
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med., 18 August 2022
Sec. Cardiac Rhythmology
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.938735
This article is part of the Research TopicCase Reports in Cardiac Rhythmology: 2022View all 19 articles
Cardiac implantable electronic devices (CIED) including pacemakers (PM), implantable cardioverter defibrillators (ICD), and cardiac resynchronized therapy (CRT) have become the mainstay of therapy for many cardiac conditions, consequently drawing attention to the risks and benefits of these procedures. Although CIED implantation is usually a safe procedure, pneumothorax remains an important complication and may contribute to increased morbidity, mortality, length of stay, and hospital costs. On the other hand, pneumopericardium and pneumomediastinum are rare but potentially fatal complications. Accordingly, a high degree of awareness about these complications is important. Pneumothorax almost always occurs on the ipsilateral side of implantation. The development of contralateral pneumothorax is uncommon and may be undetected on an initial chest radiograph. Contralateral pneumothorax with concurrent pneumopericardium and pneumomediastinum is much rarer. We describe a rare case of concurrent right-sided pneumothorax with pneumopericardium and pneumomediastinum after left-sided pacemaker implantation and highlight the risk factors, management, and possible ways to prevent the complications.
Cardiac implantable electronic devices (CIED) including pacemakers (PM), implantable cardioverter defibrillators (ICD), and cardiac resynchronized therapy (CRT) have become the mainstay of therapy for many cardiac conditions, consequently drawing attention to the risks and benefits of these procedures (1, 2). Although CIED implantation is usually safe, pneumothorax has been reported to occur in 0.51–2.24%, however this reported incidence may have been overestimated (3). In recent years, many CIED implantations have moved to outpatient settings. Outpatient CIED implantations are not included in the National Inpatient Sample (NIS) database, which is the largest publicly available all-payer inpatient care database in the United States. Since the subgroup of outpatients who developed CIED-associated pneumothorax and were later hospitalized (included in the NIS, while outpatients without pneumothorax were not), the NIS database incidence of pneumothorax may have been artificially elevated (an accurate numerator with a falsely low denominator) (3). Furthermore, it seems likely that improved medical knowledge and the development of safer procedures, would have reduced the incidence of pneumothorax over time (3). Nevertheless, pneumothorax remains an important complication of CIED implants and may contribute to increased morbidity, mortality, length of stay, and hospital costs, especially when a chest tube is required (4–7). On the other hand, pneumopericardium and pneumomediastinum are rare but potentially fatal complications (8). Accordingly, a high degree of awareness about these complications is important.
Pneumothorax almost always occurs on the ipsilateral side of implantation. The development of contralateral pneumothorax is uncommon and may be undetected on an initial chest radiograph (9, 10). Contralateral pneumothorax with concurrent pneumopericardium and pneumomediastinum is much rarer (11). We describe a rare case of concurrent right-sided pneumothorax with pneumopericardium and pneumomediastinum after left-sided pacemaker implantation and highlight the risk factors, management, and possible ways to prevent these complications.
A 76-year-old man underwent dual-chamber permanent pacemaker (PPM) implantation due to sick sinus syndrome. He was 173 cm in height, 59 kg in weight, and had a body mass index (BMI) of 20 kg/m2. He had a past medical history of heavy smoking, paroxysmal atrial fibrillation, coronary atherosclerosis, and bilateral pulmonary emphysema.
A dual-chamber pacemaker (BIOTRONIK EVIA DR) was inserted using active fixation leads (Biotronik Solia S60, Biotronik Solia S53) through the left subclavian vein into the right ventricular outflow tract (RVOT) and the right atrial appendage respectively. At implantation, the parameters of the pacemaker were satisfactory. The atrial lead pacing threshold was 0.6 V at 0.4 ms and the impedance was 532 Ω. The sensed P wave was 2.3 mV. The pacing threshold of the ventricular lead was also appropriate measuring 0.4 V at 0.4 ms and the impedance 661 Ω. The sensed R wave was 11.2 mV.
The implantation procedure was completed uneventfully. Two h after the implantation, the chest radiographs revealed acceptable lead positions and no evidence of pneumothorax. There was no pericardial effusion on echocardiography. About 5 h after the procedure, the patient suddenly reported dyspnea, severe headache, neck stiffness, and shoulder pain. A chest X-ray (CXR) revealed a 3.5 cm right-sided apical pneumothorax as well as small amounts of gas as linear or curvilinear lucencies in the mediastinum, indicating pneumomediastinum. Non-contrast computed tomography (CT) of the chest showed bilateral emphysema, right-sided pneumothorax with pneumopericardium and pneumomediastinum, a small right-sided pleural effusion, and the atrial lead crossing the cardiac contour, suggesting lead perforation through the pericardium and directly into the pleural cavity (Figure 1).
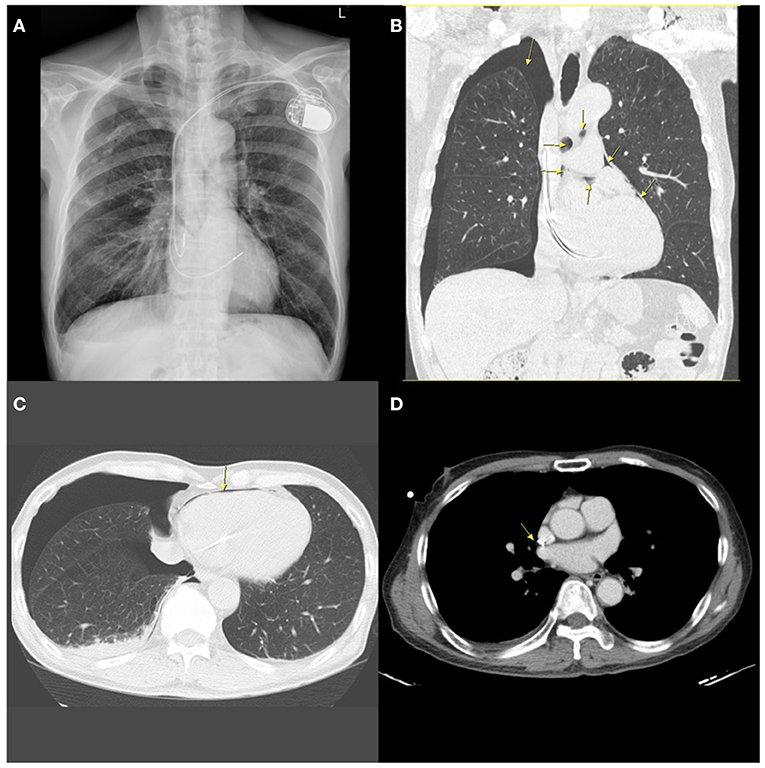
Figure 1. A chest radiograph performed 5 h after implantation showing contralateral pneumothorax, pneumopericardium, and pneumomediastinum. (A) Posterior-anterior (PA) chest X-ray demonstrating contralateral pneumothorax and mild pneumomediastinum. (B) Coronal chest non-contrast computed tomography (CT) scan image showing right-sided pneumothorax, pneumomediastinum, and pneumopericardium (arrows). (C) Horizontal CT image showing pneumopericardium (arrow) and pneumothorax. (D) Horizontal slices of CT scan suggesting possible extrusion of the atrial lead through the right atrium (arrow).
The patient's symptoms improved significantly after receiving high-flow oxygen through a nasal cannula. Nevertheless, the follow-up CXR showed no improvement. The patient, therefore, underwent insertion of a small-bore pigtail chest drain on the 3rd day, evacuating more than 80 ml of air. Serial CXRs showed significant improvement in the pneumopericardium and gradual resolution of the pneumothorax over the next few days. The electrocardiogram (ECG) showed no abnormal changes suggesting lead displacement, and interrogation of the pacemaker parameters remained fine with no significant alterations in the pacing thresholds, sensing or impedance. It was decided to leave the atrial lead in place.
During several days of in-hospital observation, the patient remained stable with no breathing difficulties, pleuritic chest discomfort, or pericardial signs and symptoms. The pigtail was then removed on day 6. The CXR verified the complete resolution of the pneumothorax and pneumopericardium before the patient was discharged on day 8. Since discharge, we have followed up on his signs and symptoms, pacemaker parameters, and ECG. The patient has remained stable for 15 months, and the pacemaker parameters and ECG have demonstrated no abnormalities.
Ipsilateral pneumothorax is commonly caused by needle penetration of the pleura during venous access (Figure 2) (5, 10, 12–14). Concurrent pneumopericardium and pneumomediastinum can occur when the leaking air passes through the lung parenchyma along the perivascular sheaths to the hilum and the mediastinum (15, 16). At its reflection enclosing the ostia of the pulmonary veins, the pericardium is most fragile, and air can thus pour into the pericardial cavity.
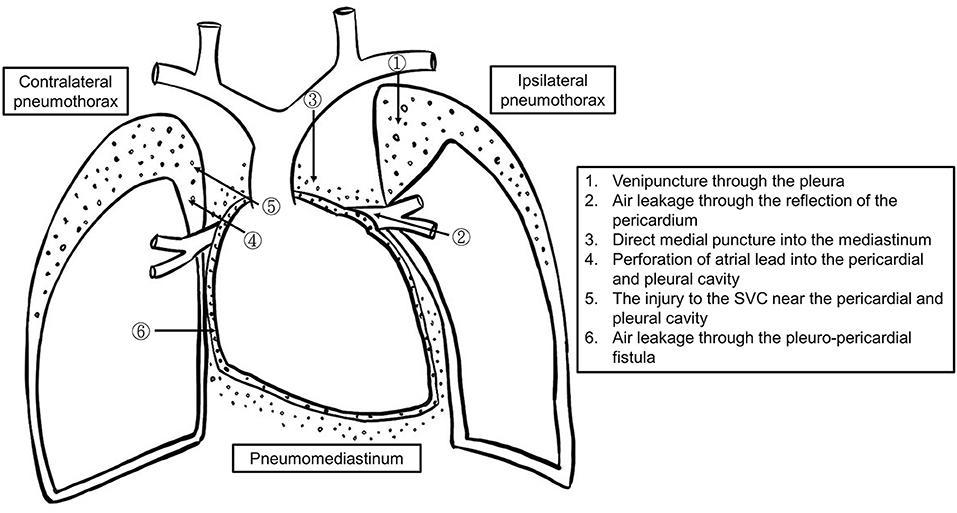
Figure 2. The schematic diagram of the mechanisms of pneumothorax, pneumopericardium, and pneumomediastinum formation.
There are two potential causes of contralateral pneumothorax and pneumopericardium. One is that the active fixation lead protrudes through the right atrium injuring the pericardium and right pleura (16). Another one could be incidental venous puncture of the right pleura while using the Seldinger technique to insert a dilator and sheath (17). An injury to the superior vena cava (SVC) near the pericardium could allow air (blood and/or intravenous fluid) to leak into the pericardial space through a congenital pericardial defect or tiny pleuro-pericardial fistulas (11).
Complications from pacemaker implantation are 30% more likely in females than in males (3). The incidence of both venipuncture related injury and cardiac perforation is higher in patients with bullous emphysema, chronic obstructive pulmonary disease (COPD), congenital defects such as persistent left superior vena cava, age >80 years, Caucasian ethnicity, steroid treatment within 7 days, anticoagulant and antiplatelet therapy, urgent surgery, low BMI (<18.5), or agitation (1, 8, 15, 18–20). Prior procedures, operation (such as sternotomies), trauma, or irradiation therapy in the affected area, clavicle/chest deformity, and previous fractures, are all significant risk factors. Difficult or lengthy procedures, large diameter (≥12 French) sheaths, more than one attempt at venipuncture, implantation of multiple electrodes, and a dual-chamber device (versus a single chamber device), are all linked to a higher risk for complications (1, 12, 14, 18, 21).
In comparison to passive fixation leads, active fixation leads provide several benefits, such as simple fixation, the capacity to be deployed at alternate pacing sites with ideal pacing and sensing parameters, decreased rates of dislodgement, and they are easier to extract (22). Because of these advantages, they are more frequently employed. Nevertheless, active fixation and over-screwing increase the risk of perforation (8, 16, 17, 20). Lead and helix design also play a role, particularly in the case of magnetic resonance imaging (MRI) compatible leads due to their greater diameter and stiffness (20). As a result, these active fixation leads should be implanted cautiously. Anatomic variations, such as multilobed or a thin-walled atrial appendage, fatty infiltration of the myocardium due to myotonic dystrophy, ischemic and dilated cardiomyopathy, are variables that increase the likelihood of atrial perforation (10, 11, 13, 15, 20, 23–29).
In our case, there were no apparent problems throughout the procedure. Over-screwing of the atrial lead seems unlikely because the requisite number of clockwise turns were performed under fluoroscopic guidance. Importantly, our patient had several risk factors including a history of longstanding smoking with bilateral emphysema. These risk factors increased the risk of pneumothorax. However, non-contrast chest CT raised suspicion of atrial lead perforation. Thus, we hypothesized that the atrial lead protruded through the pericardium directly into the pleural cavity, causing contralateral pneumothorax with concurrent pneumomediastinum and pneumopericardium.
A patient can manifest signs and symptoms during the procedure or up to 72 hours after implantation (20). Every patient should receive a chest x-ray within 4 h post-procedure (30). Patients discharged from the hospital shortly after outpatient procedures should remain in contact with the CIED center (17).
In concerning cases, fluoroscopy, chest CT with three-dimensional reconstruction, and echocardiography assist in diagnosing lead perforation (13, 31); however, they are not as sensitive for tiny perforation (15, 19, 23, 32). ECG-gated high-resolution CT (HRCT) remains the diagnostic gold standard although the perforation may be over-diagnosed (19, 25, 27). To reduce imaging distortions caused by heart motion, prospective ECG triggering and retrospective ECG gating methods are introduced (33). Prospective ECG triggering, for instance, allows the ECG signal to regulate scanning such that projection data is only collected during diastole, which is the period of the least amount of cardiac movement. Thus, HRCT maximizes spatial resolution and results in optimal delineation of the myocardium, blood, and fat interfaces (19). HRCT also aids in lead retrieval planning because it provides a reliable estimation of the orientation of important structures around the misplaced lead (23, 32).
The management depends on the presence or absence of symptoms, the hemodynamic condition, and the extent of the lesions. Although the American College of Chest Physicians (ACCP) proposed guidelines for the management of spontaneous pneumothorax (34), which were updated by the British Thoracic Society (BTS) (35), there is no consensus on the management of iatrogenic pneumothorax, let alone CIED-induced pneumothorax. We propose a flow chart for the management of CIED-associated pneumothorax (Figure 3), which was adapted from the recommended treatment for iatrogenic pneumothorax (36).

Figure 3. The management of cardiac implantable electronic device (CIED)-induced pneumothorax and concurrent pneumopericardium.
High flow (10 L) 100% nasal oxygen, which theoretically accelerates air absorption, is generally recommended as the first step despite conflicting evidence suggesting that it has probably no effect on large pneumothoraces (13, 37, 38). A clinically stable patient with a small pneumothorax (<20%) can be observed since it may resolve on its own (12, 36). After 12–24 h, further imaging should be acquired (36). If the pneumothorax is enlarging or once symptoms worsen, drainage should be considered (36).
Needle or cannula aspiration is advocated for patients with a small (<20%) pneumothorax, minimal symptoms, and no previous parenchymal disease (36). If the patients are asymptomatic after aspiration, and repeat imaging shows resolution of the pneumothorax or no progression, they can be discharged with a 48-h follow-up (36). However, observation alone may sufficient for small iatrogenic pneumothoraces. Of note, evacuated volumes >543 mL indicate the need for further intervention with a chest tube (39).
Patients with a large (>20%) pneumothorax or those presenting moderate-to-severe symptoms should be treated with a chest tube (12–16 French) for at least 24 h (11–13, 36). If the pneumothorax improves, the absence of an air leak should be confirmed before the chest tube is removed (36). Even though a pneumothorax appears to be resolved on imaging, there may still be an air leak, which is masked by an equilibrium between the air evacuation and the air flowing into the lung through the site of puncture (36). The removal of the chest tube under this condition may lead to the reoccurrence of the pneumothorax (36).
In the majority of pneumothoraces, air leakage will stop <48 h after the placement of a chest tube (40, 41). For patients with a persistent gas leak for more than 48 h, consulting a cardiothoracic surgeon or an interventional pulmonologist is recommended (42).
The symptoms, imaging findings, and lead parameters are used to determine if lead extraction or repositioning is necessary (Figure 4). The lead parameters usually alter following a lead perforation (43); however, they may remain unchanged, and the patient may be asymptomatic in certain circumstances (8, 11, 13, 20, 31). The proper management of asymptomatic lead perforation is still up for debate. Despite the uncertainty, it is generally suggested the lead be extracted or repositioned because there is a chance that it will perforate the surrounding structures over time, causing catastrophic harm (27, 28, 31, 44).
Transvenous lead extraction is effective and safe management in the majority of cases. It is conducted under fluoroscopic guidance with echocardiographic and hemodynamic monitoring. Additional precautions such as placement of a pericardial drain for emergent pericardiocentesis and having cardiac surgeons on standby help assure procedural safety (15, 23, 25, 27, 28, 31, 45). Postprocedural follow-up is recommended due to the risks of constrictive pericarditis and infections (31).
Atrial lead extraction is not always required. When there is an improvement in the pneumothorax, no pericardial effusion, minimal symptoms, and satisfactory lead parameters, it is preferable to keep the electrode in place until the fibrous tissue thickens and/or wraps around the helix, especially in the elderly and weak patients who are at higher risk for complications associated with lead extraction or repositioning. It should be noted that this strategy's long-term effectiveness is unverified (11, 46).
Figure 5 provides a clinical algorithm for early identification of individuals at high risk and appropriate preventative measures.
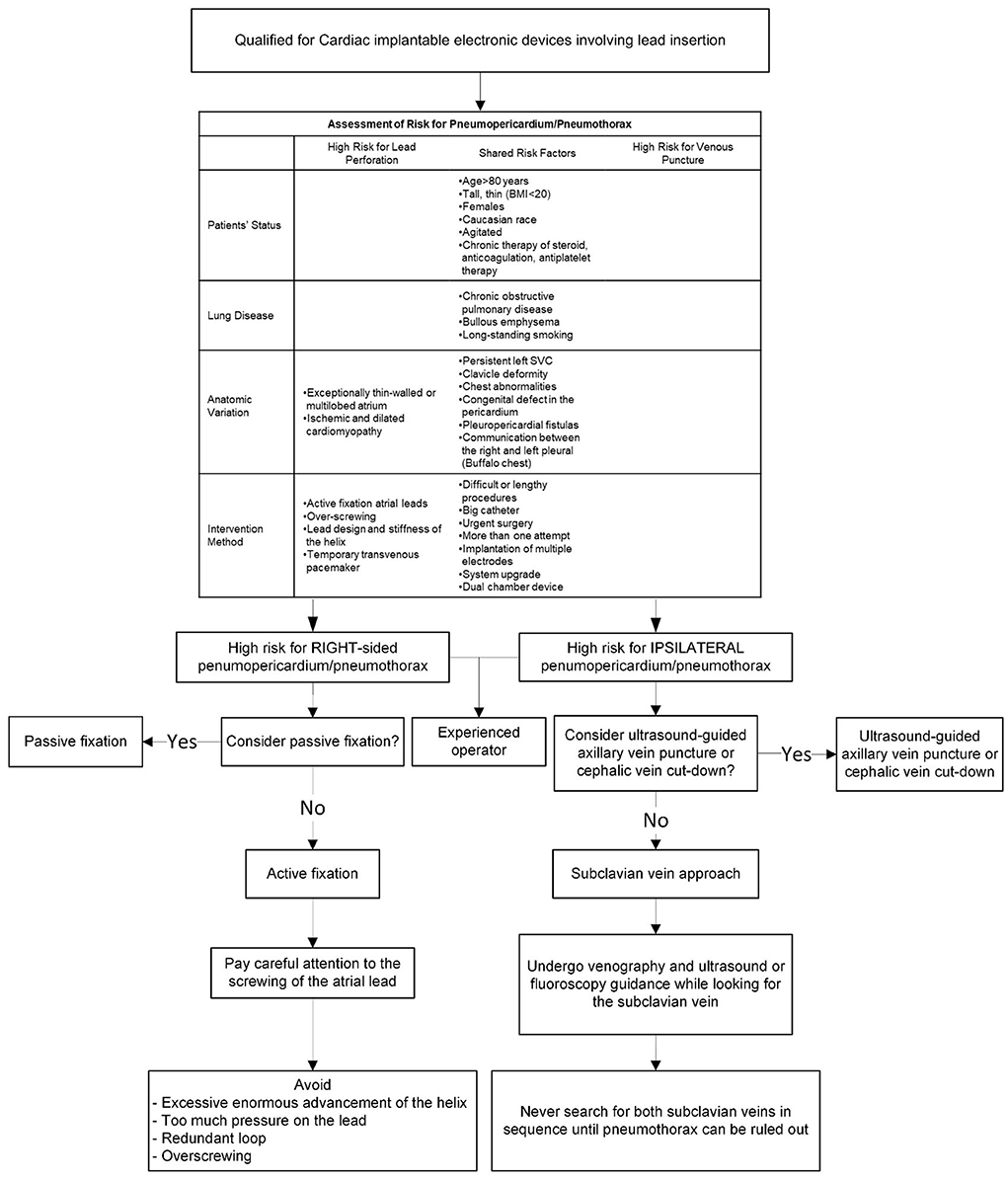
Figure 5. A clinical algorithm for early identification of patients at high risk of developing pneumothorax or pneumopericardium and corresponding preventive measures.
The subclavian vein has served as the most widely used venous access for CIEDs (47). In a patient with risk factors venography is recommended prior to subclavian vein puncture. Ultrasound guidance and/or fluoroscopic guidance may also be useful (48, 49). In a patient with unilateral pulmonary lesions, it's preferred to use the subclavian approach on the ipsilateral side of the diseased lung since there may be less severe complications (13). When venous access is not possible to establish, particularly after exhaustively looking for the subclavian vein on a specific side, the pacemaker-implanting physicians should rule out pneumothorax before shifting to the opposite side (13).
In terms of preventing pneumothorax, axillary venous access or cephalic vein cut-down is better than the subclavian vein approach (18, 47). The cephalic vein cut-down technique has been well-recognized for fewer occurrences of pneumothorax (50); however, the axillary approach is still not widely used due to inadequate training and a lack of familiarity (51).
Axillary vein access decreases the risk of complications due to its extra-thoracic anatomic location (52). Although “blind” puncture using anatomical landmarks is common, it is constrained by the variable relationship between the first rib and the axillary vein. About 5% of patients need a contrast-guided technique due to anatomical variations (52–54). As a result of inexperience, failed attempts, probable complications, and radiation exposure for both patients and physicians unavoidably increase (55).
Ultrasound guidance provides a direct view of the vessel, allowing the operator to monitor the needle's passage onto the subcutaneous tissue, assessing the depth of the vein, and preventing accidental arterial puncture, thus minimizing the probability of complications (52). A randomized clinical trial demonstrated that even when carried out by inexperienced operators, the ultrasound-guided axillary vein technique was much superior to cephalic vein cut-down (47). Therefore, we suggest ultrasound-guided axillary venous access as the preferred choice for CIED implantation.
This case highlights the risks of pneumothorax and pneumopericardium associated with CIEDs, as well as the recommended treatment. Operators should be aware of these potential, unusual complications and precautions that can be taken to avoid them. However, there is currently no definitive guidance on the management of CIED-associated pneumothorax or pneumopericardium. We recommend more multicenter randomized, trials to compare conservative vs. invasive therapy for CIED-associated pneumothorax of varying severity and to compare the long-term outcome between conservative management and lead extraction in patients with asymptomatic lead perforation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study involving human participants has been reviewed and approved by National Cheng Kung University Hospital Institutional Review Board (NCKUH IRB) on Jun 28th, 2022. IRB No: A-EC-111-023. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conception and design, data acquisition, and critical revision of the article for important intellectual content: S-WL and J-YC. Literature review and drafting and finalizing the article: S-WL. Supervised the literature search and revision and approved the final version: J-YC. All authors contributed to the article and approved the submitted version.
This study was supported by the Ministry of Science and Technology of Taiwan, China (MOST 110-2218-E-006-017 and MOST 110-2218-E-006-015) and Higher Education Sprout Project, Ministry of Education to the Headquarters of University Advancement at National Cheng Kung University (NCKU).
The authors would like to thank the Ministry of Science and Technology of the Republic of China, Taiwan, for financially supporting this research under contract MOST 110-2218-E-006-017 and MOST 110-2218-E-006-015.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. (2014) 35:1186–94. doi: 10.1093/eurheartj/eht511
2. Merenkov VV, Monahov YE, Kovalev AN, Ustinov AA. Pneumothorax after pacemaker implantation: a diagnostic value of sonography. J Cardiothorac Vasc Anesth. (2013) 27:e73–5. doi: 10.1053/j.jvca.2013.07.003
3. Ogunbayo GO, Charnigo R, Darrat Y, Morales G, Kotter J, Olorunfemi O, et al. Incidence, predictors, and outcomes associated with pneumothorax during cardiac electronic device implantation: a 16-year review in over 3. 7 million patients. Heart Rhythm. (2017) 14:1764–70. doi: 10.1016/j.hrthm.2017.07.024
4. Kirkfeldt RE, Johansen JB, Nohr EA, Moller M, Arnsbo P, Nielsen JC. Risk factors for lead complications in cardiac pacing: a population-based cohort study of 28,860 Danish patients. Heart Rhythm. (2011) 8:1622–8. doi: 10.1016/j.hrthm.2011.04.014
5. Kirkfeldt RE, Johansen JB, Nohr EA, Moller M, Arnsbo P, Nielsen JC. Pneumothorax in cardiac pacing: a population-based cohort study of 28,860 Danish patients. Europace. (2012) 14:1132–8. doi: 10.1093/europace/eus054
6. Udo EO, Zuithoff NP, van Hemel NM, de Cock CC, Hendriks T, Doevendans PA, et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm. (2012) 9:728–35. doi: 10.1016/j.hrthm.2011.12.014
7. van Rees JB, de Bie MK, Thijssen J, Borleffs CJ, Schalij MJ, van Erven L. Implantation-related complications of implantable cardioverter-defibrillators and cardiac resynchronization therapy devices: a systematic review of randomized clinical trials. J Am Coll Cardiol. (2011) 58:995–1000. doi: 10.1016/j.jacc.2011.06.007
8. Sebastian CC, Wu WC, Shafer M, Choudhary G, Patel PM. Pneumopericardium and pneumothorax after permanent pacemaker implantation. Pacing Clin Electrophysiol. (2005) 28:466–8. doi: 10.1111/j.1540-8159.2005.40063.x
9. Schorlemmer GR, Khouri RK, Murray GF, Johnson G Jr. Bilateral pneumothoraces secondary to latrogenic buffalo chest an unusual complication of median sternotomy and subclavian vein catheterization. Ann Surg. (1984) 199:372–4. doi: 10.1097/00000658-198403000-00021
10. Ayati M, Menon SD, Morillo CA, Healey J, Connolly SJ. Bilateral pneumothorax post insertion of intracardiac defibrillator, a rare condition, risk factors and prevention. Eu J Arrhyth Electrophysiol. (2016) 2:40. doi: 10.17925/EJAE.2016.02.01.40
11. Parashar NK, Deepti S, Yadav R, Sinha M, Ramakumar V. An unexpected complication of intracardiac device implantation: contralateral pneumothorax and pneumopericardium. Indian Pacing Electrophysiol J. (2019) 19:167–70. doi: 10.1016/j.ipej.2019.04.001
13. Olesen LL. Bilateral pneumothorax complicating pacemaker implantation, due to puncture of the left subclavian vein and electrode perforation of the right atrium. Cureus. (2020) 12:11302. doi: 10.7759/cureus.11302
14. Res JC, de Priester JA, van Lier AA, van Engelen CL, Bronzwaer PN, Tan PH, et al. Pneumothorax resulting from subclavian puncture: a complication of permanent pacemaker lead implantation. Neth Heart J. (2004) 12:101–5.
15. Dilling-Boer D, Ector H, Willems R, Heidbüchel H. Pericardial effusion and right-sided pneumothorax resulting from an atrial active-fixation lead. EP Europace. (2003) 5:419–23. doi: 10.1016/S1099-5129(03)00079-5
16. Oginosawa Y, Abe H, Nakashima Y. Right pneumothorax resulting from an endocardial screw-in atrial lead in an implantable cardioverter defibrillator system. Pac Clin Electrophysiol. (2002) 25:1278–9. doi: 10.1046/j.1460-9592.2002.01278.x
17. Grabowski M. Pneumothorax after pacemaker implantation localized contralaterally to the side of implantation–a rare but possible complication. Heart Beat J. (2017) 2:1611. doi: 10.24255/hbj/81611
18. Kotsakou M, Kioumis I, Lazaridis G, Pitsiou G, Lampaki S, Papaiwannou A, et al. Pacemaker insertion. Ann Trans Med. (2015) 3:42. doi: 10.3978/j.issn.2305-5839.2015.02.06
19. Saradna A, Sinha A, Abduraimova M, Rodriguez D, Yang F. Tale of a wandering lead: late atrial lead perforation into right lung following pacemaker implantation. Cureus. (2017) 9:1865. doi: 10.7759/cureus.1865
20. Srivathsan K, Byrne R, Appleton C, Scott LR. Pneumopericardium and pneumothorax contralateral to venous access site after permanent pacemaker implantation. EP Europace. (2003) 5:361–3. doi: 10.1016/S1099-5129(03)00093-X
21. Tsotsolis N, Tsirgogianni K, Kioumis I, Pitsiou G, Baka S, Papaiwannou A, et al. Pneumothorax as a complication of central venous catheter insertion. Ann Trans Med. (2015) 3:40. doi: 10.3978/j.issn.2305-5839.2015.02.11
22. Hao Y, Li Y, Liao D, Yang L, Liu F. A comparative analysis of the effectiveness of active versus passive atrial lead fixation in Chinese patients with cardiac implantable electrical devices: a long term, retrospective, observational, single-center study. Curr Med Res Opin. (2017) 33:573–8. doi: 10.1080/03007995.2016.1275938
23. Banaszewski M, Stepińska J. Right heart perforation by pacemaker leads. Archives of medical science: AMS. (2012) 8:11. doi: 10.5114/aoms.2012.27273
24. Glikson M, VON FELDT LK, Suman VJ, Hayes DL. Clinical surveillance of an active fixation, bipolar, polyurethane insulated pacing lead, part I: the atrial lead. Pacing Clin Electrophysiol. (1994) 17:1399–404. doi: 10.1111/j.1540-8159.1994.tb02459.x
25. Kumar S, Yassin H, Esan O, Budzikowski AS, Kassotis JT. Mechanism and management of pacing lead related cardiac perforation. Curr Iss Rec Adv Pacemaker Therapy: IntechOpen. (2012). doi: 10.5772/48630
26. Martos R, Khadadah S, Alsaleh H, Foley B, Dodd J. Pneumopericardium and contralateral pneumothorax to venous access site after a biventricular permanent pacemaker implantation. J Clinic Experiment Cardiol. (2011) 2:2. doi: 10.4172/2155-9880.1000150
27. Sadamatsu K. Complication of pacemaker implantation: an atrial lead perforation. Mod Pacemakers-Pre Fut: IntechOpen. (2011). doi: 10.5772/13854
28. Van Gelder BM, Verberkmoes N, Nathoe R, Bracke FA. Late asymptomatic atrial lead perforation, a fortuitous finding during lead extraction using thoracoscopic surveillance: a case report and review of the literature. EP Europace. (2016) 18:1773–8. doi: 10.1093/europace/euw054
29. Mahadevan MS, Yadava RS, Mandal M. Cardiac pathology in myotonic dystrophy type 1. Int J Mol Sci. (2021) 22:11874. doi: 10.3390/ijms222111874
30. Thomas GR, Kumar SK, Turner S, Moussa F, Singh SM. The natural history and treatment of cardiac implantable electronic device associated pneumothorax—A 10-year single-centre experience. CJC open. (2021) 3:176–81. doi: 10.1016/j.cjco.2020.10.011
31. HUANG XM FU HX, Zhong L, Osborn MJ, Asirvatham SJ, Sinak LJ, et al. Outcomes of lead revision for myocardial perforation after cardiac implantable electronic device placement. J Cardiovasc Electrophysiol. (2014) 25:1119–24. doi: 10.1111/jce.12457
32. Balabanoff C, Gaffney CE, Ghersin E, Okamoto Y, Carrillo R, Fishman JE. Radiographic and electrocardiography-gated noncontrast cardiac CT assessment of lead perforation: modality comparison and interobserver agreement. J Cardiovasc Comput Tomogr. (2014) 8:384–90. doi: 10.1016/j.jcct.2014.08.004
33. Feng Q, Yin Y, Hua X, Zhu R, Hua J, Xu J. Prospective ECG triggering versus low-dose retrospective ECG-gated 128-channel CT coronary angiography: comparison of image quality and radiation dose. Clin Radiol. (2010) 65:809–14. doi: 10.1016/j.crad.2010.05.005
34. Baumann MH, Strange C, Heffner JE, Light R, Kirby TJ, Klein J, et al. Management of spontaneous pneumothorax: an American college of chest physicians Delphi consensus statement. Chest. (2001) 119:590–602. doi: 10.1378/chest.119.2.590
35. MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British thoracic society pleural disease guideline 2010. Thorax. (2010) 65:ii18–31. doi: 10.1136/thx.2010.136986
36. Loiselle A, Parish JM, Wilkens JA, Jaroszewski DE. Managing iatrogenic pneumothorax and chest tubes. J Hosp Med. (2013) 8:402–8. doi: 10.1002/jhm.2053
37. Chadha TS, Cohn MA. Noninvasive treatment of pneumothorax with oxygen inhalation. Respiration. (1983) 44:147–52. doi: 10.1159/000194541
38. Clark S, Saker F, Schneeberger M, Park E, Sutton D, Littner Y. Administration of 100% oxygen does not hasten resolution of symptomatic spontaneous pneumothorax in neonates. J Perinatol. (2014) 34:528–31. doi: 10.1038/jp.2014.55
39. Yamagami T, Kato T, Iida S, Hirota T, Yoshimatsu R, Nishimura T. Efficacy of manual aspiration immediately after complicated pneumothorax in CT-guided lung biopsy. J Vasc Intervent Radiol. (2005) 16:477–83. doi: 10.1097/01.RVI.0000150032.12842.9E
40. Baumann MH, Strange C. The clinician's perspective on pneumothorax management. Chest. (1997) 112:822–8. doi: 10.1378/chest.112.3.822
41. Schoenenberger RA, Haefeli WE, Weiss P, Ritz RF. Timing of invasive procedures in therapy for primary and secondary spontaneous pneumothorax. Arch Surg. (1991) 126:764–6. doi: 10.1001/archsurg.1991.01410300110017
42. Schoenenberger RA, Haefeli WE, Weiss P, Ritz R. Evaluation of conventional chest tube therapy for iatrogenic pneumothorax. Chest. (1993) 104:1770–2. doi: 10.1378/chest.104.6.1770
43. Ellenbogen KA, Wood MA, Shepard RK. Delayed complications following pacemaker implantation. Pacing Clin Electrophysiol. (2002) 25:1155–8. doi: 10.1046/j.1460-9592.2002.01155.x
44. Sadamatsu K, Enomoto N, Tsuji M, Tashiro H. Progressive atrial lead perforation developed 5 years after pacemaker replacement. J Cardiol. (2009) 53:150–3. doi: 10.1016/j.jjcc.2008.07.006
45. Migliore F, Zorzi A, Bertaglia E, Leoni L, Siciliano M, De Lazzari M, et al. Incidence, management, and prevention of right ventricular perforation by pacemaker and implantable cardioverter defibrillator leads. Pacing Clin Electrophysiol. (2014) 37:1602–9. doi: 10.1111/pace.12472
46. Nantsupawat T, Li J-M, Benditt DG, Adabag S. Contralateral pneumothorax and pneumopericardium after dual-chamber pacemaker implantation: Mechanism, diagnosis, and treatment. HeartRhythm Case Reports. (2018) 4:256–9. doi: 10.1016/j.hrcr.2018.03.001
47. Tagliari AP, Kochi AN, Mastella B, Saadi RP, di Leoni Ferrari A, Saadi EK, et al. Axillary vein puncture guided by ultrasound vs cephalic vein dissection in pacemaker and defibrillator implant: a multicenter randomized clinical trial. Heart Rhythm. (2020) 17:1554–60. doi: 10.1016/j.hrthm.2020.04.030
48. Ahmed AS, Gilge JL, Clark BA, Shah A, Bagga S, Padanilam MS, et al. Predictors of successful ultrasound-guided lead implantation. Pacing Clin Electrophysiol. (2020) 43:217–22. doi: 10.1111/pace.13855
49. Harada Y, Katsume A, Kimata M, Hikosaka T, Yamanaka S, Akashi K, et al. Placement of pacemaker leads via the extrathoracic subclavian vein guided by fluoroscopy and venography in the oblique projection. Heart Vessels. (2005) 20:19–22. doi: 10.1007/s00380-004-0797-1
50. Chauhan A, Grace A, Newell S, Stone D, Shapiro L, Schofield P, et al. Early complications after dual chamber versus single chamber pacemaker implantation. Pacing Clin Electrophysiol. (1994) 17:2012–5. doi: 10.1111/j.1540-8159.1994.tb03791.x
51. Migliore F, Fais L, Vio R, De Lazzari M, Zorzi A, Bertaglia E, et al. Axillary vein access for permanent pacemaker and implantable cardioverter defibrillator implantation: fluoroscopy compared to ultrasound. Pacing Clin Electrophysiol. (2020) 43:566–72. doi: 10.1111/pace.13940
52. Migliore F, Siciliano M, De Lazzari M, Ferretto S, Valle CD, Zorzi A, et al. Axillary vein puncture using fluoroscopic landmarks: a safe and effective approach for implantable cardioverter defibrillator leads. J Intervent Card Electrophysiol. (2015) 43:263–7. doi: 10.1007/s10840-015-0011-7
53. De Sensi F, Miracapillo G, Addonisio L, Breschi M, Paneni F, Cresti A, et al. Axillary vein access with or without venography: is this the dilemma in the ultrasounds era? EP Euro. (2018) 20:1389–90. doi: 10.1093/europace/euy048
54. Antonelli D, Feldman A, Freedberg NA, Turgeman Y. Axillary vein puncture without contrast venography for pacemaker and defibrillator leads implantation. Pacing Clin Electrophysiol. (2013) 36:1107–10. doi: 10.1111/pace.12181
Keywords: contralateral pneumothorax, pneumopericardium, pneumomediastinum, cardiac implantable electronic device, pacemaker
Citation: Lo S-W and Chen J-Y (2022) Case report: A rare complication after the implantation of a cardiac implantable electronic device: Contralateral pneumothorax with pneumopericardium and pneumomediastinum. Front. Cardiovasc. Med. 9:938735. doi: 10.3389/fcvm.2022.938735
Received: 08 May 2022; Accepted: 25 July 2022;
Published: 18 August 2022.
Edited by:
Matteo Anselmino, University of Turin, ItalyReviewed by:
Federico Migliore, University of Padua, ItalyCopyright © 2022 Lo and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ju-Yi Chen, anV5aUBtYWlsLm5ja3UuZWR1LnR3
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.