- 1Department of Cardiology, First Affiliated Hospital of Shantou University Medical College, Shantou, China
- 2School of Medical and Health Sciences, Edith Cowan University, Perth, WA, Australia
- 3Clinical Research Center, First Affiliated Hospital of Shantou University Medical College, Shantou, China
Aim: The aim of this study is to evaluate the association between serum sodium concentrations at hospital admission and all-cause mortality within 365 days post-discharge in patients with atrial fibrillation (AF) without heart failure (HF).
Methods: The prospective cohort study enrolled 1,446 patients with AF without HF between November 2018 and October 2020. A follow-up was performed 30, 90, 180, and 365 days after enrollment through outpatient visits or telephone interviews. All-cause mortality was estimated in three groups according to serum sodium concentrations: hyponatremia (< 135 mmol/L), normonatremia (135–145 mmol/L), and hypernatremia (> 145 mmol/L). We estimated the risk of all-cause mortalities using univariable and multivariable Cox proportional hazards models with normonatremia as the reference.
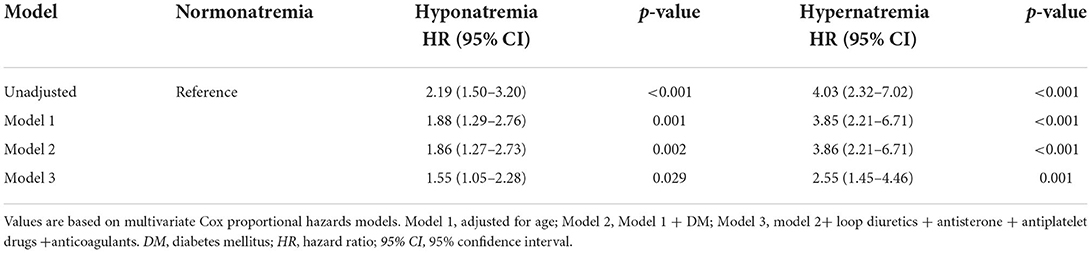
Results: The all-cause mortalities of hyponatremia, normonatremia, and hypernatremia were 20.6, 9.4, and 33.3% within 365 days post-discharge, respectively. In the univariable analysis, hyponatremia (HR: 2.19, CI 1.5–3.2) and hypernatremia (HR: 4.03, CI 2.32–7.02) increased the risk of all-cause mortality. The HRs for hyponatremia and hypernatremia were 1.55 (CI 1.05–2.28) and 2.55 (CI 1.45–4.46) after adjustment for age, diabetes mellitus, loop diuretics, antisterone, antiplatelet drugs, and anticoagulants in the patients with AF without HF. The association between serum sodium concentrations and the HRs of all-cause mortality was U-shaped.
Conclusion: Dysnatremia at hospital admission was an independent factor for all-cause mortality in patients with AF without HF within 365 days post-discharge.
Introduction
Atrial fibrillation (AF), which is the most common form of cardiac arrhythmia and is triggered in lifestyle-related conditions such as diabetes mellitus (DM) and stress (1–3), accounts for substantial morbidity and mortality (4–6), and the incidence of AF is expected to at least double by 2050 (7). AF is also associated with a nearly 5-fold increase in the risk of ischemic stroke (8) and heart failure (HF) (9, 10), and an increased financial burden (11, 12). Serum sodium is the main component of human plasma responsible for osmotic pressure and the main participant in heart electrical activity. Dysnatremia, including hyponatremia and hypernatremia, is very common in hospitalized patients (13, 14) and complications of acute diseases, and results from interventions during patient treatment (15, 16) or exists as a comorbid condition (17). Previous studies have shown that abnormal serum sodium concentrations are independent factors for poor prognosis of internal medicine patients (18–20) and patients with cardiac diseases (14, 21, 22). Studies show that patients with HF with AF frequently present with hyponatremia (23, 24), which is associated with high mortality (25). The incidence of abnormal sodium levels in non-HF patients is lower compared with patients with HF. However, it is unknown whether abnormal serum sodium levels play a role in the pathophysiology of and can be predictors of long-term poor prognosis in patients with AF without HF. In this study, we determine the association between serum sodium concentrations at the time of hospital admission and all-cause mortality over the 365 days following discharge in patients with AF without HF.
Methods
Study cohort
The data used were sub-data obtained from a single-center prospective observational cohort study in which 2011 patients with AF were enrolled from November 2018 to October 2020 at the First Affiliated Hospital of Shantou University Medical College in Shantou, China. Briefly, AF was diagnosed by a physician according to heart rhythm by ECG. The diagnostic criteria for ECG were (1) absolutely irregular RR intervals, (2) no discernible or distinct P waves, and (3) an episode lasting at least 30 s. The inclusion criteria were diagnosis of AF and age >18 years old. The exclusion criteria were pregnancy, death in the hospital, HF, and refusal of follow-up. A follow-up was performed through a clinic visit or telephone interview 30, 90, 180, and 365 days after discharge. The primary endpoint was all-cause death in participants after discharge from the hospital. The patients were grouped based on admission serum sodium levels defined as follows: hyponatremia (< 135 mmol/L), normonatremia (135–145 mmol/L), and hypernatremia (> 145 mmol/L).
Data collection
All the patients with AF received a systemic clinical evaluation at the beginning of hospitalization. Data were collected, including age, sex, comorbidities, medication, and serum sodium concentrations, from patient hospital records. The serum sodium concentrations used in the analysis were obtained within the first 24 h of admission and measured by the clinical laboratory of the First Affiliated Hospital of Shantou University Medical College (instruments: Beckman Coulter AU5800 automatic biochemical analyzer, method: direct ion-selective electrodes). Hypertension was defined as blood pressure over 140 and/or 90 mmHg measured at least two different times over 4 h or taking an antihypertensive medication. DM was defined as fasting blood glucose of ≥7 mmol/L, random blood glucose ≥11.1 mmol/L, or taking an antidiabetic medication. Coronary artery disease was diagnosed as having more than one coronary stenosis of >50% by coronary artery angiography or having a history of coronary artery disease. HF was diagnosed by a senior clinician according to an ejection fraction lower than 50% by echocardiography combined with symptoms (such as shortness of breath) and physical signs (such as edema) or elevated N-terminal pro-B-type natriuretic peptide and evidence of diastolic dysfunction (26). Medicines that the patients were taking contained amiodarone, loop diuretics, antisterone, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, antiplatelet drugs, anticoagulants, and beta-blockers.
This study complied with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Shantou University Medical College.
Statistical analyses
Non-normal variables (age, median survival time) are presented as the median and interquartile range, and a Kruskal-Wallis rank-sum test was conducted to evaluate differences. Categorical data were determined as counts or percentages, and differences were evaluated by a Pearson χ2 test. Kaplan-Meier survival curves were plotted for three sodium levels to illustrate survival. In order to investigate the association between dysnatremia and all-cause mortality within 30–365 days, we used univariable and multivariable Cox proportional hazards models. The relationship between serum sodium concentration and the unadjusted hazard ratio (HR) of mortality was assessed using a restricted cubic spline curve based on Cox proportional hazards models (27, 28). We considered a two-sided p-value below 0.05 to be statistically significant and below 0.01 to be highly statistically significant. The statistical analyses were performed using SPSS 23.0 for Windows (version 23.0; IBM Corp., Armonk, NY) and the R (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria) software.
Results
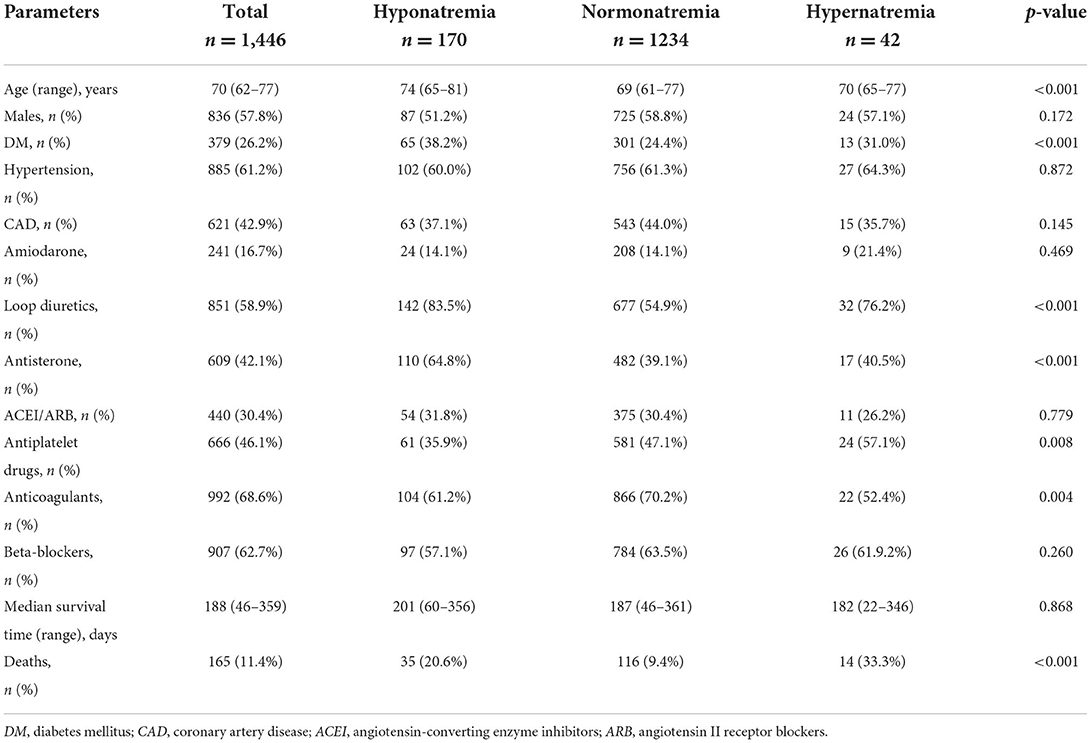
Data were selected according to the flow chart shown in Figure 1. Among 2,011 participants, 197 were excluded because of lack of follow-up data, 77 patients were excluded because of missing baseline serum sodium concentrations, and 291 were excluded because of history of heart failure. The final analysis included 1,446 patients with diagnosed AF but without HF. The baseline characteristics of the study population according to each sodium group are shown in Table 1. The prevalence rate of hypo- and hypernatremia was 11.8 and 2.9%, respectively. Of the 1,446 patients, 165 (11.4%) died between 30 and 180 days. This population was characterized by advanced age, with the hyponatremic and hypernatremic patients being older than the normonatremic patients (Table 1). The all-cause mortality in the three groups from hyponatremia, normonatremia, and hypernatremia was 35 (20.6%), 116 (9.4%), and 14 (33.3%), respectively. Significant differences among the three groups were observed for age and prevalence of DM, as well as taking loop diuretics, antisterone, antiplatelet drugs, and anticoagulants. The association between all-cause mortality and serum sodium concentrations (hyponatremia, normonatremia, and hypernatremia) is illustrated as Kaplan–Meier survival curves (Figure 2).
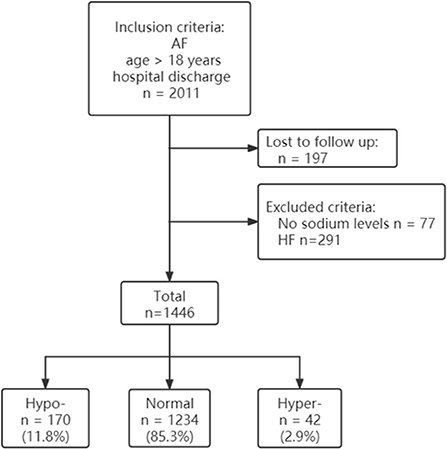
Figure 1. Participant flow diagram. AF, atrial fibrillation; HF, heart failure; Hypo, hyponatremia; Normal, normonatremia; Hyper, hypernatremia.
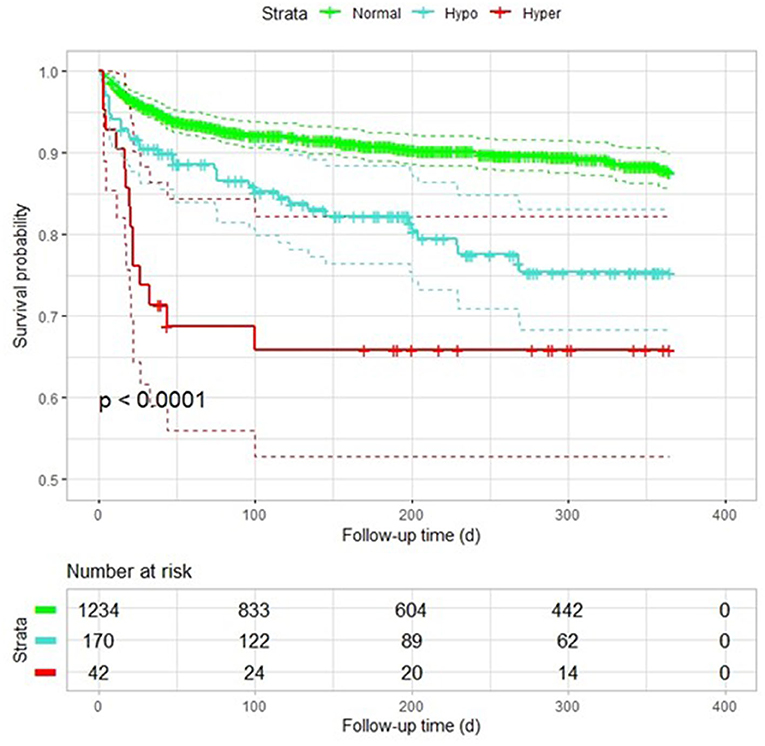
Figure 2. Kaplan–Meier plot of survival probability among patients with AF with different sodium levels. Hypo, hyponatremia; Normal, normonatremia; Hyper, hypernatremia.
The results of the multivariable-adjusted analysis with normal sodium are shown in Table 2. The all-cause mortality rates were higher in patients with hyponatremia (HR: 2.19, CI: 1.5–3.2) and hypernatremia (HR: 4.03, CI: 2.32–7.02) than in normonatremia in the univariate Cox hazards analyses. In model 1, the HR for hyponatremia was 1.88 (CI: 1.29–2.76), and the HR for hypernatremia was 3.85 (CI: 2.21–6.71) after adjustment for age. In model 2, after adding DM to model 1, the HR for hyponatremia and hypernatremia was 1.86 (CI: 1.27–2.73) and 3.86 (CI: 2.21–6.71), respectively. In model 3, the mortality rate remained significantly high in patients with hyponatremia (HR: 1.55, CI: 1.05–2.28) and hypernatremia (HR: 2.55, CI: 1.45–4.46) after adjustment for age, prevalence of DM, loop diuretics, antisterone, antiplatelet drugs, and anticoagulants.
The relationship between the unadjusted HRs of post-discharge mortality in patients with AF without HF and serum sodium levels was U-shaped (Figure 3). The spline curve showed that both high and low sodium levels increased the risk for all-cause post-discharge mortality in patients with AF with no HF within 365 days.
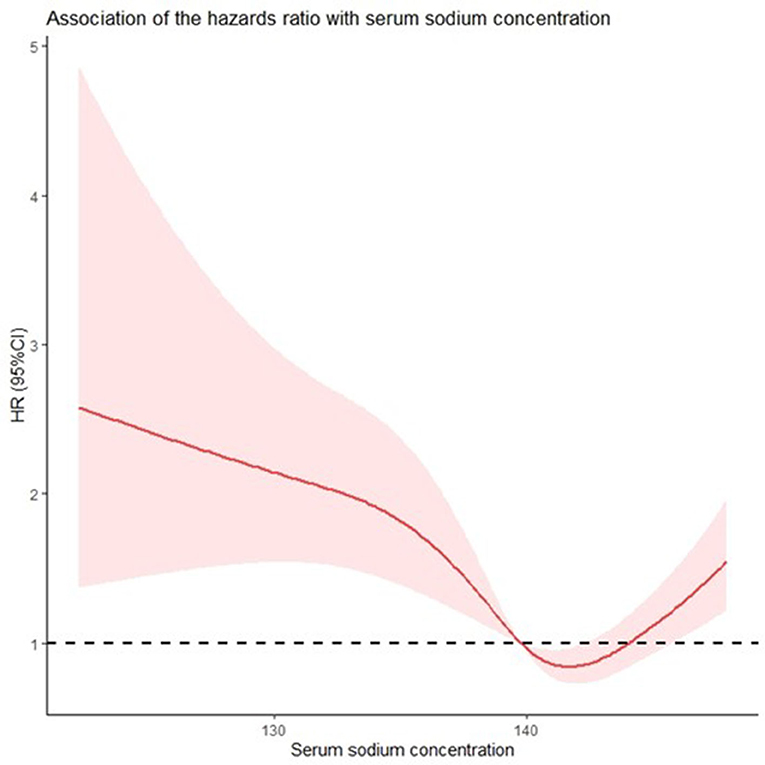
Figure 3. Both hypo- and hypernatremia affect the all-cause mortality of patients with atrial fibrillation without heart failure in a serum sodium concentration-dependent manner. Restricted cubic spline curve (RCS) showing the unadjusted hazard ratios for all-cause mortality as a function of sodium concentration according to univariate Cox proportional hazards analysis.
Discussion
Our study shows that the dysnatremia at the time of hospital admission in the patients with AF without HF is highly associated with increased risk of all-cause mortality during the 365-day period following discharge. This relationship remains after adjustment for age, comorbidities, and medication. Furthermore, the prognostic relationship between serum sodium concentrations and the unadjusted HRs for all-cause post-discharge mortality exhibits a U-shaped curve, as opposed to a nonlinear association, with a higher risk at both ends of the sodium concentration distribution. It is noteworthy that both hyponatremia and hypernatremia are the risk factors for mortality in the patients with AF without HF.
Limited studies have reported that hyponatremia increased the risk of mortality in patient with AF, mainly in patients with HF. Studies showed that hyponatremia was more common in HF with reduced ejection fraction combined with AF (23, 29). In a prospective multicenter pilot survey that included 215 participants with AF and HF, Ozierański et al. showed that the incidence of hyponatremia was 19.1%, and that hyponatremia at hospital admission was a risk for mortality in patients with AF who have had HF for 12 months (25). In our study, the prevalence of hyponatremia was 11.8% in patients with AF without HF, which was lower than before. After multivariable adjustment, the hyponatremia remained associated with decreased survival. This could be because of lower serum sodium causing decreased Na+ influx, reduced transmembrane potential, inhibition of Na+/K+ ATPase activity (30), or more triggered electrical activity and burst in the pulmonary vein (31). The main interactions among the three involve neurohormone-induced hyponatremia, renin-angiotensin-aldosterone system activation, retention of water and sodium, links to fluid overload, and atrial myocardial stretch facilitation of AF (32). Our data supplement and emphasize the prognostic importance of hyponatremia at admission in patients with AF without HF.
We also evaluated the association between hypernatremia and post-discharge mortality. Our study suggests that hypernatremia at the time of hospital admission is highly related to risk of adjusted all-cause mortality within 365 days after discharge for AF patients without HF. Studies have reported that hypernatremia indicates adverse prognosis for the medical patients (14, 33–35). Breen et al. showed the same result in a cardiac intensive care unit in a retrospective study (14). A single-center cohort study that included 55,901 patients has shown that the impact on higher 1-year mortality is more prominent for hypernatremia than for normonatremia (18). The relationship between hypernatremia and adverse prognosis could be due to decreased left ventricular contractility (36) and/or increased peripheral insulin resistance (37). A case report showed that three hypernatremic patients incurred AF during treatment for hypernatremia (38). It is possible that hypernatremia could be a risk factor for AF-related mortality because of atrial stretch during treatment-stimulated pulmonary vein electrical activity (39). The reason why hypernatremia is a risk factor for patients with AF may be associated with treatment of hypernatremia.
Limitations
First, our study is a single-center one and our participants live in the south of China, which might limit the generalizability of our results to other populations. Second, we only included the serum sodium concentration at the time of admission and did not identify the changes in the hospital. Third, we did not evaluate the association between dysnatremia and cardiac-related mortality. In future studies, we will establish the relationship between changes in serum sodium concentration and cardiac-related mortality of patients with AF.
Conclusion
In conclusion, hypo- and hypernatremia increase the mortality of patients with AF without HF. Sodium concentrations need to be monitored after discharge from the hospital, especially in patients who have dysnatremia at the time of hospital admission.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Shantou University Medical College. The patients/participants provided their written informed consent to participate in this study.
Author contributions
XT, YC, and YZ contributed to the conception and design of the study. DL, SW, JX, MY, ZX, MW, and RC organized the database. YZ and YC performed the statistical analysis. YZ wrote the first draft of the manuscript. YC, ZC, and CT wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by the Grant for Key Disciplinary Project of Clinical Medicine under the Guangdong High-level University Development Program, Guangdong University Innovation Team Project (Nature) (2019KCXTD003), 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant (2020LKSFG19B) and Dengfeng Project for the construction of high-level hospitals in Guangdong Province–the First Affiliated Hospital of Shantou University Medical College, the study of mechanism and early warning model of Hypertensive Disorders of Pregnancy–construction of cohort and specimen bank of pregnant women and fund of Science and Technology Special in Guangdong Province (Big Project + Task lists) (2021010303) and Transcriptome mechanisms of acute Stanford type A aortic dissection (STKJ2021083).
Acknowledgments
Thanks to Stanley Li Lin for the English revision.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.963103/full#supplementary-material
References
1. Brundel B, Ai X, Hills MT, Kuipers MG, Lip GYH, de Groot NMS. Atrial fibrillation. Nat Rev Dis Primers. (2022) 8:21. doi: 10.1038/s41572-022-00347-9
2. Tripp FZC, Huber NL, Mounsey JP, Naniwadekar A, Nekkanti R, Sears SF. Program planning in education and light exercise training for atrial fibrillation patients: a feasibility study. Heart Mind. (2020) 4:80–4. doi: 10.4103/hm.hm_25_20
3. Ivanovic TMB. Emotional stress-induced takotsubo cardiomyopathy, acute heart failure, and atrial fibrillation in the same patient. Heart Mind. (2019) 3:70–2. doi: 10.4103/hm.hm_41_19
4. Al-Khayatt BM, Salciccioli JD, Marshall DC, Krahn AD, Shalhoub J, Sikkel MB. Paradoxical impact of socioeconomic factors on outcome of atrial fibrillation in Europe: trends in incidence and mortality from atrial fibrillation. Eur Heart J. (2021) 42:847–57. doi: 10.1093/eurheartj/ehaa1077
5. Cai X, Zheng S, Liu Y, Zhang Y, Lu J, Huang Y. Nonalcoholic fatty liver disease is associated with increased risk of atrial fibrillation. Liver Int. (2020) 40:1594–600. doi: 10.1111/liv.14461
6. Yang Y, Guo S, Huang Z, Deng C, Chen L, Zhou G, et al. Decreased mortality with beta-blocker therapy in HFpEF patients associated with atrial fibrillation. Cardiol Res Pract. (2020) 2020:3059864. doi: 10.1155/2020/3059864
7. Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. (2021) 16:217–21. doi: 10.1177/1747493019897870
8. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. Jama. (2001) 285:2864–70. doi: 10.1001/jama.285.22.2864
9. Packer M. Is long-standing atrial fibrillation a biomarker of or contributor to the symptoms or progression of chronic heart failure? Am J Med. (2020) 133:17–8. doi: 10.1016/j.amjmed.2019.05.026
10. Jelavic KGMM, Pintaric H. Usage and safety of direct oral anticoagulants at patients with atrial fibrillation and planned diagnostic procedures, interventions, and surgery. Heart Mind. (2019) 3:1–6. doi: 10.4103/hm.hm_61_19
11. Wang Z, Chen Z, Wang X, Zhang L, Li S, Tian Y, et al. The disease burden of atrial fibrillation in china from a national cross-sectional survey. Am J Cardiol. (2018) 122:793–8. doi: 10.1016/j.amjcard.2018.05.015
12. Bai Y, Wang YL, Shantsila A, Lip GYH. The global burden of atrial fibrillation and stroke: a systematic review of the clinical epidemiology of atrial fibrillation in Asia. Chest. (2017) 152:810–20. doi: 10.1016/j.chest.2017.03.048
13. Chi C, Patel S, Cheung NW. Admission sodium levels and hospital outcomes. Int Med J. (2021) 51:93–8. doi: 10.1111/imj.14777
14. Breen T, Brueske B, Sidhu MS, Murphree DH, Kashani KB, Barsness GW, et al. Abnormal serum sodium is associated with increased mortality among unselected cardiac intensive care unit patients. J Am Heart Assoc. (2020) 9:e014140. doi: 10.1161/JAHA.119.014140
15. Ramírez E, Rodríguez A, Queiruga J, García I, Díaz L, Martínez L, et al. Severe hyponatremia is often drug induced: 10-year results of a prospective pharmacovigilance program. Clin Pharmacol Ther. (2019) 106:1362–79. doi: 10.1002/cpt.1562
16. Martens P, Ferreira JP, Vincent J, Abreu P, Busselen M, Mullens W, et al. Serum sodium and eplerenone use in patients with a myocardial infarction and left ventricular dysfunction or heart failure: insights from the EPHESUS trial. Clin Res Cardiol. (2021). doi: 10.1007/s00392-021-01853-8
17. Abebe TB, Gebreyohannes EA, Tefera YG, Bhagavathula AS, Erku DA, Belachew SA, et al. The prognosis of heart failure patients: Does sodium level play a significant role? PLoS ONE. (2018) 3:e0207242. doi: 10.1371/journal.pone.0207242
18. Thongprayoon C, Cheungpasitporn W, Petnak T, Ghamrawi R, Thirunavukkarasu S, Chewcharat A, et al. The prognostic importance of serum sodium levels at hospital discharge and one-year mortality among hospitalized patients. Int J Clin Pract. (2020) 74:e13581. doi: 10.1111/ijcp.13581
19. Thongprayoon C, Cheungpasitporn W, Yap JQ, Qian Q. Increased mortality risk associated with serum sodium variations and borderline hypo- and hypernatremia in hospitalized adults. Nephrol Dial Transplant. (2020) 35:1746–52. doi: 10.1093/ndt/gfz098
20. Akirov A, Diker-Cohen T, Steinmetz T, Amitai O, Shimon I. Sodium levels on admission are associated with mortality risk in hospitalized patients. Eur J Int Med. (2017) 46:25–9. doi: 10.1016/j.ejim.2017.07.017
21. Miles JA, Quispe R, Mehlman Y, Patel K, Lama C. Von Buchwald, et al. Racial differences and mortality risk in patients with heart failure and hyponatremia. PLoS ONE. (2019) 14:e0218504. doi: 10.1371/journal.pone.0218504
22. Plakht Y, Gilutz H, Shiyovich A. Sodium levels during hospitalization with acute myocardial infarction are markers of in-hospital mortality: soroka acute myocardial infarction II (SAMI-II) project. Clin Res Cardiol. (2018) 107:956–64. doi: 10.1007/s00392-018-1268-5
23. Cavusoglu Y, Kaya H, Eraslan S, Yilmaz MB. Hyponatremia is associated with occurrence of atrial fibrillation in outpatients with heart failure and reduced ejection fraction. Hellenic J Cardiol. (2019) 60:117–21. doi: 10.1016/j.hjc.2018.03.006
24. Su Y, Ma M, Zhang H, Pan X, Zhang X, Zhang F, et al. Prognostic value of serum hyponatremia for outcomes in patients with heart failure with preserved ejection fraction: An observational cohort study. Exp Ther Med. (2020) 20:101. doi: 10.3892/etm.2020.9231
25. Ozierański K, Kapłon-Cieślicka Kapłon-Cieślicka A, Peller M, Tymińska A, Balsam P, Galas M, et al. Clinical characteristics and predictors of one-year outcome of heart failure patients with atrial fibrillation compared to heart failure patients in sinus rhythm. Kardiol Pol. (2016) 74:251–61. doi: 10.5603/KP.a2015.0180
26. Ezekowitz JA, O'Meara E, McDonald MA, Abrams H, Chan M, Ducharme A, et al. Comprehensive update of the Canadian cardiovascular society guidelines for the management of heart failure. Can J Cardiol. (2017) 33:1342–433. doi: 10.1016/j.cjca.2017.08.022
27. Johannesen CDL, Langsted A, Mortensen MB, Nordestgaard BG. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. BMJ. (2020) 371:m4266. doi: 10.1136/bmj.m4266
28. Wu J, Zheng H, Liu X, Chen P, Zhang Y, Luo J, et al. Prognostic value of secreted frizzled-related protein 5 in heart failure patients with and without type 2 diabetes mellitus. Circ Heart Fail. (2020) 13:e007054. doi: 10.1161/CIRCHEARTFAILURE.120.007054
29. Bavishi C, Ather S, Bambhroliya A, Jneid H, Virani SS, Bozkurt B, et al. Prognostic significance of hyponatremia among ambulatory patients with heart failure and preserved and reduced ejection fractions. Am J Cardiol. (2014) 113:1834–8. doi: 10.1016/j.amjcard.2014.03.017
30. Despa S, Brette F, Orchard CH, Bers DM. Na/Ca exchange and Na/K-ATPase function are equally concentrated in transverse tubules of rat ventricular myocytes. Biophys J. (2003) 85:3388–96. doi: 10.1016/S0006-3495(03)74758-4
31. Lu YY, Cheng CC, Chen YC, Lin YK, Chen SA, Chen YJ. Electrolyte disturbances differentially regulate sinoatrial node and pulmonary vein electrical activity: a contribution to hypokalemia- or hyponatremia-induced atrial fibrillation. Heart Rhythm. (2016) 13:781–8. doi: 10.1016/j.hrthm.2015.12.005
32. Oikonomou E, Mystakidi VC, Tousoulis D. Hyponatremia in patients with atrial fibrillation and heart failure: the difficult triangle. Hellenic J Cardiol. (2019) 60, 122–3. doi: 10.1016/j.hjc.2019.03.012
33. Tzoulis P, Waung JA, Bagkeris E, Hussein Z, Biddanda A, Cousins J, et al. Dysnatremia is a predictor for morbidity and mortality in hospitalized patients with COVID-19. J Clin Endocrinol Metab. (2021) 106:1637–48. doi: 10.1210/clinem/dgab107
34. Ruiz-Sánchez JG, Núñez-Gil IJ, Cuesta M, Rubio MA, Maroun-Eid C, Arroyo-Espliguero R, et al. Prognostic impact of hyponatremia and hypernatremia in COVID-19 pneumonia. a HOPE-COVID-19 (health outcome predictive evaluation for COVID-19) registry analysis. Front Endocrinol. (2020) 11:599255. doi: 10.3389/fendo.2020.599255
35. Grim CCA, Termorshuizen F, Bosman RJ, Cremer OL, Meinders AJ, Nijsten MWN, et al. Association between an increase in serum sodium and in-hospital mortality in critically Ill patients. Crit Care Med. (2021). doi: 10.1097/ccm.0000000000005173
36. Lenz K, Gössinger H, Laggner A, Druml W, Grimm G, Schneeweiss B. Influence of hypernatremic-hyperosmolar state on hemodynamics of patients with normal and depressed myocardial function. Crit Care Med. (1986) 14:913–4. doi: 10.1097/00003246-198610000-00020
37. Bratusch-Marrain PR, DeFronzo RA. Impairment of insulin-mediated glucose metabolism by hyperosmolality in man. Diabetes. (1983) 32: 1028–34. doi: 10.2337/diab.32.11.1028
38. Timilsina S, Pata R, Timilsina S, Cherala S, Kafle P. Correction of hypernatremia due to pure dehydration could be a potential risk factor for transient atrial fibrillation. Cureus. (2019) 11:e5387. doi: 10.7759/cureus.5387
Keywords: atrial fibrillation, hyponatremia, hypernatremia, mortality, cohort study
Citation: Zhou Y, Lin D, Wu S, Xiao J, Yu M, Xiao Z, Wu M, Chen Z, Tian C, Chen R, Chen Y and Tan X (2022) Dysnatremia is associated with increased risk of all-cause mortality within 365 days post-discharge in patients with atrial fibrillation without heart failure: A prospective cohort study. Front. Cardiovasc. Med. 9:963103. doi: 10.3389/fcvm.2022.963103
Received: 07 June 2022; Accepted: 14 September 2022;
Published: 12 October 2022.
Edited by:
Daniel M. Johnson, The Open University, United KingdomReviewed by:
Tong Liu, Tianjin Medical University, ChinaYuli Huang, Southern Medical University, China
Copyright © 2022 Zhou, Lin, Wu, Xiao, Yu, Xiao, Wu, Chen, Tian, Chen, Chen and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yequn Chen, Z2RjeWN5cUAxNjMuY29t
 Yan Zhou1
Yan Zhou1 Shiwan Wu
Shiwan Wu Muli Wu
Muli Wu Yequn Chen
Yequn Chen Xuerui Tan
Xuerui Tan