- Department of Cardiovascular Medicine, Shanxi Bethune Hospital, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Shanxi Academy of Medical Sciences, Taiyuan, China
Background: Cardiovascular disease, including acute myocardial infarction (AMI), is a major global cause of mortality and morbidity. Specificity and sensitivity limit the utility of classic diagnostic biomarkers for AMI. Therefore, it is critical to identify novel biomarkers for its accurate diagnosis. Cumulative studies have demonstrated that circulating microRNAs (miRs) participate in the pathophysiological processes of AMI and are promising diagnostic biomarkers for the condition. This study aimed to ascertain the diagnostic accuracy of circulating miR-21-5p and miR-126 used as biomarkers in patients with AMI and infarct-related artery total occlusion (IR-ATO) or infarct-related blood-vessel recanalization (IR-BVR).
Methods: The expression of miR-21-5p and miR-126 was examined separately in 50 healthy subjects, 51 patients with IR-ATO AMI, and 49 patients with IR-BVR AMI using quantitative real-time polymerase chain reaction.
Results: When compared with the control group, the IR-ATO AMI group exhibited increased miR-21-5p (p < 0.0001) and miR-126 (p < 0.0001), and the IR-BVR AMI group exhibited increased miR-21-5p (p < 0.0001). However, there was no significant difference in miR-126 between the IR-BVR AMI and the control groups. A Spearman's correlation coefficient showed a strong correlation was found between miR-21-5p, miR-126, cardiac troponin-I, and creatine kinase isoenzyme in all three groups, while a receiver operating characteristic analysis revealed that miR-21-5p and miR-126 exhibited considerable diagnostic accuracy for IR-ATO AMI.
Conclusion: Circulating miR-21-5p and miR-126 may be promising prognostic biomarkers for patients with AMI and IR-ATO.
Introduction
Cardiovascular disease is the leading cause of death worldwide, and coronary artery disease (CAD) accounts for half of those deaths (1). In CAD, acute myocardial infarction (AMI) caused by the occlusion of the coronary arteries and leading to the cell necrosis of cardiomyocytes plays a major role in acute and chronic heart failure (2). The accurate diagnosis of AMI is an unmet clinical need that could help to reduce the incidence of heart failure after AMI (3). Currently, the diagnosis of AMI depends on at least two of the following detailed clinical features: Q waves in electrocardiograms (ECGs), the elevation of or decrease in cardiac markers, e.g., creatine kinase isoenzyme (CK-MB), and increased levels of serum cardiac troponin (cTn) -I and -T alone or in combination (4–6). However, CK-MB lacks sensitivity for the onset of AMI, especially within 6 h (7). In addition, elevated cTn occurs not only in patients with heart failure but also in those with chronic kidney diseases (8). Therefore, it is critical to identify sensitive and specific biomarkers for the accurate diagnosis of AMI.
MicroRNA (miR), endogenous short (20–22 nucleotides) non-coding RNA, has been shown to participate in diverse cellular processes, including cancer, cardiovascular diseases, and inflammatory diseases (9–11). The miRs perform physiological and pathological functions by regulating the expression of target genes (12). Among them, miR-21, identified initially as a tumor growth enhancer, has been shown to participate in the initiation and progression of AMI (13). Published studies found that the upregulation of miR-21 or phosphatase and tensin homolog (PTEN) silencing improved cardiomyocytes' viability and reduced the apoptotic cell rate by activating the phosphatidylinositol 3-kinase/serine/threonine kinase signaling pathway (14), and the overexpression of miR-21 reduced the infarct size and immune cell infiltration in a rat model with AMI (15). However, miR-126, which is highly enriched with endothelial cells, has been observed to increase in the plasma or serum of patients with AMI from the onset of the condition (16). Therefore, miR-21 and miR-126 are considered promising biomarkers for the diagnosis of AMI.
In the present study, we detected the expression of circulating miR-21-5p and miR-126 in patients with AMI and infarct-related artery total occlusion (IR-ATO) or infarct-related blood-vessel recanalization (IR-BVR). We looked for correlations between miRs, CK-MB, and cTn-I to investigate the sensitivity and reliability of these biomarkers in identifying AMI and its possible clinical type.
Methods
Study subjects
A total of 100 consecutive patients, aged 26–87 y (54 ± 12 y), with AMI and ST-segment elevation myocardial infarction (STEMI) were enrolled in this case-controlled study from January 2018 to January 2019.
The inclusion criteria were as follows: (1) persistent chest pain for ≥30 min, (2) significant ST-segment elevation (≥0.1 or ≥0.2 mV on ≥2 adjacent limb or precordial leads, respectively, or new left bundle-branch block), or (3) the onset of chest pain within 12 h or after more than 12 h accompanied by hemodynamic instability and hypovolemic shock.
The exclusion criteria were as follows: thrombolytic therapy for STEMI, valvular heart disease, myocarditis, cardiomyopathy, severe heart failure, severe liver and kidney dysfunction, hematological system diseases, malignant tumors, gastrectomy, acute and chronic infectious diseases, autoimmune diseases, bronchus asthma, abnormal calcium metabolism, thyroid or adrenal dysfunction, history of surgery, or trauma.
The patients were divided into the IR-ATO group (n = 51) and the IR-BVR group (n = 49) according to their coronary angiography results. In addition, 50 healthy patients without a history of heart, vascular, or other major disease and with a normal chest X-ray, ECG, liver and kidney function, and biochemical index were recruited as the control group. The baseline characteristics of all subjects were collected.
The Gensini score was used to evaluate the degree of coronary artery disease in patients, and the correlations between the Gensini score and the levels of miR-21-5p and miR-126 were analyzed. The Gensini scoring criteria were as follows: 1 point: stenosis <25%; 2 points: stenosis 25–49%, 4 points, stenosis 50–74%; 8 points, 75–89% stenosis; 16 points, 90–99% stenosis; 32 points, 100% stenosis in the lesion.
This study was approved by the ethics committee of the Shanxi Bethune Hospital, and all participants signed an informed consent form.
Sample collection
Approximately 10 ml of venous blood was collected from each participant in ethylene diamine tetra acetic acid-coated tubes. All samples were centrifuged at 3,000 rpm for 10 min at 4°C. The supernatant was removed and saved at −20°C for future use.
Measurement of cardiac troponin-I and creatine kinase isoenzyme
According to the manufacturer's protocol, the serum cTn-I concentration and CK-MB activity were measured using an enzyme-linked immunosorbent assay kit and immunoassay analyzer, respectively.
Total RNA extraction and microRNA reverse transcription
Total RNA was extracted from serum using TRNzol Universal Reagent (TIANGEN® Biotech, Beijing, China) based on the protocol provided by the manufacturer. Serum samples (0.25 ml) were homogenized in TRIzol™ (0.75 ml) and stored at room temperature for 5 min. Chloroform was added to each sample (0.2 ml) before it was shaken vigorously to ensure complete dissociation of the nucleoprotein complexes. After standing at room temperature for 10 min, the mixtures were centrifuged at 12,000 × g for 15 min at 4°C. The RNA in the aqueous phase was precipitated with cold isopropyl alcohol (0.5 ml). Following centrifugation at 12,000 × g for 15 min, the pellets, i.e., RNA, were washed with 75% ethanol and finally dissolved with diethyl pyrocarbonate H2O (10 μl). The RNA purity was determined using a NanoDrop™ 2000 RNA analyzer (Thermo Fisher Scientific, Waltham, MA, USA), and only those samples with a ratio of between 1.8 and 2.1 were used in the present study.
Reverse transcription was performed using 1 μg of total RNA in a total reaction volume of 20 μl using a First Strand cDNA Synthesis kit (KR201, TIANGEN®). The 20-μl reactions were incubated at 37°C for 1 h and terminated by heating to 85°C for 5 min. Following cDNA synthesis, all cDNA samples were stored at −20°C.
MicroRNA quantitative real-time polymerase chain reaction
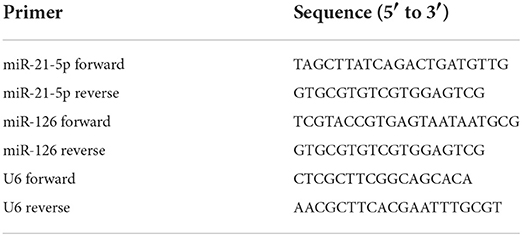
A quantitative real-time polymerase chain reaction (qPCR) was performed using a real-time PCR kit (Takara, Beijing, China) and an Applied Biosystems™ 7500 real-time PCR system (Thermo Fisher Scientific, Inc.). The sequences of all the primers used in our study are shown in Table 1. For normalization, U6 small nuclear RNA was used as an endogenous control. The 20-μl qPCR reactions were incubated at 95°C for 30 s followed by 40 cycles of 95°C for 4 s and 60°C for 40 s. The data were processed using the relative quantification method and calculated using the 2−ΔΔCt method. There were 10 replicates for each group.
Data analysis and statistics
For statistical analyses, the GraphPad Prism 6.0 software package (GraphPad Software, Inc., La Jolla, CA, USA) was utilized to visualize the data. The normality of the data was tested by the Kolmogorov–Smirnov test and Shapiro–Wilk test. Normally distributed data were presented as mean ± SD and were compared using a Student's t-test. The expressions of miR-21-5p and miR-126 were compared between groups using a one-way analysis of variance followed by Tukey's test. The Spearman's rank correlation coefficient was used to evaluate the association between miR levels and between these levels and cTn-I and CK-MB. Pearson's correlation analysis was used to analyze the correlations of the Gensini score with the plasma levels of miR-21-5p and miR-126.
Logistic regression was used to correct for confounding factors. The receiver operating characteristic (ROC) and area under the curve (AUC) were calculated to evaluate the diagnostic accuracy of miR-21-5p and miR-126. All statistical tests were two-tailed, and p < 0.05 was considered statistically significant.
Results
Baseline clinical characteristics of the study subjects
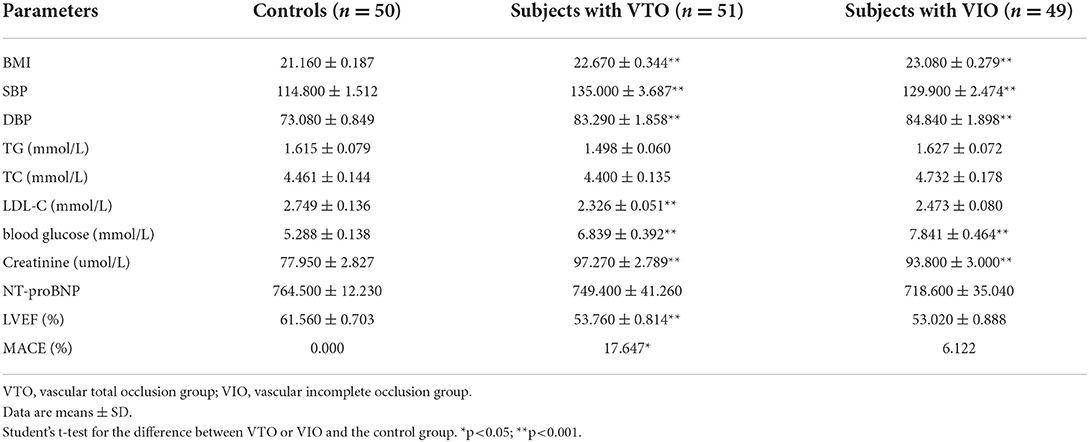
The baseline clinical characteristics of all the study subjects are listed in Table 2. Statistically significant differences existed in the values for body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), low-density lipoprotein cholesterol (LDL-C), blood glucose (GLU), creatinine (CRE), and left ventricular ejection fraction (LVEF) between the control group and the IR-ATO and IR-BVR groups. When compared with the control group, BMI, SBP, DBP, triglyceride (TG), total cholesterol (TC), LDL-C, GLU, and CRE were higher, while LDL-C, N-terminal prohormone of brain natriuretic peptide (NT-proBNP), and LVEF were lower in the IR-ATO and the IR-BVR groups.
Serum levels of cardiac troponin-I and creatine kinase isoenzyme
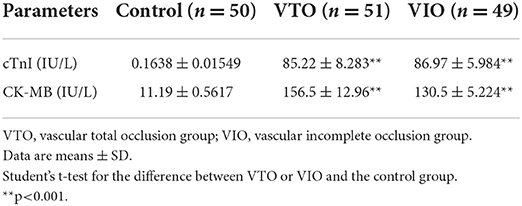
The serum levels of cTn-I and CK-MB are shown in Table 3. The average levels of cTn-I and CK-MB were 85.22 ± 8.283 and 86.97 ± 5.984 IU/L, respectively, in the IR-ATO group and 156.5 ± 12.96 and 130.5 ± 5.224 IU/L, respectively, in the IR-BVR group. These levels were significantly higher than 0.1638 ± 0.01549 and 11.19 ± 0.5617 IU/L, respectively, in the control group.
Expression levels of miR-21-5p and miR-126
The differences in the expression of miR-21-5p and miR-126 among the groups are displayed in Figure 1. There was a significant difference in the relative expression of miR-21-5p between the IR-ATO group (3.616 ± 0.1718) and the control group (1.431 ± 0.06963) and between the IR-BVR group (2.126 ± 0.1026) and the control group. In addition, the relative expression of miR-126 in the vascular group (i.e., the IR-ATO and IR-BVR groups) of 2.667 ± 0.1045 was significantly higher than in the control group (1.04 ± 0.04034). However, there was no difference in the relative expression of miR-126 between the IR-BVR (1.16 ± 0.0496) and the control groups.
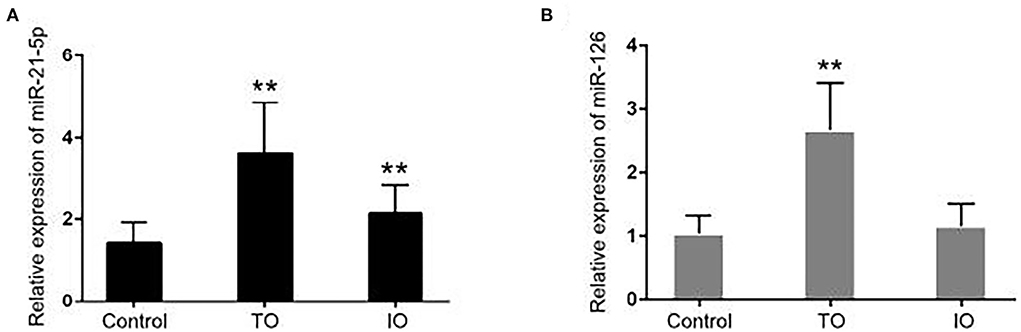
Figure 1. Expressions of miR-21-5p and miR-126 in all subjects. The bar chart shows the expressions of (A) miR-21-5p and (B) miR-126 measured by quantitative real-time polymerase chain reaction in the control group (n = 50), infarct-related artery total occlusion (IR-ATO) acute myocardial infarction (AMI) group (n = 51), and IR-blood-vessel recanalization (BVR) AMI group (n = 49). The relative microRNA expression was calculated using the 2−ΔΔCt method. The differences between the groups were compared using a one-way analysis of variance (**p < 0.001). The IR-ATO group is denoted by VO, and the IR-BVR group is denoted by VIO.
Spearman's correlation coefficient was calculated to ascertain whether the expressions of miR-21-5p and miR-126 were correlated. The Spearman's correlation coefficient between miR-21-5p and miR-126 in the IR-ATO, IR-BVR, and control groups was 0.9995 (p < 0.0001), 0.9998 (p < 0.0001), and 0.9996 (p < 0.0001), respectively (Figure 2).
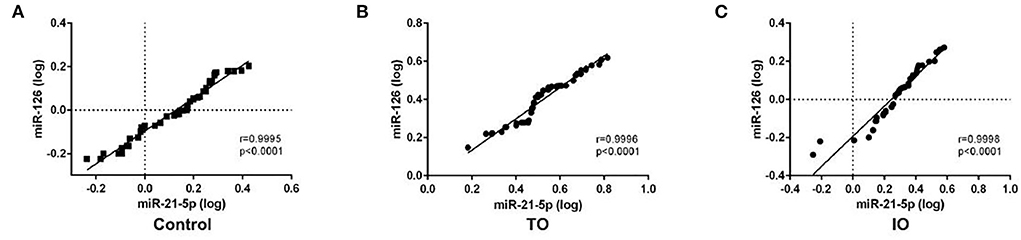
Figure 2. Spearman's correlation coefficient between circulating miR-21-5p and miR-126 in all subjects. The scatter plots show the strong correlation between miR-21-5p and miR-126 in the control group (n = 50), (A) infarct-related artery total occlusion (IR-ATO) acute myocardial infarction (AMI) group (n = 51), (B) IR blood-vessel recanalization (BVR) AMI group (n = 49), and (C) VO = IR-ATO group, and VIO = IR-BVR group.
Correlation analysis
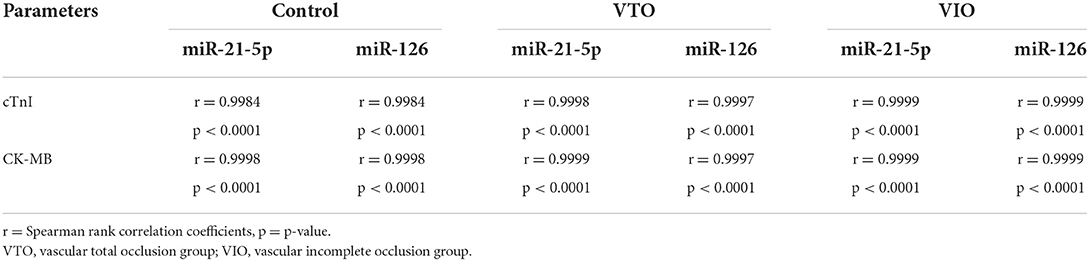
The Spearman's correlation coefficient was also calculated to ascertain the correlation between miR-21-5p and cTn-I, miR-21-5p and CK-MB, miR-126 and cTn-I, and miR-126 and CK-MB (see Table 4). A strong correlation existed between miR-21-5p and cTn-I or CK-MB and between miR-126 and cTn-I or CK-MB in all groups.
Pearson's correlation analysis was then performed to determine the correlations of the Gensini scores with miR-21-5p and miR-126 (Figures 3A,B). A strong positive correlation was observed between the Gensini score and the miR-21-5p levels and between the Gensini score and the miR-126 levels in all patients.
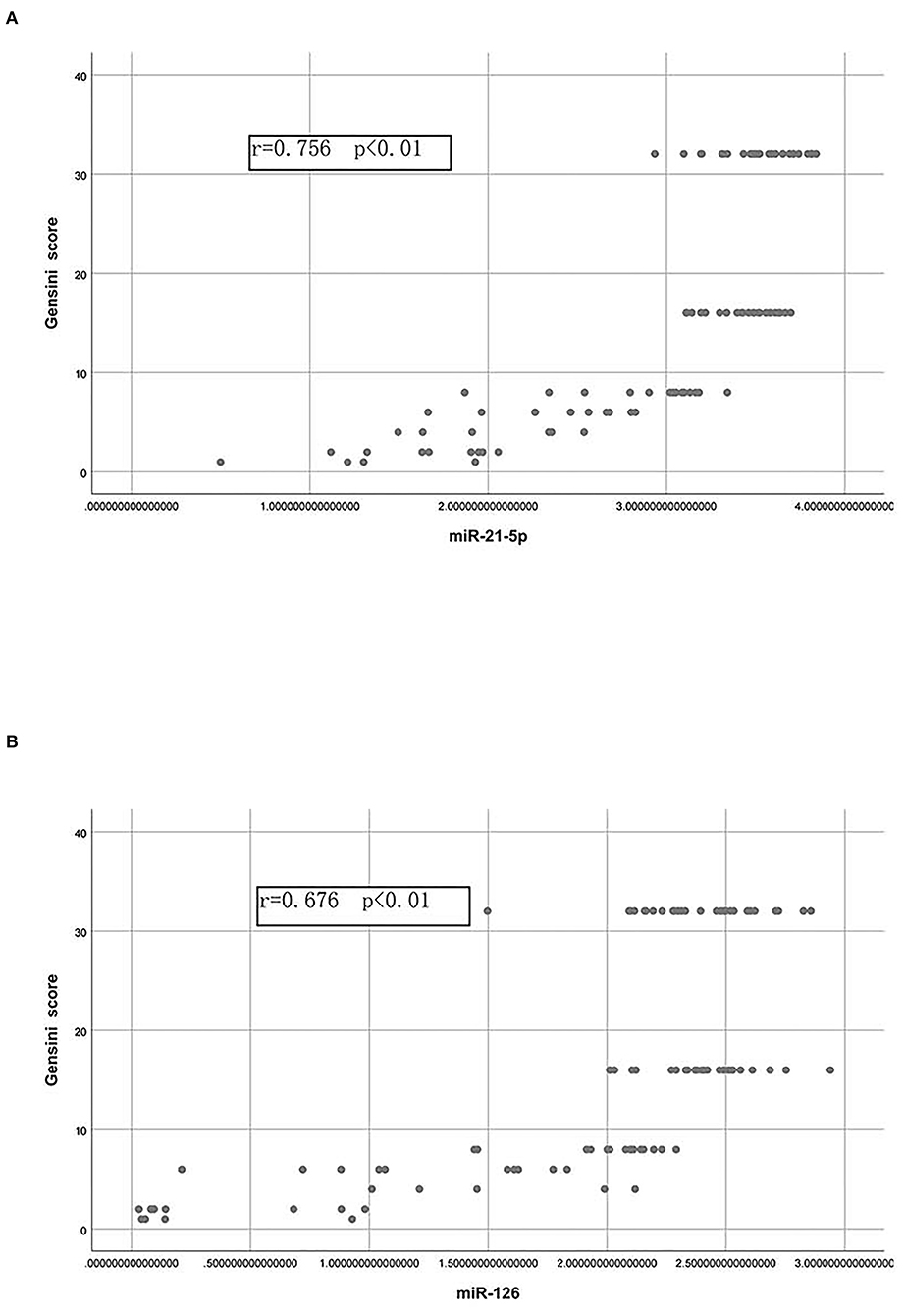
Figure 3. Correlations of the Gensini score with the plasma levels of miR-21-5p and miR-126. (A) Pearson correlation between the Gensini score and the levels of miR-21-5p. (B) Pearson correlation between the Gensini score and the levels of miR-126.
The diagnostic accuracy of miR-21-5p and miR-126
An analysis of the ROC and AUC was conducted to investigate the diagnostic accuracy of miR-21-5p and miR-126 in patients with AMI and IR-ATO or IR-BVR (Figure 4). The AUC for miR-21-5p was 0.9784 (95% CI: 0.9558–1.001, p < 0.0001) in the IR-ATO group and 0.7914 (95% CI: 0.7014–0.8814, p < 0.0001) in the IR-BVR group (Figures 4A,B). The AUC for miR-126 was 0.9973 (95% CI: 0.9913–1.003, p < 0.0001) in the IR-ATO group and 0.5988 (95% CI: 0.4866–0.7110, p = 0.0903) in the IR-BVR group (Figures 4C,D).
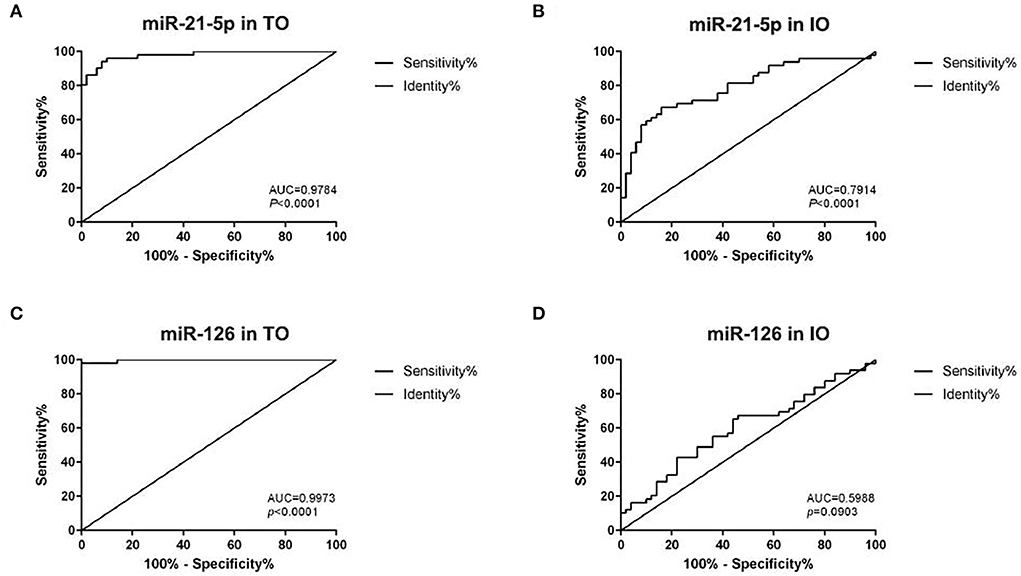
Figure 4. Receiver operating characteristic analysis of miR-21-5p and miR-126. The areas under the curves are 0.9784 (95% CI: 0.9558–1.001, p < 0.0001) for miR-21-5p in the infarct-related artery total occlusion (IR-ATO) acute myocardial infarction (AMI) group, (A) 0.7914 (95% CI: 0.7014–0.8814, p < 0.0001) for miR-21-5p in the IR blood-vessel recanalization (BVR) AMI group, (B) 0.9973 (95% confidence interval [CI]: 0.9913–1.003, p < 0.0001) for miR-126 in the IR-ATO AMI group, (C) 0.5988 (95% CI: 0.4866–0.7110, p = 0.0903) for miR-126 in the IR-BVR AMI group, and (D) VO = IR-ATO group, and VIO = IR-BVR group.
Discussion
It has been demonstrated that a reduction in myocardial blood perfusion and oxygen supply caused by coronary artery occlusion induces myocardial infarction (MI) (17). The presenting symptom of MI is chest pain or discomfort described as pain, pressure, tightness, heaviness, burning, or a squeezing or crushing sensation lasting 20 min or longer (18). In addition, the major pathological changes of MI include ischemia, inflammation, cardiomyocyte apoptosis, cardiac fibrosis, myocardial extracellular matrix lysis, and contractile dysfunction (19). Under the guidelines of the American College of Cardiology/American Heart Association, AMI covers three disorders: unstable angina, non-STEMI (formerly called non-Q wave MI), and STEMI (formerly called Q wave MI) (20). This article focuses on STEMI.
Circulating miRs are promising novel biomarkers for the diagnosis and prognosis of different types of cardiovascular diseases (21). Jaguszewski et al. (22) reported a signature of four circulating miRs as a robust biomarker to distinguish tako-tsubo syndrome from patients with AMI, which highlighted distinct characteristics of the miR profile in clinically indistinguishable cardiovascular diseases. Various miRs have been widely studied for their modulation of cardiomyocyte apoptosis, inflammation, angiogenesis, and fibrosis of AMI (23). Of these, miR-214 is highly expressed in the sera of elderly patients with AMI and may inhibit myocardial cell apoptosis by inhibiting the expression of miR-214 target genes, including the p53-upregulated modulator of apoptosis, PTEN, Bcl-2-associated X, and caspase 7 (24). The overexpression of miR-488-3p suppresses AMI-induced cardiomyocyte apoptosis by targeting zinc finger protein 791 (25). The downregulation of miR-130 expression alleviates AMI by targeting peroxisome proliferator-activated receptor-γ, which has a cardioprotective effect by inhibiting nuclear factor kappa-light-chain-enhancer of activated B cells-mediated inflammation and transforming growth factor beta 1-mediated fibrosis (26). The overexpression of miR-210 promotes angiogenesis in AMI by stimulating hepatocyte growth factor expression and inducing improved left ventricular remodeling (27). Furthermore, accumulated evidence has revealed the effectiveness of circulating miRs used as candidate biomarkers for the prognostic and diagnostic evaluation of AMI. Of these, miR-184 presents as a potential dynamic biomarker before and after percutaneous coronary intervention treatment for AMI (28). Peripheral blood miR-124 is accurate for the early diagnosis of AMI (29), and circulating miR-1 within 3 h of acute chest pain has potential prognostic and diagnostic value for AMI (30).
Both miR-126 and miR-21 are highly implicated in ischemic heart disease. Overexpression of miR-126 has been shown to protect hypoxic-reoxygenation-exposed human umbilical vein endothelial cellular injury (31). Jansen et al. reported that miR-126 correlates with ischemic heart disease in patients with coronary artery disease (32). Elevated miR-21 can be detected in patients with acute coronary syndrome who showed symptom onset within 3 h (33). The intracellular effects of miR-21 have also been shown to improve cardiac function post-AMI (34). In the present study, we delineated the difference in expression of miR-21-5p and miR-126 in patients with IR-ATO and IR-BVR. Both in the IR-ATO group and the vascular-incomplete group, circulating miR-21-5p was significantly upregulated when compared with the control group. In addition, when compared with the control group, the expression of miR-126 in patients with IR-ATO was higher, but no significant difference was found in patients with IR-BVR. The serum levels of cTn-I and CK-MB in patients with IR-ATO and IR-BVR were significantly higher than in the control group, and the expressions of miR-21-5p and miR-126 were strongly correlated with each other and with the levels of cTn-I, the levels of CK-MB, and the Gensini score separately. When compared with the control group, the ROC analysis revealed that the AUC for miR-21-5p and miR-126 in the IR-ATO group was significantly higher, miR-21-5p in the IR-BVR group was significantly higher, and miR-21-5p in the IR-BVR group showed no significant difference. This suggests that miR-21-5p is more accurate for diagnosing both IR-BVR and IR-ATO, and miR-126 is more accurate for diagnosing IR-ATO than IR-BVR.
The kinetics analysis of troponin showed that the concentration of troponin increased within 8 h after symptom onset in patients with acute myocardial infarction (35). The kinetics of circulating miRs during heart attack remains poorly understood. Few miR kinetics studies have been performed. We found a recent study showing that miR-1 and miR-133b were enriched in circulating microparticles (within a couple of hours) following primary percutaneous coronary intervention in a monophasic or biphasic pattern in patients with myocardial infarction (36). Whether miRs share the same kinetics patterns during heart attack warrants further investigation.
Despite these findings, the small sample size limits the usefulness of miR-21-5p and miR-126 as diagnostic biomarkers for patients with AMI and IR-ATO or IR-BVR. Large clinical studies are needed to confirm the results. In addition, we used only one housekeeping gene (U6) for analyzing the qPCR results. The use of other housekeeping genes will be needed to validate the current findings.
Conclusion
The present study found that miR-21-5p may be a promising diagnostic marker for patients with IR-ATO AMI and vascular-incomplete AMI, and miR-126 may be used for the diagnosis of IR-ATO AMI rather than vascular-incomplete AMI. These findings support further investigations and clinical use of circulating miR-21-5p and miR-126 as diagnostic biomarkers for IR-ATO AMI.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shanxi DAYI Hospital (SBQLL-2017-035). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conception and design of the research: X-lM and X-wL. Critical revision of the manuscript for intellectual content and obtaining financing: X-wL. Writing of the manuscript: X-lM. Statistical analysis: Z-jZ. Analysis and interpretation of the data: Y-pG. Acquisition of data: D-jH, ZX, and TL. All authors read and approved the final draft.
Funding
This work was funded by Shanxi Natural Science Foundation (Grant no. 201701D121148).
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. Ong SB, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek X-Y, Cabrera-Fuentes HA, et al. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. (2018) 186:73–87. doi: 10.1016/j.pharmthera.2018.01.001
3. Tilea I, Varga A, Serban RC. Past, present, and future of blood biomarkers for the diagnosis of acute myocardial infarction-promises and challenges. Diagnostics (Basel). (2021) 11:881. doi: 10.3390/diagnostics11050881
4. Zimetbaum PJ, Josephson ME. Use of the electrocardiogram in acute myocardial infarction. N Engl J Med. (2003) 348:933–40. doi: 10.1056/NEJMra022700
5. van der Linden N, Wildi K, Twerenbold R, Pickering JW, Than M, Cullen L, et al. Combining high-sensitivity cardiac troponin I and cardiac troponin T in the early diagnosis of acute myocardial infarction. Circulation. (2018) 138:989–99. doi: 10.1161/CIRCULATIONAHA.117.032003
6. Aydin S, Ugur K, Aydin S, Sahin I, Yardim M. Biomarkers in acute myocardial infarction: current perspectives. Vasc Health Risk Manag. (2019) 15:1–10. doi: 10.2147/VHRM.S166157
7. Ye XD, He Y, Wang S, Wong GT, Irwin MG, Xia Z. Heart-type fatty acid binding protein (H-FABP) as a biomarker for acute myocardial injury and long-term post-ischemic prognosis. Acta Pharmacol Sin. (2018) 39:1155–63. doi: 10.1038/aps.2018.37
8. Kraus D, von Jeinsen B, Tzikas S, Palapies L, Zeller T, Bickel C, et al. Cardiac troponins for the diagnosis of acute myocardial infarction in chronic kidney disease. J Am Heart Assoc. (2018) 7:e008032. doi: 10.1161/JAHA.117.008032
9. van Rooij E, Olson EN. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov. (2012) 11:860–72. doi: 10.1038/nrd3864
10. O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. (2010) 10:111–22. doi: 10.1038/nri2708
11. Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. (2011) 223:102–15. doi: 10.1002/path.2806
12. Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. (2018) 141:1202–7. doi: 10.1016/j.jaci.2017.08.034
13. Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, et al. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. (2009) 284:29514–25. doi: 10.1074/jbc.M109.027896
14. Liu Z, Wang H, Hou G, Cao H, Zhao Y, Yang B. Notoginsenoside R1 protects oxygen and glucose deprivation-induced injury by upregulation of miR-21 in cardiomyocytes. J Cell Biochem. (2019) 120:9181–92. doi: 10.1002/jcb.28194
15. Chen CH, Hsu SY, Chiu CC, Leu S. MicroRNA-21 Mediates the protective effect of cardiomyocyte-derived conditioned medium on ameliorating myocardial infarction in rats. Cells. (2019) 8. doi: 10.3390/cells8080935
16. Long G, Wang F, Duan Q, Chen F, Yang S, Gong W, et al. Human circulating microRNA-1 and microRNA-126 as potential novel indicators for acute myocardial infarction. Int J Biol Sci. (2012) 8:811–8. doi: 10.7150/ijbs.4439
17. Yao G, Su G, Li K, Li B, Dong H. Comparative study of ticagrelor and clopidogrel in therapeutic effect of acute myocardial infarction patients undergoing percutaneous coronary intervention. Saudi J Biol Sci. (2017) 24:1818–20. doi: 10.1016/j.sjbs.2017.11.020
18. Nagle B, Nee C. Recognizing and responding to acute myocardial infarction. Nursing. (2002) 32:50–4. doi: 10.1097/00152193-200210000-00045
19. Awada HK, Hwang MP, Wang Y. Towards comprehensive cardiac repair and regeneration after myocardial infarction: aspects to consider and proteins to deliver. Biomaterials. (2016) 82:94–112. doi: 10.1016/j.biomaterials.2015.12.025
20. Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc. (2009) 84:917–38. doi: 10.1016/S0025-6196(11)60674-5
21. Çakmak HA, Demir M. MicroRNA and cardiovascular diseases. Balkan Med J. (2020) 37:60–71. doi: 10.4274/balkanmedj.galenos.2020.2020.1.94
22. Jaguszewski M, Osipova J, Ghadri JR, Napp LC, Widera C, Franke J, et al. A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur Heart J. (2014) 35:999–1006. doi: 10.1093/eurheartj/eht392
23. Wu C, Liu B, Wang R, Li G. The regulation mechanisms and clinical application of MicroRNAs in myocardial infarction: a review of the recent 5 years. Front Cardiovasc Med. (2022) 8:809580. doi: 10.3389/fcvm.2021.809580
24. Yin Y, Lv L, Wang W. Expression of miRNA-214 in the sera of elderly patients with acute myocardial infarction and its effect on cardiomyocyte apoptosis. Exp Ther Med. (2019) 17:4657–62. doi: 10.3892/etm.2019.7464
25. Zheng HF, Sun J, Zou ZY, Zhang Y, Hou GY. MiRNA-488-3p suppresses acute myocardial infarction-induced cardiomyocyte apoptosis via targeting ZNF791. Eur Rev Med Pharmacol Sci. (2019) 23:4932–9. doi: 10.26355/eurrev_201906_18083
26. Chu X, Wang Y, Pang L, Huang J, Sun X, Chen X. miR-130 aggravates acute myocardial infarction-induced myocardial injury by targeting PPAR-gamma. J Cell Biochem. (2018) 119:7235–44. doi: 10.1002/jcb.26903
27. Fan ZG, Qu XL, Chu P, Gao YL, Gao XF, Chen SL, et al. MicroRNA-210 promotes angiogenesis in acute myocardial infarction. Mol Med Rep. (2018) 17:5658–65. doi: 10.3892/mmr.2018.8620
28. Liu ZH, Sun XP, Zhou SL, Wang HX. Research on the relations between the variation of miRNA-184 before and after treatment of acute myocardial infarction and prognosis. Eur Rev Med Pharmacol Sci. (2017) 21:843–7.
29. Guo ML, Guo LL, Weng YQ. Implication of peripheral blood miRNA-124 in predicting acute myocardial infarction. Eur Rev Med Pharmacol Sci. (2017) 21:1054–9. doi: 10.1016/j.jacc.2017.07.071
30. Su T, Shao X, Zhang X, Yang C, Shao X. Value of circulating miRNA-1 detected within 3h after the onset of acute chest pain in the diagnosis and prognosis of acute myocardial infarction. Int J Cardiol. (2019). doi: 10.1016/j.ijcard.2019.09.050
31. Sheikh MSA, Almaeen A, Alduraywish A, Alomair BM, Salma U, Fei L, et al. Overexpression of miR-126 protects hypoxic-reoxygenation-exposed HUVEC cellular injury through regulating LRP6 expression. Oxid Med Cell Longev. (2022) 2022:3647744. doi: 10.1155/2022/3647744
32. Jansen F, Schäfer L, Wang H, Schmitz T, Flender A, Schueler R, et al. Kinetics of circulating micro RNA s in response to cardiac stress in patients with coronary artery disease. J Am Heart Assoc. (2017) 6:e005270. doi: 10.1161/JAHA.116.005270
33. Oerlemans MI, Mosterd A, Dekker MS, de Vrey EA, van Mil A, Pasterkamp G, et al. Early assessment of acute coronary syndromes in the emergency department: the potential diagnostic value of circulating microRNAs. EMBO Mol Med. (2012) 4:1176–85. doi: 10.1002/emmm.201201749
34. Dai B, Wang F, Nie X, Du H, Zhao Y, Yin Z, et al. The cell type-specific functions of miR-21 in cardiovascular diseases. Front Genet. (2020) 11:563166. doi: 10.3389/fgene.2020.563166
35. Möckel M, Searle J. Copeptin-marker of acute myocardial infarction. Curr Atheroscler Rep. (2014) 16:421. doi: 10.1007/s11883-014-0421-5
Keywords: circulating miRNA, acute myocardial infarction, biomarker, microRNA-21-5p, microRNA-126
Citation: Mi X-l, Gao Y-p, Hao D-j, Zhang Z-j, Xu Z, Li T and Li X-w (2022) Prognostic value of circulating microRNA-21-5p and microRNA-126 in patients with acute myocardial infarction and infarct-related artery total occlusion. Front. Cardiovasc. Med. 9:947721. doi: 10.3389/fcvm.2022.947721
Received: 19 May 2022; Accepted: 26 August 2022;
Published: 18 October 2022.
Edited by:
Nazario Carrabba, Careggi Hospital, ItalyReviewed by:
Prima Hapsari Wulandari, Massachusetts General Hospital and Harvard Medical School, United StatesSalvatore De Rosa, Magna Græcia University, Italy
Copyright © 2022 Mi, Gao, Hao, Zhang, Xu, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue-wen Li, bGl4dWV3ZW5seHdAMTI2LmNvbQ==
 Xiao-long Mi
Xiao-long Mi Xue-wen Li
Xue-wen Li