, Mignogna MD and Adamo D (2023) Prevalence of hypertension and correlation with mental health in women with burning mouth syndrome: A case-control study. Front. Cardiovasc. Med. 9:969148. doi: 10.3389/fcvm.2022.969148
Received: 14 June 2022; Accepted: 27 December 2022;
Published: 20 January 2023.
Edited by:
Valeria Visco, University of Salerno, ItalyReviewed by:
Ioanina Parlatescu, Carol Davila University of Medicine and Pharmacy, RomaniaCopyright © 2023 Canfora, Calabria, Pecoraro, Leuci, Coppola, Mazzaccara, Spirito, Aria, D'Aniello, Mignogna and Adamo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Calabria,  Y2FsYWJyaWFlbGVuYTkyQGdtYWlsLmNvbQ==
Y2FsYWJyaWFlbGVuYTkyQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.