
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 11 October 2022
Sec. Heart Failure and Transplantation
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.950291
This article is part of the Research Topic Atherosclerosis and Hypertension in Heart Failure: Mechanisms, diagnosis and therapy View all 9 articles
Background: Most of coronary artery ectasia (CAE) patients have comorbid coronary atherosclerosis. It was lack of prognostic data for CAE patients with coronary heart disease (CHD) and for whom with acute myocardial infarction (AMI).
Objective: To determine the overall prognosis for CAE patients.
Materials and methods: This study was a retrospective cohort study. Fifty-one patients with CAE and comorbid AMI (CAE + AMI) and 108 patients with CAE and comorbid CHD (CAE + CHD) were enrolled and matched to non-CAE subjects at a ratio of 1:3 using a propensity score method, respectively. Controls for CAE + AMI group were 153 AMI patients, controls for CAE group were 324 CHD patients and 329 participants with relatively normal coronary arteries (CON). We followed them up to observe major cardiovascular events (MACE).
Results: The Kaplan-Meier curves showed that the prognosis in CAE + AMI group was worse than in AMI group (5-year non-MACE rate: 62.70% vs. 79.70%, P = 0.010), the prognosis in CAE group was worse than in CHD and CON groups (5-year non-MACE rate: 74.10% vs. 85.80% and 96.70%, respectively, P = 0.000). The main MACEs in CAE + AMI and CAE groups were AMI reoccurrence (19.61% vs. 4.57%, P = 0.002) and re-hospitalization due to repeated angina pectoris (14.81% vs. 8.33% and 2.74%, P = 0.000), respectively. Additionally, the COX regression analysis revealed that the protective factors for preventing MACE in CAE + AMI group included antiplatelet agents (hazard ratio = 0.234, P = 0.016) and angiotensin-converting enzyme inhibitor/angiotensin receptor inhibitor (ACEI/ARB, hazard ratio = 0.317, P = 0.037). Whereas the main factor promoting MACE in CAE group was the degree of coronary stenosis (Gensini score, hazard ratio = 1.011, P = 0.022).
Conclusion: The prognosis of patients with CAE + AMI was worse than that of those with AMI. The overall prognosis of patients with CAE was worse than that of those with CHD. CAE + AMI and CAE groups had different characteristics; the former was prone to AMI reoccurrence, and the latter was prone to repeated angina pectoris. To prevent MACE, medications, including antiplatelets and ACEI/ARBs, are indicated for patients with CAE + AMI, whereas prevention of the progression of atherosclerotic lesions is indicated for patients with CAE.
Coronary artery ectasia (CAE) refers to the dilatation of the coronary arteries to a diameter more than 1.5 times of its normal adjacent segment (1). Its prevalence is 1.2–7.4% among patients who have undergone coronary angiography (2). Comorbidity of coronary heart disease (CHD) occurred in more than 80% of patients with CAE (3). Its pathological manifestations are characterized by the extensive destruction of musculoelastic elements, particularly elastin fibers, which are dominant components of the extracellular matrix of the coronary wall (4, 5). It leads to slow blood flow and dysfunction in microcirculation (6, 7), thrombosis in the expanded coronary (3), and increased rupture risk of the expanded part (8). Therefore, the main clinical manifestations include angina, acute myocardial infarction (AMI), arrhythmia, and sudden death (2, 9, 10). In our clinical practice, some patients with CAE present with angina pectoris as the main manifestation, whereas others are more likely to present with AMI. Due to the low prevalence rate, it was still lack of the overall prognosis data for patients with CAE.
In this study, patients with coronary artery dilatation hospitalized in Beijing Friendship Hospital in recent years were followed up to observe their main cardiovascular events (MACE). These results would be useful for clinical practice and future research in this field.
This study was a retrospective cohort study, the process of subject enrollment was showed in Figure 1. Fifty-one patients with CAE and comorbid AMI (CAE + AMI group) and 108 patients with CAE and comorbid CHD (CAE group) were selected from the population of patients who underwent coronary angiography in Beijing Friendship Hospital from January 2015 to December 2020. All patients with CAE were matched with individuals in the control groups at a ratio of 1:3. They were matched using a propensity score method, according to sex, age, hypertension, diabetes, smoking, alcohol consumption, liver and renal functions, lip profiles, and medications after discharge including statins, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs), beta receptor blockers, and calcium channel antagonists (CCBs). Finally, the controls for the CAE + AMI group were 153 patients with AMI (AMI group); those for the CAE group were 324 patients with CHD (CHD group); and 329 patients with relatively normal coronary arteries (CON group). This study was approved by the ethics committee of Beijing Friendship Hospital (2017-P2-013-02) and conducted in accordance with the Declaration of Helsinki. All study participants provided written informed consent.
Coronary artery ectasia (CAE) is defined as an ectatic artery diameter exceeding 1.5 times that of adjacent normal segments (4). CHD is defined as stenosis of ≥50% in one or more major coronary arteries (11). Participants with stenosis of <20% in their coronary arteries were used as controls. According to current consensus (12), AMI is defined by an elevated cardiac biomarker and at least one of the following: (1) symptoms relating to ischemia; (2) changes on an electrocardiogram, such as ST segment changes, new left bundle branch block, or Q waves; and (3) changes in the motion of the heart wall on imaging. Since our preliminary analysis showed that CAE + AMI might be different from CAE (the formal data in this research confirmed it), patients with old myocardial infarction were not included in the CON, CHD, and CAE groups.
Several exclusion criteria were established. These were diagnosis of cardiomyopathy, valvular heart disease, heart failure, aneurysm in other vessels, collagen tissue diseases, vasculitis, syphilis, chronic obstructive lung disease, pulmonary hypertension, early menopause, documented history of hepatic diseases, renal failure, known malignancy, local or systemic infection, previous history of infection (<3 months), and other acute or chronic inflammatory diseases.
The hospital medical records were detailed and intact. Most of the baseline data in this research were extracted from the medical records; they included demographic data (age and sex), disease history (CHD, diabetes, and other diseases), history of smoking and alcohol consumption, family history of disease (hypertension, diabetes, and CHD), and medications taken before admission and after discharge. The body mass index (BMI) was calculated by dividing weight in kilograms by height in meters squared (kg/m2).
For patients with AMI (AMI and CAE + AMI groups), the myocardial infarct size could be estimated by peak blood concentrations of cardiac-specific enzymes, including creatine kinase MB fraction (CK-MB), myoglobin (Myo), and troponin I (TnI) (13). Left ventricular function was gauged by echocardiography, peak value of N-terminal pro-brain natriuretic peptide (NT-proBNP), and Killip grades.
The elbow vein blood was extracted during the next morning after admission and sent to the laboratory of Beijing Friendship Hospital to detect serum levels of glutamic pyruvic transaminase (ALT), serum creatinine, urea nitrogen, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and other serum components. For patients with AMI, serum concentrations of TnI, Myo, CK-MB, and NT-proBNP were measured at admission and at 12-h intervals during the first 5 days after presentation of AMI (from symptom onset).
Transthoracic echocardiography was performed after in-hospital admission and at a median of 5 days after AMI. All images were analyzed by a single investigator, who was blinded to all clinical data. The coronary angiography was performed using a radial artery approach or a femoral artery approach, and each image was interpreted by two independent cardiologists.
Most patients with ST-segmental elevated myocardial infarction (STEMI) received an emergency percutaneous coronary intervention (PCI) as part of reperfusion therapy within 12 h of the onset of symptoms. For most patients with non-STEMI, initial antithrombotic therapy was instituted, and subsequent coronary angiography (delayed PCI) was performed within the first week.
According to the Markis classification method (4), CAE could be classified into four groups based on the extent of ectasia in the coronary arteries: Markis type I, diffuse ectasia of two or three vessels; Markis type II, diffuse disease in one vessel and localized disease in another vessel; Markis type III, diffuse ectasia of one vessel only; and Markis type IV, localized or segmental ectasia (4).
The Gensini scoring method (14) was used to evaluate the extent of coronary stenosis, and the most severe stenotic site was considered as the stenotic site for scoring. A stenotic diameter of <25% was scored as 1 point, 25–49% as 2 points, 50–74% as 4 points, 75–89% as 8 points, 90–99% as 16 points, and total occlusion as 32 points. The above scores were multiplied by a corresponding coefficient: 5 for the left main branch (LM); 2.5 and 1.5 for the proximal and middle segment of the left anterior descending artery (LAD), respectively; 1 and 0.5 for D1 and D2 in the diagonal branches, respectively; 2.5 and 1 for proximal and distal segment lesions of the left circumflex artery (LCX), respectively; and 1 for proximal, middle, distal, and posterior descending branch lesions of the right coronary artery (RCA). The sum of the scores for each lesion was the total score of the degree of coronary artery stenosis for a patient.
Our research team followed up on participants at the outpatient department and with telephones at 1, 3, 6, 12, 24, 36, 48, and 60 months after discharge. Early or delayed follow-up was within a week of the scheduled time point. The missing data were addressed with propensity score methods. The endpoint was MACE; in this study, it referred to cerebral infarction, cerebral hemorrhage, cardiac death, major hemorrhagic events, malignant arrhythmias, cardiogenic shock, readmission, revascularization therapy, and AMI.
The SPSS 26.0 statistical software (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. Normally distributed continuous data were described using X ± SD, and their inter-group difference comparisons were performed using independent T test analysis. Discrete data were summarized as frequencies and analyzed using the χ2 test for inter-group differences. The multi-group comparisons were performed using the one-way ANOVA and rank-sum test. Non-normally distributed continuous data were summarized using the median and inter-quartile range. The 5-years’ prognostic data for all participants (AMI group, AMI + CAEgroup; CON group, CHDgroup, CAE group) was analyzed by the Kaplan-Meier curve; the COX regression analyses was used to find out factors relating to CAE (CON = 1, CHD = 2, CAE = 3) and CAE + AMI (AMI = 0, AMI + CAE = 1); the logistic regression was aimed to find out the independent factors relating to CAE + AMI, in this regression model, the dependent factor was CAE (CAE + CHD = 0, CAE + AMI = 1). P < 0.05 indicated statistically significant difference.
According to Table 1, the following baseline characteristics of all participants were observed. (1) Comparing the CAE + AMI and AMI groups, number of old cases of myocardial infarction was higher in the CAE + AMI group than in the AMI group. However, no significant differences were observed between both groups for most baseline data. (2) No significant differences in baseline characteristics were observed among the CAE, CHD, and CON groups. (3) The CAE + AMI group had higher number of males, lower hypertension rate, lower CHD rate, higher liver function and renal function, and poorer lipid profile than the CAE group.
As shown in Table 2, the electrolyte, inflammation, cardiac function, and medications were compared among all participants. (1) Comparing the CAE + AMI and AMI groups, hypersensitive C-reactive protein level was higher in the CAE + AMI group than in the AMI group. No further significant differences were observed. (2) The diastolic function indicated by E peak value to A peak (E/A) value was lower in the CAE group than the CHD and CON groups. (3) The CAE + AMI group had a lower left ventricular ejection fraction (LVEF), neutrophil-to-lymphocyte ratio (N/L ratio), and hypersensitive C-reactive protein level than the CAE group.
Table 3 shows evaluations of ectasia and stenosis in the coronary arteries. The extent of stenosis, indicated by the Gensini score, and stenosis rate of LCX in the CAE + AMI group were lower than those in the AMI group. For distribution of ectasia, the RCA had the highest involvement with dilatation, and the most common type was Markis type I. Furthermore, the extent of stenosis in the CAE group was similar to that in the CHD group, whereas the stenosis rate of LAD was lower in the CAE group. For the ectasia distribution, RCA had the highest involvement with dilatation, and the most common type was Markis type I. In comparing the CAE + AMI and CAE groups, the CAE + AMI group had a higher stent implant rate, Gensini score, and RCA stenosis and occlusion rates than the CAE group.
Figure 2 shows the 5-year prognostic analysis of all participants using the Kaplan-Meier curve. The overall prognosis of the CAE + AMI group was worse than that of the AMI group (cumulative survival rate or 5-year non-MACE rate: 62.70% vs. 79.70%, P = 0.010) (Figure 1, left). The overall prognosis of the CAE group was worse than those of the CHD and CON groups (cumulative survival rate or 5-year non-MACE rate: 74.10% vs. 85.80% and 96.70%, respectively, P = 0.000) (Figure 1, right).
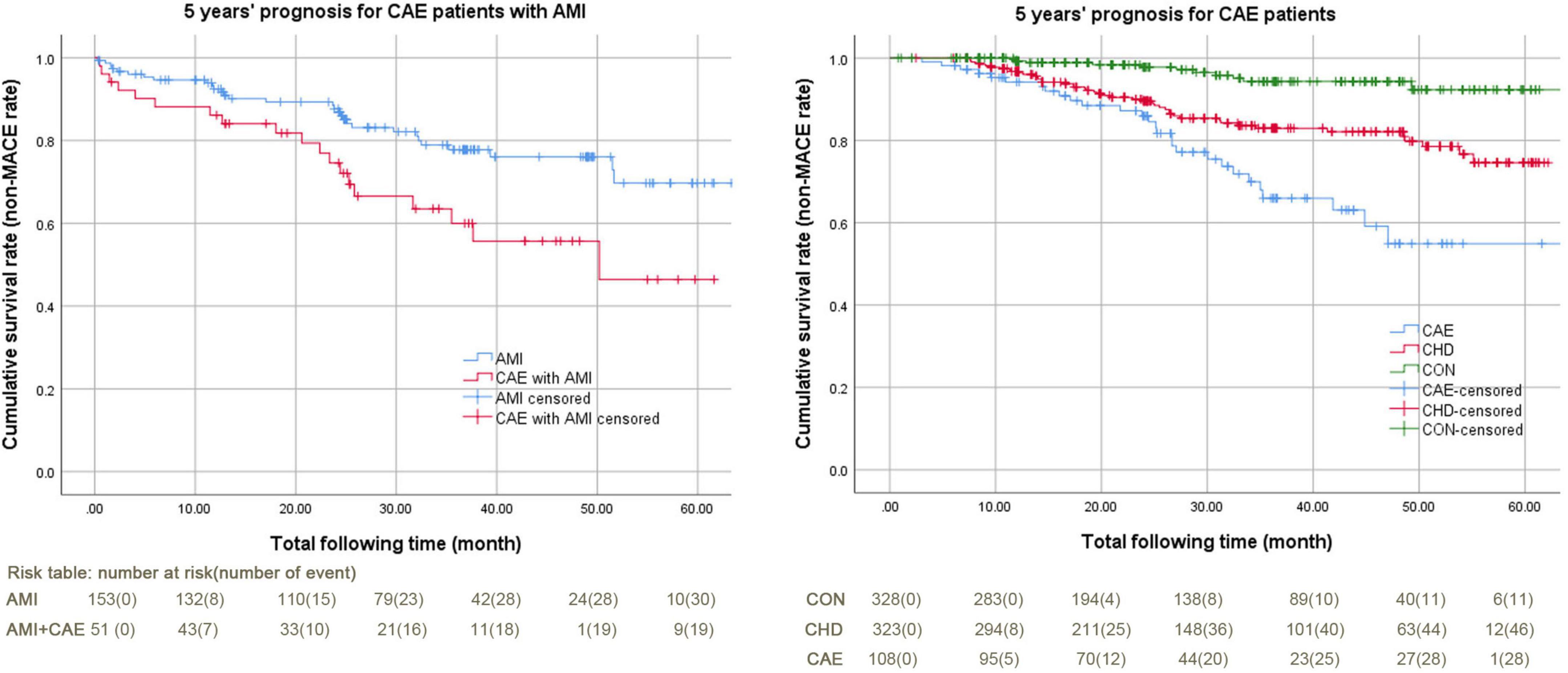
Figure 2. Kaplan-Meier curves of 5-year MACE for patients with CAE + AMI and CAE. AMI, acute myocardial infarction; CAE, coronary artery ectasia; CHD, coronary heart disease; CON, coronary artery without CAE and stenosis; MACE, major adverse cardiovascular events. The MACE in this study included cerebral infarction, cerebral hemorrhage, cardiac death, major hemorrhagic events, malignant arrhythmias, cardiogenic shock, readmission, revascularization therapy, and AMI. 1) Figure left: Kaplan-Meier curves of 5-year MACE for patients with CAE + AMI, log Rank (Mantel-Cox) P value was 0.010. The non-MACE percentages were 79.70% and 62.70%; the non-MACE time spans were 53.37 (49.66–57.08) months and 41.91 (35.14–48.67) months. The total follow-up times were 32.07 (13.20–48.87) and 25.33 (13.37–42.80) months. 2) Figure right: Kaplan-Meier curves of 5-year MACE for patients with CAE, Log Rank (Mantel-Cox) P value was 0.000. The non-MACE percentages were 96.70%, 85.80%, and 74.10%. The non-MACE time spans were 61.84 (60.38–63.29) months, 54.08 (51.96–56.20) months, and 49.75 (44.58–54.92) months. The total follow-up times were 24.10 (12.53–42.85) months, 27.50 (15.50–46.77) months, and 26.62 (15.63–36.57) months.
Regarding MACE in the CAE + AMI and AMI groups, the main MACE in the CAE + AMI group was AMI reoccurrence (19.61% vs. 4.57%, P = 0.002). Comparing MACE in the CAE, CHD, and CON groups, the main MACE in the CAE group was re-hospitalization due to repeated angina pectoris (14.81% vs. 8.33% and 2.74%, P = 0.000). The MACE rates in the CAE + AMI and CAE groups were similar, but the exact MACE events differed between the groups [AMI including STEMI and NSTEMI (3.70% vs. 19.61%) and re-hospitalization due to repeated angina pectoris (14.81% vs. 9.80%)] (Table 4).
After the COX regression analyses of the CAE + AMI and AMI groups, the group (AMI = 0, CAE + AMI = 1) was found to be a promoting factor for MACE. For the CAE, CHD, and CON groups, the group (control = 1, CHD = 2, CAE = 3) was found to be a promoting factor for MACE (Table 5).
Based on the COX regression analyses, 1) the protective factors preventing MACE in the CAE + AMI group were antiplatelet agents (standardization coefficient beta or hazard ratio = 0.234, P = 0.016) and ACEI/ARB (standardization coefficient beta or hazard ratio = 0.317, P = 0.037). 2) For the CAE group, the main factor promoting MACE was the degree of coronary stenosis (Gensini score, hazard ratio = 1.011, P = 0.022) (Table 6).
The purpose of logistic regression was aimed to find out the independent factors relating to CAE + AMI (CAE = 0, CAE + AMI = 1). It revealed that N/L ratio, LDL-c, and hypersensitive C-reactive protein level were positively associated with the CAE + AMI, whereas Markis type was negatively associated with the CAE + AMI (Table 7).
It was lack of the overall prognosis for patients with CAE and those with CAE + AMI (15). Therefore, this study enrolled a consecutive series of 51 patients with CAE + AMI and 108 patients with CAE + CHD. They were matched with controls at a 1:3 ratio using the propensity score method and were followed up. From the study findings, the Kaplan-Meier curve showed that the MACE of participants in the CAE + AMI group was higher than those in the AMI group. Furthermore, the MACE of participants in the CAE group was higher than that of those in the CHD and CON groups. Regarding MACE, the main MACEs in the CAE + AMI and CAE groups were AMI reoccurrence and re-hospitalization due to repeated angina pectoris, respectively. Additionally, the COX regression analysis revealed that the protective factors for preventing MACE in the CAE + AMI group included antiplatelet agents and ACEI/ARB while the main factor promoting MACE in the CAE group was the degree of coronary stenosis. The logistic regression analysis showed that the factors with positive association with CAE + AMI were higher inflammatory status and poorer lipid profile, whereas that with negative association with CAE + AMI was Markis types.
First, the overall prognosis of patients with CAE was poorer than that of patients with CHD. Additionally, the prognosis of patients with CAE + AMI was poorer than patients with AMI. The relationship between CAE and CHD remained unclear, although more than 80% of patients with CAE had comorbid CHD (16). According to some studies, CAE is a variation of CHD because the two diseases have similar features, including risk factors, clinical symptoms, and pathological findings (16, 17). Both diseases have obvious atherosclerotic changes in the intima and media of the coronary artery. However, the damage of the middle coronary artery is an important difference between CHD and CAE (4, 5). An obvious slow coronary blood flow and microcirculation are present in dilated coronary arteries (6). Therefore, the overall prognosis of patients with CAE was understandably poorer than that of patients with CHD, because those extra characters might contribute to the extra MACE in patients with CAE. The prognostic data were useful for designing the treatment strategy, although no consensus on the treatment for patients with CAE was not reached. Currently, most of the treatment proposals for CAE are based on CHD treatment (18, 19). Therefore, to decrease MACE in patients with CAE, this study indicated the need for more aggressive treatments for patients with CAE than for those with CHD.
Second, the CAE and CAE + AMI groups were found to be different populations. Patients with CAE had a higher risk of repeated angina pectoris than patients with AMI. Patients with CAE + AMI had a higher risk of AMI reoccurrence but not angina pectoris (20–22). The underlying mechanism contributing to these differences was unclear. The logistic regression in this study revealed that the factors positively associated with AMI in the CAE population were higher inflammatory status and poor lipid profiles. As known, CAE is an inflammatory disease (6, 23); therefore, a higher inflammatory status might be predispose patients to occurrence of thrombotic events. Other underlying factors relating to AMI in patients with CAE should be explore further. In this study, for patients with CAE, more aggressive treatment was needed to relieve ischemia and ischemic symptoms but not to prevent thrombosis. Whereas for patients with CAE + AMI, more aggressive treatment was needed to prevent thrombosis but not to relieve ischemia and ischemic symptoms. These findings are helpful in categorizing patients with CAE and formulating specific treatment strategies in future clinical practice, because for a long period, CAE was not categorized into different subgroups according to their different prognoses.
Third, the detailed treatment strategy for patients with CAE was explored in this study. From the perspective of MACE prevention, the progression of atherosclerotic lesions should be prevented in patients with CAE (23, 24) while the anti-thrombotic treatments, are indicated for patients with CAE + AMI (25, 26). As reported in our previous study, patients with CAE and CHD had minimal ectasia progression but the atherosclerosis progressed gradually (3). The dynamic change of CAE might be mainly manifested by the development of atherosclerotic changes but not the extent of ectasia; therefore, for patients with CAE, the main treatment might be to prevent the progression of atherosclerotic lesions; for CAE + AMI patients, the thrombotic events might be related to ectasia itself (higher inflammatory status and poor lipid profiles) or other unknown factors (23), and the remodeling of left ventricular could be improved by ACEI/ARBs. Most medicines for CAE are based on the current treatment options for CHD, such as antithrombotic therapy, statins, and trimetazidine (10). According to our findings, anti-atherosclerotic agents, including statins, should be more aggressively used in patients with CAE. For patients with CAE + AMI, anti-thrombotic treatment options, including double anti-platelet agents, or a more aggressive strategy by combination of anti-platelet agents and anti-coagulation agents should be considered (27, 28). Additionally for patients with poor response to medications, coronary artery interventional therapy and coronary artery bypass graft should be considered (1).
This study had the following limitations. First, the small sample size was due to the difficulty in recruiting participants, owing to the low prevalence of CAE and use of special diagnostic methods, such as coronary angiography, but not other routine methods. Second, this study was a single-center study. Therefore, further multi-center collaborations are needed in the future to produce more representative results.
The prognosis of patients with CAE + AMI were worse than that of patients with AMI, and the overall prognosis for patients with CAE patients was worse than that of patients with CHD. The CAE + AMI and CAE groups had different characteristics; the former was prone to AMI reoccurrence, and the latter was prone to repeated angina pectoris. To prevent MACE, medications, including antiplatelets and ACEI/ARBs, are indicated for patients with CAE + AMI, whereas prevention of the progression of atherosclerotic lesions is indicated for patients with CAE.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics committee of Beijing Friendship Hospital Affiliated to Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
RL and HZ: conceive the study and its design, conceptualization, formal analysis, resources, and software. HZ: data curation. RL, XG, SL, and HZ: investigation and validation. XG: methodology. RL, SL, and HZ: project administration. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (Grant No. 81600276).
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewers WL and LZ declared a shared parent affiliation with several of the authors to the handling editor at the time of the review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Damay V, Pranata R, Wiharja W. Recurrent acute coronary syndrome in a patient with right coronary artery ectasia: a case report. J Med Case Rep. (2019) 13:78.
2. Eitan A, Roguin A. Coronary artery ectasia: new insights into pathophysiology, diagnosis, and treatment. Coron Artery Dis. (2016) 27:420–8.
3. Esposito L, Di Maio M, Silverio A, Cancro FP, Bellino M, Attisano T, et al. Treatment and outcome of patients with coronary artery ectasia: current evidence and novel opportunities for an old dilemma. Front Cardiovasc Med. (2022) 8:805727. doi: 10.3389/fcvm.2021.805727
4. Fan CH, Hao Y, Liu YH, Li XL, Huang ZH, Luo Y, et al. Anti-inflammatory effects of rosuvastatin treatment on coronary artery ectasia patients of different age groups. BMC Cardiovasc Disord. (2020) 20:330. doi: 10.1186/s12872-020-01604-z
5. Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. (2021) 144:e368–454. doi: 10.1161/CIR.0000000000001030
6. Hajsadeghi S, Chitsazan M, Chitsazan M, Haghjoo M, Babaali N, Norouzzadeh Z, et al. Metabolic syndrome is associated with higher wall motion score and larger infarct size after acute myocardial infarction. Res Cardiovasc Med. (2015) 4:e25018. doi: 10.5812/cardiovascmed.25018
7. Kaplan M, Abacıoğlu ÖÖ, Yavuz F, Kaplan GI, Topuz M. Slow flow phenomenon impairs the prognosis of coronary artery ectasia as well as coronary atherosclerosis. Braz J Cardiovasc Surg. (2021) 36:346–53. doi: 10.21470/1678-9741-2020-0618
8. Lee NY, Park HY, Park CK, Ahn MD. Analysis of systemic endothelin-1, matrix metalloproteinase-9, macrophage chemoattractant protein-1, and high-sensitivity C-reactive protein in normal-tension glaucoma. Curr Eye Res. (2012) 37:1121–6. doi: 10.3109/02713683.2012.725798
9. Liu R, Zhao H, Gao X, Liang S. Is coronary artery ectasia a progressive disease? A self-controlled retrospective cohort study. Front Cardiovasc Med. (2021) 8:774597. doi: 10.3389/fcvm.2021.774597
10. Markis JE, Joffe CD, Cohn PF, Feen DJ, Herman MV, Gorlin R. Clinical significance of coronary arterial ectasia. Am J Cardiol. (1976) 37:217–22. doi: 10.1016/0002-9149(76)90315-5
11. Matta AG, Yaacoub N, Nader V, Moussallem N, Carrie D, Roncalli J. Coronary artery aneurysm: a review. World J Cardiol. (2021) 13:446–55. doi: 10.4330/wjc.v13.i9.446
12. Matte BDS, de Araujo GN, Valle FH, Krepsky AMR. Multiple culprit coronary artery thrombosis in a patient with coronary ectasia. Case Rep Cardiol. (2018) 2018:6148470. doi: 10.1155/2018/6148470
13. Molina-Martin de Nicolas J, Jurado Roman A, Rubio Alonso B, Garcia Tejada J. Recurrent myocardial infarctions due to thrombosis of a coronary aneurysm in neurofibromatosis type 1: is antiplatelet treatment enough? JACC Cardiovasc Interv. (2015) 8:e55–7. doi: 10.1016/j.jcin.2014.10.031
14. Osevala MA, Heleotis TL, DeJene BA. Successful treatment of a ruptured mycotic coronary artery aneurysm. Ann Thorac Surg. (1999) 67:1780–2. doi: 10.1016/s0003-4975(99)00329-x
15. Ozbay Y, Akbulut M, Balin M, Kayancicek H, Baydas A, Korkmaz H. The level of hs-CRP in coronary artery ectasia and its response to statin and angiotensin-converting enzyme inhibitor treatment. Mediators Inflamm. (2007) 2007:89649. doi: 10.1155/2007/89649
16. Perlman PE, Ridgeway NA. Thrombosis and anticoagulation therapy in coronary ectasia. Clin Cardiol. (1989) 12:541–2. doi: 10.1002/clc.4960120912
17. Reji R, Nguyen M. Medically managed coronary artery aneurysm without concomitant stenosis. BMJ Case Rep. (2018) 2018:bcr2018224244. doi: 10.1136/bcr-2018-224244
18. Sheikh AS, Hailan A, Kinnaird T, Choudhury A, Smith D. Coronary artery aneurysm: evaluation, prognosis, and proposed treatment strategies. Heart Views. (2019) 20:101–8. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_1_19
19. Sheng Q, Zhao H, Wu S, Liu R. Underlying factors relating to acute myocardial infarction for coronary artery ectasia patients. Medicine. (2020) 99:e21983. doi: 10.1097/MD.0000000000021983
20. Tomioka T, Takeuchi S, Ito Y, Shioiri H, Koyama J, Inoue K. Recurrent acute myocardial infarction in a patient with severe coronary artery ectasia: implication of antithrombotic therapy. Am J Case Rep. (2016) 17:939–43. doi: 10.12659/ajcr.900474
21. Uyarel H, Okmen E, Tartan Z, Kasikcioglu H, Dayi SU, Karabulut A, et al. The role of angiotensin converting enzyme genotype in coronary artery ectasia. Int Heart J. (2005) 46:89–96. doi: 10.1536/ihj.46.89
22. Virmani R, Robinowitz M, Atkinson JB, Forman MB, Silver MD, McAllister HA. Acquired coronary arterial aneurysms: an autopsy study of 52 patients. Hum Pathol. (1986) 17:575–83. doi: 10.1016/s0046-8177(86)80129-0
23. Wang KY, Zheng YY, Wu TT, Ma YT, Xie X. Predictive value of gensini score in the long-term outcomes of patients with coronary artery disease who underwent PCI. Front Cardiovasc Med. (2022) 8:778615. doi: 10.3389/fcvm.2021.778615
24. Waqas M, Bizzocchi LL, Menegus MA, Faillace RT. Coronary artery ectasia: an insight into intraprocedural and postprocedural management strategies. Cureus. (2019) 11:e3928. doi: 10.7759/cureus.3928
25. Xi Z, Qiu H, Guo T, Wang Y, Dou K, Xu B, et al. Prevalence, predictors, and impact of coronary artery ectasia in patients with atherosclerotic heart disease. Angiology. (2022) [Online ahead of print]. doi: 10.1177/00033197221091644
26. Yang Z, Yuan J, Cui J, Guan H, Qiao S. Association of the lymphocyte-to-monocyte ratio, mean diameter of coronary arteries, and uric acid level with coronary slow flow in isolated coronary artery ectasia. BMC Cardiovasc Disord. (2021) 21:156. doi: 10.1186/s12872-021-01952-4
27. Yao YA, Zhang SY, Wu W, Chen LF. The clinical manifestations and angiographic characteristics of coronary artery ectasia. Zhonghua Nei Ke Za Zhi. (2010) 49:389–91.
Keywords: coronary artery ectasia (CAE), coronary heart disease (CHD), acute myocardial infarction (AMI), major cardiovascular events (MACE), prognosis
Citation: Liu R, Gao X, Liang S and Zhao H (2022) Five-years’ prognostic analysis for coronary artery ectasia patients with coronary atherosclerosis: A retrospective cohort study. Front. Cardiovasc. Med. 9:950291. doi: 10.3389/fcvm.2022.950291
Received: 22 May 2022; Accepted: 23 September 2022;
Published: 11 October 2022.
Edited by:
Sufang Li, Peking University People’s Hospital, ChinaReviewed by:
Lin Zhao, Capital Medical University, ChinaCopyright © 2022 Liu, Gao, Liang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiqiang Zhao, aHVpcWlhbmd6aGFvMTIzNDU2QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.