- 1Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
Objective: Gastrointestinal bleeding (GIB) post acute myocardial infarction (AMI) is a severe clinical condition with a poor prognosis. The purpose of the study was to evaluate the rate of in-hospital mortality in patients with GIB post-AMI and to identify the potential risk factors of this situation.
Methods: In this single-center retrospective study, a total of 154 patients diagnosed with AMI who subsequently suffered GIB were enrolled from October 2013 to December 2021. Demographic, laboratory, and clinical data were collected. The in-hospital mortality was the outcome of interest. Logistic regression analysis was used to investigate the potential risk factors of in-hospital mortality.
Results: Among the 154 subjects included in the final analysis, the mean age was 65.58 ± 11.20 years, and 104 (67.53%) were males. GIB occurred in 11 patients after thrombolytic therapy, 50 patients after percutaneous coronary intervention (PCI), and 93 patients during drug conservative treatment. A total of 41 patients died in the hospital. The in-hospital mortality rate of the thrombolysis group, PCI group, and drug conservative treatment group was 27.27% (3/11), 28.00% (14/50), and 25.81% (24/93), respectively. There was no difference in the in-hospital mortality among the three groups. The multivariate logistic regression analysis showed that the peak levels of TnI (OR 1.07, 95% CI 1.02–1.12, P = 0.011), condition of cardiogenic shock after admission (OR 14.52, 95% CI 3.36–62.62, P < 0.001), and the use of the mechanical ventilator (OR 8.14, 95% CI 2.03–32.59, P = 0.003) were significantly associated with in-hospital mortality.
Conclusion: Regardless of the treatment strategy for AMI, once GIB occurred, the prognosis was poor. High in-hospital mortality in patients with GIB post-AMI was independently associated with the peak levels of TnI, condition of cardiogenic shock, and the use of a mechanical ventilator.
Introduction
Acute myocardial infarction (AMI) is a category of disease associated with the greatest mortality and morbidity (1, 2). Meanwhile, gastrointestinal bleeding (GIB) is a common medical condition that may lead to substantial morbidity and mortality (3–5). Once patients diagnosed with AMI subsequently suffer GIB, the situation will worsen.
Dual antiplatelet therapy (DAPT) with aspirin and a thienopyridine derivative is the common anti-platelet strategy after AMI irrespective of conservative or invasive treatment (6, 7). While the combined use of antithrombotic medications may reduce the risk of cardiovascular events, they increase the risk of hemorrhage events. GIB is a common cause of hemorrhage in AMI patients (8). Studies have reported GIB rates from 0.6 to 3.9% in AMI patients, which is associated with an increased risk of poor prognosis (9–13). A retrospective analysis of patients undergoing primary percutaneous coronary intervention (PCI) found that GIB was an independent predictor of all-cause mortality (14). Multiple factors, such as treatment contradiction and complicated conditions, may lead to adverse clinical outcomes for patients with GIB post-AMI.
At present, there are no guidelines to define the etiology, risk factors, and treatment principles in patients with GIB post-AMI. Considering the poor prognosis of patients with GIB post-AMI, it is critical to identify patients with an increased risk of in-hospital mortality, thereby increasing vigilance for these patients. Therefore, the purpose of the study was to evaluate the rate of in-hospital mortality in patients with GIB post-AMI and to identify the potential risk factors of this situation.
Materials and Methods
Study Population
This present study was a single-center retrospective analysis of hospitalized patients with AMI and GIB at the First Affiliated Hospital of Zhengzhou University, Henan, China. Patients admitted because of GIB post-AMI were retrospectively enrolled between October 2013 and December 2021. The inclusion criterion was confirmed admission diagnosis of AMI with subsequent GIB. Patients with a positive fecal occult blood test but no visible melena or without any other clinical evidence of GIB were excluded. In addition, subjects with GIB who subsequently suffered an AMI were also excluded.
According to the timing of GIB and different managements of AMI, we divided the AMI patients into three groups: thrombolysis group, PCI group, and drug conservative treatment group. The patients in the thrombolysis group had GIB after thrombolysis, patients in the PCI group suffered GIB after emergency PCI, and the drug conservative treatment group had GIB in simple antithrombotic therapy during the acute stage of AMI. This research was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Data Collection
We retrospectively collected data concerning patients’ demographic information, timing and main manifestations of GIB, types of AMI, comorbidities, medication history, admission features, physiological data, echocardiographic features, laboratory data, and clinical characteristics. Laboratory data were recorded at admission and rechecked (24, 48, 72 h, and then every 2–3 days) during hospitalization, including minimum values of hemoglobin, and peak values of creatine kinase muscle B (CK-MB), cardiac troponin I (TnI), and N-terminal pro-B-type natriuretic peptide (NT-pro BNP) for all patients. In this analysis, the markers of myocardial injury (CK-MB, TnI, and NT-pro BNP) were the peak values, and hemoglobin and red blood cells were the minimum values. Other laboratory data were based on admission data.
Antithrombotic Therapy and Gastrointestinal Bleeding Treatment
In this study, patients diagnosed with AMI received a loading dose of aspirin (300 mg) plus clopidogrel (300 or 600 mg) or ticagrelor (180 mg), followed by a maintenance dose of aspirin (100 mg once a day) plus clopidogrel (75 mg once a day) or ticagrelor (90 mg twice a day). All patients with AMI in our center routinely used proton pump inhibitors (PPIs) to reduce the risk of GIB.
Once the diagnosis of GIB was clear, antithrombotic drugs would often be stopped, and the patients would increase the dosage of PPIs and use other mucosal protective drugs. Depending on the amount of blood loss and whether the bleeding continues, patients might require a blood transfusion. If possible, endoscopy is helpful in the treatment of GIB in patients with AMI. Meanwhile, in order to stop active GIB, some patients underwent interventional embolization. Once GIB is controlled, antithrombotic therapy will be resumed as soon as possible.
Definition
Acute myocardial infarction was diagnosed according to the fourth universal definition (15), containing the ST-segment elevation AMI and the non-ST-segment elevation AMI. GIB was defined as clinically evident GIB (hematemesis, coffee-ground emesis and melena, and bloody stool) accompanied by decreased hemoglobin levels. Major GIB was defined as clinically evident GIB with a decrease in hemoglobin level of ≥2 g/dL from baseline (16–18). Each variable was defined in accordance with cardiovascular data standards (19). The primary outcome was in-hospital mortality from all causes, including cardiac death, multiple organ failure, massive hemorrhage/intracranial hemorrhage, and sudden death.
Statistical Analysis
Categorical variables were shown as frequencies and percentages, whereas continuous variables were presented as mean ± standard deviations (SD). Continuous variables were compared using the one-way ANOVA analysis or Kruskal–Wallis test, whereas categorical variables were compared using the Chi-square test. Logistical regression was performed to evaluate the odds ratio (OR) and 95% confidence interval (CI) for the association between risk factors and in-hospital mortality. Variables significantly related to in-hospital mortality in univariate analysis (P < 0.05) and clinically relevant factors (age, sex, severity of GIB, and comorbidities) were input into one multiple logistic regression model to determine the risk factors of in-hospital mortality. The factors entered into the multivariate logistical regression analysis were as follows: age, sex, severity of GIB, hypertension, diabetes mellitus, coronary artery disease, previous stroke, admission heart rate, admission systolic blood pressure, left ventricular ejection fraction (LVEF), white blood cell, estimated glomerular filtration rate (eGFR), CK-MB, TnI, C-reactive protein (CRP), cardiogenic shock, mechanical ventilator, extracorporeal membrane oxygenation (ECMO), continuous renal replacement therapy (CRRT). In addition, cumulative incidence rates of in-hospital mortality were estimated by the Kaplan–Meier method and compared with the log-rank test. Statistical analyses were performed using SPSS 23.0 software. A two-sided P-value < 0.05 was considered statistically significant.
Results
General Characteristics of Study Patients
Between October 2013 to December 2021, there were 238 patients diagnosed with GIB post-AMI in our center. A total of 154 patients were included after excluding 71 subjects diagnosed with GIB who subsequently suffered AMI, eight patients with a positive fecal occult blood test but without any other clinical evidence of GIB, and five patients with missing data (Figure 1).
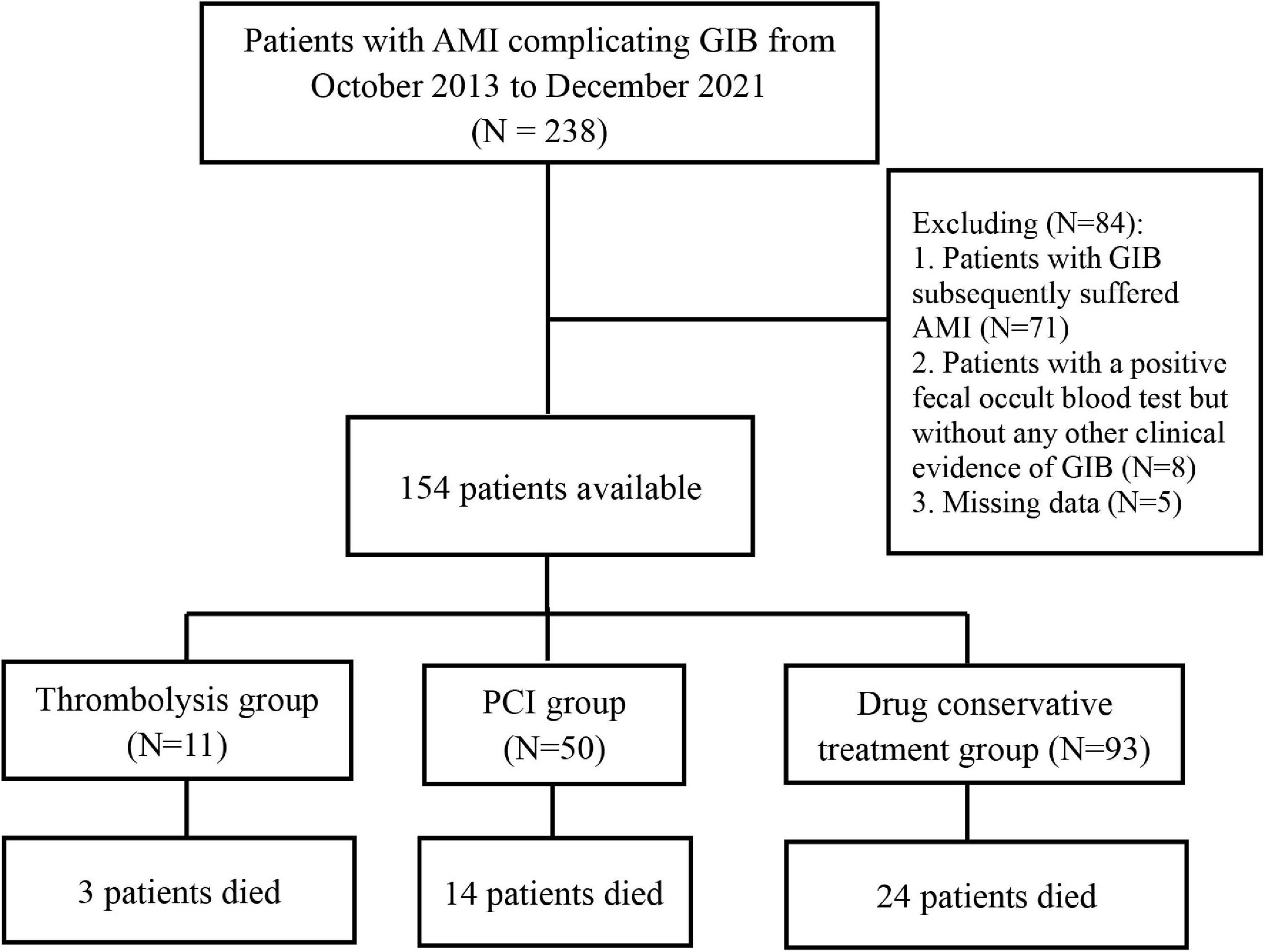
Figure 1. Flow chart for selection of study population. AMI, acute myocardial infarction; GIB, gastrointestinal bleeding; PCI, percutaneous coronary intervention.
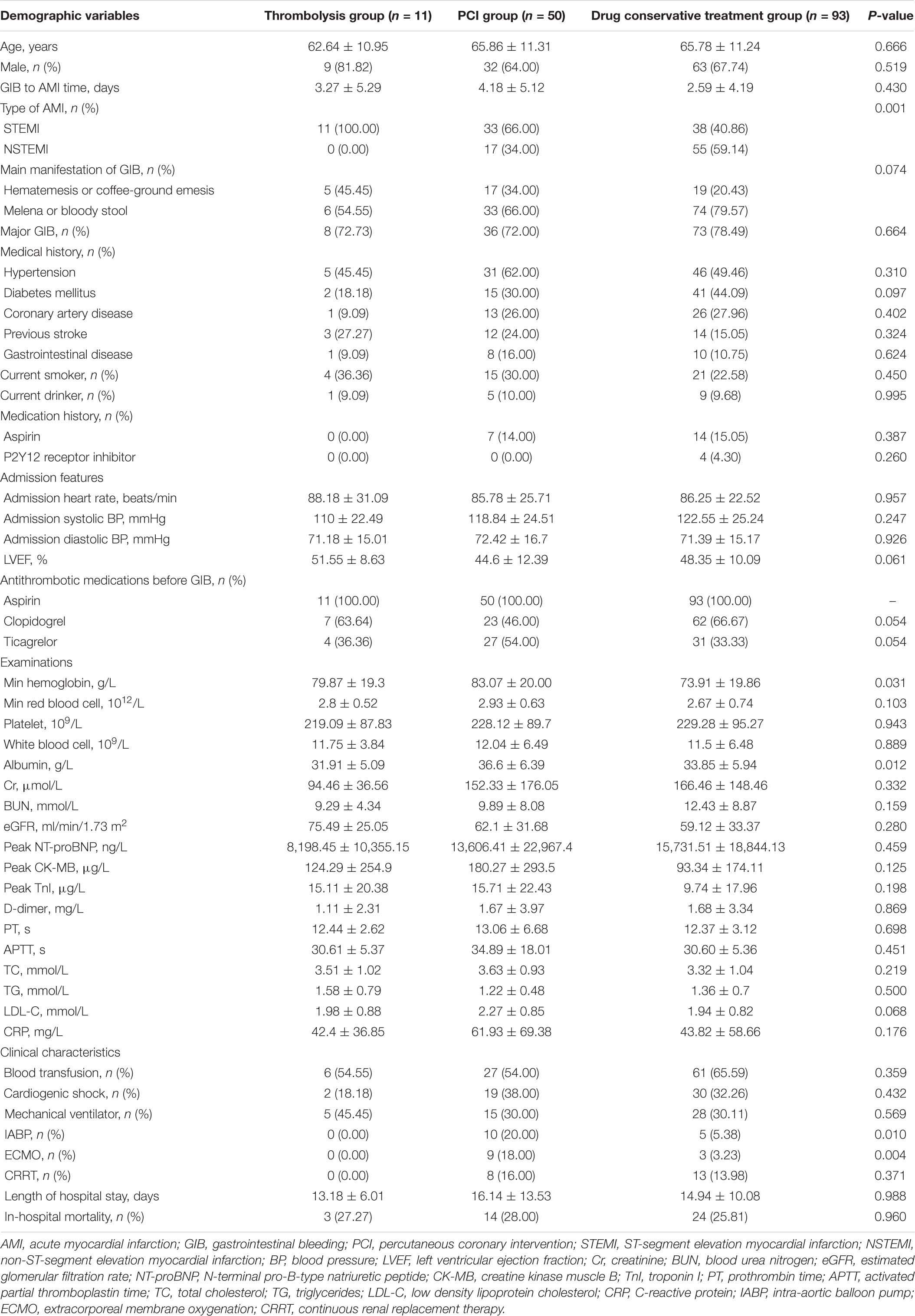
Among the 154 subjects included in this study, 104 (67.53%) were males, and the mean age was 65.58 ± 11.20 years. Besides, GIB occurred in 11 patients after thrombolytic therapy (thrombolysis group), 50 patients after PCI (PCI group), and 93 patients during the drug conservative treatment (conservative treatment group).
Baseline characteristics according to the timing of GIB and different managements of AMI were illustrated in Table 1. Most characteristics had no difference among the three groups. The mean ages of the thrombolysis group, PCI group, and drug conservative group were 62.64 ± 10.95, 65.86 ± 11.31, and 65.78 ± 11.24, respectively. In addition, among the three groups, 9 (81.82%), 32 (64.00%), and 63 (67.74%) were males; 11 (100.00%), 33 (66.00%), and 38 (40.86%) were STEMI; 8 (72.73%), 36 (72%), and 73 (78.49%) had major GIB. The levels of hemoglobin and albumin in the PCI group were higher, and the proportion of patients using IABP and ECMO was higher in the PCI group.
Independent Risk Factors of In-Hospital Mortality
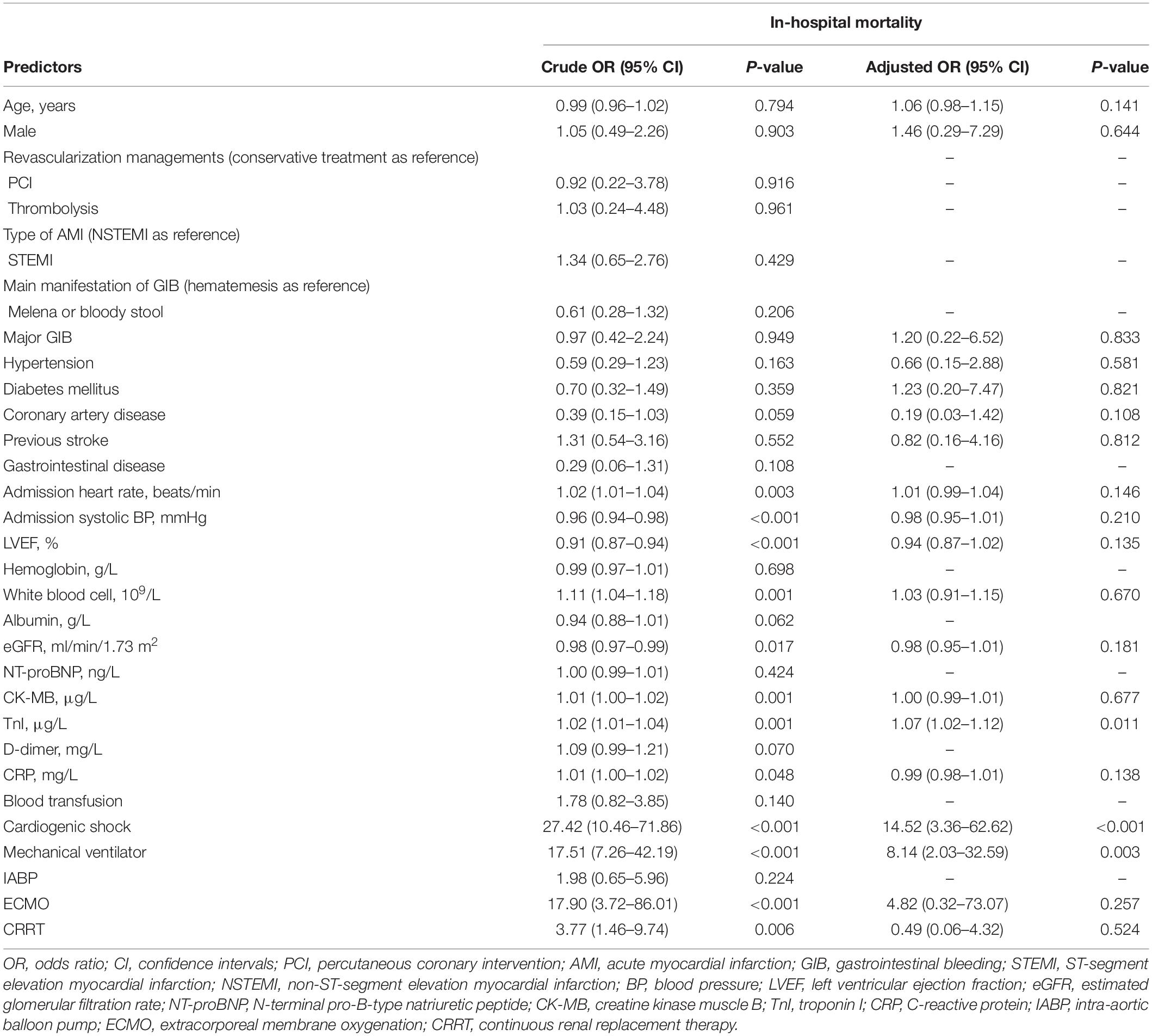
We conducted the regression analysis of factors associated with in-hospital mortality. Univariate logistic regression analysis revealed that admission heart rate (OR 1.02, 95% CI 1.01–1.04, P = 0.003), admission systolic blood pressure (OR 0.96, 95% CI 0.94–0.98, P < 0.001), LVEF (OR 0.91, 95% CI 0.87–0.94, P < 0.001), white blood cell (OR 1.11, 95% CI 1.04–1.18, P = 0.001), eGFR (OR 0.98, 95% CI 0.97–0.99, P = 0.017), CK-MB (OR 1.01, 95% CI 1.00–1.02, P = 0.001), TnI (OR 1.02, 95% CI 1.01–1.04, P = 0.001), CRP (OR 1.01, 95% CI 1.00–1.02, P = 0.048), cardiogenic shock (OR 27.42, 95% CI 10.46–71.86, P < 0.001), mechanical ventilator (OR 17.51, 95% CI 7.26–42.19, P < 0.001), ECMO (OR 17.90, 95% CI 3.72–86.01, P < 0.001), and CRRT (OR 3.77, 95% CI 1.46–9.74, P = 0.006) were predictors of the in-hospital mortality. In addition, the multivariate logistic regression analysis showed that the peak levels of TnI (OR 1.07, 95% CI 1.02–1.12, P = 0.011), condition of cardiogenic shock after admission (OR 14.52, 95% CI 3.36–62.62, P < 0.001), and the use of a mechanical ventilator (OR 8.14, 95% CI 2.03–32.59, P = 0.003) were significantly associated with in-hospital mortality (Tables 2, 3).
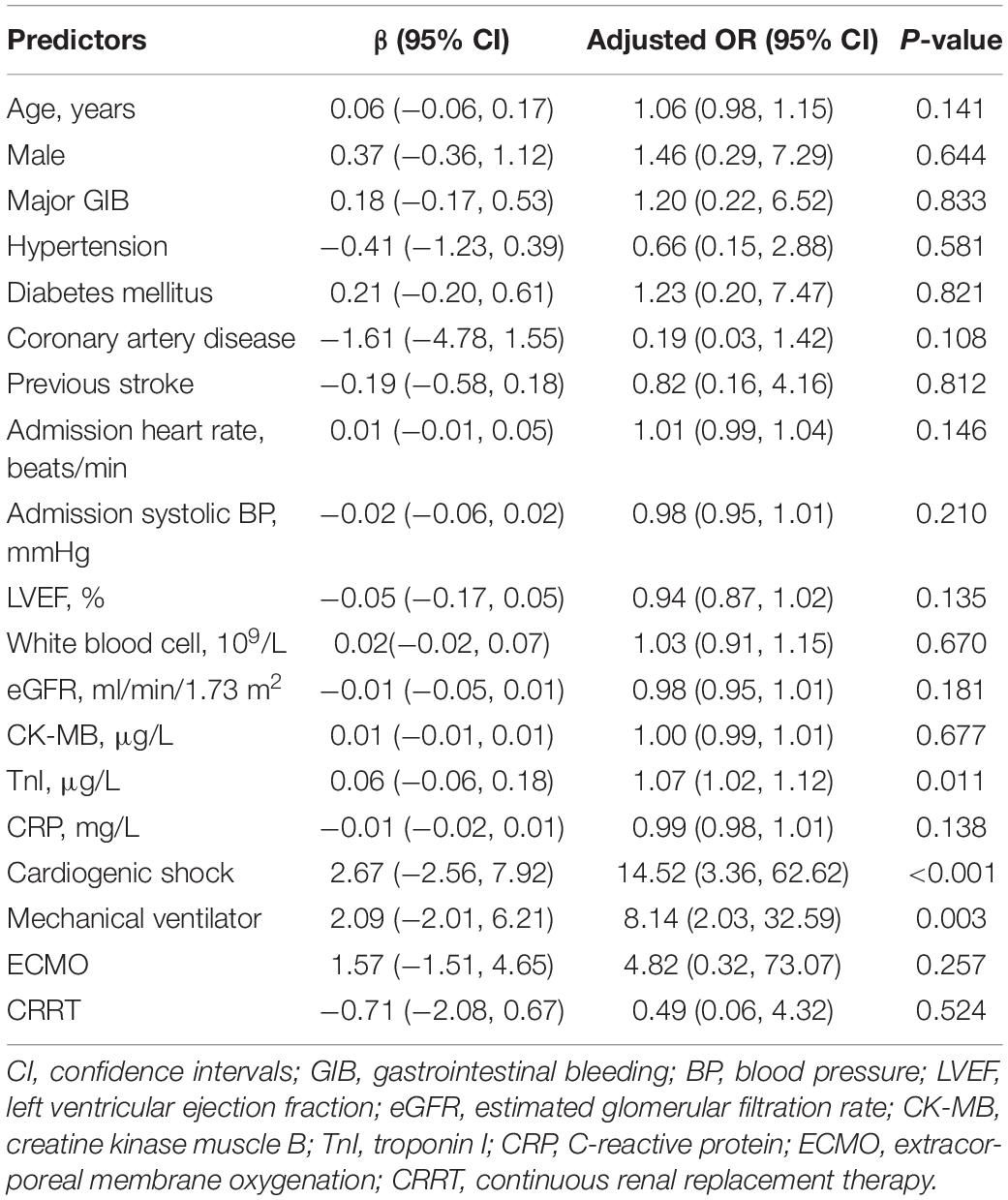
Table 3. The multivariate logistic regression of in-hospital mortality with the beta coefficient and the confident interval.
Cumulative In-Hospital Mortality Rates
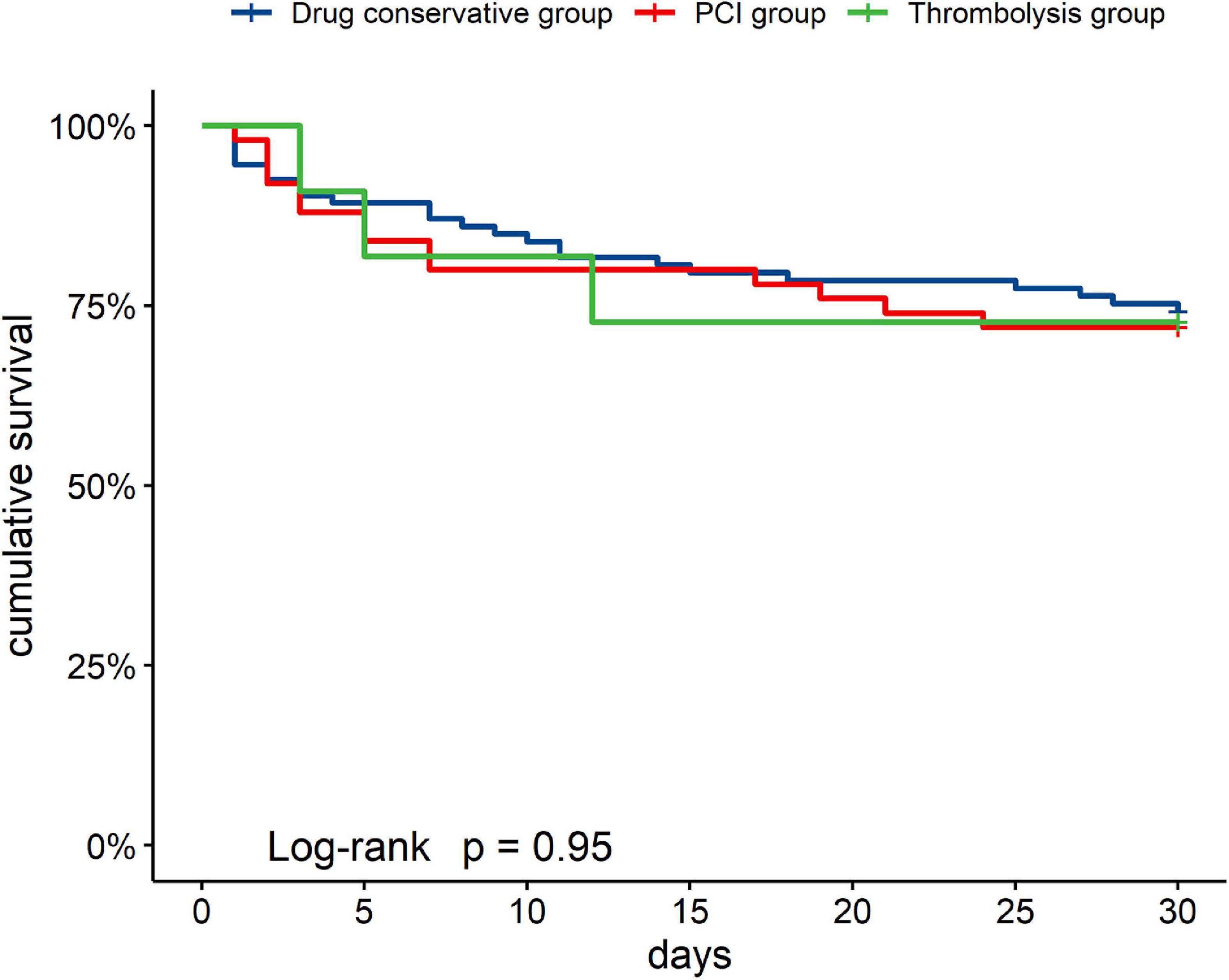
A total of 41 (26.62%) patients died in the hospital. The in-hospital mortality rate of the thrombolysis group, PCI group, and drug conservative treatment group was 27.27% (3/11), 28.00% (14/50), and 25.81% (24/93), respectively. Causes of death included refractory heart failure (25, 60.98%), hemorrhage event (4, 9.75%), multiple system organ failure (10, 24.39%), and unknown reasons (2, 4.88%).
Figure 2 showed the cumulative in-hospital survival rates of patients with different managements of AMI. There was no significant difference in the cumulative in-hospital mortality rates among the groups [thrombolysis group (27.27%, 3/11), PCI group (28.00%, 14/50), and drug conservative treatment group (25.81%, 24/93), Log-rank P = 0.950].
Discussion
In this single-center retrospective study, we found that the in-hospital mortality of patients with AMI who subsequently suffered GIB was extremely high. In addition, regardless of the treatment strategy of AMI, once GIB occurred, the high in-hospital mortality was consistent. The peak levels of TnI, condition of cardiogenic shock, and the use of a mechanical ventilator were found to be independent predictors of poor prognosis.
To our knowledge, this study is one of the studies with the largest number of patients with GIB post-AMI, including individuals receiving invasive treatment strategies and non-invasive treatment strategies.
Dual antiplatelet therapy with aspirin and a thienopyridine derivative is the common antithrombotic strategy after AMI irrespective of conservative or invasive therapeutic methods (6, 7). This treatment reduces ischemic events but will be offset by increased bleeding events (20). Antiplatelet therapy will be limited once a bleeding event occurs. In addition, thrombolysis, as a treatment strategy for AMI, also has a high risk of bleeding. A multi-center study, data from the TIMI (Thrombolysis in Myocardial Infarction) trial, found that 0.4–1.8% of AMI patients receiving thrombolytic therapy suffered GIB events (21).
Gastrointestinal bleeding, including both lower and upper gastrointestinal origins, was independently associated with increased mortality in different conditions (11, 13, 14, 22). The ACUITY study illustrated that GIB was strongly associated with 30-day all-cause mortality [hazard ratio (HR) 4.87, interquartile range 2.61–9.08, P < 0.0001] (13). Sarajlic et al. demonstrated that upper GIB in AMI patients was associated with an increased risk of all cause-death (HR 2.86, 95% CI 2.583.1–6) and stroke (HR 1.80, 95% CI 1.32–2.45) (11). Meanwhile, Shalev et al. found that upper GIB in individuals with acute coronary syndrome (ACS) was associated with a markedly increased 30-day mortality (33%) (22).
In our study, the in-hospital mortality of AMI patients who subsequently suffered GIB was 26.62%. In one single-center retrospective study that included only patients diagnosed with non-ST-segment elevation AMI, the in-hospital mortality of individuals with GIB and AMI was 24.7% (23). Another retrospective study, data from the CAMI (China Acute Myocardial Infarction) Registry, noted that subjects with GIB had a significantly higher risk for death (HR 1.392; 95% CI 1.105–1.764, P = 0.0071), with an in-hospital mortality rate of 22% (10). In addition, Gaglia et al. identified GIB in 0.72% of 20,621 patients who underwent PCI, and the 30-day mortality rate of patients with GIB was 20.5% (24). Overall, our results are consistent and extend the evidence in patients with AMI who subsequently suffered GIB.
The mechanism of the poor prognosis in patients with GIB post-AMI may be multi-factorial. Firstly, GIB may lead to bleeding-related hemodynamic instability and aggravate ischemia, leading to stroke and reinfarction. Secondly, blood transfusion after GIB may have indirect effects, leading to systemic inflammation in the prethrombotic state, increasing oxidative stress, and paradoxically reducing oxygen delivery, all of which could lead to worse results (25). Besides, even mild bleeding without blood transfusion may result in the interruption of antithrombotic therapy, which could indirectly affect the prognosis (26). When antithrombotic therapy is suspended, the risk of acute stent thrombosis is extremely high, especially in patients undergoing primary PCI. Moreover, patients with GIB post-AMI had poor baseline clinical characteristics, such as older age and more comorbidities, which might be associated with poor outcomes. Based on the combined effect of the above reasons, the mortality of GIB post-AMI is higher than that of each situation.
The purpose of our study is to describe and explore potential predictors of in-hospital mortality in patients with GIB post-AMI. Univariate logistic regression analysis revealed that admission heart rate, admission systolic blood pressure, LVEF, white blood cell, eGFR, CK-MB, TnI, CRP, cardiogenic shock, mechanical ventilator, ECMO, and CRRT were predictors of the in-hospital mortality. Rapid heart rate and low blood pressure are the manifestations of cardiogenic shock. A retrospective study suggested that low systolic blood pressure and rapid heart rate at admission of STEMI patients were associated with a higher risk of in-hospital death (27). White blood cell counts and CRP as inflammatory markers in patients with AMI are independently associated with mortality (28, 29). Lower levels of eGFR and the use of CRRT mean that patients have acute or chronic kidney disease. Patients with chronic kidney disease experienced poor outcomes after AMI, while acute kidney injury was also associated with mortality in AMI patients (30, 31).
In the meantime, the multivariate logistic regression analysis showed that the peak levels of TnI, condition of cardiogenic shock, and the use of a mechanical ventilator were independent risk predictors of in-hospital mortality in AMI patients with GIB. To some extent, higher levels of TnI represent greater ranges of myocardial necrosis, which might be associated with poor prognosis. Widmer et al. found that TnI values ≥0.1 ng/ml were associated with higher in-patient mortality and 30-day readmission rates in myocardial injury patients (32). In addition, patients with cardiogenic shock often present with poor cardiac function and serious conditions, and these patients tend to have poor outcomes (33). A cardiogenic shock complicating AMI cohort study noted that the presence of respiratory failure and mechanical ventilator was associated with higher in-hospital mortality (34).
This research helps to identify patients with an increased risk of in-hospital mortality, thereby increasing vigilance for these patients. Patients with GIB post-AMI are in serious condition, and most of them are admitted to the intensive care unit for treatment. In the course of treatment, close monitoring should be carried out to prevent the complications of AMI and GIB.
Previous studies have found that factors such as previous bleeding, more intensive antithrombotic therapy, old age, and low hemoglobin are predictors of GIB in AMI patients (11, 12). Given the poor prognosis of GIB in individuals with AMI, it is more critical to prevent GIB in high-risk patients. PPIs or other mucosal protective agents could be commonly used in these patients to reduce the risk of GIB associated with antithrombotic therapy (8, 35). Besides, the intensity of antithrombotic therapy could be adjusted according to the risk of bleeding and ischemic event. Considering the adverse outcome of ischemic and hemorrhagic complication, the best treatment strategy must balance the risks of these events.
This clinical study has several limitations. Firstly, this was one single-center retrospective study, with the common shortcomings of analysis of the prerecorded data. Secondly, few patients underwent endoscopy in our study. Although some studies have pointed out that endoscopic treatment of GIB in patients with AMI is relatively safe (36–38), doctors usually choose relatively conservative treatment in order to avoid medical disputes. In addition, since we do not have the endoscopy results of these patients, the origin (lower and upper) of the GIB is unclear. Moreover, in our study, the severity of the disease and treatment methods are not consistent, but the treatment principles are consistent.
Conclusion
The in-hospital mortality of AMI patients who subsequently suffered GIB was extremely high. Regardless of the treatment strategy of AMI, once GIB occurred, the prognosis was poor. The peak levels of TnI, condition of cardiogenic shock, and the use of a mechanical ventilator were found to be independent risk factors for poor prognosis.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Human Research Ethics Committee of The First Affiliated Hospital of Zhengzhou University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XS, HS, and JD contributed to study planning and analysis, and were responsible for the overall content as guarantors. YW, SP, ZZ, YZ, PZ, and HL conducted the study and performed the examinations. XS and YW performed the statistical analysis and wrote the manuscript. All authors have read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: a report from the American heart association. Circulation. (2016) 133:e38–360. doi: 10.1161/CIR.0000000000000350
2. Johansson S, Rosengren A, Young K, Jennings E. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: a systematic review. BMC Cardiovasc Disord. (2017) 17:53. doi: 10.1186/s12872-017-0482-9
3. Hearnshaw SA, Logan RFA, Lowe D, Travis SPL, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the uk: patient characteristics, diagnoses and outcomes in the 2007 Uk audit. Gut. (2011) 60:1327–35. doi: 10.1136/gut.2010.228437
4. El-Tawil AM. Trends on gastrointestinal bleeding and mortality: Where are we standing? World J Gastroenterol. (2012) 18:1154–8. doi: 10.3748/wjg.v18.i11.1154
5. Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KAA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. (2006) 114:774–82. doi: 10.1161/CIRCULATIONAHA.106.612812
6. Collet J-P, Thiele H. The ‘ten commandments’ for the 2020 esc guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation. Eur Heart J. (2020) 41:3495–7. doi: 10.1093/eurheartj/ehaa624
7. Valgimigli M, Bueno H, Byrne RA, Collet J-P, Costa F, Jeppsson A, et al. 2017 Esc focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with eacts: the task force for dual antiplatelet therapy in coronary artery disease of the european society of cardiology (esc) and of the european association for cardio-thoracic surgery (Eacts). Eur Heart J. (2018) 39:213–60. doi: 10.1093/eurheartj/ehx419
8. Sehested TSG, Carlson N, Hansen PW, Gerds TA, Charlot MG, Torp-Pedersen C, et al. Reduced risk of gastrointestinal bleeding associated with proton pump inhibitor therapy in patients treated with dual antiplatelet therapy after myocardial infarction. Eur Heart J. (2019) 40:1963–70. doi: 10.1093/eurheartj/ehz104
9. Albeiruti R, Chaudhary F, Alqahtani F, Kupec J, Balla S, Alkhouli M. Incidence, predictors, and outcomes of gastrointestinal bleeding in patients admitted with st-elevation myocardial infarction. Am J Cardiol. (2019) 124:343–8. doi: 10.1016/j.amjcard.2019.05.008
10. Shi W, Fan X, Yang J, Ni L, Su S, Yu M, et al. In-hospital gastrointestinal bleeding in patients with acute myocardial infarction: incidence, outcomes and risk factors analysis from china acute myocardial infarction registry. BMJ Open. (2021) 11:e044117. doi: 10.1136/bmjopen-2020-044117
11. Sarajlic P, Simonsson M, Jernberg T, Bäck M, Hofmann R. Incidence, associated outcomes, and predictors of upper gastrointestinal bleeding following acute myocardial infarction: a swedeheart-based nationwide cohort study. Eur Heart J Cardiovasc Pharmacother. (2021) [Online ahead of print]. doi: 10.1093/ehjcvp/pvab059
12. Moukarbel GV, Signorovitch JE, Pfeffer MA, McMurray JJV, White HD, Maggioni AP, et al. Gastrointestinal bleeding in high risk survivors of myocardial infarction: the valiant trial. Eur Heart J. (2009) 30:2226–32. doi: 10.1093/eurheartj/ehp256
13. Nikolsky E, Stone GW, Kirtane AJ, Dangas GD, Lansky AJ, McLaurin B, et al. Gastrointestinal bleeding in patients with acute coronary syndromes: incidence, predictors, and clinical implications: analysis from the acuity (acute catheterization and urgent intervention triage strategy) trial. J Am Coll Cardiol. (2009) 54:1293–302. doi: 10.1016/j.jacc.2009.07.019
14. Koskinas KC, Räber L, Zanchin T, Wenaweser P, Stortecky S, Moschovitis A, et al. Clinical impact of gastrointestinal bleeding in patients undergoing percutaneous coronary interventions. Circ Cardiovasc Interv. (2015) 8:e002053. doi: 10.1161/CIRCINTERVENTIONS.114.002053
15. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Circulation. (2018) 138:e618–51. doi: 10.1161/CIR.0000000000000617
16. Kim BSM, Li BT, Engel A, Samra JS, Clarke S, Norton ID, et al. Diagnosis of gastrointestinal bleeding: a practical guide for clinicians. World J Gastrointest Pathophysiol. (2014) 5:467–78. doi: 10.4291/wjgp.v5.i4.467
17. Barkun AN, Almadi M, Kuipers EJ, Laine L, Sung J, Tse F, et al. Management of nonvariceal upper gastrointestinal bleeding: guideline recommendations from the international consensus group. Ann Intern Med. (2019) 171:805–22. doi: 10.7326/M19-1795
18. Aisenberg J, Chatterjee-Murphy P, Friedman Flack K, Weitz JI, Ruff CT, Nordio F, et al. Gastrointestinal bleeding with edoxaban versus warfarin: results from the engage af-timi 48 trial (effective anticoagulation with factor xa next generation in atrial fibrillation-thrombolysis in myocardial infarction). Circ Cardiovasc Qual Outcomes. (2018) 11:e003998. doi: 10.1161/CIRCOUTCOMES.117.003998
19. Anderson HV, Weintraub WS, Radford MJ, Kremers MS, Roe MT, Shaw RE, et al. Standardized cardiovascular data for clinical research, registries, and patient care: a report from the data standards workgroup of the national cardiovascular research infrastructure project. J Am Coll Cardiol. (2013) 61:1835–46. doi: 10.1016/j.jacc.2012.12.047
20. Valgimigli M, Costa F, Lokhnygina Y, Clare RM, Wallentin L, Moliterno DJ, et al. Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: lessons from the thrombin receptor antagonist for clinical event reduction in acute coronary syndrome (tracer) randomized trial. Eur Heart J. (2017) 38:804–10. doi: 10.1093/eurheartj/ehw525
21. Bovill EG, Terrin ML, Stump DC, Berke AD, Frederick M, Collen D, et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. results of the thrombolysis in myocardial infarction (timi), phase ii trial. Ann Intern Med. (1991) 115:256–65. doi: 10.7326/0003-4819-115-4-256
22. Shalev A, Zahger D, Novack V, Etzion O, Shimony A, Gilutz H, et al. Incidence, predictors and outcome of upper gastrointestinal bleeding in patients with acute coronary syndromes. Int J Cardiol. (2012) 157:386–90. doi: 10.1016/j.ijcard.2010.12.081
23. He L, Zhang J, Zhang S. Risk factors of in-hospital mortality among patients with upper gastrointestinal bleeding and acute myocardial infarction. Saudi J Gastroenterol. (2018) 24:177–82. doi: 10.4103/sjg.SJG_492_17
24. Gaglia MA, Torguson R, Gonzalez MA, Ben-Dor I, Maluenda G, Collins SD, et al. Correlates and consequences of gastrointestinal bleeding complicating percutaneous coronary intervention. Am J Cardiol. (2010) 106:1069–74. doi: 10.1016/j.amjcard.2010.06.011
25. Doyle BJ, Rihal CS, Gastineau DA, Holmes DR. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol. (2009) 53:2019–27. doi: 10.1016/j.jacc.2008.12.073
26. Grines CL, Bonow RO, Casey DE, Gardner TJ, Lockhart PB, Moliterno DJ, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American heart association, American college of cardiology, society for cardiovascular angiography and interventions, American college of surgeons, and American dental association, with representation from the American college of physicians. Catheter Cardiovasc Interv. (2007) 69:334–40.
27. Bordejevic DA, Caruntu F, Mornos C, Olariu I, Petrescu L, Tomescu MC, et al. Prognostic impact of blood pressure and heart rate at admission on in-hospital mortality after primary percutaneous intervention for acute myocardial infarction with st-segment elevation in western romania. Ther Clin Risk Manag. (2017) 13:1061–8. doi: 10.2147/TCRM.S141312
28. Shiyovich A, Gilutz H, Plakht Y. White blood cell subtypes are associated with a greater long-term risk of death after acute myocardial infarction. Tex Heart Inst J. (2017) 44:176–88. doi: 10.14503/THIJ-16-5768
29. Ziakas A, Gavrilidis S, Giannoglou G, Souliou E, Gemitzis K, Kalampalika D, et al. In- hospital and long-term prognostic value of fibrinogen, Crp, and Il-6 levels in patients with acute myocardial infarction treated with thrombolysis. Angiology. (2006) 57:283–93. doi: 10.1177/000331970605700304
30. Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Wiviott SD. Short-term outcomes of acute myocardial infarction in patients with acute kidney injury: a report from the national cardiovascular data registry. Circulation. (2012) 125:497–504. doi: 10.1161/CIRCULATIONAHA.111.039909
31. Shroff GR, Frederick PD, Herzog CA. Renal failure and acute myocardial infarction: clinical characteristics in patients with advanced chronic kidney disease, on dialysis, and without chronic kidney disease. a collaborative project of the united states renal data system/national institutes of health and the national registry of myocardial infarction. Am Heart J. (2012) 163:399–406. doi: 10.1016/j.ahj.2011.12.002
32. Widmer RJ, Wilson G, Haneke T, Lee M, Fan J, Davis A, et al. Inpatient mortality and 30-day readmission rates associated with troponin testing in patients without acute myocardial infarction. Clin Med Res. (2020) 18:82–8. doi: 10.3121/cmr.2020.1513
33. Jentzer JC, van Diepen S, Barsness GW, Henry TD, Menon V, Rihal CS, et al. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. (2019) 74:2117–28. doi: 10.1016/j.jacc.2019.07.077
34. Vallabhajosyula S, Kashani K, Dunlay SM, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, et al. Acute respiratory failure and mechanical ventilation in cardiogenic shock complicating acute myocardial infarction in the USA, 2000-2014. Ann Intensive Care. (2019) 9:96. doi: 10.1186/s13613-019-0571-2
35. Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. (2010) 363:1909–17.
36. Lim RG, Cobell WJ, Theivanayagam S, Kilgore TW, Matteson ML, Puli SR, et al. Endoscopy after acute myocardial infarction: an evaluation of safety. South Med J. (2013) 106:545–9. doi: 10.1097/SMJ.0000000000000001
37. Cena M, Gomez J, Alyousef T, Trohman RG, Pierko K, Agarwal R. Safety of endoscopic procedures after acute myocardial infarction: a systematic review. Cardiol J. (2012) 19:447–52. doi: 10.5603/cj.2012.0083
Keywords: acute myocardial infarction, gastrointestinal bleeding, in-hospital mortality, thrombolysis, percutaneous coronary intervention
Citation: Su X, Wei Y, Pang S, Zhang Z, Zhang Y, Zheng P, Li H, Sang H and Dong J (2022) Clinical Characteristics and Risk Factors of In-Hospital Mortality in Patients With Acute Myocardial Infarction With Subsequent Gastrointestinal Bleeding: A Single-Center Experience. Front. Cardiovasc. Med. 9:942467. doi: 10.3389/fcvm.2022.942467
Received: 12 May 2022; Accepted: 06 June 2022;
Published: 13 July 2022.
Edited by:
Sy Duong-Quy, Lam Dong Medical College, VietnamReviewed by:
Tien Hoang Anh, Hue University of Medicine and Pharmacy, VietnamThu Nguyen Ngoc Phuong, Pham Ngoc Thach University of Medicine, Vietnam
Copyright © 2022 Su, Wei, Pang, Zhang, Zhang, Zheng, Li, Sang and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiqiang Sang, ZmNjc2FuZ2hxQHp6dS5lZHUuY24=
 Xin Su
Xin Su Yuzhen Wei1
Yuzhen Wei1 Shuo Pang
Shuo Pang Zeqing Zhang
Zeqing Zhang Peipei Zheng
Peipei Zheng Haiqiang Sang
Haiqiang Sang Jianzeng Dong
Jianzeng Dong