
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 24 October 2022
Sec. Cardiovascular Therapeutics
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.966331
This article is part of the Research Topic Drug-induced Cardiotoxicity: Identification, assessment, prevention and management View all 14 articles
Objective: This study aimed to identify the most common and top drugs associated with the risk of torsades de pointes (TdP) based on the United States Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database.
Materials and methods: We used OpenVigil 2.1 to query FAERS database and data from the first quarter of 2004 to the third quarter of 2021 were retrieved. The Medical Dictionary for Regulatory Activities (MedDRA) was used to identify TdP cases. We listed the most common drugs associated with the reported TdP cases. Then, the reporting odds ratio (ROR) and the proportional reporting ratio (PRR) for the reporting association between different drugs and TdP risk were calculated. Meanwhile, comparisons were conducted with the QT drug lists of CredibleMeds® in an attempt to identify drugs with a potential risk of TdP that were not on the list.
Results: A total of 9,217,181 adverse event reports were identified, of which 3,807 (0.04%) were related to TdP. TdP was more likely to occur in the elderly and females. Amiodarone (464 cases) was associated with most cases of TdP. According to the disproportionality analysis, the top five drugs with the highest ROR and PRR were tolazoline (ROR 1615.11, 95% confidence interval [CI] 455.59–5725.75, PRR 969.46, χ2 2960.10), levomethadyl (ROR 1211.01, 95% CI 302.75–4844.04, PRR 807.67, χ2 1677.03), ibutilide (ROR 1118.74, 95% CI 425.00–2944.91, PRR 765.77, χ2 3845.27), halofantrine (ROR 660.55, 95% CI 184.21–2368.69, PRR 519.22, χ2 1076.31), and isoproterenol (ROR 352.20, 95% CI 227.19–546.00, PRR 307.82, χ2 6692.53). Approximately half of the top 50 drugs (22 for ROR, 30 for PRR) were not outlined on the QT drug lists of CredibleMeds®.
Conclusion: Approximately half of the top risk drugs (22 for ROR, 30 for PRR) were not outlined in the QT drug lists of CredibleMeds®. Notably, potential risks are of great importance and should be closely monitored in clinical practice. Also, further research is needed to investigate the association between these drugs and TdP.
Torsades de pointes (TdP) is a type of polymorphic ventricular tachycardia (1). The incidence of TdP is from 0.0032‰/year to 0.16‰/year (2–4), which is very low but often life-threatening. The mortality of TdP is approximately 10–20% (5). In general, TdP is associated with prolonged QT interval, for every 10 ms increase in QT interval, the risk of TdP increases by approximately 5–7% (6). Long QT interval can be congenital or acquired. The latter is most often drug-induced. In clinical practice, multiple drugs may cause TdP (7, 8), and several of them have been withdrawn from the market due to TdP, such as cisapride, droperidol, and terfenadine (9, 10).
It is particularly essential to assess drug-induced TdP. The International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) released a guideline for clinical evaluation of QT/QTc interval prolongation. This guideline could help us identify drugs that may cause QT prolongation before marketing (11). However, due to the limited sample size and follow-up time, it is difficult to observe rare adverse events (AEs) in such studies. Therefore, post-marketing surveillance is also very important, especially for rare but clinically significant AEs, such as TdP. Food and Drug Administration Adverse Event Reporting System (FAERS) database is believed to be a powerful tool for mining new or rare AEs and could reflect profiles of AEs in real-world clinical settings. It is a publicly accessible database that includes a large number of AE reports submitted by healthcare professionals, consumers, and manufacturers (12). Previous researchers have used the FAERS database to investigate TdP and have comprehensively assessed the specific class drugs in terms of TdP, mainly including H1-antihistamines, antipsychotics, and antibiotics (13–16).
Increasing new drugs have been approved on the market in recent years, and it is of great significance to update the risk of TdP in currently available drugs based on spontaneous reporting AEs data. Therefore, this study aimed to comprehensively investigate the risk of drug-induced TdP across all drugs and identify drugs with a potential risk of TdP that were not on the QT drug lists of CredibleMeds®.
This retrospective pharmacovigilance study was conducted based on the FAERS database. FAERS is a spontaneous reporting AEs database which is available to the public. A large amount of information of AEs can be found in this database, such as demographic and administrative information, drug information and reaction information (17, 18).
OpenVigil 2.11 was used to retrieve FAERS data. OpenVigil 2.1 is a validated pharmacovigilance data extraction, cleaning, and mining tool of the FAERS database (19, 20). For this study, the AEs of TdP were searched from the first quarter of 2004 to the third quarter of 2021 using preferred terms (PTs) in the Medical Dictionary for Regulatory Activities (MedDRA) Dictionary (Version 24.0). We used Torsade de Pointes (PT: 10044066) to search.
First, the descriptive analysis was performed to summarize the clinical features of cases of TdP, including patients’ gender, age and reporting country. According to the counts of reports, the top 50 drugs associated with TdP were selected.
Second, a disproportionality analysis was conducted. Disproportionality analysis is largely used to generate hypotheses on possible associations between drugs and AEs. It is based on the contrast between observed and expected numbers of reports, for any given drug and AE (21). Reporting odds ratio (ROR) and proportional reporting ratio (PRR) were two measures of disproportionality analysis. We calculated ROR and PRR to detect each drug’s TdP risk signal. The equations and criteria for the algorithms were listed in Table 1 (22–24). ROR and PRR offer a rough indication of the strength of the signal. A relatively higher ROR or PRR indicates a stronger signal between the drug and TdP. The analyses were performed using the Microsoft EXCEL 2019.
Credible Meds (accessible at www.crediblemeds.org) has established a list of drugs that increase risk of QT prolongation and TdP. And these drugs were classified into three categories, “known risk,” “possible risk,” or “conditional risk” of TdP. In our study, comparisons were conducted with the QT drug lists of CredibleMeds® in an attempt to identify drugs with a potential risk of TdP that are not on the list (25).
From 2004 Q1 to 2021 Q3, there were 9,217,181 AEs in FAERS, of which 3,807 (0.04%) were TdP cases. The characteristics of cases were listed in Table 2. TdP was more likely observed in female patients (56.82%) and most frequently reported in the United States (41.27%). When stratified by age group, the majority of AE reports were distributed to the elderly (≥65 years) (33.44%).
Based on the counts of AE reports, the 50 most common drugs associated with TdP are summarized in Figure 1. Among the most frequently reported drugs, amiodarone (464 cases) was associated with the most cases of TdP, followed by furosemide (412 cases), methadone (292 cases), citalopram (260 cases) and loperamide (259 cases). Of these 50 drugs, 30 were included on the QT drug lists of CredibleMeds®.
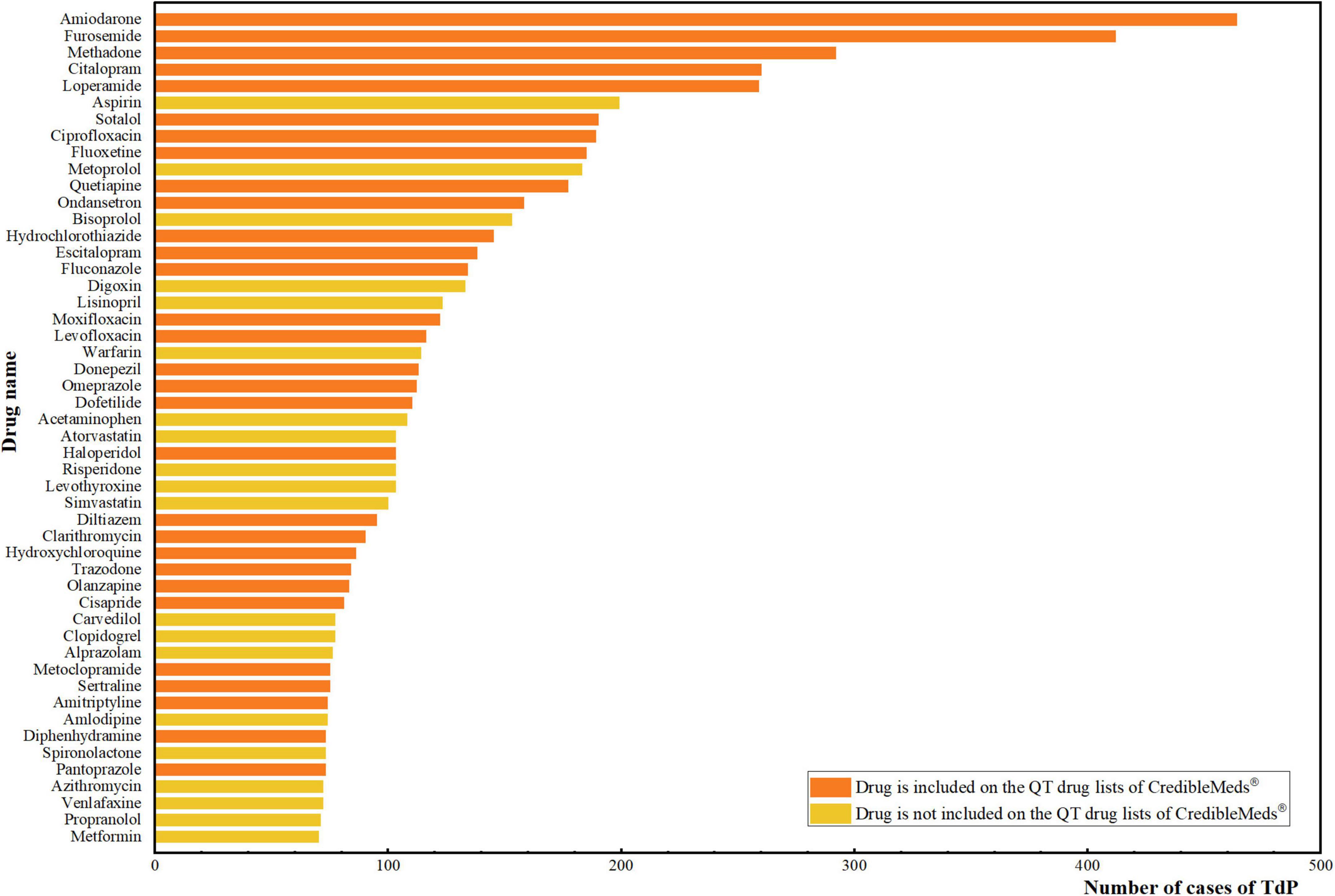
Figure 1. Top 50 drugs with the most number of cases of TdP. The vertical axis is the name of the drugs, and the horizontal axis is the corresponding number of cases of TdP for each drug. The orange color represents that the drug is included on the QT drug lists of CredibleMeds®. The yellow color represents that the drug is not included on the QT drug lists of CredibleMeds®.
Based on the criteria for ROR, a total of 306 signals were detected for TdP. Drugs with the top 50 highest RORs are listed in Table 3. Tolazoline (ROR 1615.11, 95%CI 455.59–5725.75) reported the highest ROR for TdP, followed by levomethadyl (ROR 1211.01, 95% CI 302.75–4844.04), ibutilide (ROR 1118.74, 95% CI 425.00–2944.91), halofantrine (ROR 660.55, 95% CI 184.21–2368.69) and isoproterenol (ROR 352.20, 95% CI 227.19–546.00). Of the top 50 drugs, 28 were included on the QT drug lists of CredibleMeds®. According to the risk categories of CredibleMeds®, 19 drugs were classified as known risk of TdP, 5 drugs as conditional risk, 3 drugs as special risk, and the other one drug as possible risk of TdP.
The estimated PRRs for each of the individual drugs associated with TdP are summarized in the Supplementary Table 1. A total of 253 signals were detected, and the top five drugs with the highest PRRs were consistent with the results of RORs, including tolazoline (PRR 969.46, χ2 2960.10), levomethadyl (PRR 807.67, χ2 1677.03), ibutilide (PRR 765.77, χ2 3845.27), halofantrine (PRR 519.22, χ2 1076.31), and isoproterenol (PRR 307.82, χ2 6692.53). Thirty drugs were included on the QT drug lists of CredibleMeds®. According to the risk categories, 22 drugs were classified as known risk of TdP, 5 drugs as conditional risk, 2 drugs as special risk, and the other one drug as possible risk of TdP.
This study comprehensively assessed the AEs of drug-induced TdP in the real world based on the FAERS database. The results indicated that TdP was more likely to occur in the elderly and females. Amiodarone was associated with most cases of TdP. According to the disproportionality analysis, the top five drugs with the highest ROR and PRR were tolazoline, levomethadyl, ibutilide, halofantrine and isoproterenol. Approximately half of the top 50 drugs (22 for ROR, 30 for PRR) were not outlined on the QT drug lists of CredibleMeds®.
Drug-induced TdP is a non-negligible AE in pharmaceutical treatments, which can lead to cardiac sudden death. Previous studies have found multiple risk factors for TdP, including female, age (≥65 years), etc. (26–28), which was also observed in our study. Sex-related differences in TdP are increasingly recognized, but the mechanisms remain uncertain. Sex hormones may be a possible factor for sex-related differences. Testosterone appears to shorten the QT interval, and estrogen may lengthen QT interval. Thus, females have a longer QT interval compared to males after puberty, and the QT interval in males gradually lengthens and approximates that of females with aging (29, 30). In addition, QTc > 500 ms is considered a risk factor of TdP. Females have a higher baseline QT interval and are more likely to prolong over 500 ms when challenged by QT-prolonging drugs (7). In terms of the elderly, their prescriptions have more QT-prolonging drugs. A study conducted on 5,319 elderly outpatients in North Jordan showed that 58.5% of patients were consuming drugs that carry the risk of TdP (31). Therefore, the elderly are more likely to be exposed to high risk of TdP.
Based on data mining, we listed the top 50 drugs with the strongest signals, and compared these drugs to the QT drug lists of CredibleMeds®. Interestingly, about half of the drugs (22 for ROR, 30 for PRR) were not outlined in lists, representing potential new signals. The drugs involved in new signals include antihistamines (e.g., clemastine), antibiotics (e.g., cloxacillin), inhalation anesthetics (e.g., isoflurane), etc. Although some of these drugs have been on the market for a long time, there is inadequate attention to TdP. Therefore, further attention is needed to determine whether these drugs are needed to be included on the QT drug lists of CredibleMeds®. On the other hand, the post-marketing safety monitoring of newly marketed drugs is also an imperative topic. In our study, of the top 50 drugs, viloxazine was the most recently marketed (32). Due to the short time on the market, only two cases of TdP were reported, but with significantly higher ROR and PRR. As a result, physicians and pharmacists should be alert for viloxazine-induced TdP in clinical practice.
CredibleMeds is one of the most reliable sources of information on drug-induced QT prolongation, with close monitoring of the FAERS database as well. Our results are slightly different from the QT drug lists of CredibleMeds® may be due to different methods. The CredibleMeds team used the Bayesian method to estimate the relative reporting ratio and we used the frequentist method. These two methods have their own strengths. The frequentist method has higher sensitivity, while the Bayesian method has higher specificity (33). Therefore, we hope our results could be a supplement to detect TdP of drugs.
Our study has several strengths. Above all, FARES is one of the largest public pharmacovigilance databases, and the sample size is sufficient to detect rare AEs of TdP and provide valuable suggestions to guide clinical decision-making. Further, we compared the 50 drugs with the strongest signals to the QT drug lists of CredibleMeds® and mined some new signals, which can be considered hypotheses to stimulate further research. Last but not least, given their simplicity and sensitivity, the choice of ROR and PRR for signal detection helps to detect new AEs signals more quickly.
Our study also has several limitations. First of all, due to the missing denominator data, the true incidence of TdP of each drug was not estimated based on the FAERS database (34). Second, considering that FAERS is a spontaneous reporting system, the causal relationship between AEs and drugs may not be determined. Thus, our research can only give clinicians and researchers some hints about which drugs may have a high potential risk of TdP, and further studies with more reliable methods are needed to verify. Third, due to the insufficient information in the FAERS database, it is hard to assess the effects of drug-drug interactions or route of administration (e.g., oral vs. intravenous) on TdP. Finally, underreporting may not be avoided because prolongation of the QT intervals is judged by an electrocardiogram, which is not routinely equipped in clinical practice. Nevertheless, the FAERS database remains an important tool for post-marketing surveillance.
In conclusion, we comprehensively assessed TdP reports and associated drugs using the FAERS database. Approximately half of the top risk drugs (22 for ROR, 30 for PRR) were not outlined on the QT drug lists of CredibleMeds®. Notably, the potential risks are of great importance and should be closely monitored in clinical practice. Also, further research is needed to investigate the association between these drugs and TdP.
Publicly available datasets were analyzed in this study. This data can be found here: http://h2876314.stratoserver.net:8080/OV2/search/.
ZW and PZ extracted and analyzed the data and drafted the manuscript. SZ provided pharmacological guidance. NH supported data analysis. All authors participated in the study design, contributed to the revision of the manuscript, and approved the final version.
We would like to thank Yanxing Zhang for the figure editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor FS declared a shared affiliation with the authors at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.966331/full#supplementary-material
1. Li S, Xu Z, Guo M, Li M, Wen Z. Drug-induced QT prolongation atlas (DIQTA) for enhancing cardiotoxicity management. Drug Discov Today. (2022) 27:831–7. doi: 10.1016/j.drudis.2021.10.009
2. Novotný T. Mechanisms and Incidence of Torsades de Pointes Tachycardia. Sex and Cardiac Electrophysiology. London: Academic Press (2020). p. 303–8.
3. Molokhia M, Pathak A, Lapeyre-Mestre M, Caturla L, Montastruc JL. Case ascertainment and estimated incidence of drug-induced long-QT syndrome: study in Southwest France. Br J Clin Pharmacol. (2008) 66:386–95. doi: 10.1111/j.1365-2125
4. Vandael E, Vandenberk B, Vandenberghe J, Pincé H, Willems R, Foulon V. Incidence of torsade de pointes in a tertiary hospital population. Int J Cardiol. (2017) 243:511–5. doi: 10.1016/j.ijcard.2017.05.072
5. Shah RR. Drug-induced QT interval prolongation: does ethnicity of the thorough QT study population matter? Br J Clin Pharmacol. (2013) 75:347–58. doi: 10.1111/j.1365-2125.2012.04415.x
6. Trinkley KE, Page RL II, Lien H, Yamanouye K, Tisdale JE. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin. (2013) 29:1719–26. doi: 10.1185/03007995.2013.840568
7. Tisdale JE, Chung MK, Campbell KB, Hammadah M, Joglar JA, Leclerc J, et al. American Heart Association clinical pharmacology committee of the council on clinical cardiology and council on cardiovascular and stroke nursing. Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. (2020) 142:e214–33. doi: 10.1161/CIR.0000000000000905
8. Zhou P, Wang X, Zhang X, Xu B, Tong X, Zhou W, et al. Recommendations on off-label use of intravenous azithromycin in children. Int J Clin Pract. (2021) 75:e14010. doi: 10.1111/ijcp.14010
10. Wilke RA, Lin DW, Roden DM, Watkins PB, Flockhart D, Zineh I, et al. Identifying genetic risk factors for serious adverse drug reactions: current progress and challenges. Nat Rev Drug Discov. (2007) 6:904–16. doi: 10.1038/nrd2423
11. Darpo B. The thorough QT/QTc study 4 years after the implementation of the ICH E14 guidance. Br J Pharmacol. (2010) 159:49–57. doi: 10.1111/j.1476-5381.2009.00487.x
12. Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci. (2013) 10:796–803. doi: 10.7150/ijms.6048
13. Teng C, Walter EA, Gaspar DKS, Obodozie-Ofoegbu OO, Frei CR. Torsades de pointes and QT prolongation associations with antibiotics: a pharmacovigilance study of the FDA adverse event reporting system. Int J Med Sci. (2019) 16:1018–22. doi: 10.7150/ijms.34141
14. Poluzzi E, Raschi E, Godman B, Koci A, Moretti U, Kalaba M, et al. Pro-arrhythmic potential of oral antihistamines (H1): combining adverse event reports with drug utilization data across Europe. PLoS One. (2015) 10:e0119551. doi: 10.1371/journal.pone.0119551
15. Poluzzi E, Raschi E, Koci A, Moretti U, Spina E, Behr ER. Antipsychotics and torsadogenic risk: signals emerging from the US FDA adverse event reporting system database. Drug Saf. (2013) 36:467–79. doi: 10.1007/s40264-013-0032-z
16. Poluzzi E, Raschi E, Moretti U, De Ponti F. Drug-induced torsades de pointes: data mining of the public version of the FDA adverse event reporting system (AERS). Pharmacoepidemiol Drug Saf. (2009) 18:512–8. doi: 10.1002/pds.1746
17. FDA.Data Mining at FDA: White Paper. (2018). Available online at: https://www.fda.gov/science-research/data-mining/data-mining-fda-white-paper (accessed May 12, 2022).
18. Duggirala HJ, Tonning JM, Smith E, Bright RA, Baker JD, Ball R, et al. Use of data mining at the food and drug administration. J Am Med Inform Assoc. (2016) 23:428–34. doi: 10.1093/jamia/ocv063
19. Böhm R, Höcker J, Cascorbi I, Herdegen T. OpenVigil–free eyeballs on AERS pharmacovigilance data. Nat Biotechnol. (2012) 30:137–8. doi: 10.1038/nbt.2113
20. Böhm R, Bulin C, Waetzig V, Cascorbi I, Klein HJ, Herdegen T. Pharmacovigilance-based drug repurposing: the search for inverse signals via OpenVigil identifies putative drugs against viral respiratory infections. Br J Clin Pharmacol. (2021) 87:4421–31. doi: 10.1111/bcp.14868
21. Caster O, Aoki Y, Gattepaille LM, Grundmark B. Disproportionality analysis for pharmacovigilance signal detection in small databases or subsets: recommendations for limiting false-positive associations. Drug Saf. (2020) 43:479–87. doi: 10.1007/s40264-020-00911-w
22. Ooba N, Kubota K. Selected control events and reporting odds ratio in signal detection methodology. Pharmacoepidemiol Drug Saf. (2010) 19:1159–65. doi: 10.1002/pds.2014
23. Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. (2001) 10:483–6. doi: 10.1002/pds.677
24. CredibleMeds.CredibleMeds Process for Evaluating Evidence and Assigning Risk. Tucson, AZ: CredibleMeds (2022).
25. Ansermot N, Bochatay M, Schläpfer J, Gholam M, Gonthier A, Conus P, et al. Prevalence of ECG abnormalities and risk factors for QTc interval prolongation in hospitalized psychiatric patients. Ther Adv Psychopharmacol. (2019) 9:2045125319891386. doi: 10.1177/2045125319891386
26. Krumpholz L, Wiśniowska B, Polak S. Open-access database of literature derived drug-related torsade de pointes case. BMC Pharmacol Toxicol. (2022) 23:7. doi: 10.1186/s40360-021-00548-0
27. Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R, et al. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. (1992) 8:690–5.
28. Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. (2004) 350:1013–22. doi: 10.1056/NEJMra032426
29. Huo J, Wei F, Cai C, Lyn-Cook B, Pang L. Sex-related differences in drug-induced QT prolongation and torsades de pointes: a new model system with human iPSC-CMs. Toxicol Sci. (2019) 167:360–74. doi: 10.1093/toxsci/kfy239
30. Vink AS, Clur SB, Wilde AAM, Blom NA. Effect of age and gender on the QTc-interval in healthy individuals and patients with long-QT syndrome. Trends Cardiovasc Med. (2018) 28:64–75. doi: 10.1016/j.tcm.2017.07.012
31. Al-Azayzih A, Gharaibeh S, Jarab AS, Mukattash TL. Prevalence of torsades de pointes inducing drugs usage among elderly outpatients in North Jordan hospitals. Saudi Pharm J. (2018) 26:1146–54. doi: 10.1016/j.jsps.2018.07.002
32. Findling RL, Candler SA, Nasser AF, Schwabe S, Yu C, Garcia-Olivares J, et al. Viloxazine in the management of CNS disorders: a historical overview and current status. CNS Drugs. (2021) 35:643–53. doi: 10.1007/s40263-021-00825-w
33. Chen Y, Guo JJ, Steinbuch M, Lin X, Buncher CR, Patel NC. Comparison of sensitivity and timing of early signal detection of four frequently used signal detection methods. Pharm Med. (2008) 22:359–65. doi: 10.1007/bf03256733
Keywords: long QT syndrome, torsades de pointes, FAERS, disproportionality analysis, pharmacovigilance
Citation: Wu Z, Zhou P, He N and Zhai S (2022) Drug-induced torsades de pointes: Disproportionality analysis of the United States Food and Drug Administration adverse event reporting system. Front. Cardiovasc. Med. 9:966331. doi: 10.3389/fcvm.2022.966331
Received: 10 June 2022; Accepted: 30 September 2022;
Published: 24 October 2022.
Edited by:
Feng Sun, Peking University, ChinaReviewed by:
Bert Vandenberk, University Hospitals Leuven, BelgiumCopyright © 2022 Wu, Zhou, He and Zhai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suodi Zhai, emhhaXN1b2RpQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.