- Department of Pediatric Intensive Care Unit, The First Hospital of Jilin University, Changchun, China
Primary cardiac tumors in children are exceedingly rare overall, which benign account for most part. The onset of the disease is occult, while the clinical manifestations are non-specific-patients may be asymptomatic or show a range of obstructive, arrhythmic, embolic or systemic symptoms. The clinical presentations generally depend on the tumors’ size, localization, and pace of growth of the tumor. Moreover, the diagnosis needs comprehensive judgment based on imaging results and pathological examination. With advances in cardiac imagining and the introduction of cardiopulmonary support, the diagnosis and treatment of these rare tumors have improved the prognosis and outlook for benign tumors. To sum up the above, we sought to integrate articles from recent years for the latest comprehensive review of the clinical manifestations, imaging characteristics, clinic pathologic features and treatment of benign cardiac tumors in children to provide a broader idea for pediatricians to recognize and treat such diseases.
Highlights
– In this review, we systematically summarize the clinical characteristics and auxiliary examination characteristics of benign cardiac tumors, which is particularly important for improving the clinical thinking of pediatric physicians
– Previously published literature on cardiac tumors has focused more on summarizing treatment approaches and survival times. We summarize the latest technical characteristics
– The critical point of this review is to improve the systematic understanding of pediatricians and medical technicians on benign cardiac tumors.
Key message
The clinician’s ability to recognize and diagnose the disease is critical in diagnosing and treatment of primary cardiac tumors in children. Using non-invasive examination and accurate judgment can reduce the misdiagnosis rate of children; reduce the psychological burden of invasive examination and family. Therefore, developing compassionate diagnosis and treatment methods is more important than treatment methods.
Background
Primary cardiac tumors are exceedingly rare in all age groups, with an approximate incidence of 0.0017–0.17% in autopsy series or during the clinical examination (1–3), it is difficult to obtain accurate statistics because of the unbalanced means of medical examination globally. Due to the development of non-invasive imaging modalities in recent years, the reports of primary cardiac tumors have gradually increased. Among these diagnosed cardiac tumors, benign tumors account for approximately 90% (3, 4). In a retrospective analysis by Morka et al. (1) in Poland, 95.1% of children diagnosed with a heart tumor were determined to be benign primary cardiac tumors in multiple centers over 15 years. Coincidentally, in another retrospective analysis by Wang et al. (5) it was mentioned that among the 52 children with primary cardiac tumors treated in the past 30 years in China, 48 (92.3%) cases were benign tumors. However, benign tumors can adversely affect children’s health, such as syncope, seizures, heart failure, arrhythmia and sudden death (5, 6). With the advancement of clinical diagnostic technology, the detection rate of children’s cardiac tumors has increased, and clinicians’ awareness of primary cardiac tumors has gradually improved. Based on the improvement of diagnostic technology for primary cardiac tumors in recent years. This systematic review aims to improve the thinking of pediatricians in the systematic diagnosis and treatment of children with primary benign cardiac tumors.
Nomenclature and classification
The current nomenclature and classification of cardiac tumors mainly adopt the World Health Organization (WHO) classification of cardiac tumors (Fourth Edition) (7). Unlike the incidence in adults, the most frequent tumors reported in children were rhabdomyoma, followed by fibroma, myxoma, teratoma, and hemangioma (1, 4, 8). When analyzing the age-adjusted prevalence of tumors, rhabdomyoma, hemangioma, and fibroma were more common among infant, while myxoma was more common among children over 1 year old (8).
Clinical manifestation
Children with cardiac tumors can be consists of non-specific symptoms-just like fever, and anemia, which make the diagnosis challenging, or present with a variety of clinical traits depending on the tumors’ size, localization, and pace of growth (9, 10). The clinical presentation generally does not depend on the histopathology of the tumor, so there was no significant difference in mass effect between benign and malignant cardiac tumors. Tumors can occur in the myocardium, atrium (chamber) cavity, and heart valve. When the tumor is small, there may be no apparent symptoms; when the tumor is massive, the obstruction is severe and narrowing of the cardiac chamber. The extensive tumor infiltration into the ventricular wall will decrease the effective myocardial beat, resulting in weakened cardiac systolic and diastolic functions. Arrhythmias may occur when tumors involve the conduction system, including premature beats, supraventricular tachycardia, pre-excitation syndrome, bundle branch block, etc.; Among these, atrioventricular block and ventricular tachycardia are not uncommon and may even be related to sudden death. When the tumor is located around the biventricular inflow (outlet) tract, it may cause obstruction, and the blood flow velocity increases at the location of the obstruction, resulting in a heart murmur. Atrial mass is more likely to block the flow of atrioventricular blood flow than ventricular mass, similar to valve stenosis, and ventricular mass blocking the outflow tract may lead to chest pain, dyspnea, and syncope. In addition, tumor infiltration of surrounding organs or tissues may cause the corresponding dysfunction. It should be emphasized that cardiac myxomas that easily dislodge fragments in older patients may cause multi-organ embolism (11–13). The possible clinical manifestations of each tumor will be discussed in detail.
Diagnostic approach
All examination methods are widely used in adult patients or older children. At present, the diagnosis of cardiac tumors still mainly relies on echocardiography. Most clinical reports mentioned that echocardiography was complemented with magnetic resonance imaging, computed tomography, and histopathological examination for diagnosing cardiac tumors. Although percutaneous or catheter biopsy can directly determine the histological type, its was not suitable for pediatric patients due to its shortcomings, such as invasiveness and poor repeatability (Table 1). It is recommended to explore new methods suitable for children-specific examinations in the process of diagnosis and operation. A complete and consistent examination and high-quality imaging are essential for improving diagnostic accuracy. The tumor type and mass effect should be clarified for children with suspected cardiac tumors to guide the follow-up treatment.
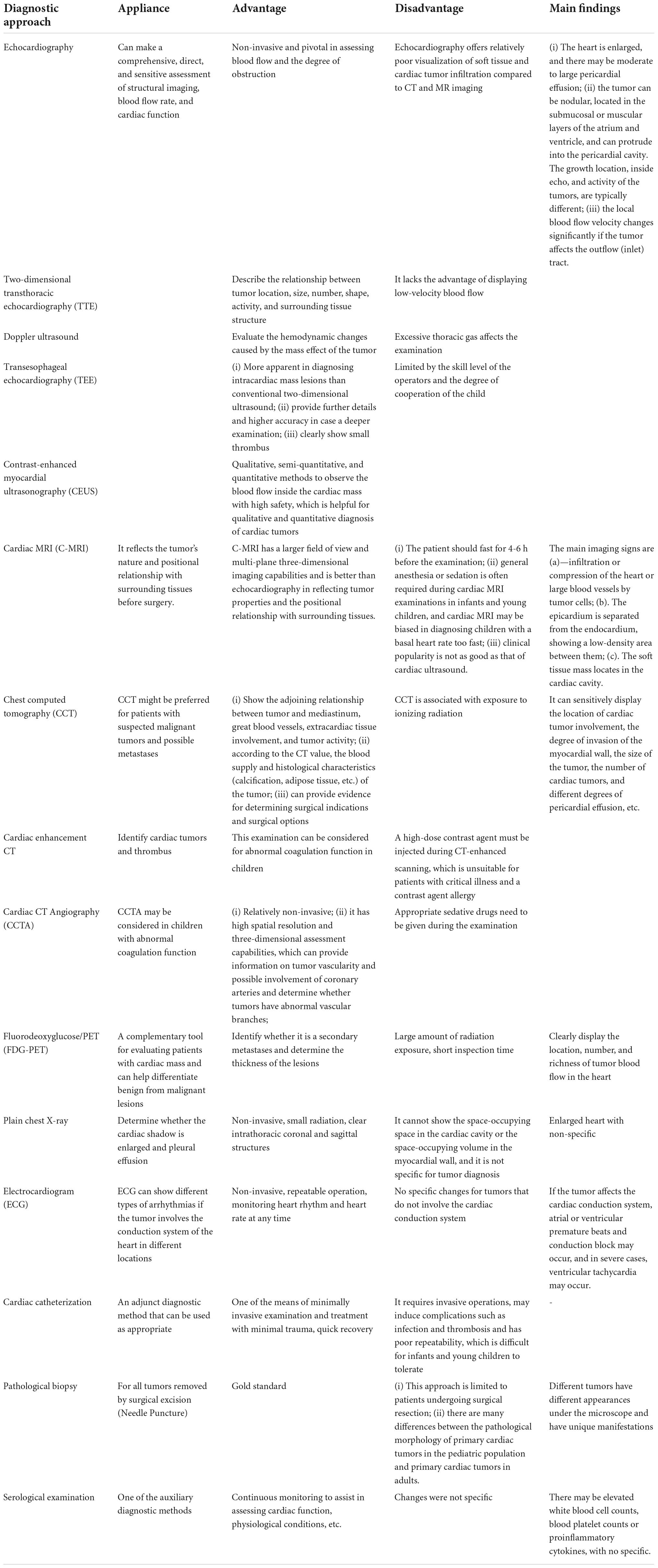
Table 1. Outline advantages/disadvantages, indications, and main findings of different investigative modalities.
Echocardiography
Workup of a suspected cardiac tumor should begin with echocardiography since it is non-invasive and pivotal in assessing blood flow and the degree of obstruction. Echocardiography has high value for diagnosing cardiac tumors-can make a comprehensive, direct, and sensitive assessment of structural imaging, blood flow rate, and cardiac function, where it is the most frequent imaging modality employed (14), so its application runs through the entire treatment and follow-up process of children with cardiac tumors. The main imaging findings under ultrasound: (i) The heart is enlarged, and there may be moderate to large pericardial effusion; (ii) the tumor can be nodular, located in the submucosal or muscular layers of the atrium and ventricle, and can protrude into the pericardial cavity. The growth location, inside echo, and activity of the tumors, are typically different (Figure 1); (iii) the local blood flow velocity changes significantly if the tumor affects the outflow (inlet) tract.
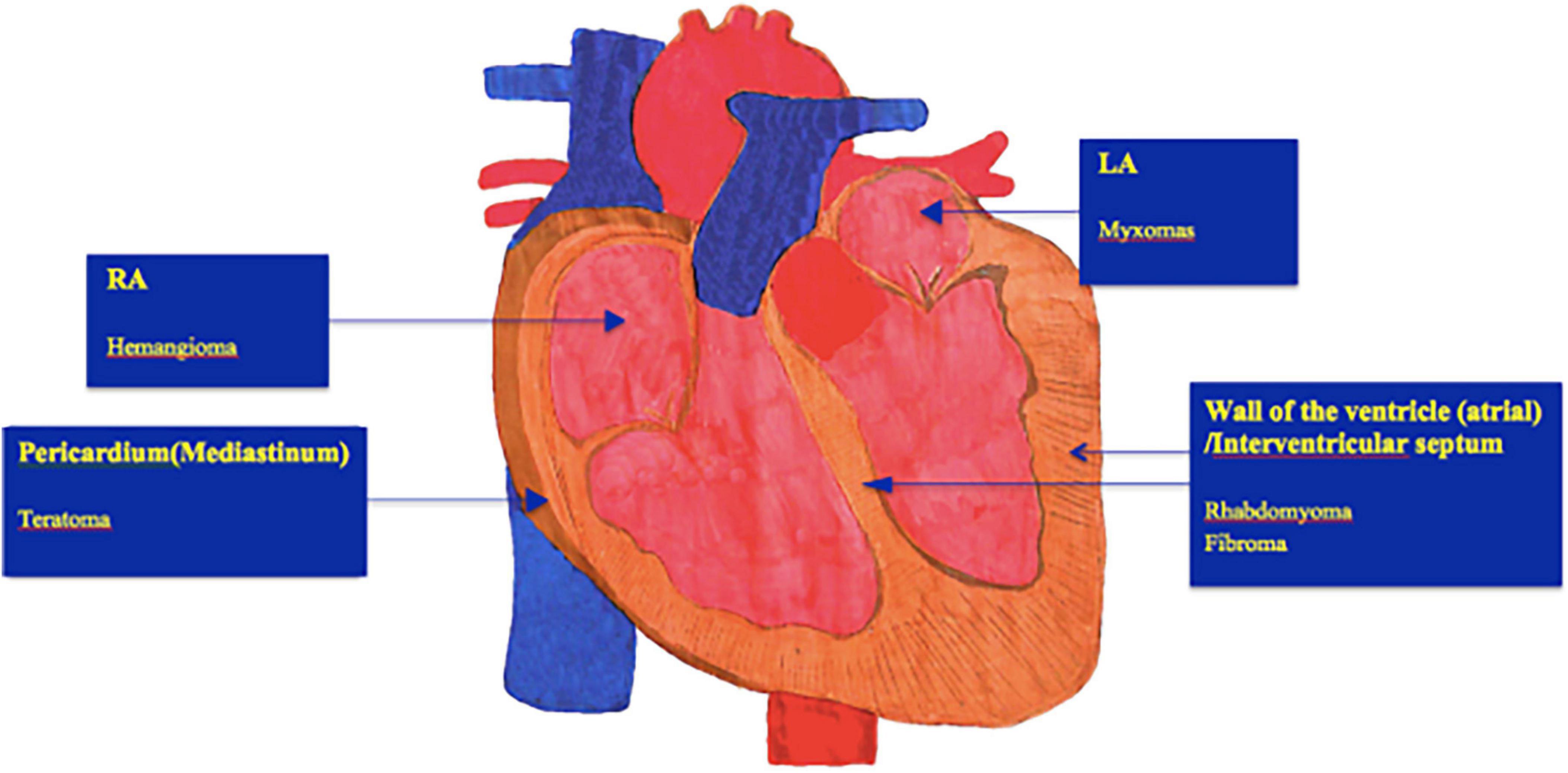
Figure 1. Show typical locations of different subtypes of benign cardiac tumors. However, many heart tumors can occur in any chamber. LA, Left atrium; RA, Right atrium.
Two-dimensional transthoracic echocardiography (TTE) can accurately describe the relationship between tumor location, size, number, shape, activity and surrounding tissue structure, but it lack the advantage of displaying low-velocity blood flow. If the tumor is located in the pericardium, once pericardial effusion occurs, the tumor needs to be differentiated from the extrusion of extra-pericardial lesions (15). Doppler ultrasound can evaluate the hemodynamic changes caused by the mass effect of the tumor; transesophageal echocardiography (TEE) is more apparent in diagnosing intracardiac mass lesions than conventional two-dimensional ultrasound (16). TEE can provide further details and higher accuracy in case a deeper examination of the mass is needed. Moreover, TEE can also clearly show small thrombus that TTE cannot. However, its application in the pediatric patient group is limited by the skill level of the operators (17) and the degree of cooperation of the child; In addition, echo contrast agents are helpful to confirm the presence of an intracardiac mass and to characterize it further under the extent of contrast enhancement. There are qualitative, semi-quantitative, and quantitative methods to observe the blood flow inside the cardiac mass by contrast-enhanced myocardial ultrasonography (CEUS), with high safety (16, 18), which is helpful for qualitative and quantitative diagnosis of cardiac tumors. It has a high application value in diagnosing cardiac tumors (19). It is emphasized that echocardiography offers relatively poor visualization of soft tissue and cardiac tumor infiltration compared to CT and MR imaging. So, if it is difficult to determine the nature of the mass under ultrasonic cardiogram (UCG), further cardiac magnetic resonance imaging (MRI) or cardiac contrast-enhanced CT can be considered for supplementary diagnosis. Therefore, CT and MRI are increasingly important in diagnosing, characterizing, and planning treatment strategies for cardiac tumors.
Cardiac magnetic resonance imaging
MRI is the most sensitive imaging technique. With the continuous development of new MRI technologies, various scanning sequences have been gradually applied in clinical practice. These technologies include 2D and 3D SSFP sequences and ECG-gated spin-echo T1W SEEPI sequences, significantly improving the time of MRI images and spatial resolution, multi-directional display of cardiac tumor morphology and its impact on cardiac function, which is of great significance for the clinical diagnosis of cardiac tumors. C-MRI has a larger field of view and multi-plane three-dimensional imaging capabilities and is better than echocardiography in reflecting tumor properties and the positional relationship with surrounding tissues (20, 21). The main imaging signs are: (a) Infiltration or compression of the heart or large blood vessels by tumor cells. (b) The epicardium is separated from the endocardium, showing a low-density area between them. (c) The soft tissue mass locate in the cardiac cavity.
A multicenter retrospective study by Beroukhim et al. (22) concluded that cardiac MRI has a 97% accuracy in predicting tumor histology, which is critical in establishing the best-individualized treatment plan and determining the need for surgical intervention. Thence, tissue characterization of the tumor by cardiac MRI is strongly recommended before cardiac surgery (23). However, the patient should fast for 4–6 h before the examination, general anesthesia or sedation is often required during cardiac MRI examinations in infants and young children, and cardiac MRI may be biased in diagnosing children with a basal fast heart rate. Clinical popularity is not as good as that of cardiac ultrasound.
Chest computed tomography
Chest CT can see the space-occupying lesions in the heart, which has a definite diagnosis and differential diagnosis significance and can show the adjoining relationship between tumor and mediastinum, great blood vessels, extracardiac tissue involvement, and tumor activity. According to the CT value, the blood supply and histological characteristics (calcification, adipose tissue, etc.) of the tumor can also be inferred to provide better guidance for clinical treatment. Whereas CT is associated with exposure to ionizing radiation, it might be preferred for patients with suspected malignant tumors and possible metastases. This examination can provide evidence for determining surgical indications and surgical options.
Cardiac enhancement CT is mainly used to identify cardiac tumors and thrombus. This examination can be considered for abnormal coagulation function in children (24). However, a high-dose contrast agent must be injected during CT-enhanced scanning, which is unsuitable for patients with critical illness and a contrast agent allergy (25). Cardiac CT Angiography (CCTA) is relatively non-invasive. It has high spatial resolution and three-dimensional assessment capabilities, which can provide information on tumor vascularity and possible involvement of coronary arteries and determine whether tumors have abnormal vascular branches. During this examination, appropriate sedative drugs need to be given. When the child is relatively stable, the pediatrician injects the contrast agent into the child’s cubital vein, and the radiologist uses the obtained original slices to perform multiplanar reconstruction (MPR), maximum intensity projection (MIP), minimum density projection (Min IP). Thin-layer reconstruction, volume rendering (VR), and other techniques for image post-processing can further detect intracardiac malformations and extracardiac vascular abnormalities. For children who cannot tolerate long-term sedation, C-MRI examination, CCTA combined with echocardiography can also make a systematic judgment for the disease. In addition, the results of CCTA examination are more meaningful in differentiating cardiac tumors and thrombus. CCTA may be considered in children with abnormal coagulation function.
Fluorodeoxyglucose/PET
In recently years, FDG-PET has been a complementary tool for evaluating patients with cardiac mass and can help differentiate benign from malignant lesions (26). However, this examination is only an optional auxiliary examination, and its clinical application is not common in some areas.
Plain chest X-ray
This examination can determine whether the cardiac shadow is enlarged and pleural effusion. However, it cannot show the space-occupying space in the cardiac cavity or the space-occupying volume in the myocardial wall, and it is not specific for tumor diagnosis.
Electrocardiogram
ECG shows no specific changes. However, if the tumor affects the cardiac conduction system, atrial or ventricular premature beats and conduction block may occur, and in severe cases, ventricular tachycardia may occur.
Cardiac catheterization
Cardiac catheterization is one of the main techniques for detecting heart disease (27). However, it requires invasive operations, may induce complications such as infection and thrombosis and has poor repeatability, which is difficult for infants and young children to tolerate, so it limits its use in infants and young children.
Pathological biopsy
Though visualization methods have improved continuously, the most vital part of an assessment of prognosis is a histopathological analysis of the tumors, which remains the gold standard. Nevertheless, this approach is limited to patients undergoing surgical resection. There are many differences between the pathological morphology of primary cardiac tumors in the pediatric population and primary cardiac tumors in adult (28).
Serological examination
There are elevated white blood cell counts and blood platelet counts in laboratory tests. It is common for heart tumors to increase the levels of proinflammatory cytokines (interleukin 6, especially in myxoma) (1). However, these changes were not specific.
Characteristics of common primary cardiac tumors in children
Each type of tumor has its specificity, and we have made a summary according to the frequency of tumor occurrence (Table 2).
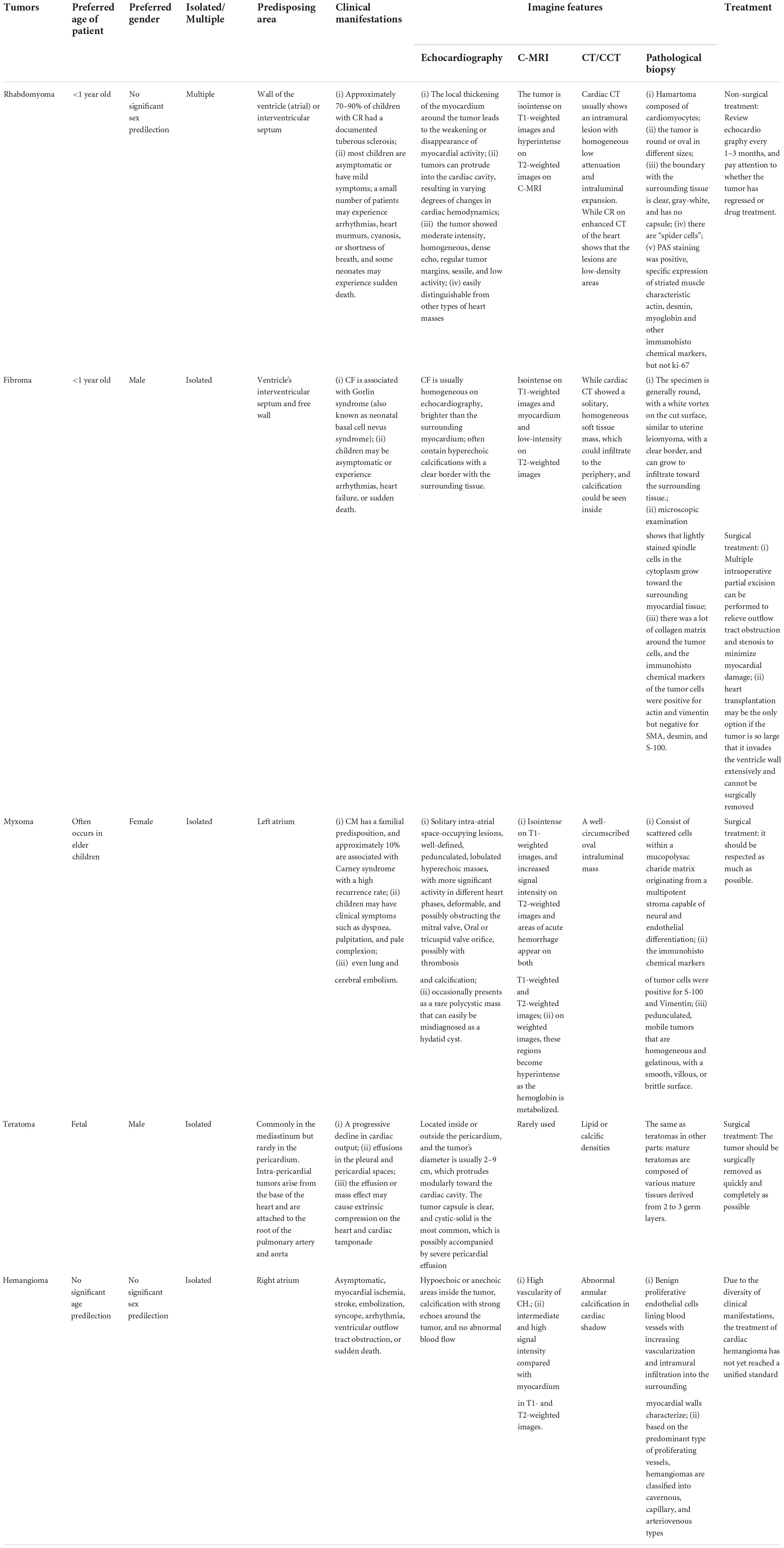
Table 2. Characteristics, imaging results, and treatment options of different benign cardiac tumors.
Cardiac rhabdomyoma
CR occurs almost exclusively in children, mainly before age 1, and can also be found in fetus, with no significant sex predilection (2, 4, 10). In a retrospective analysis by Słowińska et al. (29) CR was detected prenatally in 71/100 cases (71%), and 82/100 cases (82%) were diagnosed within 4 months after birth. Approximately 70–90% of children with CR had a documented tuberous sclerosis (TSC) diagnosis in recent years (29–31), also called Bourneville disease. The discovery of CR can guide the early diagnosis of TSC; in other words, a high level of clinical suspicion of CR should be maintained when a child is diagnosed with TSC. Tumors seen on echocardiography are primarily found in the ventricular wall and interventricular septum, but also the atrial wall and involving valves. The local thickening of the myocardium around the tumor leads to the weakening or disappearance of myocardial activity, and tumors can protrude into the cardiac cavity, resulting in varying degrees of changes in hemodynamics and affecting cardiac systolic and diastolic function. The tumor presents with moderate intensity, homogeneous, and dense echoes, with regular tumor margins, sessile, and low activity; multiple tumors are a prominent feature of CR, and it is not difficult to distinguish them from other types of cardiac mass under ultrasound. It is easier to diagnose CR if there is a history of TSC; Cardiac CT usually shows an intramural lesion with homogeneous low attenuation and intraluminal expansion. While CR on enhanced CT of the heart shows that the lesions are low-density areas, C-MRI isointense on T1-weighted images and hyperintense on T2-weighted images. Histopathologically, it is a hamartoma composed of cardiomyocytes. The tumor body varies in size and is round or oval. It has a clear boundary with the surrounding tissue, is grayish-white, and has no capsule. There are “spider cells” in some areas conducive to disease diagnosis. At the same time, cardiac rhabdoid tumor cells were positive by PAS staining and specifically expressed rhabdoid characteristic actin, desmin, myoglobin, and other immunohistochemical markers but did not express ki-67. Most children with CR have asymptomatic or mild symptoms, and most of them are found during the physical examination; a few patients will have clinical manifestations such as arrhythmia, heart murmur, cyanosis, or shortness of breath, and very few may also experience sudden death, which is more common in neonates.
Cardiac fibroma
CF is children’s second most common benign cardiac tumor after CR, and is also more common in infants (32, 33). Male has a slight predominance, and usually single tumor. CF is associated with Gorlin syndrome (also known as neonatal basal cell nevus syndrome) (34), an autosomal dominant disorder caused by mutations in the PTCH1 gene. CF is usually homogeneous on echocardiography, brighter than the surrounding myocardium, often contain hyperechoic calcifications, and is usually single tumors with a clear border with the surrounding tissue. The tumors prefer to be located in the ventricle’s interventricular septum and free wall, and fewer occur in the atrium. While cardiac CT showed a solitary, homogeneous soft tissue mass, which could infiltrate to the periphery, and calcification could be seen inside, CF in MRI showed isointense on T1-weighted images and myocardium and low-intensity on T2-weighted images. Markedly enhancing borders and hypointense in the core are characteristic signs of fibroids. The specimen is generally round, with a white vortex on the cut surface, similar to uterine leiomyoma, with a clear border, and can grow to infiltrate toward the surrounding tissue. Microscopic examination shows that lightly stained spindle cells in the cytoplasm grow toward the surrounding myocardial tissue. There was a lot of collagen matrix around the tumor cells, and the immunohistochemical markers of the tumor cells were positive for actin and vimentin but negative for SMA, desmin, and S-100. CF may occupy any heart structure and were primarily seen to grow with a predisposition to the ventricles. The tumor bulk is intramural, occupying the chamber cavity, but its margins were found to interdigitate with ventricular muscles, replacing the functional muscle mass. In this way, they can extend into the ventricular conduction system, interfere with electrical conduction, and cause arrhythmia. Boston Children’s Hospital reviewed 40 years of experience treating primary cardiac tumors and found that ventricular arrhythmias in patients with CF were about 64%, and the recurrence rate of arrhythmias after tumor resection was low (35). Since the base of a CF tumor often infiltrates the free wall of the ventricle and occupies most of the cardiac chambers, it can cause a decrease in the adequate cardiac chamber volume and myocardial contractility, leading to congestive heart failure.
Cardiac myxoma
CM is another one of the typical primary cardiac tumors in children (36). Dr. Ding’s study (4) reviewed a single-institute 12 years of experience, which showed myxoma often occurs in elder children. CM has a familial predisposition, and approximately 10% are associated with Carney syndrome with a high recurrence rate (37, 38). There is predominance in female, with the incidence ranging from 1.5 to 2 times that in men (39). About 80% of myxomas originate from the left atrium; (1, 40, 41) the rest are primarily located in the right atrium, and the ventricle is rare, accounting for 3–4% and 8% of the left and right ventricles, respectively. Echocardiography is the primary test for diagnosing CM with high accuracy. Ultrasonographic manifestations of CM are solitary intra-atrial space-occupying lesions, well-defined, pedunculated, lobulated hyperechoic masses, with more significant activity in different heart phases, deformable, and possibly obstructing the mitral valve, Oral or tricuspid valve orifice, possibly with thrombosis and calcification. Occasionally presents as a rare polycystic mass that can easily be misdiagnosed as a hydatid cyst; Due to the unique advantages of color ultrasound diagnosis, cardiac CT and cardiac MRI are less used in clinical practice. Contrast-enhanced cardiac CT may present as a well-circumscribed oval intraluminal mass; on MRI, CM is usually isointense on T1-weighted images, and increased signal intensity on T2-weighted images and areas of acute hemorrhage appear on both T1-weighted and T2-weighted images. On weighted images, these regions become hyperintense as the hemoglobin is metabolized. Histologically, tumors consist of scattered cells within a mucopolysaccharide matrix originating from a multipotent stroma capable of neural and endothelial differentiation. The immunohistochemical markers of tumor cells were positive for S-100 and Vimentin. Typical CM is pedunculated, mobile tumors that are homogeneous and gelatinous, with a smooth, villous or brittle surface. The effect of the so-called “wrecking ball” is known. CM often prolapses to varying degrees to the atrioventricular valve opening, causing symptoms and signs of atrioventricular valve stenosis and insufficiency. Children may have clinical symptoms such as dyspnea, palpitation, and pale complexion (1, 3, 41); when heart tumor fragments fall off, lung and cerebral embolism may occur, manifested as limb hemiplegia, impaired speech, etc. Individual children may have acute cerebral embolism as the first presentation (39, 42). Symptoms in some children were abrupt, intermittent, and position-related. Because of the different clinical characteristics of CM, the misdiagnosis rate is high.
Several other rare primary cardiac tumors
(i) Cardiac Teratoma: Myocardial germ cell tumors are rare, with scattered reports (43). More than half are diagnosed in utero, most of the remainder in children under 15 years of age. There is a slight predominance in males. Teratomas are more commonly reported in the mediastinum, but rarely in the pericardium. Intra-pericardial teratomas arise from the base of the heart and are attached to the root of the pulmonary artery and aorta, most of which are mature type (44). Ultrasonographic manifestations show that teratoma can be located inside or outside the pericardium, and the tumor’s diameter is usually 2–9 cm, which protrudes modularly toward the cardiac cavity, The tumor capsule is clear, and cystic-solid is the most common, which is possibly accompanied by severe pericardial effusion; Lipid or calcific densities can also be present on CT, which often provide valuable clues to the diagnosis. The pathological manifestations are the same as teratomas in other parts: mature teratomas are composed of various mature tissues derived from 2 to 3 germ layers. Tumor growth was extraordinarily rapid and associated with a progressive decline in cardiac output (45). Rupture of the cyst in the pericardium leads to the development of effusions in the pleural and pericardial spaces. The effusion or mass effect may cause extrinsic compression on the heart and cardiac tamponade. (ii) Cardiac Hemangioma (CH): CH accounts for 2–3% of the primary cardiac tumors with no significant sex predilection (46). The right atrium is the most common location for fetal and neonatal CH, whereas the left ventricle is the most common site for CH in the adulthood (46, 47). Imaging is helpful in the preoperative screening and diagnosis of CH. Plain chest X-ray and electrocardiography are normal or non-specific in most of cases. Abnormal annular calcification in cardiac shadow is occasionally seen on chest X-ray or cardiac CT. Echocardiography enables real-time observation of CH, which showed that there were hypoechoic or anechoic areas inside the tumor, calcification with strong echoes around the tumor, and no abnormal blood flow. MRI is good at describing the high vascularity of CH. Typically, CH shows intermediate and high signal intensity compared with myocardium in T1- and T2-weighted images. In addition, histopathology is still the gold standard for diagnosing cardiac hemangioma. Histopathologic features of CH are identical to those of hemangiomas elsewhere in the body. Examination shows that benign proliferative endothelial cells lining blood vessels with increasing vascularization and intramural infiltration into the surrounding myocardial walls characterize. Based on the predominant type of proliferating vessels, hemangiomas are classified into cavernous, capillary, and arteriovenous types (48). Hemangioms are histopathologically benign and clinically dangerous tumors with various presentations, including asymptomatic, myocardial ischemia, stroke, embolization, syncope, arrhythmia, ventricular outflow tract obstruction, or sudden death.
Choice of treatment mode
There is still no unified standard for surgical correction of primary cardiac tumors in infants and young children. Treatment options for pediatric primary cardiac tumors should be performed individually whether children with primary cardiac tumors need surgery and the choice of surgery methods and timing need to be comprehensively dependent mainly on the size, location, nature of the tumor, and its impact on hemodynamics. In the case of benign tumors, the most commonly reported indication for surgical intervention was “clinical symptomatology,” which usually representing severe obstruction with hemodynamic compromise or arrhythmias, such as significant blood flow disorder, hypotension, the need for inotropic drugs, or even unable to escape ECMO treatment.
Non-surgical treatment
When the cardiac tumor is small in size, slow in growth, and does not affect the vital signs of the child, regular follow-up can be performed, and echocardiography can be reviewed in 1–3 months to evaluate the cardiac function of the child and monitor the changes of the tumor. For example, CR cells have no mitotic ability, so some cases can spontaneously regress with the prolongation of the disease course (49, 50), and drug therapy (such as Rapamycin) may reduce the tumor, so the current treatment for CR is symptomatic drug therapy. If there is no continuous remission, consider complete or partial tumor resection by surgery.
Surgical treatment and surgical approach
Surgery is the traditional treatment method, but the indications for surgery need to be carefully grasped. The principle purpose of surgical treatment is to restore normal hemodynamics and protect important structures and cardiac tissue. When surgical intervention is deemed necessary, the appropriate surgical approach selection depends on the tumor’s location. It aims for an adequate exposure that would permit a complete resection with adequate margins and minimal disruption of healthy cardiac tissue and anatomy. It is worth noting that compared with adults, infants and young children have smaller and thinner heart walls, increased load after tumor resection, decreased contractility, and are more prone to hemodynamic complications. Therefore, the surgical plan needs to be individually formulated (4, 51); Gentle manipulation of the heart is required during the procedure to prevent the mass from breaking up and embolizing. Tumors should be resected through the natural channel whenever possible, avoiding ventricular incision, especially the left ventricle. This minimizes myocardial damage, avoids damage to coronary arteries, valves, and conduction systems. In recent years, thoracoscopy and small-incision cardiac surgery have gradually matured, significantly reducing the trauma of children. Patients with large tumors may require heart transplantation (52).
For instance, CF is generally not likely to regress spontaneously (51) and require a limited period of surgical treatment; the base of CF is often broad, and the boundary with the myocardial tissue is unclear. Multiple intraoperative partial excision can be performed to relieve outflow tract obstruction and stenosis to minimize myocardial damage. Heart transplantation may be the only option if the tumor is so large that it invades the ventricle wall extensively and cannot be surgically removed (53); CM is associated with a high risk of embolism and is prone to recurrence, so it should be respected as much as possible. Most CMs are pedunculated, have clear borders, and are easy to distinguish from normal myocardial tissue, and most of them can be wholly resected (54); teratoma proliferates and has apparent compression to the heart. Once found, it should be surgically removed as soon as possible (45), and some cases were reported underwent open fetal surgery. At the same time, complete surgical resection is the preferred treatment. Chemotherapy and radiotherapy are not very useful in teratoma. Due to the diversity of clinical manifestations, the treatment of cardiac hemangioma has not yet reached a unified standard; accurate localization of the tumor location before surgery is crucial (47). Surgical treatment of atrial hemangioma is the best, the recurrence rate is low, and the patient’s symptoms are significantly improved or completely relieved (55).
Surgical results
Good results may be obtained after complete resection of early benign cardiac tumors with a low recurrence rate. Postoperative complications are mainly cardiac insufficiency (low cardiac output), infection, and damage to adjacent structures or nerves. Although surgical treatment carries a high risk of death and postoperative complications in the perioperative period, most patients eventually survive. The patient survived well at postoperative follow-up. Death and postoperative complications tend to appear in younger children (23). Therefore, when conditions permit, younger children should be treated conservatively and followed close until there is sufficient cardiac reserve to tolerate surgery.
Precautions after operation
However, most tumors cannot be completely removed (10, 46, 51). For patients with incomplete resection, dynamic echocardiography is required to monitor whether there is recurrence or whether the residual tumor volume continues to grow, hemodynamic changes, and myocardial beating. If there is a transient low output state of low cardiac output, continuous blood purification (CBP) or extracorporeal membrane oxygenation (ECMO) can be applied to improve perfusion.
Outcomes and follow-up
A higher percentage of tumor relapse was noted for myxoma and teratoma (4, 8). In recent years, literature reports have shown that with the advancement of medical technology, the detection rate and successful treatment rate of cardiac tumors have increased year by year, and surgical techniques have been improved. The patients’ quality of life has improved.
Conclusion
Most primary cardiac tumors in children are benign; most were diagnosed in infancy, with various clinical manifestations: dyspnea and chest pain are reported among the most common symptoms. There are many types of tumors, all of which have specific typical imaging and pathological features. Although echocardiography has provided a consistent assessment of anatomy and function, CT and MRI currently allow more extensive diagnoses. Diagnosing a heart tumor in children is not synonymous with a fatal prognosis; complete surgical resection is the most valuable treatment. Ensuring cardiac function during surgery and long-term follow-up has a good prognosis.
Author contributions
CQ-S conceptualized the study, drafted the initial manuscript, and reviewed and revised the manuscript. CF-Y and YK-C collected literatures. YM-L critically reviewed the manuscript. All authors have read and approved the final manuscript as submitted, agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investing and resolved.
Funding
This study was supported by the Department of Science and Technology of Jilin Province (Grant No. 20210204134YY).
Acknowledgments
We want to thank Yuanyuan Wang from the Pediatric Ultrasound Department of the First Hospital of Jilin University for her guidance in the relevant professional parts during the writing of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CBP, Continuous blood purification; CCT, Chest computed tomography; CCTA, Cardiac CT angiography; CEUS, Contrast-enhanced myocardial ultrasonography; CF, Cardiac Fibromas; CH, Cardiac Hemangioma; CM, Cardiac Myxomas; C-MRI, Cardiac magnetic resonance imaging; CR, Cardiac Rhabdomyoma; ECG, Electrocardiogram; ECMO, Extracorporeal membrane oxygenation; FDG-PET, Fluorodeoxyglucose/PET; MIP, Maximum intensity projection; Min IP, Minimum density projection; MPR, Multi-plane reconstruction; TEE, Transesophageal echocardiography; TTE, Transthoracic echocardiography; TSC, Tuberous sclerosis; UCG, Ultrasonic cardiogram; VR, Volume rendering; WHO, World Health Organization.
References
1. Morka A, Kohut J, Radzymińska-Chruściel B, Mroczek T, Gładki M, Weryński P, et al. Echocardiography and newer imaging techniques in diagnosis and long-term follow-up of primary heart tumors in children. Int J Environ Res Public Health. (2020) 17:5471. doi: 10.3390/ijerph17155471
2. Yuan SM. Fetal primary cardiac tumors during perinatal period. Pediatr Neonatol. (2017) 58:205–10. doi: 10.1016/j.pedneo.2016.07.004
3. Lu X, Liu Y, Liu Q, Liu D, Cui L, Zhu Q, et al. Diagnosis and treatment of primary cardiac tumors in pediatric patients. Chin J Appl Clin Pediatr. (2021) 36:33–5. doi: 10.3760/cma.j.cn101070-20190415-00319
4. Ding P, Qi J, Mo R, Sun J, Pen W, Wu K, et al. Clinical treatment of pediatric primary cardiac tumors: a single-institute 12-year experience. J Pediatr Hematol Oncol. (2020) 42:488–94. doi: 10.1097/MPH.0000000000001520
5. Wang, H, Li L, Lue F, Song L, Ruan Y, Zhao H, et al. Clinical-pathological analysis of primary cardiac neoplasms in 52 cases of children and adolescents: a report of 52 cases. Chin J Practi Pediatr. (2008) 2:125–9. doi: 10.3969/j.issn.1005-2224.2008.02.015
6. Shi L, Wu L, Fang H, Han B, Yang J, Ma X, et al. Identification and clinical course of 166 pediatric cardiac tumors. Eur J Pediatr. (2017) 176:253–60. doi: 10.1007/s00431-016-2833-4
7. Burke A, Tavora F. The 2015 WHO classification of tumors of the heart and pericardium. J Thorac Oncol. (2016) 11:441–52. doi: 10.1016/j.jtho.2015.11.009
8. Svobodov AA, Glushko LA, Ergashov AY. Surgical treatment of primary cardiac tumors in children systematic review and meta-analysis. Pediatr Cardiol. (2022) 43:251–66. doi: 10.1007/s00246-022-02814-2
9. Siurana JM, Fernández J, Navarro A, Akel G, Abella RF, Albert DC. A venous malformation: an unusual primary cardiac tumor in children. Ann Thorac Surg. (2019) 108:e325–7. doi: 10.1016/j.athoracsur.2019.02.047
10. Kwiatkowska J, Wałdoch A, Meyer-Szary J, Potaż P, Grzybiak M. Cardiac tumors in children: a 20-year review of clinical presentation, diagnostics and treatment. Adv Clin Exp Med. (2017) 26:319–26. doi: 10.17219/acem/62121
11. Shabab S, Erfanzadeh M, Ahmadian S, Mahmoudabady M, Mazloum N. A case report of left atrial myxoma presenting with amnesia. BMC Cardiovasc Disord. (2021) 21:225. doi: 10.1186/s12872-021-02036-z
12. Liu Y, Wang J, Guo L, Ping L. Risk factors of embolism for the cardiac myxoma patients: a systematic review and metanalysis. BMC Cardiovasc Disord. (2020) 20:348. doi: 10.1186/s12872-020-01631-w
13. Szerszyńska A, Nowak R, Łaskawski G, Fijałkowski M. Recurrent pneumonia and pulmonary embolism in a young patient as a presentation of right ventricular myxoma. Kardiol Pol. (2019) 77:63. doi: 10.5603/KP.2019.0008
14. Ma H, Niu Y, Tian M, Liu L, Gong W, Zheng M. A study of 399 cardiac tumors: characteristics of echocardiography and pathological features. Echocardiography. (2022) 39:37–45. doi: 10.1111/echo.15249
15. Grant MD, Mann RD, Kristenson SD, Buck RM, Mendoza JD, Reese JM, et al. Transthoracic echocardiography: beginner’s guide with emphasis on blind spots as identified with CT and MRI. Radiographics. (2021) 41:1022–42. doi: 10.1148/rg.2021200142
16. Ren M, Huang L, Ye X, Xv Z, Ouyang C, Han Z. Evaluation of cardiac space-occupying lesions by myocardial contrast echocardiography and transesophageal echocardiography. J Healthc Eng. (2022) 2022:2066033. doi: 10.1155/2022/2066033
17. Johri AM, Picard MH, Newell J, Marshall JE, King ME, Hung J. Can a teaching intervention reduce interobserver variability in LVEF assessment: a quality control exercise in the echocardiography lab. JACC Cardiovasc Imaging. (2011) 4:821–9. doi: 10.1016/j.jcmg.2011.06.004
18. Porter TR, Xie F. Contrast echocardiography: latest developments and clinical utility. Curr Cardiol Rep. (2015) 17:569. doi: 10.1007/s11886-015-0569-9
19. Wang D, Zhang J, Liu Y, Sun R, Tian J, Zhao W, et al. Diagnostic value of transthoracic echocardiography combined with contrast-enhanced ultrasonography in mediastinal masses. J Ultrasound Med. (2019) 38:415–22. doi: 10.1002/jum.14704
20. Fathala A, Abouzied M, AlSugair AA. Cardiac and pericardial tumors: a potential application of positron emission tomography-magnetic resonance imaging. World J Cardiol. (2017) 9:600–8. doi: 10.4330/wjc.v9.i7.600
21. Rathi VK, Czajka AT, Thompson DV, Doyle M, Tewatia T, Yamrozik J, et al. Can cardiovascular MRI be used to more definitively characterize cardiac masses initially identified using echocardiography? Echocardiography. (2018) 35:735–42. doi: 10.1111/echo.14017
22. Beroukhim RS, Prakash A, Buechel ER, Cava JR, Dorfman AL, Festa P, et al. Characterization of cardiac tumors in children by cardiovascular magnetic resonance imaging: a multicenter experience. J Am Coll Cardiol. (2011) 58:1044–54. doi: 10.1016/j.jacc.2011.05.027
23. Liu X, Hong H, Zhang H, Xu Z, Liu J, Qiu L. Treatment strategies for primary tumors of the heart in children: a 10-year experience. Ann Thorac Surg. (2015) 100:1744–9. doi: 10.1016/j.athoracsur.2015.06.030
24. Chen FM, Zhang L, Ni JM, Cao JN, You CY. A case report of tricuspid stenosis, right artium and pulmonary artery therombosis caused by primary cardiac Burkitt lymphoma. Chin J Cardiol. (2019) 47:921–2. doi: 10.3760/cma.j.issn.0253-3758.2019.11.014
25. Asadian S, Rezaeian N, Hosseini L, Toloueitabar Y, Hemmati Komasi MM. The role of cardiac CT and MRI in the diagnosis and management of primary cardiac lymphoma: a comprehensive review. Trends Cardiovasc Med. (2022) 32:408–20. doi: 10.1016/j.tcm.2021.08.010
26. Martineau P, Dilsizian V, Pelletier-Galarneau M. Incremental value of FDG-PET in the evaluation of cardiac masses. Curr Cardiol Rep. (2021) 23:78. doi: 10.1007/s11886-021-01509-z
27. Jiang K, Chen J, Zhu X, Xiao H, Ran T, Tang Y, et al. Rupture of sinus of Valsalva aneurysm: a case report in a child. BMC Cardiovasc Disord. (2022) 22:158. doi: 10.1186/s12872-022-02603-y
28. Tao J, He N. Clinicopathological analysis of 11 children with primary cardiac tumors. Chin J Heart Heart Rhythm. (2018) 6:143–5. doi: 10.3877/cma.j.issn.2095-6568.2018.03.005
29. Słowińska M, Jóźwiak S, Peron A, Borkowska J, Chmielewski D, Sadowski K, et al. Early diagnosis of tuberous sclerosis complex: a race against time. How to make the diagnosis before seizures? Orphanet J Rare Dis. (2018) 13:25. doi: 10.1186/s13023-018-0764-z
30. Wilbur C, Sanguansermsri C, Chable H, Anghelina M, Peinhof S, Anderson K, et al. Manifestations of tuberous sclerosis complex: the experience of a provincial clinic. Can J Neurol Sci. (2017) 44:35–43. doi: 10.1017/cjn.2016.311
31. Gu X, Han L, Chen J, Wang J, Hao X, Zhang Y, et al. Antenatal screening and diagnosis of tuberous sclerosis complex by fetal echocardiography and targeted genomic sequencing. Medicine. (2018) 97:e0112. doi: 10.1097/MD.0000000000010112
32. Nathan M, Fabozzo A, Geva T, Walsh E, del Nido PJ. Successful surgical management of ventricular fibromas in children. J Thorac Cardiovasc Surg. (2014) 148:2602–8. doi: 10.1016/j.jtcvs.2013.11.052
33. Qian T, Wu Z, Yang Y, Xie L, Yin N, Lu T, et al. Surgery for primary cardiac tumors in children: successful management of large fibromas. Front Cardiovasc Med. (2022) 9:808394. doi: 10.3389/fcvm.2022.808394
34. Szczałuba K, Makuła E, Piórecka-Makuła A, Sicińska J, Rydzanicz M, Gasperowicz P, et al. Intracardiac tumor as a rare manifestation of genetic syndromes-presentation of a family with Gorlin syndrome and a literature review. J Appl Genet. (2020) 61:559–65. doi: 10.1007/s13353-020-00582-4
35. Jones JP, Ramcharan T, Chaudhari M, Bhole V, Mcleod K, Sadagopan S, et al. Ventricular fibromas in children, arrhythmia risk, and outcomes: a multicenter study. Heart Rhythm. (2018) 15:1507–12. doi: 10.1016/j.hrthm.2018.06.018
36. Griborio-Guzman AG, Aseyev OI, Shah H, Sadreddini M. Cardiac myxomas: clinical presentation, diagnosis and management. Heart. (2022) 108:827–33. doi: 10.1136/heartjnl-2021-319479
37. Kong LY, Cui XZ, Xiang W, Chen LL, Li L, Liu F. Case report: two myxomas of different echodensities on transthoracic echocardiography in one patient. Front Cardiovasc Med. (2021) 8:770228. doi: 10.3389/fcvm.2021.770228
38. Xiaoyan Z, Zhen Z, Yumei L. Carney syndrome: a case report and literature review. Chin Pediatr Emerg Med. (2020) 27:153–7. doi: 10.3760/cma.j.issn.1673-4912.2020.02.018
39. Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics. (2000) 20:1073–112. doi: 10.1148/radiographics.20.4.g00jl081073
40. Schiele S, Maurer SJ, Pujol Salvador C, Vitanova K, Weirich G, Meierhofer C, et al. Left atrial myxoma. Circ Cardiovasc Imaging. (2019) 12:e008820. doi: 10.1161/CIRCIMAGING.118.008820
41. Wu KH, Mo XM, Liu YL. Clinical analysis and surgical results of cardiac myxoma in pediatric patients. J Surg Oncol. (2009) 99:48–50. doi: 10.1002/jso.21175
42. Vega RA, Chan JL, Anene-Maidoh TI, Grimes MM, Reavey-Cantwell JF. Mechanical thrombectomy for pediatric stroke arising from an atrial myxoma: case report. J Neurosurg Pediatr. (2015) 15:301–5. doi: 10.3171/2014.10.PEDS14292
43. Tao Z, Li Y, Zhao Y, Liu D. Incidental finding of an adult intracardiac teratoma. J Card Surg. (2021) 36:3441–4. doi: 10.1111/jocs.15724
44. Nakra T, Chandrashekhara SH, Rajashekar P, Ray R, Arava S. Intracardiac teratoma in a child: a rare site of a common tumor. J Pediatr Hematol Oncol. (2021) 43:e697–701. doi: 10.1097/MPH.0000000000001929
45. Rychik J, Khalek N, Gaynor JW, Johnson MP, Adzick NS, Flake AW, et al. Fetal intrapericardial teratoma: natural history and management including successful in utero surgery. Am J Obstet Gynecol. (2016) 215:780.e1–7. doi: 10.1016/j.ajog.2016.08.010
46. Malakan Rad E, Radmehr H, Vasei M, Rahimi Rastgoo B. Giant congenital right atrial epithelioid-capillary hemangioma with prolonged QT interval: case report and practical surgical treatment strategy for primary cardiac tumors in children based on 25-year review of 299 cases. Echocardiography. (2018) 35:1471–81. doi: 10.1111/echo.14105
47. Li W, Teng P, Xu H, Ma L, Ni Y. Cardiac hemangioma: a comprehensive analysis of 200 cases. Ann Thorac Surg. (2015) 99:2246–52. doi: 10.1016/j.athoracsur.2015.02.064
48. Rathore K, Yussouf R, Teh M, Jindal S, Wong D, Newman M. Left atrial anastomosing hemangioma causing recurrent pericardial effusion. Ann Thorac Surg. (2020) 109:e157–9. doi: 10.1016/j.athoracsur.2019.06.082
49. Chang JS, Chiou PY, Yao SH, Chou IC, Lin CY. Regression of neonatal cardiac rhabdomyoma in two months through low-dose everolimus therapy: a report of three cases. Pediatr Cardiol. (2017) 38:1478–84. doi: 10.1007/s00246-017-1688-4
50. Lee KA, Won H-S, Shim J-Y, Lee PR, Kim A. Molecular genetic, cardiac and neurodevelopmental findings in cases of prenatally diagnosed rhabdomyoma associated with tuberous sclerosis complex. Ultrasound Obstet Gynecol. (2013) 41:306–11. doi: 10.1002/uog.11227
51. Delmo Walter EM, Javier MF, Sander F, Hartmann B, Ekkernkamp A, Hetzer R. Primary cardiac tumors in infants and children: surgical strategy and long-term outcome. Ann Thorac Surg. (2016) 102:2062–9. doi: 10.1016/j.athoracsur.2016.04.057
52. Cai J, Li Y, Qiu L, Liu X, Yu X, Liu J. A single-institution systematic review of 135 patients with pediatric primary cardiac tumors. Clin J Appl Clin Pediatr. (2020) 35:1790–3. doi: 10.3760/cma.j.cn101070-20190522-00441
53. Rodriguez-Gonzalez M, Pérez-Reviriego AA, Gómez-Guzmán E, Tejero-Hernández MÁ, Sanz AZ, Valverde I. Primary cardiac fibroma in infants: a case report and review of cases of cardiac fibroma managed through orthotopic heart transplant. Ann Pediatr Cardiol. (2021) 14:224–7. doi: 10.4103/apc.APC_78_20
54. Salem M, Hillmer J, Friedrich C, Panholzer B, Saad M, Salem M, et al. Cardiac myxomas resembling malignant neoplasia: incidentally diagnosed vs. cerebral embolized myxomas. Cancers. (2022) 14:1111. doi: 10.3390/cancers14051111
Keywords: primary cardiac tumors, cardiovascular imaging (CV imaging), cardiovascular pathology, children, benign cardiac tumors
Citation: Sheng C, Yang C, Cheng Y and Li Y-M (2022) Current status of diagnosis and treatment of primary benign cardiac tumors in children. Front. Cardiovasc. Med. 9:947716. doi: 10.3389/fcvm.2022.947716
Received: 08 June 2022; Accepted: 05 October 2022;
Published: 21 October 2022.
Edited by:
Emanuele Monda, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Gaetano Thiene, University of Padua, ItalySupreet Marathe, Children’s Health Queensland, Australia
Copyright © 2022 Sheng, Yang, Cheng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Mei Li, eW1fbGlAamx1LmVkdS5jbg==
†ORCID: Chuqiao Sheng, orcid.org/0000-0002-5591-7196
 Chuqiao Sheng
Chuqiao Sheng Chunfeng Yang
Chunfeng Yang Yongkang Cheng
Yongkang Cheng Yu-Mei Li
Yu-Mei Li