- 1Department of Cardiovascular Surgery, School of Medicine, The First Affiliated Hospital of Zhejiang University, Hangzhou, China
- 2Department of Cardiology, School of Medicine, The First Affiliated Hospital of Zhejiang University, Hangzhou, China
Background: The use of cannabis has increased globally due to more regions decriminalizing marijuana use for therapeutic and recreational aims. Several observational studies have revealed that cannabis use is associated with an increased risk of adverse cardiovascular pathologies and diseases. Nevertheless, the causal associations between cannabis use and cardiovascular diseases remain unclear. Hence, we performed single-variable and multivariable Mendelian randomization (MR) to evaluate the association between cannabis use disorder and various cardiovascular diseases.
Materials and methods: Summary statistics were collected from the largest-to-date genome-wide association studies (GWAS) of cannabis use disorder. The 12 SNPs for cannabis use disorder were used as instrumental variables in this study. MR estimates were pooled using a random-effects inverse-variance weighted (IVW) method. Simple median and weighted median methods were conducted as sensitivity analyses.
Results: The genetic liability to cannabis use disorder was associated with an augmented risk of coronary artery disease, myocardial infarction, atrial fibrillation, heart failure, deep venous thrombosis, pulmonary embolism, and stroke. Except for stroke, the results were inconsistent in the sensitivity analyses. The overall patterns for the associations of cannabis use disorder with atrial fibrillation, heart failure, pulmonary embolism and stroke remained in multivariable MR analyses adjusting for potential mediators, including smoking, alcohol, body mass index, blood lipid, type 2 diabetes, hypertension, and depression. However, the association with coronary artery disease, myocardial infarction, and deep venous thrombosis did not persist in multivariable MR analyses. Mediation analysis demonstrated that smoking, body mass index, low-density lipoprotein, hypertension, and depression have more significant mediation effects, which suggests that these factors partly mediate the link from cannabis use disorder to coronary artery disease, myocardial infarction, and deep venous thrombosis.
Conclusion: The genetic liability to cannabis use disorder was associated with a higher risk of atrial fibrillation, heart failure, pulmonary embolism, and stroke. The evidence for the association between cannabis use disorder, coronary artery disease, myocardial infarction, and deep venous thrombosis was weak. Hence, future use of cannabis for therapeutic and recreational aims should consider its potential impact on cardiovascular diseases.
Introduction
In United Nations drug treaties, the admission of cannabis use has been a disputed issue for some time as it induces less damage than illegal opioids. However, with the rise in more legitimate markets, Cannabis sativa is the most smoked substance after cigarettes, and its popularity is growing. According to statistics, in 2018, more than 192 million or 3.9% of the global adult population consumed cannabis (1). Compared with cannabis usage in low-income or middle-income countries, it is much more popular in high-income countries such as North America, Europe and Oceania (2). The prevalence of cannabis use was low but has increased in low-income and middle-income countries (3). In addition, cannabis use patterns in adults have altered in America. In the 1980s, the use of cannabis began in adolescence, with the highest consumption between the ages of 20 and 25 years, but it sharply decreased after 28 years (4). However, after 2008, adults over 30 in America frequently consumed cannabis.
Since 1996, only patients who suffer from nausea, weight loss, muscle spasm, and chronic neuropathic pain caused by multiple sclerosis were permitted to use cannabis for medical purposes in California. However, as of July 30, 2021, thirty-three American States and the District of Columbia have legalized cannabis for medicinal purposes, and the District of Columbia and eleven states have passed laws legalizing marijuana for recreational use (5). Generally, legalization will make cannabis products cheaper and more accessible for people to obtain cannabis, which is likely to increase cannabis use; in the long term, legalization may lead to an increase in marijuana-related harm (6). Although the concentration of tetrahydrocannabinol (THC) and cannabidiol (CBD) vary due to the heterogeneity of cannabis use patterns and regions, potency monitoring programs in America and Europe have shown that over the last couple of decades, THC dosage in cannabis has markedly increased from approximately 5% to more than 15%, and the mean THC: CBD ratio has also increased substantially from 23 in 2008 to 104 in 2017 (4, 6, 7). At present, even though reliable statistical data on the average THC dose are lacking, cannabis users apparently receive a higher dose of THC (8).
According to several observational clinical studies, cannabis consumption was related to various cardiovascular diseases (CVDs) such as coronary artery disease, myocardial infarction (9, 10), atrial fibrillation (11), stroke (12–15) and heart failure (16, 17). In a multicenter, interview-based study, marijuana smoking was a trigger for the onset of acute myocardial infarction. Marijuana use was associated with an increased risk of myocardial infarction onset by 4.8-fold compared to baseline non-use (10). Simple logistic regression analysis showed that teenagers (13–19 years old) who used cannabis were at a higher risk of acute myocardial infarction in a retrospective analysis using the “2012 Kids” Inpatient Database (18). The use of marijuana was also a significant risk factor for acute myocardial infarction in multiple adult-related studies, even when the patients had no other cardiac risk factors (19). An observational study based on the National Inpatient Sample Database in the USA showed that after a multivariable regression analysis of several risk factors, cannabis use remained an independent predictor of both HF and stroke in individuals between 18 and 55 years old (20). Simultaneously, according to a clinical study using a large national administrative database in the USA from 2003 to 2016, 0.5% of hospitalized teenagers (13–20 years old) with cannabis use disorder suffered arrhythmias, and the most frequent arrhythmia was atrial fibrillation (11). Furthermore, several case reports have linked marijuana smoking to atrial fibrillation (21, 22).
In epidemiological studies, however, insufficient evidence was found for CVDs because these studies included a small number of cannabis-only consumers and were not evaluated for optimal exposures. Unfortunately, most of the previous epidemiological data were short-term, retrospective and observational. As up to 70–90% of cannabis consumers smoke cigarettes simultaneously, we could not evaluate directly if differences in cannabis use caused CVD outcomes, were explained by cigarette smoking factors or were a consequence of worsening symptoms (23). As a result of simultaneous marijuana and cigarette consumption, on the basis of traditional observational studies, it was difficult to segregate the separate effects of cannabis use on CVDs. Mendelian randomization (MR) is a technique that examines the causal relationship between exposures and results by using genetic variants as instrumental variables (24). Genetic variants are inherited randomly in MR so that it can be conceptualized as a natural randomized controlled trial (25). When randomized control trials are not feasible, MR became an alternative method for exploring causal relationships between exposure and outcomes (24, 26). Recently, several studies explored the effects of substance use (cannabis use, alcohol consumption, tobacco use) on CVDs or other physical health by using the MR strategy (27–29). Multivariable Mendelian randomization is a method that incorporates genetic variants from each exposure into the same model, thus enabling simultaneous assessment of several correlated exposures (30). Multivariable MR analysis is beneficial where genetic variants are pleiotropic—that is, associated with several risk factors. Confounders that could produce erroneous relationships between exposures and outcomes can be minimized by using multivariable MR analysis. Given the potential confounding and limited causal inference obtained from observational data, we used MR and multivariable MR methods to evaluate relationships between cannabis use disorder and CVDs.
Materials and methods
Study design
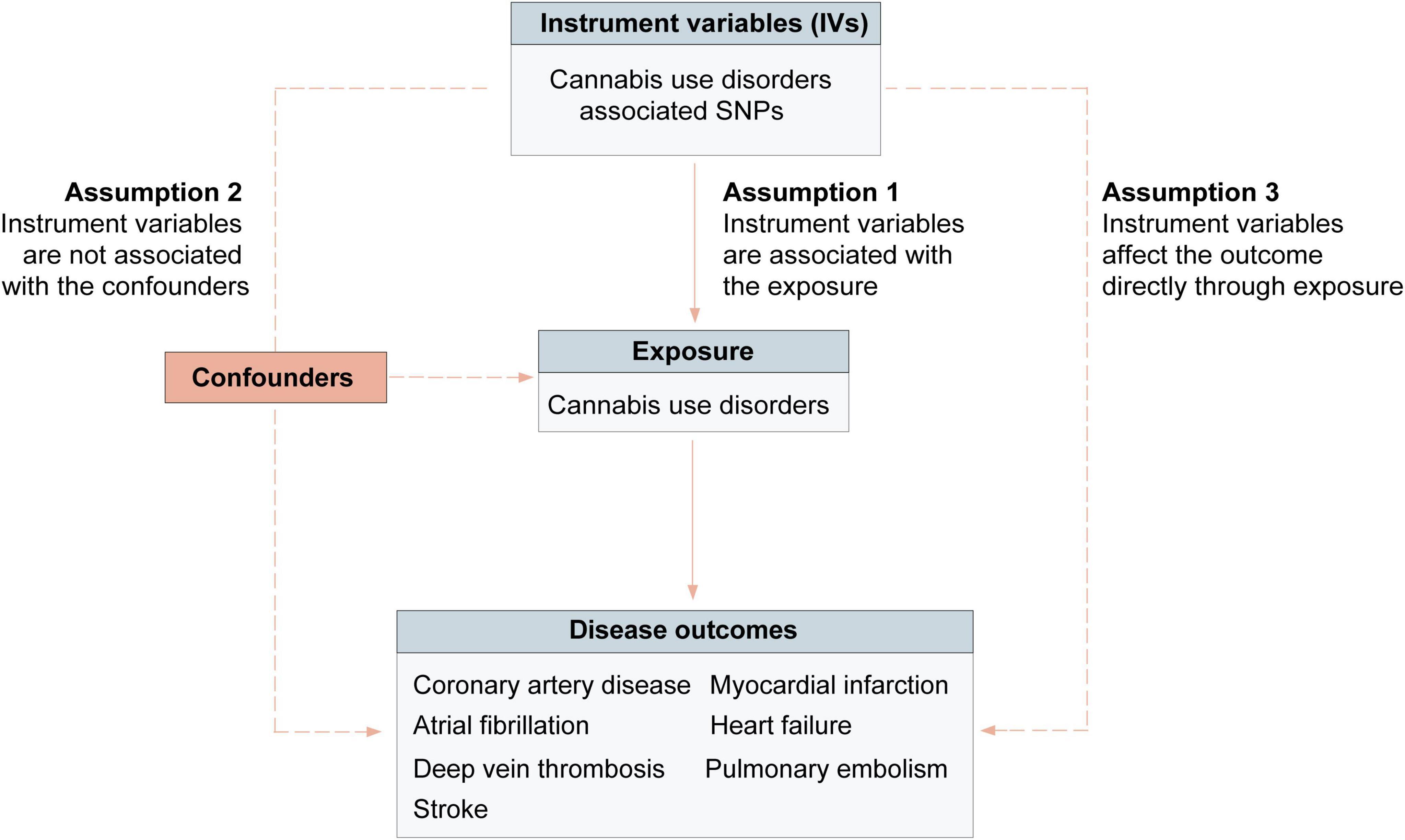
We conducted a two-sample MR analysis using summary-level data for exposures and outcomes from the publicly available genome-wide association studies (GWAS) to assess the relationships of cannabis use disorder with cardiovascular diseases. Based on three assumptions, genetic variants were used as instrumental variables (IVs) to evaluate the association between cannabis use disorder and outcomes (Figure 1). First, genetic variants are associated with the risk factor of interest (cannabis use disorder); Second, the genetic variants considered as IVs are independent from biologically plausible confounders; Third, the genetic variants should affect the outcome directly through the risk factor of interest (24). These publicly available GWAS data used in this study were presented in Supplementary Tables 1, 3. Participants in all original studies provided informed consent and ethical review approval.
Data sources and instruments selection
Instrumental variables for cannabis use disorder
The GWAS summary-level data for cannabis use disorder were obtained from a GWAS meta-analysis involving 384,032 participants from Psychiatric Genomics Consortium Substance Use Disorders working group, Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH), and deCODE (31). The summary statistics of European ancestry case-control individuals were used in our study (14,080 cases and 343,726 controls). The linkage disequilibrium analysis among exposure-associated SNPs was conducted in RStudio with the TwoSampleMR package, using the clump function (r2 < 0.01 and clump window was 10,000 kb) based on the 1000 genomes LD reference panel of only Europeans (CEU, TSI, FIN, GBR and IBS) (23). Only 2 SNPs at p-value < 5 × 10–8 were associated with cannabis use disorder after linkage disequilibrium analysis. We selected 12 SNPs at p-value < 5 × 10–7 associated with cannabis use disorder as instrumental variables (Supplementary Table 4). The LD Link1 was used to select proxies (r2 > 0.60) if no matching SNPs were available in an outcome GWAS (23). One SNP (rs72818514) for cannabis use disorder was unavailable in the pulmonary embolism GWAS, and no proxies could be used instead.
Genome-wide association studies summary statistics for cardiovascular outcomes
The GWAS summary statistics for coronary artery disease and myocardial infarction were obtained from the Coronary Artery Disease Genome-Wide Replication and Meta-analysis plus the Coronary Artery Disease Genetics (CardiogramplusC4D) consortium, which contained 60,801 cases (43,676 cases with myocardial infarction) and 123,504 controls (32). The majority (77%) of the participants were of European ancestry. The GWAS summary statistics of atrial fibrillation were obtained from a large GWAS meta-analysis of 65,446 cases and 522,744 controls using more than 50 studies (33). The sample was composed of 84.2% Europeans, and the data of European ancestry individuals were used in our study (55,114 cases and 482,295 controls). The GWAS summary statistics of heart failure were extracted from a recent GWAS meta-analysis containing 977,323 European participants (47,309 cases and 930,014 controls) (34). The GWAS summary statistics of stroke were obtained from a multiancestry GWAS, including 67,162 cases and 454,450 controls (35). The majority of participants were European, including 40,585 cases and 406,111 controls. We retrieved GWAS summary statistics of deep vein thrombosis and pulmonary embolism from the IEU Open GWAS Project2 : ukb-b, which includes GWAS summary statistics output from the GWAS pipeline using Phesant-derived variables from the UK Biobank. The sample size for deep vein thrombosis was 462,933 (9,241 cases and 453,692 controls) and pulmonary embolism was 462,933 (3,823 cases and 459,110 controls). In addition to the data for coronary artery disease and myocardial infarction being obtained from mixed populations, we used datasets of other cardiovascular diseases (atrial fibrillation, heart failure, deep vein thrombosis, pulmonary embolism, and stroke) only included participants of European descent. There was no participant overlap between the cannabis use disorder dataset and outcomes datasets. Detailed information for the GWAS of exposure and outcomes were presented in Supplementary Table 2.
Moreover, we used replication datasets of cardiovascular diseases to validate our results further. The descriptive information of replication datasets was presented in Supplementary Table 3. The summary-level data of coronary artery disease, myocardial infarction, atrial fibrillation, heart failure, deep vein thrombosis, and pulmonary embolism were derived from the UK biobank. The summary-level data for stroke were obtained from a GWAS meta-analysis of 12 case-control studies (36). The meta-analysis comprised 10,307 cases and 19,326 controls, which contained a small proportion of south Asia individuals (2,385 cases and 5,193 controls).
Genome-wide association studies summary statistics for risk factors
The GWAS summary data for smoking and alcohol intake were derived from a large GWAS involving 1.2 million individuals (37). We chose smoking initiation phenotypes as our instrument for smoking, which indicated whether an individual had ever smoked regularly. Alcohol intake was measured with drinks per week. The GWAS summary data for body mass index were obtained from a large GWAS meta-analysis involving 339,224 individuals (38). Body mass index was measured or self-reported weight in kg per height in meters squared. The data for blood lipid, including low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and total cholesterol, was obtained from a GWAS meta-analysis containing 94,595 European individuals (39). The summary data for type 2 diabetes was obtained from a GWAS meta-analysis, including 26,488 cases and 83,964 controls (40). The summary statistics of hypertension were derived from the UK biobank, which contained 463,010 individuals. The summary data for depression were extracted from the largest GWAS mate-analysis containing 807,553 individuals (excluding 23andme data because of restricted access) (41).
Statistical analysis
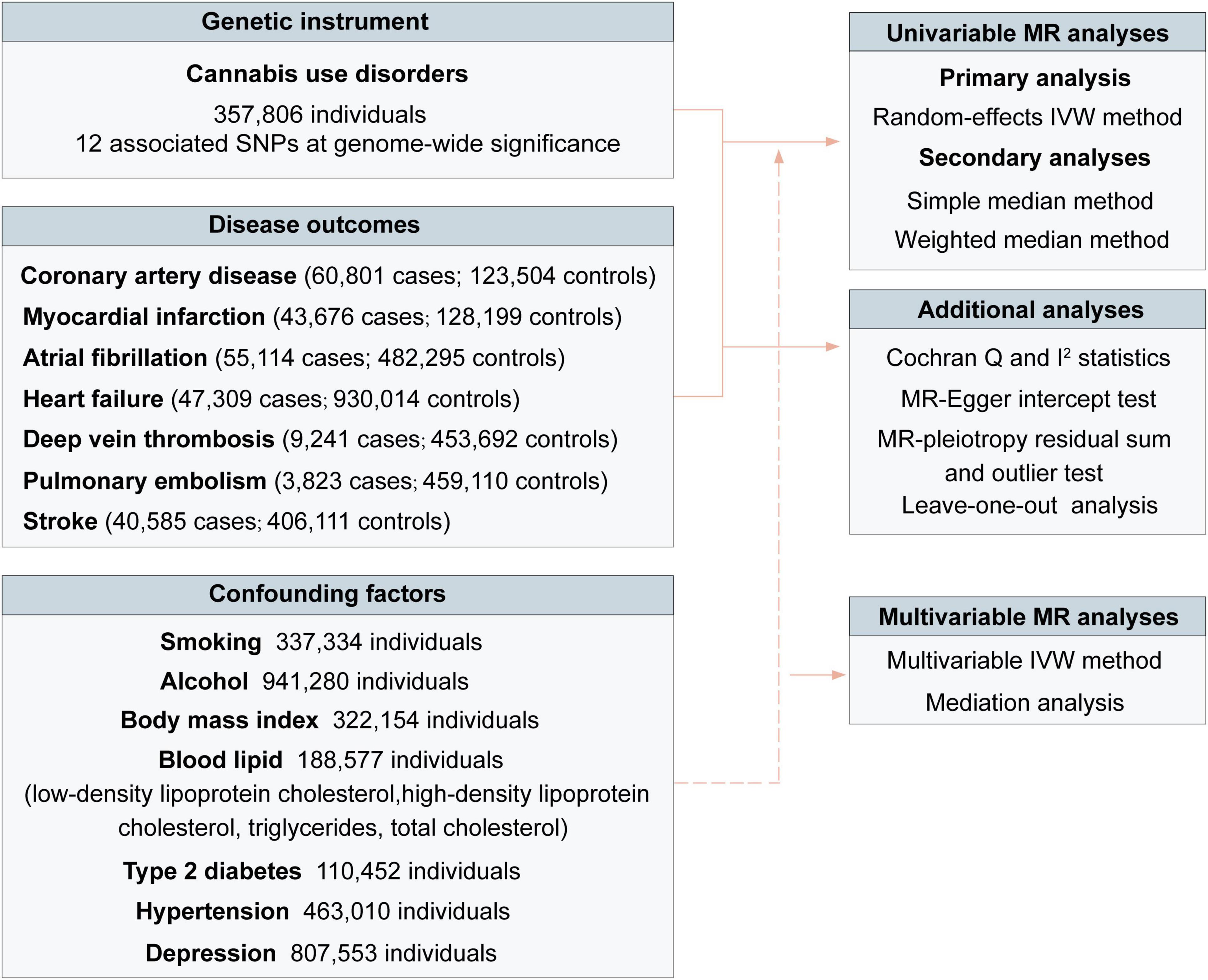
The workflow of performed analyses was presented in Figure 2. For each significant SNP, the R2 was calculated as follows: (2 × EAF × (1-EAF) × beta2)/(2 × EAF × (1-EAF) × beta2 + 2 × EAF × (1-EAF) × N × se2) (42), where EAF was the effect allele frequency, N was the sample size, and beta was the estimated effect on cannabis use disorder. The F-statistic was calculated to estimate the strength of genetic instruments using the formula: F-statistic = R2 × (N - 2)/(1 - R2) (42). The R2 and F-statistics for each SNP were presented in Supplementary Table 4. We used the online tool3 to calculate a priori statistical power. The 12 SNPs for cannabis use disorder explained an estimated 0.1% of phenotypic variability. Power estimates for the 12 SNPs of cannabis use disorder classified by outcomes were presented in Supplementary Table 5.
Following the extraction of estimates and the harmonization of estimates via the effect alleles, we used the Wald Estimator to generate main effect estimates and the Delta method to calculate standard error (43). The MR estimates were combined as standard analysis using the multiplicative random-effects inverse-variance weighted (IVW) method (44). We pooled estimates using complementary simple and weighted median methods as a sensitivity analysis. When at least 50% of the weight is derived from valid instrumental variables, the median method can generate consistent estimates (44). The Cochran Q and I2 statistics were performed to assess heterogeneity among estimates across individual SNPs (44, 45). Heterogeneity was considered if the p-value < 0.05 and I2 were used to quantify heterogeneity (I2 ≤ 25%: low heterogeneity; 25% < I2 ≤ 50%: moderate heterogeneity; I2 ≥ 50%: high heterogeneity). A random-effects model was more suitable if the heterogeneity was high. We also performed the MR-Egger intercept test to investigate horizontal pleiotropy (44, 46). The MR-pleiotropy residual sum and outlier test was adopted to detect and correct for horizontal pleiotropic outliers in the IVW method (SNPs > 10) (47). The detected outliers will be excluded and corrected. Furthermore, we conducted a leave-one-out sensitivity analysis to preclude the possibility that the causal inference was driven by a single SNP. Multivariable MR analyses were performed to evaluate the direct effect of cannabis use disorder on CVD outcomes whilst accounting for potential mediation effects by smoking, alcohol, body mass index, blood lipid, type 2 diabetes, and hypertension, which were the common cardiovascular risk factors (48). Given the strong genetic correlation between cannabis use disorder and depression (31), we also conducted a multivariable MR analysis adjusting for genetic liability to depression. Confounders were subsequently explored via mediation analysis, as previously described, to estimate the mediation effects on the pathway from cannabis use disorder to CVDs (49, 50).
A p-value < 0.05 was considered statistically significant. We adjusted the p-value by performing FDR correction (q value) using the Benjamini-Hochberg method with an FDR threshold q < 0.05. MR analyses were conducted using the TwoSampleMR (version 0.5.5), MendelianRandomization (version 0.4.3), MVMR (version 0.3) and MRPRESSO (version 1.0) in R. All data analyses were conducted with R version 3.6.1.
Results
Association of genetic liability to cannabis use disorder with cardiovascular diseases
The minimum F statistic of the genetic variants was 25.5. The standard IVW analyses showed that cannabis use disorder was positively associated with several cardiovascular diseases (Figure 3 and Supplementary Figure 1). The odds ratios were 1.057 (95% CI: 1.008–1.107, p-value = 0.021, q-value = 0.021) for coronary artery disease, 1.056 (95% CI: 1.014–1.099, p-value = 0.008, q-value = 0.014) for myocardial infarction, 1.062 (95% CI: 1.016–1.111, p-value = 0.008, q-value = 0.012) for atrial fibrillation, 1.096 (95% CI: 1.043–1.151, p-value = 2.64 × 10–4, q-value = 0.001) for heart failure 1.147 (95% CI: 1.029–1.279, p-value = 0.013, q-value = 0.015) for deep vein thrombosis, 1.367 (95% CI: 1.173–1.593, p-value = 5.99 × 10–5, q-value = 4.19 × 10–4) for pulmonary embolism, and 1.096 (95% CI: 1.032–1.164, p-value = 0.003, q-value = 0.007) for stroke. The IVW estimates and 95% CIs were broadly consistent with estimates from the simple median and weighted median sensitivity analyses (Supplementary Table 6). Nevertheless, except for stroke, the q-value of coronary artery disease, myocardial infarction, atrial fibrillation, heart failure, deep vein thrombosis, and pulmonary embolism failed to reach statistical significance in the sensitivity analyses (Supplementary Table 6).
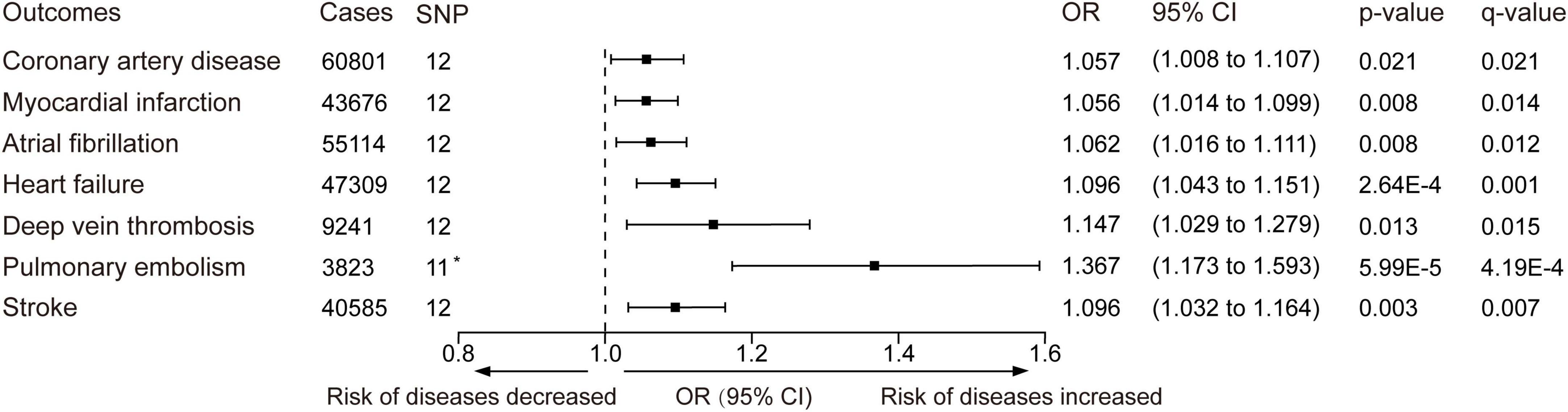
Figure 3. Inverse-variance–weighted Mendelian randomization was performed to determine whether the genetic liability to cannabis use disorder is related to cardiovascular disease. OR, odds ratio. Estimates and p-value were from the random-effect inverse variance-weighted method. CI, confidence interval. The q-values represent Benjamini-Hochberg’s FDR-corrected p-value. The horizontal line represented the odds ratio and 95% confidence interval, OR < 1.0 indicated the risk of diseases decreased and OR > 1.0 indicated the risk of diseases increased. *One SNP (rs72818514) was excluded in the pulmonary embolism outcome because no available proxy was found.
There was no heterogeneity for cannabis use disorder with coronary artery disease, myocardial infarction, or atrial fibrillation. However, there was slight heterogeneity for cannabis use disorder with heart failure (I2 = 2.7%, P = 0.416) and pulmonary embolism (I2 = 7.4%, p-value = 0.377), and moderate heterogeneity for cannabis use disorder with deep vein thrombosis (I2 = 28.1%, p-value = 0.171) and stroke (I2 = 30.4%, p-value = 0.149) (Supplementary Table 7). Similarly, the MR-Egger intercept analysis did not detect directional pleiotropy (Supplementary Table 7). No outlier SNPs were detected with the MR-pleiotropy residual sum and outlier test (Supplementary Table 6). The leave-one-out sensitivity analysis revealed that no single SNP influenced the IVW estimate for each outcome except coronary artery disease. Three SNPs (rs12536335, rs1392816, and rs55986679) influenced the estimate for coronary artery disease (Supplementary Figure 2). We further analyzed the effect of cannabis use disorders on cardiovascular diseases by using replication datasets. The effect of cannabis use disorders on atrial fibrillation, deep vein thrombosis, pulmonary embolism and stroke were robust. However, the association between cannabis use disorders and coronary artery disease, myocardial infarction, and heart failure was not positive when using datasets with smaller sample numbers (Supplementary Table 8).
We also evaluated the effects of those CVDs on cannabis use disorder. However, genetic ability to CVDs was not associated with cannabis use disorder (Supplementary Table 9).
Multivariable Mendelian randomization analysis
In the multivariable MR analysis adjusting for common cardiovascular risk factors (smoking, alcohol, body mass index, blood lipid, type 2 diabetes, hypertension) and depression, the overall patterns for the associations of cannabis use disorder with atrial fibrillation, heart failure, pulmonary embolism, and stroke remained (Table 1). The association with deep venous thrombosis did not persist in the multivariable MR analysis adjusting for smoking, alcohol, hypertension, and depression. Likewise, the pattern of the association between cannabis use disorder with coronary artery disease and myocardial infarction did not persist in the multivariable MR analysis adjusting for cardiovascular risk factors and depression. Mediation analysis were subsequently conducted to investigate the mediation effects of those confounders (Supplementary Table 10). The mediation analysis results showed that smoking, body mass index, low-density lipoprotein, hypertension, and depression have more significant mediation effects than other confounders, which suggested these factors might partly mediate the link from cannabis use disorder to these CVDs.
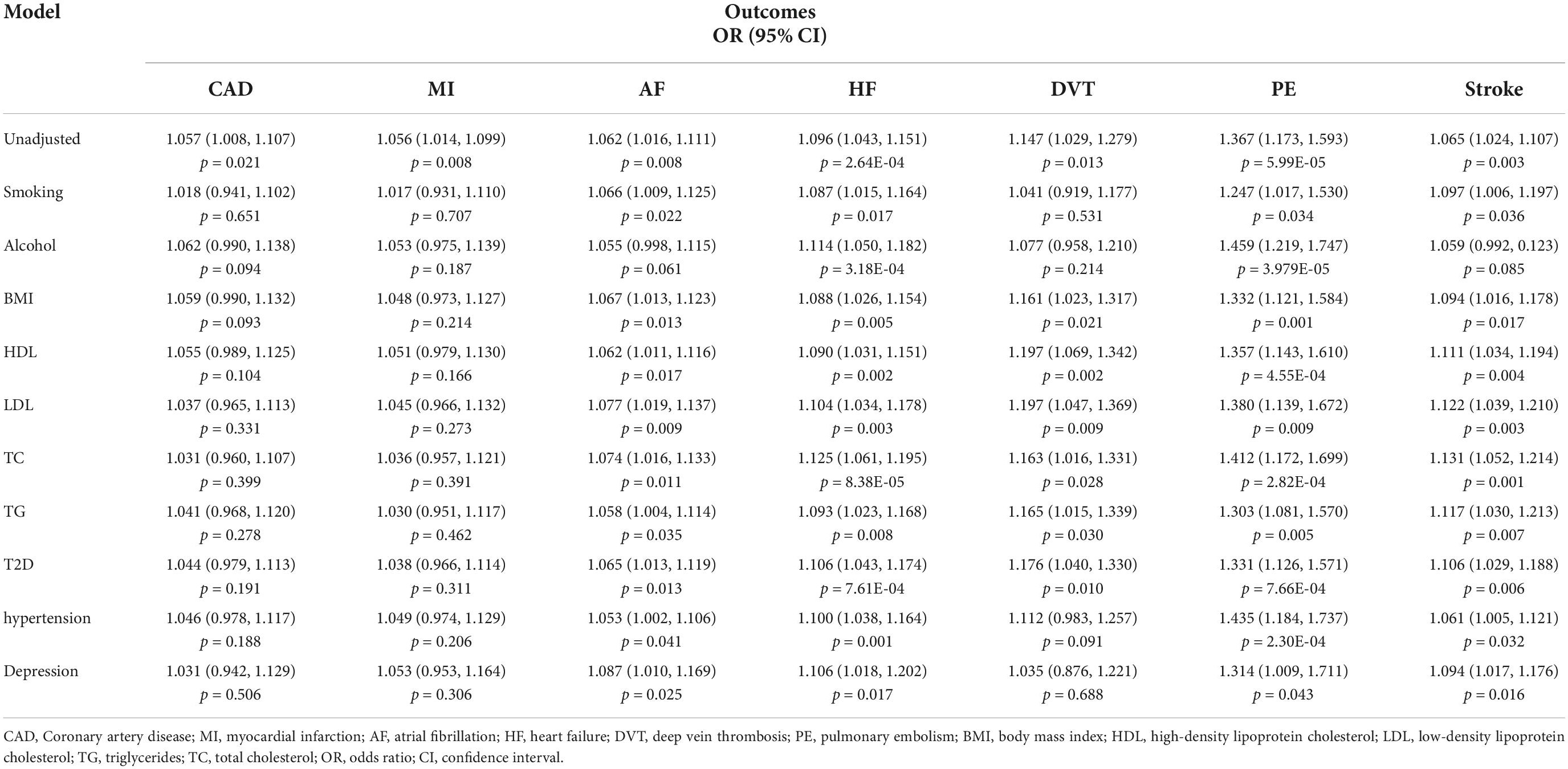
Table 1. Multivariable Mendelian randomization associations of cannabis use disorder with cardiovascular diseases adjusting for risk factors.
Discussion
Throughout the last couple of decades, emerging data have proposed a relationship between cannabis use and the risk of CVDs such as myocardial infarction (9), cardiomyopathy, arrhythmias (21, 22) and cardiac arrest (5, 51). However, prior observational clinical studies may be restricted to unexplained confounding factors (tobacco use) (31), reverse causality (52), and measurement error (inaccurate memory or society’s expectations) (17). In an in vivo animal study, the protective effect of CBD was illustrated in myocardial infarction, stroke, doxorubicin-induced and diabetic cardiomyopathies, and autoimmune myocarditis (16, 53–56). Further clarification of whether cannabis use increases the risk of cardiovascular outcomes may promote the regulated and scientific use of cannabis. Therefore, we performed an MR analysis using SNPs reported to be associated with cannabis use disorder to investigate the connection of cannabis use disorder on the risk of cardiovascular outcomes.
Our MR results provided clues for the relationship between cannabis use disorder and several cardiovascular outcomes. These results aroused our curiosity and interest in cannabis use. Even after adjusting for several common cardiovascular risk factors in multivariable MR analysis, the overall patterns for the associations of cannabis use disorder with atrial fibrillation, heart failure, pulmonary embolism, and stroke remained. The mediation analysis results suggested that smoking, body mass index, low-density lipoprotein, hypertension, and depression might partly mediate the link from cannabis use disorder to coronary artery disease, myocardial infarction, and deep venous thrombosis. Cannabis consumption was often accompanied by smoking. It was not surprising that the mediation effect of smoking was substantial. Cannabis use was also related to depression development in adolescents (57). However, there was no strong evidence for the association between cannabis use, body mass index, blood lipid, and hypertension (58–60). The results of the IVW analysis were inconsistent with the results in the simple and weighted median sensitivity analyses. The inconsistency of the estimates from different methods suggested that the genome-wide significant SNPs for cannabis use disorder are not all valid instrumental variables (p-value of most SNPs > 5 × 10–8) (44), although the F-statistics were all greater than 10. A survey reported that over 2 million Americans with diagnosed cardiovascular diseases currently consumed or have consumed cannabis (17). The genetic liability to CVDs may cause heavier cannabis use; thus, we evaluated the effects of these CVDs on cannabis use disorder. However, no evidence was found for the genetic liability to CVDs with cannabis use disorder. The result demonstrated no reverse causality, which supported the causal interpretation.
The latest study investigated the association between lifetime cannabis use and cardiovascular diseases by using MR analysis (61). Their results did not indicate a causal effect of genetic predisposition to lifetime cannabis use on several cardiovascular diseases. Lifetime cannabis use was defined as any use of cannabis during the lifetime, even if only used once or was a long time ago (62); however, cases in the GWAS of cannabis use disorder met the criteria for cannabis abuse or dependence (31), so cannabis use disorder was regarded as an exposure reflecting heavy lifetime use (23). It was reasonable to believe that the cannabis use disorder phenotype reflected more cannabis use than the lifetime cannabis use phenotype. Hence, we considered that the discrepancy between lifetime cannabis use and cannabis use disorder on cardiovascular diseases might be due to the difference in exposure time. The genetic liability to cannabis use and cannabis use disorder were also distinguished (31). Besides, the composition of the plant and the route of administration influenced the cardiovascular effects of cannabis (16).
Several studies, including case reports, have described the relationship between the cardiac electrophysiologic effect and marijuana use, including atrial fibrillation/atrial flutter, atrioventricular block, sick sinus syndrome, ventricular tachycardia, and Brugada pattern (11, 22, 63, 64). In theory, THC stimulation would increase the content of catecholamines and b-adrenaline in cardiac tissue, which may promote arrhythmogenesis. In both rabbit and dog ventricular papillary muscle, CBD increased action potential duration, decreased the rapid delayed rectifier potassium currents, the slow delayed rectifier potassium currents and the transient outward rectifier potassium currents (65). Consistent with clinical studies, the MR analysis showed that cannabis use disorder was causally associated with atrial fibrillation; however, lifetime cannabis use had no causality with atrial fibrillation. According to our analysis and the clinical observational study, the electrophysiological effect of cannabis use disorder should be considered carefully. However, in medical use, occasional marijuana use did not affect the incidence of atrial fibrillation.
Evidence from a previous observational study indicated that patients with cannabis use disorder have significantly higher incidence of venous thromboembolism, deep vein thromboses, and pulmonary embolism, consistent with our results (66). These associations may be supported by some mechanistic findings. Long-term cannabis use can lead to the deterioration of hematopoietic cells and change red cell indices, which are risk factors for venous thromboembolism (67, 68). A vitro study showed that THC could lead to platelet aggregation and Factor VII activation, which facilitate the process of coagulation (69).
A longitudinal cohort study based on a general population survey of Australians aged 20–24 years, 40–44 years and 60–64 years revealed that compared with non-cannabis users, the rate of stroke/transient ischemic attack in heavy cannabis users was 3.3 times higher than in non-cannabis users, after adjusting for age. Following adjustment for covariates such as tobacco smoking, the rate of non-fatal strokes or transient ischemic attacks among those who used cannabis weekly was higher than that of non-users (12). According to Behavioral Risk Factor Surveillance System Survey Analysis, the odds of stroke for young adults who had recently used marijuana were 1.82 times higher than those without recent marijuana use and 2.45 times higher for frequent marijuana users (> 10 days/month) (70). A longitudinal cohort study in parous women also revealed that cannabis use disorders might increase the long-term risk of CVDs in women, particularly hemorrhagic stroke (15). In addition, our study provided evidence that genetically determined cannabis use disorder had a detrimental effect on stroke. In rats, short-term exposure to secondhand marijuana smoke significantly impaired endothelial function for at least one hour. The duration of such impairment was significantly longer than comparable impairment caused by secondhand smoke (71). A prospective study of 48 consecutive young patients showed that multifocal intracranial stenosis was related to cannabis use in 21% of patients, which suggested that multifocal angiopathy related to cannabis consumption may be a significant cause of ischemic stroke in young people (72).
It is essential to consider how to interpret a causal effect estimate of a binary exposure when performing two-sample MR. The legal status of cannabis makes cannabis exposure uncommon, so the effect of the exposure cannot always be attributed to the exposure itself. In GWAS of cardiovascular diseases, individuals may carry the risk allele but have never been exposed to cannabis. Under the circumstances, the causal effect estimate should be interpreted as the effect of genetic liability to cannabis (23).
Strengths, limitations, and prospection
Our study has several strengths. In contrast to observational studies, MR analysis, particularly multivariable MR analysis, reduce the bias that may occur in observational studies due to confounders. Because of the legal status of cannabis, it is hard to conduct cannabis-related studies. The present MR study leveraged population-scale human genetics to support evidence for a causal association between cannabis use disorder and several cardiovascular diseases, which is a supplement to the existing research. We only included participants of European descent in the exposure and outcome datasets (except for coronary artery disease and myocardial infarction), which can reduce the population stratification bias.
There are some limitations in our study. First, the statistical power of our study seemed inadequate to determine the effects of cannabis use on cardiovascular health. The availability of more extensive GWAS of cannabis use disorder in the future will enhance the accuracy of our MR estimates by providing more exposure variables. Second, the results from some sensitivity analyses were inconsistent with the main findings, which suggested that our results were unstable and may be biased by horizontal pleiotropy. Third, we performed LD analysis using the 1000 Genomes panel of Europeans as the LD reference, but LD could be different in populations even in one ancestry. Fourth, cannabis usually produces deleterious effects based on potency, which is influenced by the amount of psychoactive THC in it. However, our MR study did not examine the differential health effects of THC and the potential offset due to the co-administration of CBD on different diseases. Moreover, genetic variants used as instruments may vary over age in their relationship with the exposure, leading to a risk of bias in MR analyses. We considered this bias in our MR analyses minor for some reasons: First, the exposures in this study were binary exposure variables. Cannabis use disorder included cannabis abuse or dependence that reflected heavy lifetime use. These phenotypes may change small over time. Besides, cannabis use often starts in the middle to late teenage years and peaks in the early and middle 20s. After employment, marriage, and having children, cannabis use declines steeply (73). The bias tended to decrease when exposure windows were short (74).
The levels of evidence from previous observative studies have not been robust, and the present study provides novel additional evidence. The cannabis plant contains more than 60 compounds with varying pharmacological properties (75). More research should be conducted to investigate the different effects of those compounds so that safe and effective products can be developed. Moreover, the GWAS of biomarkers of cannabis exposure, such as 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid or DNA methylation markers could also be used in further MR studies (76).
Conclusion
The present MR study supported a potentially causal association between cannabis use disorder with higher risks of atrial fibrillation, heart failure, pulmonary embolism, and stroke. The evidence for the association between cannabis use disorder, coronary artery disease, myocardial infarction, and deep venous thrombosis was weak. Additional studies, especially clinical studies, are required to verify the effects of cannabis on cardiovascular. Our results suggest caution and alertness for potential public health hazards in cannabis use.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
ZW and LM conceived and designed the experiments. MC and X-FC performed the experiments, analyzed the data, and revised manuscript. Y-LL wrote the manuscript. All authors read and approved the manuscript.
Funding
This study was supported by the Natural Science Foundation for Youth of China (82000345) and Zhejiang Provincial Natural Science Foundation of China (LQ19H020008).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.966707/full#supplementary-material
Footnotes
- ^ https://analysistools.cancer.gov/LDlink
- ^ https://gwas.mrcieu.ac.uk
- ^ https://shiny.cnsgenomics.com/mRnd/
References
1. Connor JP, Stjepanovic D, Foll B Le, Hoch E, Budney AJ, Hall WD. Cannabis use and cannabis use disorder. Nat Rev Dis Primers. (2021) 7:16. doi: 10.1038/s41572-021-00247-4
2. Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction. (2018) 113:1905–26. doi: 10.1111/add.14234
3. Carvalho AF, Stubbs B, Vancampfort D, Kloiber S, Maes M, Firth J, et al. Cannabis use and suicide attempts among 86,254 adolescents aged 12-15 years from 21 low- and middle-income countries. Eur Psychiatry. (2019) 56:8–13. doi: 10.1016/j.eurpsy.2018.10.006
4. Kilmer B. Recreational cannabis – minimizing the health risks from legalization. N Engl J Med. (2017) 376:705–7. doi: 10.1056/NEJMp1614783
5. Kaufman TM, Fazio S, Shapiro MD. Brief commentary: marijuana and cardiovascular disease-what should we tell patients? Ann Intern Med. (2019) 170:119–20. doi: 10.7326/m18-3009
6. Hall W, Stjepanović D, Caulkins J, Lynskey M, Leung J, Campbell G, et al. Public health implications of legalising the production and sale of cannabis for medicinal and recreational use. Lancet. (2019) 394:1580–90. doi: 10.1016/s0140-6736(19)31789-1
7. Chandra S, Radwan MM, Majumdar CG, Church JC, Freeman TP, ElSohly MA. New trends in cannabis potency in USA and Europe during the last decade (2008-2017). Eur Arch Psychiatry Clin Neurosci. (2019) 269:5–15. doi: 10.1007/s00406-019-00983-5
8. Pacher P, Kogan NM, Mechoulam R. Beyond THC and endocannabinoids. Annu Rev Pharmacol Toxicol. (2020) 60:637–59. doi: 10.1146/annurev-pharmtox-010818-021441
9. Nawrot TS, Perez L, Künzli N, Munters E, Nemery B. Public health importance of triggers of myocardial infarction: a comparative risk assessment. Lancet. (2011) 377:732–40. doi: 10.1016/s0140-6736(10)62296-9
10. Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation. (2001) 103:2805–9. doi: 10.1161/01.cir.103.23.2805
11. Umapathi KK, Thavamani A, Dhanpalreddy H, Nguyen HH. Prevalence of cardiac arrhythmias in cannabis use disorder related hospitalizations in teenagers from 2003 to 2016 in the United States. Europace. (2021) 23:1302–9. doi: 10.1093/europace/euab033
12. Hemachandra D, McKetin R, Cherbuin N, Anstey KJ. Heavy cannabis users at elevated risk of stroke: evidence from a general population survey. Aust N Z J Public Health. (2016) 40:226–30. doi: 10.1111/1753-6405.12477
13. Hackam DG. Cannabis and stroke: systematic appraisal of case reports. Stroke. (2015) 46:852–6. doi: 10.1161/strokeaha.115.008680
14. McGuinness B, Goel A, Elias F, Rapanos T, Mittleman MA, Ladha KS. Cannabis use disorder and perioperative outcomes in vascular surgery. J Vasc Surg (2021) 73:1376–87.e3. doi: 10.1016/j.jvs.2020.07.094
15. Auger N, Paradis G, Low N, Ayoub A, He S, Potter BJ. Cannabis use disorder and the future risk of cardiovascular disease in parous women: a longitudinal cohort study. BMC Med. (2020) 18:328. doi: 10.1186/s12916-020-01804-6
16. Pacher P, Steffens S, Haskó G, Schindler TH, Kunos G. Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat Rev Cardiol. (2018) 15:151–66. doi: 10.1038/nrcardio.2017.130
17. DeFilippis EM, Bajaj NS, Singh A, Malloy R, Givertz MM, Blankstein R, et al. Marijuana use in patients with cardiovascular disease: JACC review topic of the week. J Am Coll Cardiol. (2020) 75:320–32. doi: 10.1016/j.jacc.2019.11.025
18. Ramphul K, Mejias SG, Joynauth J. Cocaine, amphetamine, and cannabis use increases the risk of acute myocardial infarction in teenagers. Am J Cardiol. (2019) 123:354. doi: 10.1016/j.amjcard.2018.10.019
19. Patel RS, Kamil SH, Bachu R, Adikey A, Ravat V, Kaur M, et al. Marijuana use and acute myocardial infarction: a systematic review of published cases in the literature. Trends Cardiovasc Med. (2020) 30:298–307. doi: 10.1016/j.tcm.2019.08.003
20. Kalla A, Krishnamoorthy PM, Gopalakrishnan A, Figueredo VM. Cannabis use predicts risks of heart failure and cerebrovascular accidents: results from the National Inpatient Sample. J Cardiovasc Med. (2018) 19:480–4. doi: 10.2459/JCM.0000000000000681
21. Korantzopoulos P, Liu T, Papaioannides D, Li G, Goudevenos JA. Atrial fibrillation and marijuana smoking. Int J Clin Pract. (2008) 62:308–13. doi: 10.1111/j.1742-1241.2007.01505.x
22. Richards JR, Blohm E, Toles KA, Jarman AF, Ely DF, Elder JW. The association of cannabis use and cardiac dysrhythmias: a systematic review. Clin Toxicol. (2020) 58:861–9. doi: 10.1080/15563650.2020.1743847
23. Baumeister SE, Baurecht H, Nolde M, Alayash Z, Gläser S, Johansson M, et al. Cannabis use, pulmonary function, and lung cancer susceptibility: a Mendelian randomization study. J Thorac Oncol. (2021) 17:1127–35. doi: 10.1016/j.jtho.2021.03.025
24. Smith GD, Ebrahim S. Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32:1–22. doi: 10.1093/ije/dyg070
25. Davey Smith G, Ebrahim S. What can mendelian randomisation tell us about modifiable behavioural and environmental exposures? BMJ. (2005) 330:1076–9. doi: 10.1136/bmj.330.7499.1076
26. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
27. Rosoff DB, Smith G Davey, Mehta N, Clarke TK, Lohoff FW. Evaluating the relationship between alcohol consumption, tobacco use, and cardiovascular disease: a multivariable Mendelian randomization study. PLoS Med. (2020) 17:e1003410. doi: 10.1371/journal.pmed.1003410
28. Rosoff DB, Yoo J, Lohoff FW. Smoking is significantly associated with increased risk of COVID-19 and other respiratory infections. Commun Biol. (2021) 4:1230. doi: 10.1038/s42003-021-02685-y
29. Levin MG, Klarin D, Assimes TL, Freiberg MS, Ingelsson E, Lynch J, et al. Genetics of smoking and risk of atherosclerotic cardiovascular diseases: a Mendelian randomization study. JAMA Netw Open. (2021) 4:e2034461. doi: 10.1001/jamanetworkopen.2020.34461
30. Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. (2015) 181:251–60. doi: 10.1093/aje/kwu283
31. Johnson EC, Demontis D, Thorgeirsson TE, Walters RK, Polimanti R, Hatoum AS, et al. A large-scale genome-wide association study meta-analysis of cannabis use disorder. Lancet Psychiatry. (2020) 7:1032–45. doi: 10.1016/s2215-0366(20)30339-4
32. Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. (2015) 47:1121–30. doi: 10.1038/ng.3396
33. Roselli C, Chaffin MD, Weng LC, Aeschbacher S, Ahlberg G, Albert CM, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. (2018) 50:1225–33. doi: 10.1038/s41588-018-0133-9
34. Shah S, Henry A, Roselli C, Lin H, Sveinbjörnsson G, Fatemifar G, et al. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. (2020) 11:163. doi: 10.1038/s41467-019-13690-5
35. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. (2018) 50:524–37. doi: 10.1038/s41588-018-0058-3
36. Malik R, Traylor M, Pulit SL, Bevan S, Hopewell JC, Holliday EG, et al. Low-frequency and common genetic variation in ischemic stroke: the METASTROKE collaboration. Neurology. (2016) 86:1217–26. doi: 10.1212/wnl.0000000000002528
37. Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. (2019) 51:237–44. doi: 10.1038/s41588-018-0307-5
38. Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. (2015) 518:197–206. doi: 10.1038/nature14177
39. Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. (2013) 45:1274–83. doi: 10.1038/ng.2797
40. DIAbetes Genetics Replication And Meta-analysis (Diagram) Consortium, Asian Genetic Epidemiology Network Type 2 Diabetes (Agen-T2D) Consortium, South Asian Type 2 Diabetes (SAT2D) Consortium, Mexican American Type 2 Diabetes (MAT2D) Consortium, Type 2 Diabetes Genetic Exploration by Nex-generation sequencing in muylti-Ethnic Samples (T2D-Genes) Consortium, Mahajan A, et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet. (2014) 46:234–44. doi: 10.1038/ng.2897
41. Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. (2019) 22:343–52. doi: 10.1038/s41593-018-0326-7
42. Papadimitriou N, Dimou N, Tsilidis KK, Banbury B, Martin RM, Lewis SJ, et al. Physical activity and risks of breast and colorectal cancer: a Mendelian randomisation analysis. Nat Commun. (2020) 11:597. doi: 10.1038/s41467-020-14389-8
43. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
44. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology. (2017) 28:30–42. doi: 10.1097/ede.0000000000000559
45. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
46. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. (2017) 36:1783–802. doi: 10.1002/sim.7221
47. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
48. Yuan S, Mason AM, Burgess S, Larsson SC. Genetic liability to insomnia in relation to cardiovascular diseases: a Mendelian randomisation study. Eur J Epidemiol. (2021) 36:393–400. doi: 10.1007/s10654-021-00737-5
49. Burgess S, Daniel RM, Butterworth AS, Thompson SG, Epi Consortium. Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol. (2015) 44:484–95. doi: 10.1093/ije/dyu176
50. Lu Y, Wang Z, Georgakis MK, Lin H, Zheng L. Genetic liability to depression and risk of coronary artery disease, myocardial infarction, and other cardiovascular outcomes. J Am Heart Assoc. (2021) 10:e017986. doi: 10.1161/JAHA.120.017986
51. Richards JR, Bing ML, Moulin AK, Elder JW, Rominski RT, Summers PJ, et al. Cannabis use and acute coronary syndrome. Clin Toxicol (Phila). (2019) 57:831–41. doi: 10.1080/15563650.2019.1601735
52. Pastor A, Conn J, MacIsaac RJ, Bonomo Y. Alcohol and illicit drug use in people with diabetes. Lancet Diabetes Endocrinol. (2020) 8:239–48. doi: 10.1016/s2213-8587(19)30410-3
53. Rajesh M, Mukhopadhyay P, Bátkai S, Patel V, Saito K, Matsumoto S, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. (2010) 56:2115–25. doi: 10.1016/j.jacc.2010.07.033
54. Fouda MA, Ghovanloo MR, Ruben PC. Cannabidiol protects against high glucose-induced oxidative stress and cytotoxicity in cardiac voltage-gated sodium channels. Br J Pharmacol. (2020) 177:2932–46. doi: 10.1111/bph.15020
55. Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, et al. Cannabidiol: state of the art and new challenges for therapeutic applications. Pharmacol Ther. (2017) 175:133–50. doi: 10.1016/j.pharmthera.2017.02.041
56. Jouanjus E, Lapeyre-Mestre M, Micallef J. Cannabis use: signal of increasing risk of serious cardiovascular disorders. J Am Heart Assoc. (2014) 3:e000638. doi: 10.1161/jaha.113.000638
57. Gobbi G, Atkin T, Zytynski T, Wang S, Askari S, Boruff J, et al. Association of cannabis use in adolescence and risk of depression, anxiety, and suicidality in young adulthood: a systematic review and meta-analysis. JAMA Psychiatry. (2019) 76:426–34. doi: 10.1001/jamapsychiatry.2018.4500
58. Lazarte J, Hegele RA. Cannabis effects on lipoproteins. Curr Opin Lipidol. (2019) 30:140–6. doi: 10.1097/mol.0000000000000575
59. Haleem A, Hwang YJ, Elton-Marshall T, Rehm J, Imtiaz S. The longitudinal relationship between cannabis use and hypertension. Drug Alcohol Rev. (2021) 40:914–9. doi: 10.1111/dar.13266
60. Alayash Z, Nolde M, Meisinger C, Baurecht H, Baumeister SE. Cannabis use and obesity-traits: a Mendelian randomization study. Drug Alcohol Depend. (2021) 226:108863. doi: 10.1016/j.drugalcdep.2021.108863
61. Zhao J, Chen H, Zhuo C, Xia S. Cannabis use and the risk of cardiovascular diseases: a Mendelian randomization study. Front Cardiovasc Med. (2021) 8:676850. doi: 10.3389/fcvm.2021.676850
62. Pasman JA, Verweij KJH, Gerring Z, Stringer S, Sanchez-Roige S, Treur JL, et al. GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal influence of schizophrenia. Nat Neurosci. (2018) 21:1161–70. doi: 10.1038/s41593-018-0206-1
63. DeFilippis EM, Singh A, Divakaran S, Gupta A, Collins BL, Biery D, et al. Cocaine and marijuana use among young adults with myocardial infarction. J Am Coll Cardiol. (2018) 71:2540–51. doi: 10.1016/j.jacc.2018.02.047
64. Singh D, Huntwork M, Shetty V, Sequeira G, Akingbola O. Prolonged atrial fibrillation precipitated by new-onset seizures and marijuana abuse. Pediatrics. (2014) 133:e443–6. doi: 10.1542/peds.2013-1831
65. Topal L, Naveed M, Orvos P, Pászti B, Prorok J, Bajtel Á, et al. The electrophysiological effects of cannabidiol on action potentials and transmembrane potassium currents in rabbit and dog cardiac ventricular preparations. Arch Toxicol. (2021) 95:2497–505. doi: 10.1007/s00204-021-03086-0
66. Vakharia RM, Sodhi N, Anis HK, Ehiorobo JO, Mont MA, Roche MW. Patients who have cannabis use disorder have higher rates of venous thromboemboli, readmission rates, and costs following primary total knee arthroplasty. J Arthroplasty. (2020) 35:997–1002. doi: 10.1016/j.arth.2019.11.035
67. Guzel D, Yazici AB, Yazici E, Erol A. Alterations of the hematologic cells in synthetic cannabinoid users. J Clin Lab Anal. (2017) 31:e22131. doi: 10.1002/jcla.22131
68. Ozsu S, Abul Y, Gunaydin S, Orem A, Ozlu T. Prognostic value of red cell distribution width in patients with pulmonary embolism. Clin Appl Thromb Hemost. (2014) 20:365–70. doi: 10.1177/1076029612464901
69. Deusch E, Kress HG, Kraft B, Kozek-Langenecker SA. The procoagulatory effects of delta-9-tetrahydrocannabinol in human platelets. Anesth Analg. (2004) 99:1127–30. doi: 10.1213/01.Ane.0000131505.03006.74
70. Parekh T, Pemmasani S, Desai R. Marijuana use among young adults (18-44 years of age) and risk of stroke: a behavioral risk factor surveillance system survey analysis. Stroke. (2020) 51:308–10. doi: 10.1161/strokeaha.119.027828
71. Wang X, Derakhshandeh R, Liu J, Narayan S, Nabavizadeh P, Le S, et al. One minute of marijuana secondhand smoke exposure substantially impairs vascular endothelial function. J Am Heart Assoc. (2016) 5:e003858. doi: 10.1161/jaha.116.003858
72. Wolff V, Lauer V, Rouyer O, Sellal F, Meyer N, Raul JS, et al. Cannabis use, ischemic stroke, and multifocal intracranial vasoconstriction: a prospective study in 48 consecutive young patients. Stroke. (2011) 42:1778–80. doi: 10.1161/strokeaha.110.610915
73. Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. (2009) 374:1383–91. doi: 10.1016/s0140-6736(09)61037-0
74. Labrecque JA, Swanson SA. Age-varying genetic associations and implications for bias in Mendelian randomization. medRXiv[Preprint]. (2021). Available online at: https://doi.org/10.1101/2021.04.28.21256235 (accessed on April 30, 2021).
75. Choo EK, Emery SL. Clearing the haze: the complexities and challenges of research on state marijuana laws. Ann N Y Acad Sci. (2017) 1394:55–73. doi: 10.1111/nyas.13093
Keywords: cannabis use disorder, cardiovascular diseases, Mendelian randomization study, GWAS - genome-wide association study, cardiovascular genetics
Citation: Chen M, Lu Y-l, Chen X-f, Wang Z and Ma L (2022) Association of cannabis use disorder with cardiovascular diseases: A two-sample Mendelian randomization study. Front. Cardiovasc. Med. 9:966707. doi: 10.3389/fcvm.2022.966707
Received: 11 June 2022; Accepted: 12 September 2022;
Published: 06 October 2022.
Edited by:
Wayne Denis Hall, The University of Queensland, AustraliaReviewed by:
Joëlle Pasman, Karolinska Institutet (KI), SwedenMasahiro Yoshikawa, Nihon University School of Medicine, Japan
Yury Loika, Duke University, United States
Copyright © 2022 Chen, Lu, Chen, Wang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Wang, MTE3MTgxOTBAemp1LmVkdS5jbg==; Liang Ma, TUwxNDAyQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
 Miao Chen
Miao Chen Yun-long Lu
Yun-long Lu Xiao-fan Chen1
Xiao-fan Chen1 Zhen Wang
Zhen Wang Liang Ma
Liang Ma