- 1Department of Research, Weill Cornell Medicine-Qatar, Doha, Qatar
- 2Heart Hospital, Hamad Medical Corporation, Doha, Qatar
- 3The Michael E. DeBakey VA Medical Centre, Baylor College of Medicine, Houston, TX, United States
- 4Joan and Sanford I. Weill Department of Medicine, Weill Cornell Medicine, New York, NY, United States
Aims: We aimed to assess the impact of hypoglycemia in ST-elevation myocardial infarction (STEMI).
Background: Hypoglycemia increases the risk of mortality in patients with diabetes and high cardiovascular risk.
Methods: We used the National Inpatient Sample (2005–2017) database to identify adult patients with STEMI as the primary diagnosis. The secondary diagnosis was hypoglycemia. We compared cardiovascular and socio-economic outcomes between STEMI patients with and without hypoglycemia and assessed temporal trends.
Results: Hypoglycemia tends to complicate 0.17% of all cases hospitalized for STEMI. The mean age (±SD) of STEMI patients hospitalized with hypoglycemia decreased from 67 ± 15 in 2005 to 63 ± 12 in 2017 (p = 0.046). Mortality was stable with time, but the prevalence of ventricular tachycardia, ventricular fibrillation, acute renal failure, cardiogenic shock, total charges, and length of stay (LOS) increased with time (p < 0.05 for all). Compared to non-hypoglycemic patients, those who developed hypoglycemia were older and more likely to be black; only 6.7% had diabetes compared to 28.5% of STEMI patients (p = 0.001). Cardiovascular events were more likely to occur in hypoglycemia: mortality risk increased by almost 2.5-fold (adjusted OR = 2.625 [2.095–3.289]). There was a higher incidence of cardiogenic shock (adjusted OR = 1.718 [1.387–2.127]), atrial fibrillation (adjusted OR = 1.284 [1.025–1.607]), ventricular fibrillation (adjusted OR = 1.799 [1.406–2.301]), and acute renal failure (adjusted OR = 2.355 [1.902–2.917]). Patients who developed hypoglycemia were less likely to have PCI (OR = 0.596 [0.491–0.722]) but more likely to have CABG (OR = 1.792 [1.391–2.308]). They also had a longer in-hospital stay and higher charges/stay.
Conclusion: Hypoglycemia is a rare event in patients hospitalized with STEMI. However, it was found to have higher odds of mortality, arrhythmias, and other comorbidities, irrespective of diabetes.
Introduction
Despite all the advances in the management of coronary heart disease (CHD) in general and reperfusion therapy in particular, ST-elevation myocardial infarction (STEMI) still represents a significant medical, social, and economic burden worldwide (1). This burden is governed by many predisposing modifiable and non-modifiable risk factors for cardiovascular disease (CVD). For instance, in patients with first myocardial infarction, in-hospital mortality ranges between 3.6 and 14.9% and varies according to the number of risk factors (2).
Glycemic control is a significant risk factor for CVD. In patients with pre-diabetes or diabetes, poor control is associated with an excess of cardiovascular events, including STEMI (3). However, better control is associated with fewer in-hospital and 2-year major cardiovascular events in STEMI patients undergoing primary coronary intervention (4, 5).
While severe hyperglycemia encountered in diabetic ketoacidosis or hyperglycemic hyperosmolar states increases mortality and morbidity in STEMI, the impact of hypoglycemia is unknown. We, therefore, assessed the impact of hypoglycemia in patients hospitalized for STEMI and examined its trend.
Materials and methods
Study database
Data from the National Inpatient Sample (6) was analyzed. The NIS is the largest North American public inpatient database in healthcare, developed by the Healthcare Cost and Utilization Project (HCUP) and financed by the Agency for Healthcare Research and Quality (AHRQ) (NIS). NIS collects discharge level data from roughly 1,000 hospitals in the United States, with over 7 million hospital admissions added each year. Discharge weights are used to determine national estimates per the HCUP recommendation (NIS). The weighted data amounts to over 35 million hospital records representing a large national sample. NIS has no identifiable patient information, and all medical diagnoses and interventions are coded using the international classification of disease, 9th version (ICD-9) up till 2014, and the 10th version (ICD-10) 10 codes afterward. The codes have been validated and used in similar NIS studies (7–9) (Supplementary Table 1).
Study population and outcomes
Patients ≥18-year-old with a primary discharge diagnosis of STEMI between 2005 and 2017 were included in our study. The secondary diagnosis was in-hospital hypoglycemia. Exclusion criteria were unknown age, gender, length of stay, in-hospital outcome, and hospital cost. The primary outcome was in-hospital mortality. Secondary outcomes included: in-hospital ventricular tachycardia, ventricular fibrillation, atrial fibrillation, acute heart failure, stroke, acute renal failure, and procedural outcomes: primary coronary intervention (PCI), thrombolysis, and coronary artery bypass grafts (CABG).
Statistical analysis
Descriptive statistics were presented as frequencies distributions with percentages for categorical variables, while mean (standard deviation) or medians (interquartile range). Temporal changes were assessed using a trend test based on generalized linear models. A comparison of baseline characteristics of STEMI patients with vs. without hypoglycemia was performed using a Student’s t-test or a χ2 test. We first assessed temporal trends in characteristics and cardiovascular outcomes for every group, then combined data for all years to compare both groups. Multivariable logistic regression analysis was performed to look for predictors of mortality in patients with STEMI and diabetes; an interaction test was also used to look for an association between diabetes and hypoglycemia. Cardiovascular events were adjusted for baseline characteristics that are different among both groups, including age, gender, obesity, hypertension, dyslipidemia, peripheral vascular disease, diabetes, coronary artery disease, renal failure, hospital region, and the primary expected payer. We further used the receiver operating characteristic (ROC) curve to assess the predictive ability of our statistical model for the different outcomes after adjustments (10). Costs were corrected for inflation using rates provided by the US Bureau of labor statistics. A p-value <0.05 was considered statistically significant. Analyses were performed using SPSS (IBM, version 22.0) and STATA (version 15).
Results
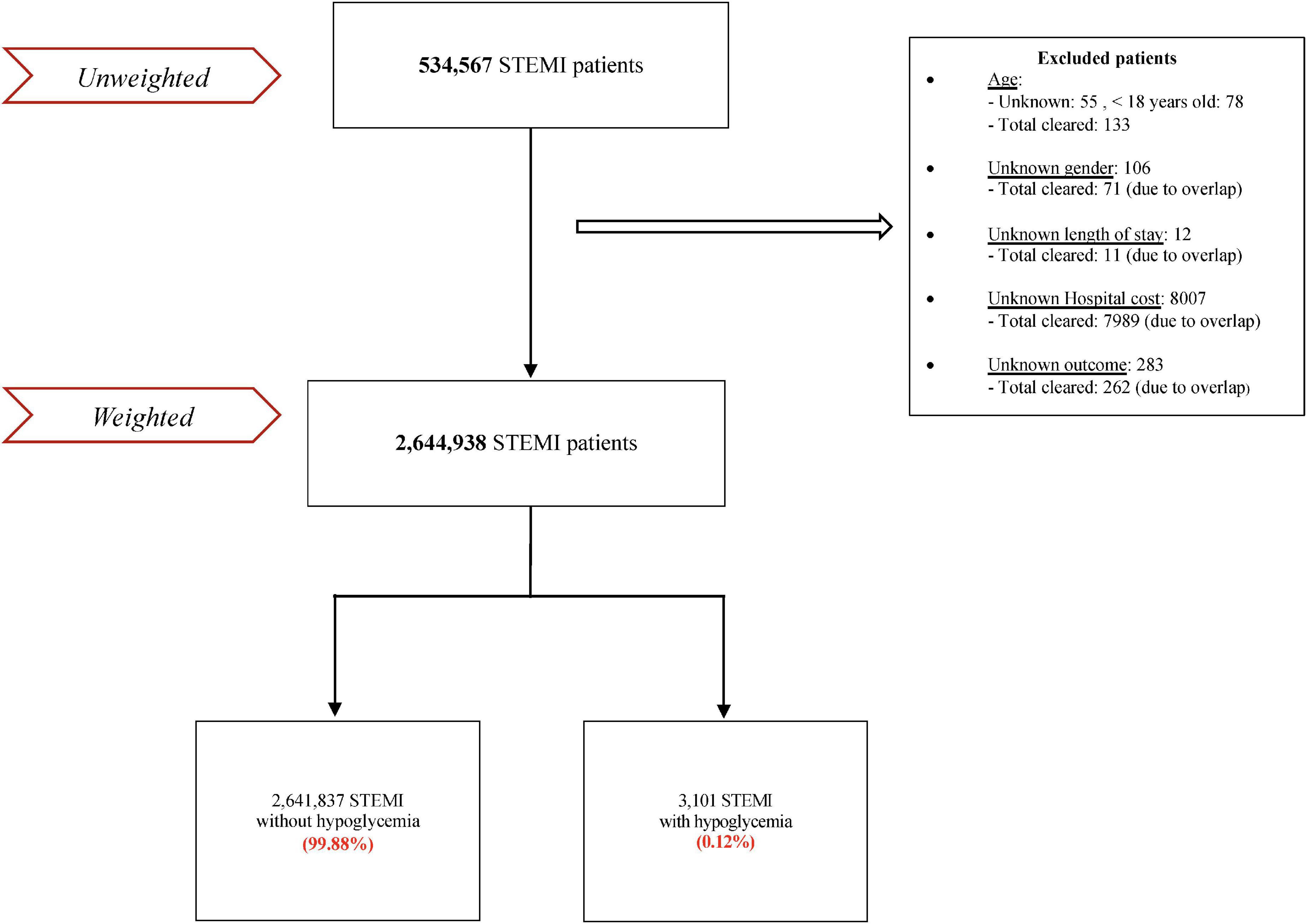
Five hundred thirty-four thousand five hundred sixty-seven patients were admitted for STEMI between January 1, 2005, and December 31, 2017. After excluding patients with missing data (8,466) and subsequently weighting, the total number of patients was 2,644,938, of which 3,101 had hypoglycemia (0.17%) (Figure 1).
STEMI patients with hypoglycemia
The number of patients with STEMI and hypoglycemia was almost unchanged during the follow-up period (Table 1). The mean age (±SD) dropped from 67 ± 15 in 2005 to 63 ± 12 in 2017 (p = 0.046). Among age categories, the percentage of patients aged 55-64 significantly increased with time, whereas patients older than 84 years decreased. The prevalence of CAD decreased from 56.3% in 2005 to 53% in 2017 (p = 0.005), whereas dyslipidemia followed an upward trend (p = 0.008). However, there were no changes in the temporal trend of the rest of the cardiovascular risk factors, including diabetes. Ventricular tachycardia, ventricular fibrillation, acute renal failure, and cardiogenic shock increased with time (p < 0.05 for all). Acute heart failure developed in at least 20% of patients, but no temporal trend was observed. The number of patients who had primary coronary intervention (PCI) increased by almost 2-fold between 2005 and 2017 (p < 0.001), but the prevalence of coronary artery bypass graft (CABG) and thrombolysis were unchanged.
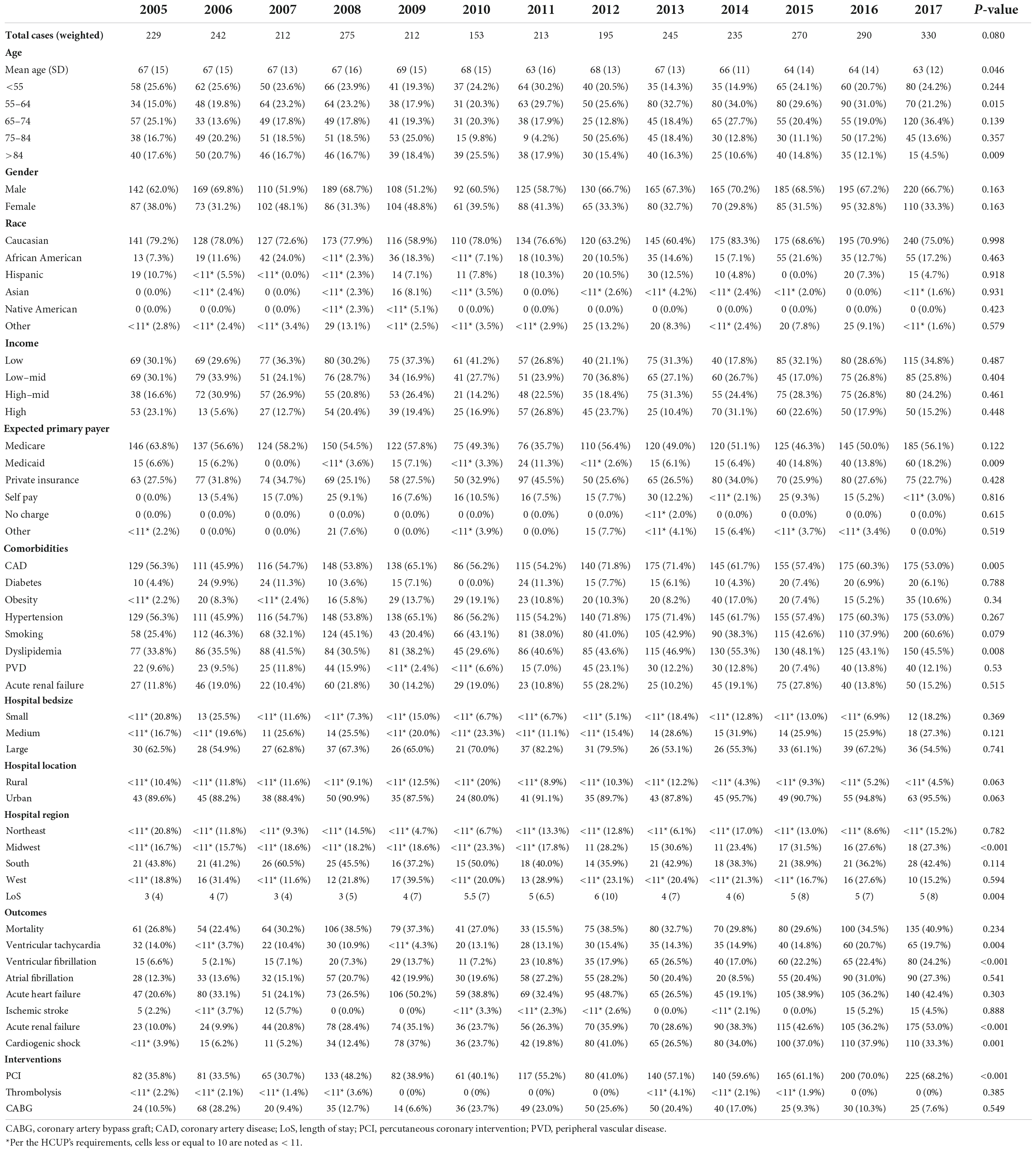
Table 1. Baseline characteristics, outcomes, and temporal trend of patients hospitalized for STEMI with a secondary diagnosis of hypoglycemia in the NIS database, between 2005 and 2017.
A drop in age was also observed in STEMI patients without hypoglycemia (66 ± 14 in 2005 to 66 ± 13 in 2017, p < 0.001) (Supplementary Table 1). All cardiovascular risk factors, including diabetes, followed a statistically significant upward trend. The prevalence of ventricular tachycardia, ventricular fibrillation, acute renal failure, and cardiogenic shock increased during observation, concordant with findings reported in STEMI patients with hypoglycemia. However, mortality decreased from 9.6 to 7.9% (p < 0.001).
Total charges increased in both groups, from 29,140 to 136,453 USD/stay in STEMI patients with hypoglycemia and from 39,032 to 82,134 USD/stay in those without hypoglycemia (Figure 2). Interestingly, the length of stay (LoS) increased from 3 (4) to 5 (8) in patients with hypoglycemia (p = 0.004) but was unchanged in those without hypoglycemia.
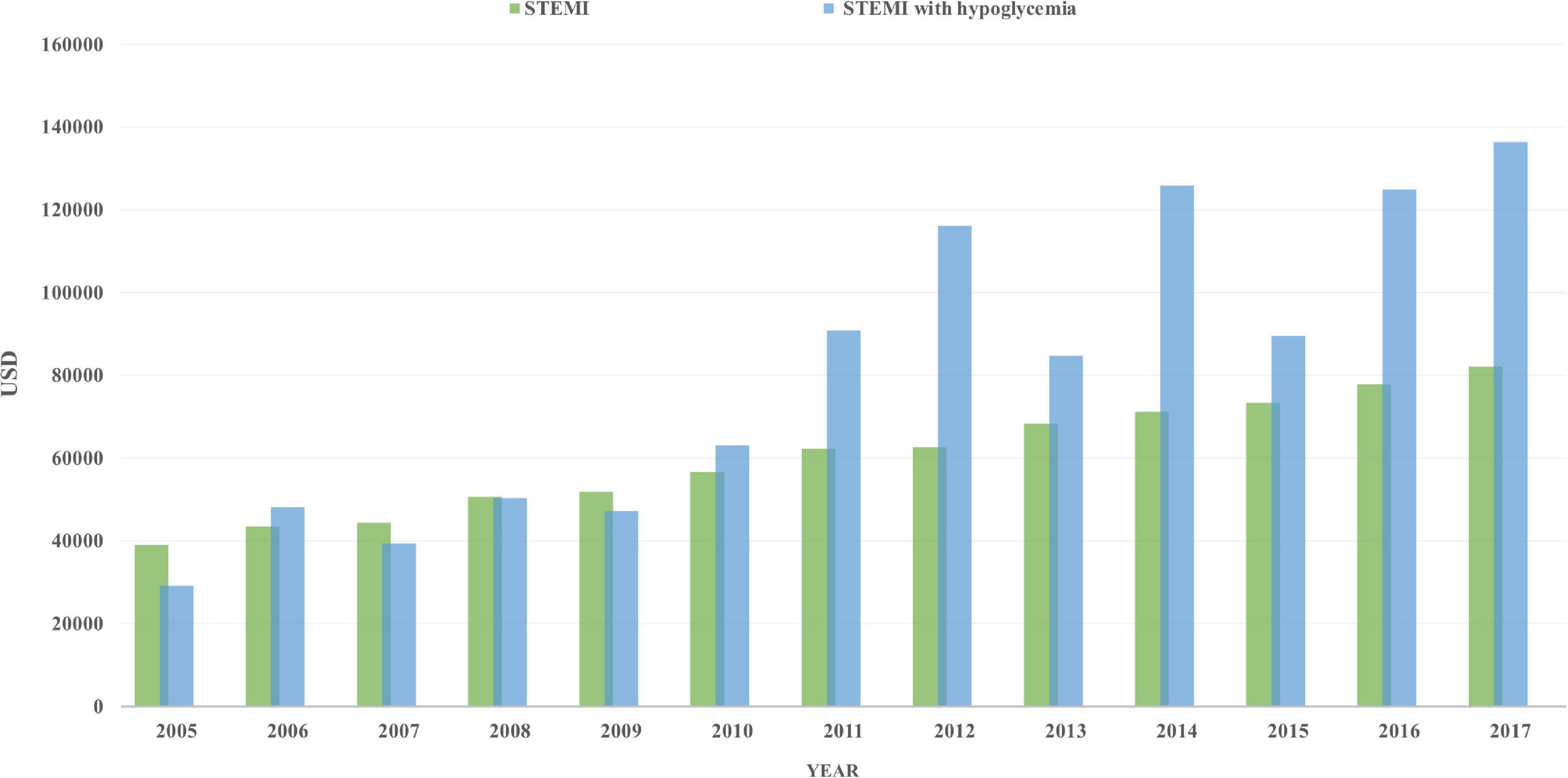
Figure 2. Total charges/stay (after adjustment for inflation) in STEMI patients with hypoglycemia (blue) and those without hypoglycemia (green), from 2005 to 2017.
Comparison of baseline characteristics and outcomes of both groups
Trend
We combined all STEMI patients with hypoglycemia from 2005 to 2017, then compared them to those without hypoglycemia for the same period. As seen in Table 2, patients with hypoglycemia were, on average, two years older (66 vs. 64 years old in STEMI without hypoglycemia), with a higher proportion of patients >84 years old (p < 0.001 for both). The male gender was dominant in both. Patients with STEMI and hypoglycemia were more likely Blacks and less likely to be Whites and private insurance (p < 0.001 for all). Surprisingly, only 6.7% of patients who developed hypoglycemia had diabetes compared to 28.5% of those who didn’t develop hypoglycemia (p < 0.001). Obesity, hypertension, and dyslipidemia were less prevalent in the hypoglycemia group, while renal failure and PVD were more prevalent.
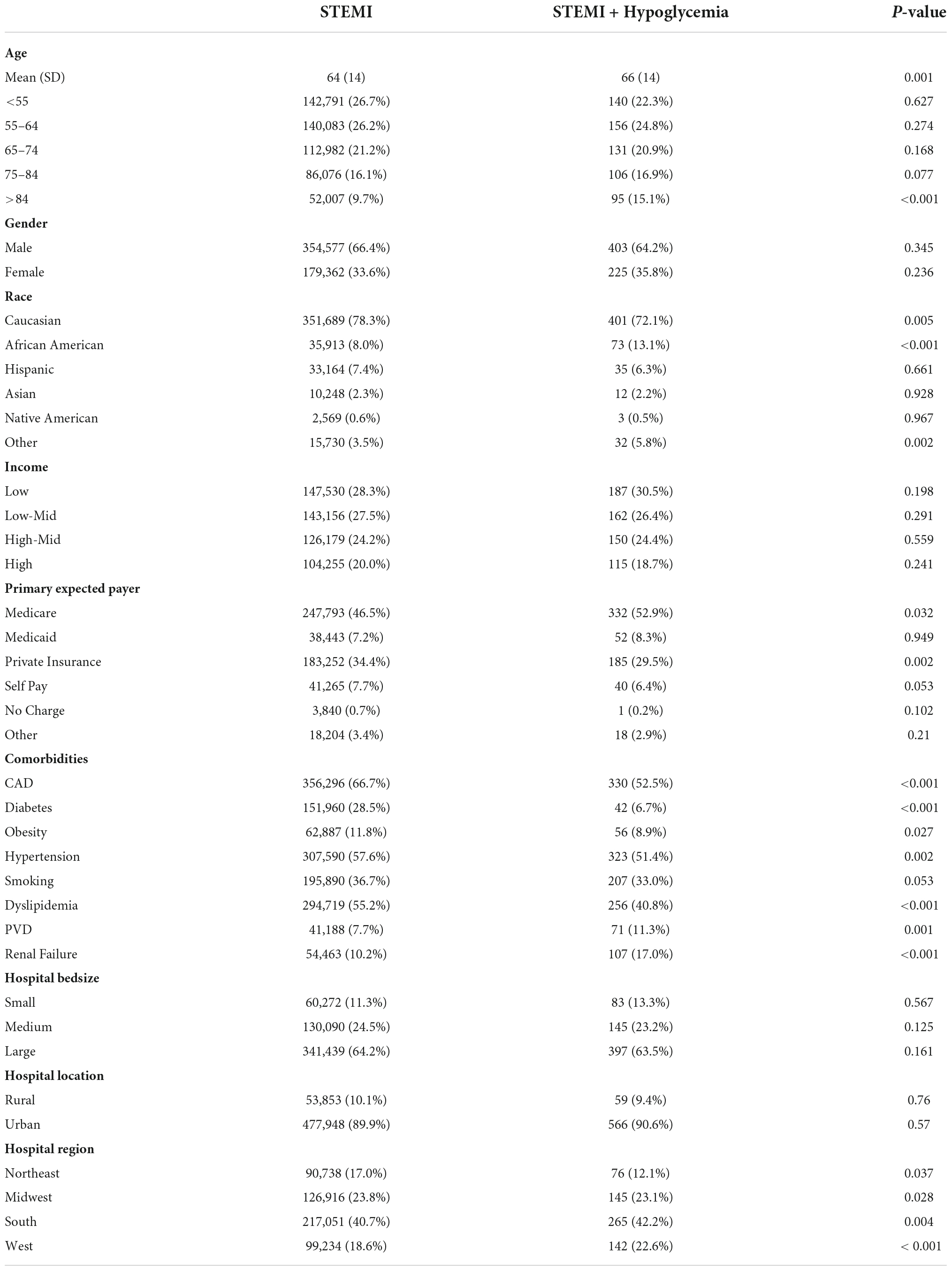
Table 2. Comparison of baseline characteristics of STEMI patients according to the presence of hypoglycemia.
Comparison of both groups
Cardiovascular events were more likely to occur in the presence of hypoglycemia (Table 3). Mortality risk increased by almost 4-fold and remained high after multivariable adjustment (adjusted OR = 2.625 [2.095–3.289]). There was a higher incidence of cardiogenic shock (adjusted OR = 1.718 [1.387–2.127]), atrial fibrillation (adjusted OR = 1.284 [1.025–1.607]), ventricular fibrillation (adjusted OR = 1.799 [1.406–2.301]), and acute renal failure (adjusted OR = 2.355 [1.902–2.917]). The ROC curve showed that all models were adequate and fit. The multivariable model had an excellent prediction of mortality (AUC = 0.84, p < 0.001) (Figure 3) and acute renal failure (AUC = 0.82, p < 0.001) (Figure 4A), a good prediction for cardiogenic shock (AUC = 0.72, p < 0.001) (Figure 4B), atrial fibrillation (AUC = 0.73, p < 0.001) (Figure 4C), but a moderate prediction for ventricular fibrillation (AUC = 0.65, p < 0.001) (Figure 4D).
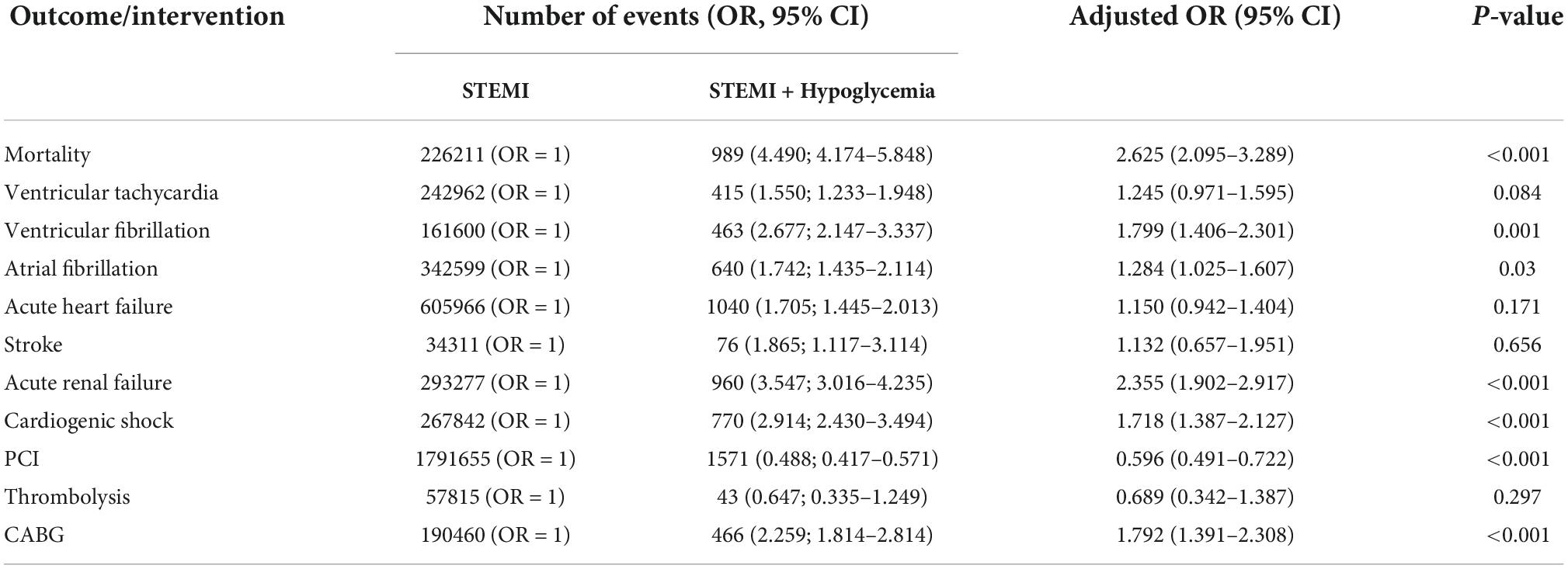
Table 3. Cardiovascular outcomes and interventions STEMI patients according to the presence of hypoglycemia.
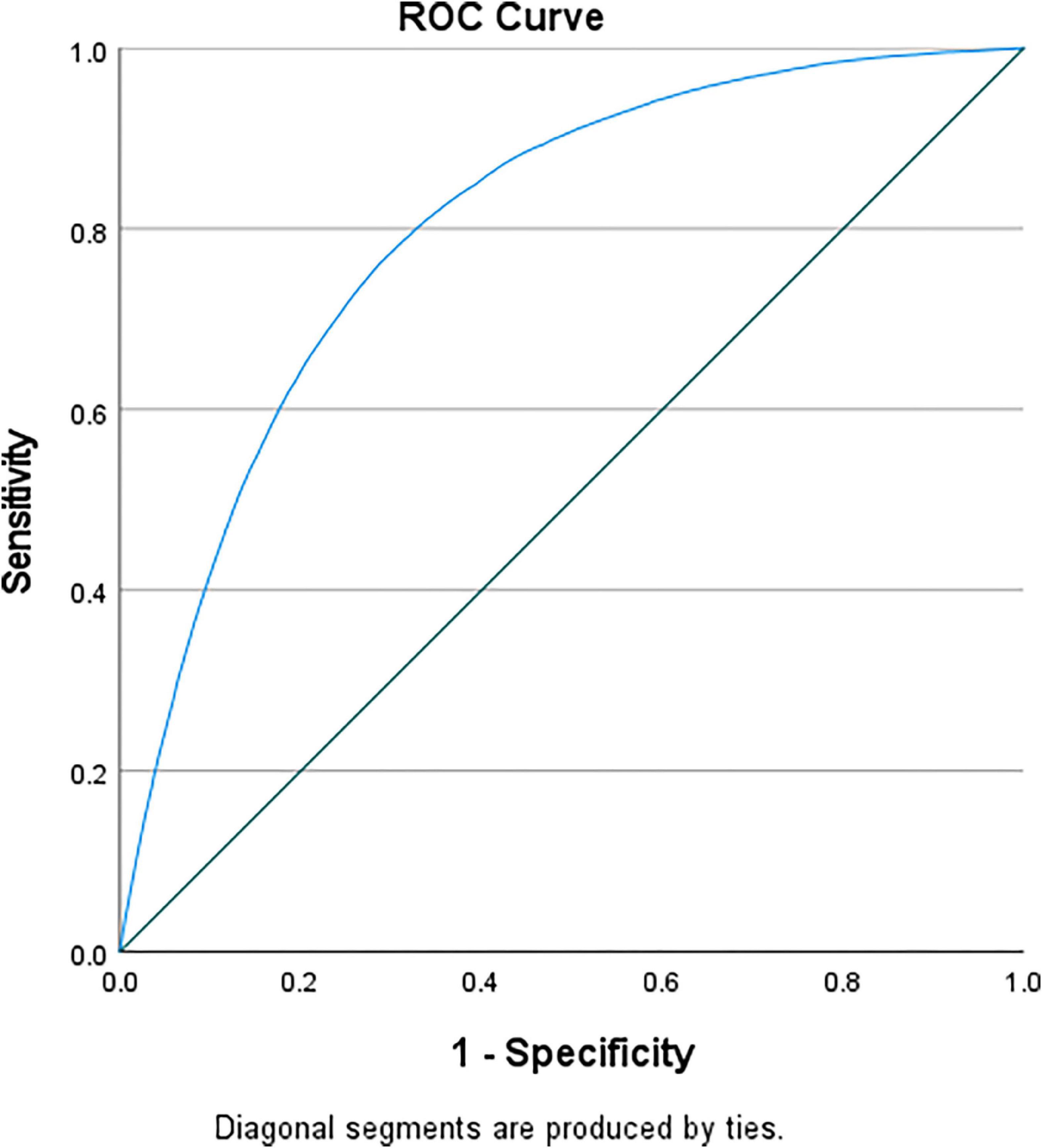
Figure 3. ROC curve testing the prediction of mortality of the multivariable regression model in patients with STEMI and diabetes.
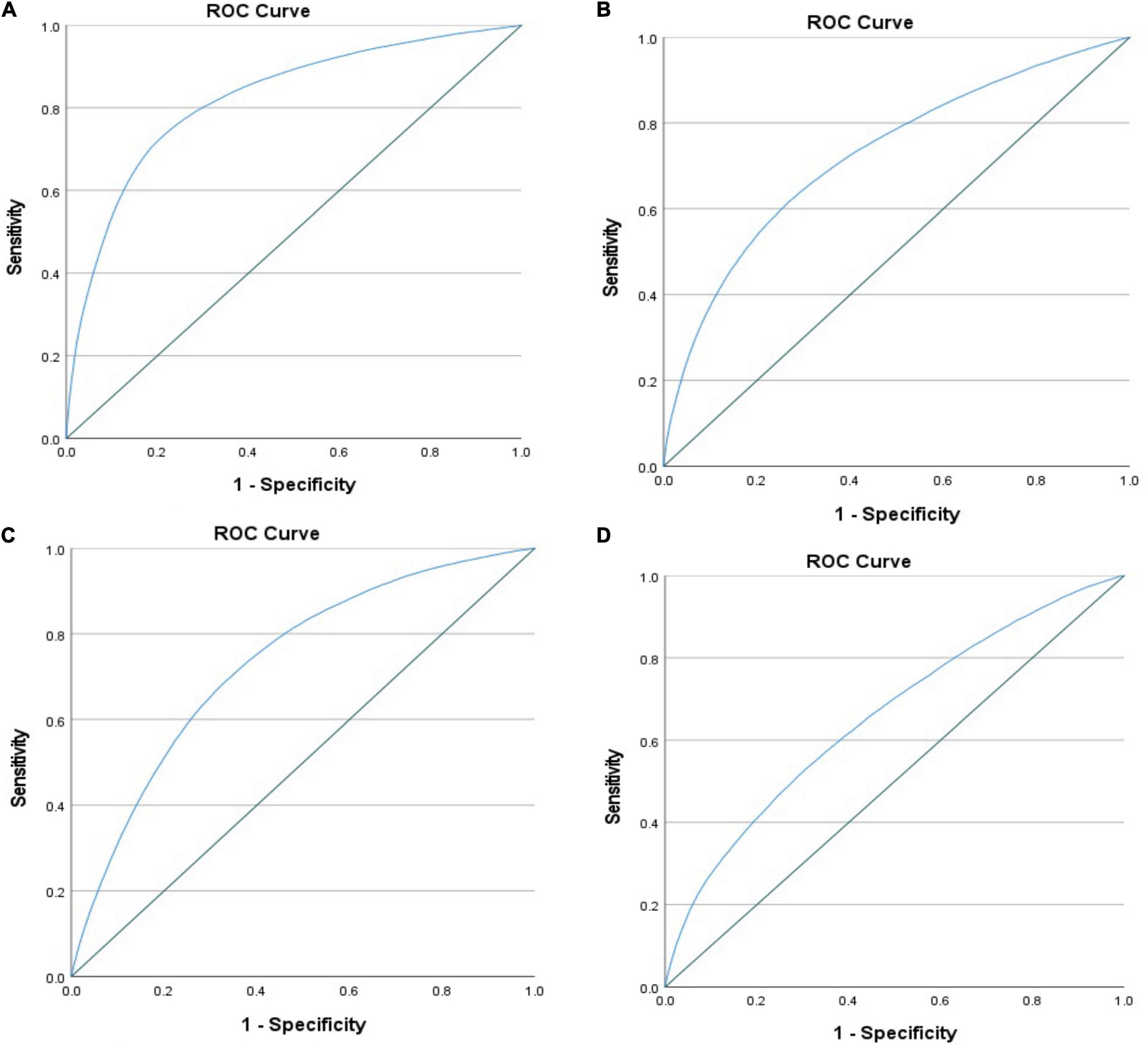
Figure 4. ROC curve testing the prediction of the multivariable regression model in patients with STEMI and diabetes regarding (A) acute renal failure, (B) cardiogenic shock, mortality, (C) atrial fibrillation, and (D) ventricular fibrillation.
A higher incidence of ventricular tachycardia, acute heart failure, and stroke was observed in STEMI patients with hypoglycemia, but events were not statistically significant after adjustment. Patients with STEMI and diabetes were less likely to have PCI (OR = 0.596 [0.491–0.722]) but more likely to have CABG (OR = 1.792 [1.391–2.308]). Total charges/stay were higher in patients with hypoglycemia than those without (80,466 vs. 60,319 USD; respectively; p < 0.001). Unsurprisingly, patients who developed hypoglycemia had a slightly longer mean LoS (4[3] vs. 3[3] days, hypoglycemia versus non-hypoglycemia, p = 0.01).
Predictors of mortality in STEMI patients with hypoglycemia
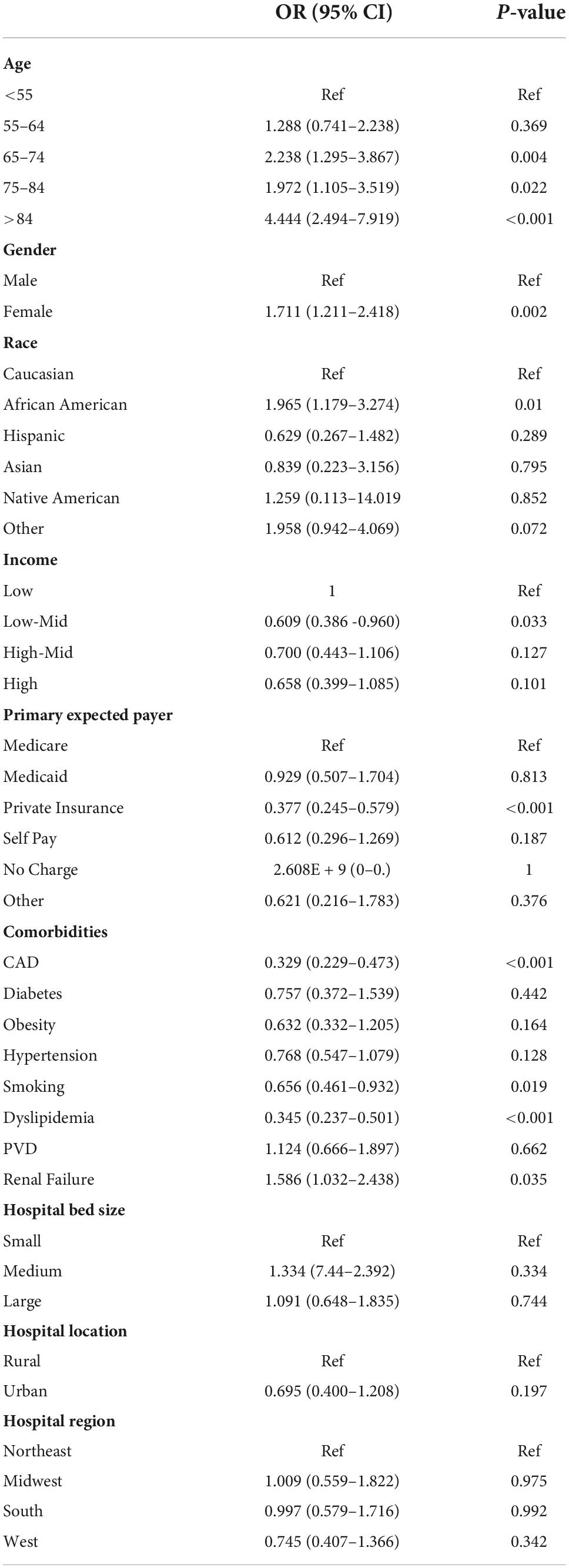
We further looked for predictors of mortality in STEMI patients with hypoglycemia. As expected, old age is associated with higher mortality risk. For instance, patients over 84 years have an almost 4.5-fold higher risk of dying than those 55 years of age or younger (Table 4). Females are more predisposed than males (OR = 1.711 [1.211–2.418]), and Blacks have a doubled risk as compared to Whites (OR = 1.965 [1.179–3.274]). Valvular heart disease, renal failure, and peripheral vascular disease – but not coronary artery disease- increased the risk significantly. Interestingly, cardiometabolic risk factors such as obesity, hypertension, dyslipidemia, and smoking were associated with lower mortality risk. Surprisingly, diabetes did not predict mortality (OR = 0.757 [0.372–1.539]). To validate our findings, we tested the interaction between diabetes and hypoglycemia and did not observe any (p interaction = 0.318).
Discussion
Hypoglycemia is consistently associated with mortality in critically ill patients admitted to the intensive care unit. In a retrospective analysis of US and Dutch cohorts, Krinsley et al. reported an excess of hospital mortality in ICU patients who developed hypoglycemia (11). Those findings were confirmed in an Australian study of almost 5,000 ICU patients. Interestingly, the mortality risk was significantly higher even in patients with mild to moderate hypoglycemia (12). In the NICE-SUGAR study, tight glucose control in intensive care patients led to more hypoglycemic events and higher mortality (13). A higher incidence of hypoglycemia was also reported by the investigators of the CHiP study in pediatric critically ill patients (14).
Data assessing the cardiovascular outcomes of hypoglycemia in STEMI is missing. To our knowledge, we are the first to explore trends across risk factors and outline excess mortality and morbidity in STEMI patients irrespective of diabetes. However, it has already been shown in smaller-scale experimental models showed that hypoglycemia is associated with ischemic changes in patients with diabetes. Using a 3-day continuous glucose monitoring in 19 patients with diabetes and CAD, Desouza et al. reported chest pain and non-specific ECG changes on Holter monitoring during hypoglycemia (15). Similar studies revealed the presence of ectopic atrial beats and QTc prolongation; findings were confirmed in a meta-analysis that included 15 of those studies (16). Changes in the ST segment have also been reported in case reports from Israel (17) and the United Kingdom (18).
Our data aligns with several epidemiological cohorts and ad hoc analyses of trials that showed an increased mortality risk with hypoglycemia in the non-ICU diabetes population. Earlier in the ’70s, a British cohort reported an excess mortality risk of 4% with hypoglycemia in diabetes patients under 50 years of age (19). In a recent Norwegian study, the mortality risk was 8% in type 1 diabetes patients younger than 56 years old of age (20). In the ORIGIN trial that included over 12,000 patients with type 2 diabetes, mortality risk was increased by almost 2-fold in the presence of severe hypoglycemia. Similar findings have been reported with type 2 diabetes patients in the DEVOTE 3 and EXAMINE trials (21, 22). In a recent post hoc analysis of the ACCORD study, severe hypoglycemia increased the risk of heart failure by almost 50% (23).
Strict glycemic control in patients with diabetes, particularly in long-standing diabetes with complications, is not recommended by the American Diabetes Association and similar cardiac societies for almost a decade now (24, 25). Three large trials—ACCORD (26), VADT (27), and ADVANCE (28)—failed to show any survival benefit with an intensive glycemic control approach in high-risk patients. Further, the ACCORD study revealed a deleterious effect of aggressive anti-hyperglycemic therapy as mortality increased. Furthermore, the three studies showed an association between hypoglycemic events and mortality, although causality was not established. In a meta-analysis that regrouped those trials and the older UKPDS study, Kelly et al. showed that intensive treatment did not reduce the risk of all-cause or cardiovascular mortality but increased episodes of severe hypoglycemia (29). However, newer anti-diabetes medications are safer (30). DDP-IV inhibitors improve HBA1C without inducing hypoglycemia; nevertheless, they do not reduce cardiovascular events in the long term (31). GLP-1 agonists do not cause hypoglycemia when administered to non-diabetes individuals or those with diabetes not otherwise treated with other classes (32, 33). However, hypoglycemic episodes have been reported when GLP-1 agonists were combined with insulin or sulfonylureas (34), which is usually handled by decreasing the dose of the latter two classes.
Similarly, SGLT-2 inhibitors decrease glycemia by blocking the reabsorption of filtered glucose in the presence of hyperglycemia. However, the excretion of glucose almost ceases in the presence of normoglycemia (35). Thus, they do not usually induce hypoglycemia in the absence of therapies that otherwise cause hypoglycemia.
It is not entirely understood why hypoglycemia increases cardiovascular risk. Further, a clear causality role between hypoglycemia and cardiovascular events could not be established. In the ADVANCE trial, repeated episodes of severe hypoglycemia did not correlate with the cardiovascular outcome; hence, hypoglycemia could have been only a marker of vulnerability (36). Nevertheless, several experimental studies suggest a direct novice effect of hypoglycemia on endothelial function, cardiac repolarization, and blood coagulability. Hypoglycemia activates the sympathoadrenal system, which results in a release of catecholamines. In experimental models, catecholamines induce blood coagulation abnormalities, including platelets and factor VII activation, resulting in a pro-inflammatory status mediated by CRP, VEGF, and interleukin 6 (37). Rana et al. reported a decreased myocardial blood flow reserve in healthy volunteers and type 1 diabetes subjects following a hypoglycemic clamp (38). Human umbilical vein endothelial cells exposed to hypoglycemia exhibit decreased nitric oxide and increased superoxide, both pathways leading to endothelial dysfunction (39).
Interestingly, hypoglycemia or related mortality did not decrease during the observation study. However, hospitalizations and mortality decreased in STEMI patients without hypoglycemia, which is concordant with the current bibliography pertinent to cardiovascular disease trends and patterns. An average 3% yearly reduction in MI-related mortality was reported in a 10-year analysis of the Medicare (40). We have recently shown that diabetes-related mortality is in a descending slot in patients with heart failure (41), stroke (42), and aortic valve replacement (43) despite the increase in the prevalence of diabetes and associated risk factors. Surprisingly, some of the classically harmful cardiometabolic parameters, such as hypertension, dyslipidemia, and smoking, were associated with decreased mortality in our study, findings reported in previous cardiovascular studies from the NIS pertinent to myocardial infarction, heart failure, stroke, and aortic valve replacement (41–45).
Contrary to our expectations, smoking was associated with lower mortality risk. Although correlation does not equate to causation, one plausible mechanism behind this paradoxical finding is that smokers usually receive more cardioprotective drugs known to decrease mortality, such as anti-platelets. In a sub-analysis of the CHARISMA trial, clopidogrel reduced mortality and major cardiovascular events in current smokers but not in former or never smokers (46). It might also be possible that smokers are relatively younger and have fewer cardiovascular comorbidities on admission, which has been recently reported in ST-elevation myocardial infarction patients who had lower mortality risk when undergoing PCI (47).
We acknowledge the presence of limitations in this study. Our cohort is an administrative database that was not designed to assess cardiovascular events or show causality. The time-to-first event is missing in the NIS database, making creating a Cox proportional hazards regression model, rather than a multivariable logistic regression model, impossible. It might also be possible that the multivariable logistic regression model subjects the same data set to multiple testing, increasing the risk of false positives. Other important parameters of hypoglycemia are missing, such as baseline medications, glycemic control, and diabetes duration, knowing that those factors are strong predictors of hypoglycemia-related mortality; hence, we could not account for those factors in our regression model. Further, it is unknown whether hypoglycemic events were symptomatic or silent, nor their severity. Finally, all diagnoses and outcomes were based on ICD-9 and ICD-10, so we cannot exclude erroneous coding or misclassification, including in 2015 when the transition between the 9th and 10th versions occurred.
Conclusion
In this large observational cohort, the prevalence of hypoglycemia in patients hospitalized for ST-elevation myocardial infarction is rare. Nevertheless, it was associated with increased mortality, atrial fibrillation, ventricular fibrillation, and acute renal failure, irrespective of diabetes at baseline. Although our results need to be duplicated in different cohorts, particularly cardiac cohorts of myocardial infarction, the findings illustrated in this work stress the importance of avoiding hypoglycemia in patients presenting with an acute coronary syndrome, particularly in vulnerable patients. With mounting data supporting the deleterious effect of hyperglycemia and hypoglycemia in critically ill cardiac patients, reaching normoglycemia is an important goal that cardiologists and endocrinologists should aim for.
Data availability statement
All datasets generated for this study are included in the article/Supplementary material.
Ethics statement
The study received administrative IRB approval from Weill Cornell Medicine-Qatar as it contains only de-identified data (record number 18-00017). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
CAK made conception and design and was the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. BH made acquisition of data and prepared figures. ZM and SD performed the statistical analyses. BH, JA, HJ, and CAK made analysis and interpretation of the data. BH and CAK wrote the manuscript. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.940035/full#supplementary-material
References
1. Myerson M, Coady S, Taylor H, Rosamond WD, Goff DC Jr, Investigators A. Declining severity of myocardial infarction from 1987 to 2002: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. (2009) 119:503–14. doi: 10.1161/CIRCULATIONAHA.107.693879
2. Canto JG, Kiefe CI, Rogers WJ, Peterson ED, Frederick PD, French WJ, et al. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA. (2011) 306:2120–7. doi: 10.1001/jama.2011.1654
3. Huang D, Refaat M, Mohammedi K, Jayyousi A, Al Suwaidi J, Abi Khalil C. Macrovascular complications in patients with diabetes and prediabetes. Biomed Res Int. (2017) 2017:7839101. doi: 10.1155/2017/7839101
4. Marfella R, Sasso FC, Siniscalchi M, Paolisso P, Rizzo MR, Ferraro F, et al. Peri-procedural tight glycemic control during early percutaneous coronary intervention is associated with a lower rate of in-stent restenosis in patients with acute ST-elevation myocardial infarction. J Clin Endocrinol Metab. (2012) 97:2862–71. doi: 10.1210/jc.2012-1364
5. Li Y, Li X, Zhang Y, Zhang L, Wu Q, Bai Z, et al. Impact of glycemic control status on patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention. BMC Cardiovasc Disord. (2020) 20:36. doi: 10.1186/s12872-020-01339-x
6. National Inpatient Sample [NIS]. Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality (2019).
7. Lipska KJ, Ross JS, Wang Y, Inzucchi SE, Minges K, Karter AJ, et al. National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med. (2014) 174:1116–24. doi: 10.1001/jamainternmed.2014.1824
8. Issa M, Alqahtani F, Berzingi C, Al-Hajji M, Busu T, Alkhouli M. Impact of acute diabetes decompensation on outcomes of diabetic patients admitted with ST-elevation myocardial infarction. Diabetol Metab Syndr. (2018) 10:57. doi: 10.1186/s13098-018-0357-y
9. Patel N, Gupta A, Doshi R, Kalra R, Bajaj NS, Arora G, et al. In-hospital management and outcomes after ST-segment-elevation myocardial infarction in medicaid beneficiaries compared with privately insured individuals. Circ Cardiovasc Qual Outcomes. (2019) 12:e004971. doi: 10.1161/CIRCOUTCOMES.118.004971
10. Zou KH, O’malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. (2007) 115:654–7. doi: 10.1161/CIRCULATIONAHA.105.594929
11. Krinsley JS, Schultz MJ, Spronk PE, Harmsen RE, Van Braam Houckgeest F, Van Der Sluijs JP, et al. Mild hypoglycemia is independently associated with increased mortality in the critically ill. Crit Care. (2011) 15:R173. doi: 10.1186/cc10322
12. Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, et al. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc. (2010) 85:217–24. doi: 10.4065/mcp.2009.0394
13. NICE-SUGAR Study Investigators, Finfer S, Chittock DR, Su SY, Blair D, Foster D, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. (2009) 360:1283–97. doi: 10.1056/NEJMoa0810625
14. Macrae D, Grieve R, Allen E, Sadique Z, Morris K, Pappachan J, et al. A randomized trial of hyperglycemic control in pediatric intensive care. N Engl J Med. (2014) 370:107–18. doi: 10.1056/NEJMoa1302564
15. Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care. (2003) 26:1485–9. doi: 10.2337/diacare.26.5.1485
16. Fitzpatrick C, Chatterjee S, Seidu S, Bodicoat DH, Ng GA, Davies MJ, et al. Association of hypoglycaemia and risk of cardiac arrhythmia in patients with diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab. (2018) 20:2169–78. doi: 10.1111/dom.13348
17. Markel A, Keidar S, Yasin K. Hypoglycaemia-induced ischaemic ECG changes. Presse Med. (1994) 23:78–9.
18. Saunders FM, Llewellyn T. Silent myocardial infarction during hypoglycaemic coma. J Accid Emerg Med. (1996) 13:357–8. doi: 10.1136/emj.13.5.357
19. Tunbridge WM. Factors contributing to deaths of diabetics under fifty years of age. On behalf of the Medical Services Study Group and British Diabetic Association. Lancet. (1981) 2:569–72. doi: 10.1016/s0140-6736(81)90950-8
20. Gagnum V, Stene LC, Jenssen TG, Berteussen LM, Sandvik L, Joner G, et al. Causes of death in childhood-onset type 1 diabetes: long-term follow-up. Diabet Med. (2017) 34:56–63. doi: 10.1111/dme.13114
21. ORIGIN Trial Investigators, Mellbin LG, Ryden L, Riddle MC, Probstfield J, Rosenstock J, et al. Does hypoglycaemia increase the risk of cardiovascular events? A report from the ORIGIN trial. Eur Heart J. (2013) 34:3137–44. doi: 10.1093/eurheartj/eht332
22. Pieber TR, Marso SP, Mcguire DK, Zinman B, Poulter NR, Emerson SS, et al. DEVOTE 3: temporal relationships between severe hypoglycaemia, cardiovascular outcomes and mortality. Diabetologia. (2018) 61:58–65. doi: 10.1007/s00125-017-4422-0
23. Echouffo-Tcheugui JB, Kaze AD, Fonarow GC, Dagogo-Jack S. Severe hypoglycemia and incident heart failure among adults with type 2 diabetes. J Clin Endocrinol Metab. (2022) 107:e955–62. doi: 10.1210/clinem/dgab794
24. American Diabetes Association. 6 Glycemic targets: standards of medical care in diabetes-2020. Diabetes Care. (2020) 43:S66–76. doi: 10.2337/dc20-S006
25. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. (2020) 41:255–323.
26. Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. (2005) 65:385–411. doi: 10.2165/00003495-200565030-00005
27. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. (2009) 360:129–39. doi: 10.1056/NEJMoa0808431
28. ADVANCE Collaborative Group, Patel A, Macmahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. (2008) 358:2560–72. doi: 10.1056/NEJMoa0802987
29. Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. (2009) 151:394–403. doi: 10.7326/0003-4819-151-6-200909150-00137
30. Nasrallah MP, Abi Khalil C, Refaat MM. The landscape of glucose-lowering therapy and cardiovascular outcomes: from barren land to metropolis. Biomed Res Int. (2017) 2017:9257930. doi: 10.1155/2017/9257930
31. Karagiannis T, Boura P, Tsapas A. Safety of dipeptidyl peptidase 4 inhibitors: a perspective review. Ther Adv Drug Saf. (2014) 5:138–46. doi: 10.1177/2042098614523031
32. Lerche S, Soendergaard L, Rungby J, Moeller N, Holst JJ, Schmitz OE, et al. No increased risk of hypoglycaemic episodes during 48 h of subcutaneous glucagon-like-peptide-1 administration in fasting healthy subjects. Clin Endocrinol. (2009) 71:500–6. doi: 10.1111/j.1365-2265.2008.03510.x
33. Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. (2009) 32:84–90. doi: 10.2337/dc08-1355
34. Huthmacher JA, Meier JJ, Nauck MA. Efficacy and safety of short- and long-acting glucagon-like peptide 1 receptor agonists on a background of basal insulin in type 2 diabetes: a meta-analysis. Diabetes Care. (2020) 43:2303–12. doi: 10.2337/dc20-0498
35. List JF, Whaley JM. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int Suppl. (2011) 120:S20–7. doi: 10.1038/ki.2010.512
36. Zoungas S, Patel A, Chalmers J, De Galan BE, Li Q, Billot L, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. (2010) 363:1410–8. doi: 10.1056/NEJMoa1003795
37. International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. (2019) 7:385–96. doi: 10.1016/S2213-8587(18)30315-2
38. Rana O, Byrne CD, Kerr D, Coppini DV, Zouwail S, Senior R, et al. Acute hypoglycemia decreases myocardial blood flow reserve in patients with type 1 diabetes mellitus and in healthy humans. Circulation. (2011) 124:1548–56. doi: 10.1161/CIRCULATIONAHA.110.992297
39. Wang J, Alexanian A, Ying R, Kizhakekuttu TJ, Dharmashankar K, Vasquez-Vivar J, et al. Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arterioscler Thromb Vasc Biol. (2012) 32:712–20. doi: 10.1161/ATVBAHA.111.227389
40. Setoguchi S, Glynn RJ, Avorn J, Mittleman MA, Levin R, Winkelmayer WC. Improvements in long-term mortality after myocardial infarction and increased use of cardiovascular drugs after discharge: a 10-year trend analysis. J Am Coll Cardiol. (2008) 51:1247–54. doi: 10.1016/j.jacc.2007.10.063
41. Mekhaimar M, Dargham S, El-Shazly M, Al Suwaidi J, Jneid H, Abi Khalil C. Diabetes-related cardiovascular and economic burden in patients hospitalized for heart failure in the US: a recent temporal trend analysis from the National Inpatient Sample. Heart Fail Rev. (2021) 26:289–300. doi: 10.1007/s10741-020-10012-6
42. Tabbalat A, Dargham S, Al Suwaidi J, Aboulsoud S, Al Jerdi S, Abi Khalil C. Mortality and socio-economic outcomes among patients hospitalized for stroke and diabetes in the US: a recent analysis from the National Inpatient Sample. Sci Rep. (2021) 11:8204. doi: 10.1038/s41598-021-87320-w
43. Khan S, Dargham S, Al Suwaidi J, Jneid H, Abi Khalil C. Trends and outcomes of aortic valve replacement in patients with diabetes in the US. Front Cardiovasc Med. (2022) 9:844068. doi: 10.3389/fcvm.2022.844068
44. Ahmed B, Davis HT, Laskey WK. In-hospital mortality among patients with type 2 diabetes mellitus and acute myocardial infarction: results from the national inpatient sample, 2000-2010. J Am Heart Assoc. (2014) 3:e001090. doi: 10.1161/JAHA.114.001090
45. Ali B, Dargham S, Al Suwaidi J, Jneid H, Abi Khalil C. Temporal trends in outcomes of st-elevation myocardial infarction patients with heart failure and diabetes. Front Physiol. (2022) 13:803092. doi: 10.3389/fphys.2022.803092
46. Berger JS, Bhatt DL, Steinhubl SR, Shao M, Steg PG, Montalescot G, et al. Smoking, clopidogrel, and mortality in patients with established cardiovascular disease. Circulation. (2009) 120:2337–44. doi: 10.1161/CIRCULATIONAHA.109.866533
Keywords: myocardial infarction, hypoglycemia, diabetes, mortality, arrhythmias, cardiovascular disease, ST-elevation myocardial infarction
Citation: Humos B, Mahfoud Z, Dargham S, Al Suwaidi J, Jneid H and Abi Khalil C (2022) Hypoglycemia is associated with a higher risk of mortality and arrhythmias in ST-elevation myocardial infarction, irrespective of diabetes. Front. Cardiovasc. Med. 9:940035. doi: 10.3389/fcvm.2022.940035
Received: 09 May 2022; Accepted: 31 August 2022;
Published: 10 October 2022.
Edited by:
Rajeev Gupta, Emirates International Hospital, United Arab EmiratesReviewed by:
Raman Kutty, Amala Cancer Research Centre, IndiaDijana Stojanovic, University of Niš, Serbia
Copyright © 2022 Humos, Mahfoud, Dargham, Al Suwaidi, Jneid and Abi Khalil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charbel Abi Khalil, Y2hhMjAyMkBtZWQuY29ybmVsbC5lZHU=
 Basel Humos
Basel Humos Ziyad Mahfoud
Ziyad Mahfoud Soha Dargham
Soha Dargham Jassim Al Suwaidi
Jassim Al Suwaidi Hani Jneid3
Hani Jneid3 Charbel Abi Khalil
Charbel Abi Khalil