
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 27 July 2022
Sec. Atherosclerosis and Vascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.936817
This article is part of the Research Topic Oxidative Stress, Inflammation and Atherosclerosis-Related Diseases: From basic to clinical research View all 11 articles
 Jie Zhang1,2†
Jie Zhang1,2† Xinyi Wang1,3†
Xinyi Wang1,3† Wende Tian1,3
Wende Tian1,3 Tongxin Wang1,2
Tongxin Wang1,2 Jundi Jia1,2
Jundi Jia1,2 Runmin Lai1,3
Runmin Lai1,3 Tong Wang1,2
Tong Wang1,2 Zihao Zhang1,2
Zihao Zhang1,2 Luxia Song1,2
Luxia Song1,2 Jianqing Ju1*
Jianqing Ju1* Hao Xu1*
Hao Xu1*Objective: The objective of this study was to measure the efficacy of various types and dosages of statins on C-reactive protein (CRP) levels in patients with dyslipidemia or coronary heart disease.
Methods: Randomized controlled trials were searched from PubMed, Embase, Cochrane Library, OpenGray, and ClinicalTrials.gov. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for data extraction and synthesis. The pairwise meta-analysis compared statins and controls using a random-effects model, and a network meta-analysis compared the types and dosages of statins using the Bayesian random-effects model. The PROSPERO registration number is CRD42021242067.
Results: The study included 37 randomized controlled trials with 17,410 participants and 20 interventions. According to the pairwise meta-analysis, statins significantly decreased CRP levels compared to controls (weighted mean difference [WMD] = −0.97, 95% confidence interval [CI] [−1.31, −0.64], P < 0.0001). In the network meta-analysis, simvastatin 40 mg/day appeared to be the best strategy for lowering CRP (Rank P = 0.18, WMD = −4.07, 95% CI = [−6.52, −1.77]). The same was true for the high-sensitivity CRP, non-acute coronary syndrome (ACS), <12 months duration, and clear measurement subgroups. In the CRP subgroup (rank P = 0.79, WMD = −1.23, 95% CI = [−2.48, −0.08]) and ≥12-month duration subgroup (Rank P = 0.40, WMD = −2.13, 95% CI = [−4.24, −0.13]), atorvastatin 80 mg/day was most likely to be the best. There were no significant differences in the dyslipidemia and ACS subgroups (P > 0.05). Node-splitting analysis showed no significant inconsistency (P > 0.05), except for the coronary heart disease subgroup.
Conclusion: Statins reduced serum CRP levels in patients with dyslipidemia or coronary heart disease. Simvastatin 40 mg/day might be the most effective therapy, and atorvastatin 80 mg/day showed the best long-term effect. This study provides a reference for choosing statin therapy based on LDL-C and CRP levels.
Atorvastatin (PubChem CID: 60823); Pravastatin (PubChem CID: 54687); Pitavastatin (PubChem CID: 5282452); Rosuvastatin (PubChem CID: 446157); Simvastatin (PubChem CID: 54454).
Dyslipidemia is the primary risk factor and a prerequisite for atherosclerotic cardiovascular disease (ASCVD) (1). Long-term prospective epidemiological studies have consistently demonstrated the critical role of managing dyslipidemia in reducing ASCVD morbidity and mortality (2). Nevertheless, cardiovascular events continue to occur even with a substantial reduction in low-density lipoprotein cholesterol (LDL-C) (3). Coronary heart disease (CHD) is a chronic inflammatory disease in which inflammation involves the entire process from plaque formation to rupture (4, 5). Recently, clinical trials using anti-inflammatory drugs [e.g., canakinumab (6) and colchicine (7, 8)] confirmed the direct vasculo-protective effects of primarily targeting inflammation, which may partly explain the residual risk after the normalization of LDL-C. These findings suggest that anti-inflammatory therapy provides insights into treating CHD in addition to lipid-lowering.
Statins (i.e., 3-hydroxy-methylglutaryl coenzyme A [HMG-CoA] reductase inhibitors) are used to lower cholesterol in the primary and secondary prevention of ASCVD (9). Their primary effect is to lower serum cholesterol levels by competitively inhibiting HMG-CoA reductase, thereby inhibiting hepatic cholesterol biosynthesis (9, 10). Furthermore, statins exert cardiovascular protective effects independent of lowering LDL-C (called “pleiotropic” effects) with anti-inflammatory effects that are attracting attention (11).
C-reactive protein (CRP) is a pentameric protein consisting of five identical non-covalently bound subunits of 206 amino acid residues (12). It is a major acute-phase protein in humans, a multifunctional component of the human innate host defense mechanism (12), and an indicator and predictor of ASCVD risk associated with inflammation (13, 14). CRP and high-sensitivity CRP (hs-CRP) were used for measuring the same substance, while hs-CRP is more sensitive than CRP at low CRP levels (15). The measurements include immunoturbidimetry, nephelometry, enzyme-linked immunosorbent assay, chemiluminescent enzyme immunometric assay, and radial immunodiffusion assay; a meta-analysis showed that these multiple methods could not influence the CRP results (16).
The initial statin prescription is generally based on the lipid-lowering intensity (9). It might be more beneficial if clinicians considered statins' anti-inflammatory and lipid-lowering effects (11). Previous clinical and experimental studies have shown that statins effectively reduced CRP levels (14, 17); however, comparisons of various types and doses of statins for CRP-lowering effects are inconsistent. A docking experiment in silico showed that rosuvastatin, fluvastatin, pitavastatin, and atorvastatin had the most substantial interactions with CRP (18). Some clinical trials demonstrated that various types and dosages of statins showed differing effects on lowering CRP levels (19, 20). In contrast, other trials found no significant differences among several statin therapies (21–23). Therefore, this study aimed to assess the effect of different types and dosages of statins on lowering CRP levels using a pairwise and network meta-analysis (NMA).
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (24) for this study, and the PRISMA checklist is listed in Supplementary Table 1. This study was registered on PROSPERO (CRD42021242067).
We included studies that met the following criteria: (1) randomized controlled trials (RCTs); (2) participants with dyslipidemia and/or CHD (including stable angina pectoris and acute coronary syndromes); (3) studies comparing patients treated with statin vs. placebo, blank, or other types or doses of statins; (4) studies providing sufficient information on the baseline and follow-up CRP or hs-CRP level; (5) participants who were taking statins with a fixed-dose once a day; and (6) studies published in English.
The exclusion criteria were as follows: (1) Participants suffering from autoimmune diseases, malignant tumors, liver failure, kidney failure, acute inflammation, or during a perioperative period; (2) participants who were taking statins before enrollment and did not experience a washout period; (3) intervention duration of <8 weeks; and (4) fewer than 30 people per arm in the study.
We searched PubMed, Embase, Cochrane Library databases, OpenGray, and ClinicalTrials.gov for eligible studies from the inception to April 1, 2021. We used a combination strategy of keywords and MeSH keywords, including “dyslipidemia,” “hypertriglyceridemia,” “hypercholesterolemia,” “coronary heart disease,” “atherosclerosis,” “atherosclerotic,” “hydroxymethylglutaryl-CoA reductase inhibitors,” “atorvastatin,” “fluvastatin,” “lovastatin,” “pravastatin,” “rosuvastatin,” “simvastatin,” “pitavastatin,” “C reactive protein,” “CRP,” and “hs-CRP.” We also scanned the references of included studies and published systematic reviews to avoid omissions. Supplementary Text 1 displays the detailed search strategy.
Two authors (WT and TXW) independently screened studies and extracted data. Disagreements were resolved through discussions with a third investigator (HX). We recorded the publication information (first author's name and year), characteristics of trials (design, location, and registration), participants (age, sex, sample size, and disease), interventions (types, dosage, and duration), other treatments, and outcomes (CRP/hs-CRP and its measurement). If possible, we extracted the results from the intention-to-treat analysis.
Two authors (WT and TXW) independently evaluated the risk of bias according to the Cochrane Collaboration Recommendations assessment tools (25). Because CRP is an objective indicator uninfluenced by allocation concealment and blinding, it is rated as low risk regardless of allocation concealment and blinding (25). Thus, we assessed the risk of bias from the following categories: sequence generation, incomplete outcome data, selective outcome reporting, and other biases (e.g., whether or not to specify the method for measuring CRP/hs-CRP). Discrepancies were resolved by discussions with a third investigator (HX). The quality of evidence for each outcome in the pairwise meta-analysis and significant results in the network meta-analyses were evaluated based on the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) process.
The outcome was plasma CRP/hs-CRP level at the final measured point. Data were standardized to mean and standard deviation (SD) (26), and units of CRP were converted to mg/L. Placebo and no-interventions were combined as the control groups to provide more evidence for comparisons among statins. We presented the pooled results as weighted mean differences (WMD) and 95% confidence intervals (CI) (16, 27). P-values of <0.05 were considered significant.
We performed a pairwise meta-analysis to compare the efficacy of statins and control on serum CRP levels using the Review Manager 5.4 software (The Cochrane Collaboration, Software Update, Oxford, UK). Multi-arm studies were split into comparisons between statins and controls, and the number of participants in the control group was proportionally divided into a new control group to ensure that the total number of participants was exact (25, 28). Due to the high heterogeneity of included studies, we chose a random-effects model to estimate the overall effect, which might provide more conservative results (29). For sensitivity analyses, the robustness of the pooled results was tested by leave-one-out influence analysis.
For NMA, we produced an evidence network plot using the Stata 16 software (STATA Corporation, College Station, TX, USA). The aggregate data drug information system ADDIS 1.16.5 software (Drug Information System, Groningen, The Netherlands) was used to generate the Bayesian random-effects model. We generated a Markov Chain Monte Carlo (MCMC) model to incorporate the efficacy of direct and indirect comparisons and rank the interventions with ranking probabilities (30, 31). The convergence of the MCMC model was assessed using the Brooks-Gelman-Rubin method, which compares within-chain and between-chain variance to calculate the potential scale reduction factor (PSRF) (32). The closer PSRF approaches 1, the better the convergence. Typically, an acceptable PSRF is <1.05 (33). To evaluate the inconsistency of NMA, we used a node-split model to assess the consistency of direct and indirect comparisons. The consistency model was adopted if the P-value of >0.05; otherwise, the inconsistent model was used (34).
CRP and hs-CRP have different measurement accuracies, and some studies did not mention the actual measurement clearly; moreover, participants with different diseases, especially those with acute coronary syndromes (ACS), might have higher inflammation levels (35). Therefore, we performed subgroup analyses for CRP measurement (including CRP, hs-CRP, and CRP/hs-CRP with a clear measurement method), population (including CHD, dyslipidemia, ACS, and non-ACS), and treatment duration (less than or more than 12 months). If a study included a population with ACS and dyslipidemia, it would belong to the CHD and ACS subgroups. If there were inconsistencies in the evidence, conclusions were treated cautiously.
According to the search strategy, we retrieved 2,804 potential eligible papers from the three databases. After screening, 37 studies (19–23, 36–69) with 17,410 participants and 20 interventions were included (Figure 1). Among them, nine studies directly compared a statin with control, 22 compared two different types or doses of statins, and six were multi-arm trials that performed a comparison between at least two different types or dosages of statins and control. The RCTs included five statins (atorvastatin, rosuvastatin, pitavastatin, simvastatin, and pravastatin) at varying dosages. Table 1 shows the baseline characteristics of each included study.
The assessments for bias risk are summarized in Figure 2. All 37 trials reported random assignment, while only 16 studies (20, 21, 36, 38, 39, 42, 49, 52–54, 60–64, 67) explicitly mentioned appropriate random sequence generation methods. Because CRP is an objective measure, the risk of allocation concealment and blinding was low for all studies (25). Concerning incomplete outcome data, five trials (40, 43, 45, 51, 66) did not report the number or reason of loss to follow-up; one trial (65) had a loss to follow-up rate higher than 35%. Regarding selective outcome reporting, 11 studies (19, 39, 42–44, 53, 55, 58, 59, 61, 64) published the protocols and reported the complete results, whereas others were unclear. In addition, 25 studies (20–23, 36–38, 40, 41, 43, 45–52, 54–58, 60, 61) described the detailed methods of measuring CRP/hs-CRP, while the remaining 12 (19, 39, 42, 44, 53, 59, 62–67) were unclear. In the pairwise meta-analysis, the quality of evidence for comparison between statins and non-statin controls was rated as high. In network meta-analyses, the quality of evidence for significant results was rated as high or moderate (Supplementary Table 2).
The pairwise meta-analysis compared the effects of statins and control on serum CRP levels. As shown in Figure 3, compared with control, statins significantly reduced CRP levels (WMD = −0.97, 95% CI [−1.31, −0.64], P < 0.0001, I2 = 95%). The leave-one-out influence analyses showed that the associations between statins and CRP levels were not determined by any individual study (Supplementary Table 3).
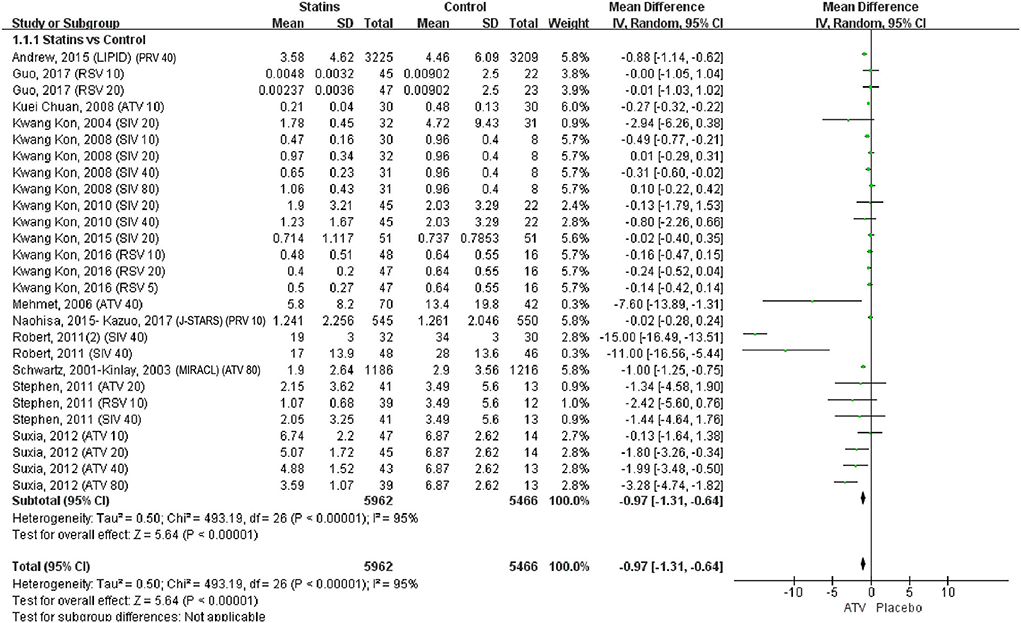
Figure 3. Forest plot of pairwise meta-analysis between statins and control. Note: PRV 40: Pravastatin 40 mg/d; RSV 10: Rosuvastatin 10 mg/d; Rosuvastatin 20 mg/d; ATV 10: Atorvastatin 10 mg/d; SIV 20: Simvastatin 20 mg/d; SIV 10: Simvastatin 10 mg/d; SIV 40: Simvastatin 40 mg/d; SIV 80: Simvastatin 80 mg/d; RSV 5: Rosuvastatin 5 mg/d; ATV 40: Atorvastatin 40 mg/d; PRV 10: Pravastatin 10 mg/d; ATV 80: Atorvastatin 80 mg/d; ATV 20: Atorvastatin 20 mg/d.
Figure 4 shows the network evidence of 19 statin therapies and control (placebo and no intervention). As shown in Figure 4, control was the most used intermediary comparator. The most common comparisons occurred between pravastatin 40 mg/day and control, followed by atorvastatin 80 mg/day vs. control.
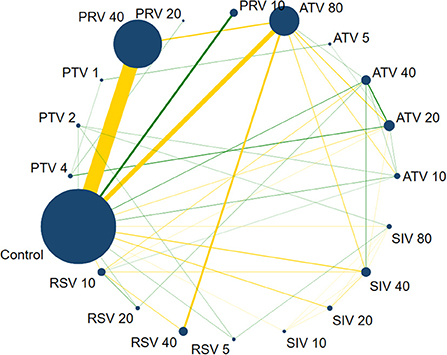
Figure 4. Network evidence plot on CRP. The size of nodes is directly proportional to the number of studies. The lines link two direct-comparison interventions, their thickness is proportional to the number of comparisons, and the green links represent at least one double-blind comparison. PTV 4: Pitavastatin 4 mg/d; PTV 2: Pitavastatin 2 mg/d; PTV 1: Pitavastatin 1 mg/d; PRV 40: Pravastatin 40 mg/d; PRV 20: Pravastatin 20 mg/d; PRV 10: Pravastatin 10 mg/d; ATV 80: Atorvastatin 80 mg/d; ATV 5: Atorvastatin 5 mg/d; ATV 40: Atorvastatin 40 mg/d; ATV 20: Atorvastatin 20 mg/d; ATV 10: Atorvastatin 10 mg/d; SIV 80: Simvastatin 80 mg/d; SIV 40: Simvastatin 40 mg/d; SIV 20: Simvastatin 20 mg/d; SIV 10: Simvastatin 10 mg/d; RSV 5: Rosuvastatin 5 mg/d; RSV 40: Rosuvastatin 40 mg/d; RSV 20: Rosuvastatin 20 mg/d; RSV 10: Rosuvastatin 10 mg/d.
All 37 studies were included in the NMA (Supplementary Table 4). Overall, only simvastatin 40 mg/day (WMD = −4.07, 95% CI= [−6.52, −1.77]) and atorvastatin 80 mg/day (WMD = −3.32, 95% CI= [−6.02, −0.83]) were significantly better than control among 19 statin therapies. We performed the rank-possibility of statins on lowering CRP. Rank 1 was the worst, and rank 20 was the best (70). Supplementary Figure 1A shows that simvastatin 40 mg/day has the highest P-value of rank 20; therefore, it also indicates that simvastatin 40 mg/day might be the best method for lowering CRP (rank P = 0.18).
We performed a subgroup analysis based on the measurement method of CRP, including CRP, hs-CRP, and CRP/hs-CRP with a clear measurement method (Supplementary Table 4). The CRP subgroup contained three studies with three interventions and 1,679 participants. We performed both consistency model and inconsistency model because there were no closed loops to conduct node-splitting analysis for assessing inconsistency. Atorvastatin 80 mg/day might be the best at lowering CRP levels (rank P = 0.79) among atorvastatin 80 mg/day, pravastatin 40 mg/day, and rosuvastatin 40 mg/day (Supplementary Figure 1B). Moreover, atorvastatin 80 mg/day was significantly better than pravastatin 40 mg/day in both the consistency model (WMD = −1.23, 95% CI = [−2.48, −0.08]) and the inconsistency model (WMD = −1.25, 95% CI= [−2.53, −0.08]). However, owing to the limited studies, the results should be interpreted with caution. The hs-CRP subgroup included 33 studies with 20 interventions. According to the ranking possibility, the best statins for reducing CRP might be pravastatin 40 mg/day (rank P = 0.15), simvastatin 40 mg/day (rank P = 0.12), or rosuvastatin 40 mg/day (rank P = 0.10) (Supplementary Figure 1C). Nevertheless, only simvastatin 40 mg/day (WMD = −4.10, 95% CI= [−6.83, −1.60]) and atorvastatin 80 mg/day (WMD = −3.66, 95% CI = [−7.01, −0.58]) were significantly better than control. Comprehensive considering the ranking and P-value, simvastatin 40 mg/day might be the best therapy for lowering CRP levels. The subgroup of CRP/hs-CRP with a clear measurement method contained 23 studies with 14 interventions. Among them, simvastatin 40 mg/day appeared to be the best (rank P = 0.20) (Supplementary Figure 1D). Furthermore, only simvastatin 40 mg/day showed a statistically significant difference compared to control (WMD = −4.28, 95% CI = [−7.21, −1.43]).
We conducted another subgroup analysis in terms of population, including CHD, dyslipidemia, ACS, and non-ACS subgroups. The CHD subgroup included 21 studies with 14 interventions. However, there are two inconsistent comparisons; thus, we used the inconsistency model (Supplementary Table 4), which should be interpreted cautiously. In total, 14 studies were included in the dyslipidemia subgroup. The results showed that pitavastatin 2 mg/day tends to be the best (rank P = 0.18) (Supplementary Figure 1E); however, there were no significant differences among the 15 interventions (P > 0.05). The ACS subgroup included 11 studies. Compared with other interventions, atorvastatin 80 mg/day might be the most effective strategy to reduce the CRP levels (rank P = 0.30). However, the comparisons among the nine interventions also showed no significant difference (P > 0.05) (Supplementary Figure 1F). Although 29 studies met the criteria of the non-ACS subgroup, only 27 studies were available for indirect comparisons. Simvastatin 40 mg/day has the highest probability of being the best for reducing CRP levels (rank P = 0.21) (Supplementary Figure 1G). Furthermore, simvastatin 40 mg/day (WMD = −4.34, 95% CI= [−7.10, −1.76]) was also significantly better than control among 16 interventions (P < 0.05).
We conducted the third subgroup analysis according to the treatment duration. For the <12-month duration subgroup, simvastatin 40 mg/day (rank P = 0.21) appeared to be the best strategy (Supplementary Figure 1H). Moreover, simvastatin 40 mg/day (WMD = −4.29, 95% CI = [−7.18, −1.55]) and atorvastatin 80 mg/day (WMD = −3.66, 95% CI = [−7.37, −0.19]) were significantly better than control for reducing CRP levels. Although 9 studies were eligible for the ≥12-month duration subgroup, only six studies were available for NMA analysis. Given that there were no closed loops to assess inconsistency, we conducted consistency and inconsistency models. In the consistency model, atorvastatin 80 mg/day (rank P = 0.40) and simvastatin 40 mg/day (rank P = 0.36) were most likely to be the best for reducing CRP levels (Supplementary Figure 1I). Atorvastatin 80 mg/day was significantly better than pravastatin 40 mg/day (WMD = −1.27, 95% CI = [−2.56, −0.11]) and control (WMD = −2.13, 95% CI = [−4.24, −0.13]) in the consistency and inconsistency models (Supplementary Table 4).
We performed node-splitting analysis to evaluate inconsistency by comparing direct and indirect effects (Supplementary Table 5). In ten comparisons, seven showed no significant inconsistency, suggesting that the consistency model is reliable. The CRP and ≥12-month duration subgroups could not form closed loops to conduct node-splitting analysis; therefore, we conducted both consistency and inconsistency models; the results of the two models were consistent, suggesting that the results are reliable. The CHD subgroup had two inconsistent comparisons between direct effect and indirect effect (P < 0.05); therefore, we generated an inconsistency model; however, the results of these two subgroups should be interpreted with caution (34, 71). In addition, the PSRF was between 1.00 and 1.05, indicating that the analysis had good convergence (70).
To the best of our knowledge, this is the first study to compare the effects of different types and doses of statins on plasma CRP levels in patients with dyslipidemia or CHD.
The pairwise meta-analysis showed that, compared with control, statins decreased CRP levels, which was consistent with previous studies. The JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) was an RCT investigating the anti-inflammatory effects of rosuvastatin in apparently healthy people with elevated hs-CRP levels (72). It showed that rosuvastatin reduced LDL-C levels by 50% and hs-CRP levels by 37%; rosuvastatin significantly reduced the occurrence of major adverse cardiovascular events. Similarly, the HOPE-3 (Heart Outcomes Prevention Evaluation-3) study on intermediate-risk participants without cardiovascular disease supported the hs-CRP-lowering effect of rosuvastatin regardless of CRP and lipid levels at baseline (73). Other systematic reviews supported the role of statins in reducing hs-CRP in patients with cardiovascular diseases (74), stroke (75), and apparently healthy people or patients with chronic diseases (76). Inconsistently, a meta-analysis (77) showed no significant difference between statins and control in lowering the hs-CRP level in atherosclerosis (WMD = −1.61, P = 0.09); however, this subgroup only included three trials with 236 participants, which was too few to obtain reliable results.
Dyslipidemia and inflammation are closely interconnected drivers of atherosclerotic heart disease (78). Correspondingly, statins are pleiotropic drugs that lower serum cholesterol by inhibiting hepatic cholesterol biosynthesis and exert cardiovascular protective effects such as anti-inflammation (10). It remains inconclusive whether the anti-inflammatory effects of statins are independent of their lipid-lowering efficacy. Labos et al. used Egger regression to reanalyze the available previous RCT data of statins (79). The study showed that each 1 mmol/L change in LDL-C with statin therapy was associated with a hazard ratio of 0.77 in cardiovascular endpoints with an intercept indistinguishable from zero, suggesting that statins' cardiovascular benefits were entirely derived from LDL-C lowering. Fernando et al. suggested that this analysis should use multivariable (and not “standard”) Egger regression (80). In contrast, in the Cholesterol and Recurrent Events (CARE) trial that investigated inflammation and coronary events after myocardial infarction, statins reduced CRP levels independently of LDL-C (81). Subsequently, a post-hoc analysis of the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) reported that compared to individuals with low levels of LDL-C and CRP, those with low LDL-C but elevated CRP levels benefited markedly from lovastatin, suggesting anti-inflammatory activity independent of lipid-lowering (82). The Pravastatin Inflammation/CRP Evaluation (PRINCE) trial demonstrated that pravastatin 40 mg/day significantly reduced plasma CRP levels independent of any changes in LDL levels (83). Unlike clinical studies with inconsistent conclusions, experimental studies assessed the anti-inflammatory effects of statins independent of their lipid-lowering action (17).
C-reactive protein is considered a nonspecific marker of inflammation, produced in response to the action of IL-6, IL-1, or TNF-α (17). It remains unclear whether patients would benefit if CRP were a therapeutic target, although CRP has attracted attention for its applications in screening and risk stratification (84–86). Nevertheless, anti-inflammatory therapies have shown compelling effects in preventing cardiovascular events recently. The Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) demonstrated that canakinumab, an IL-1β blocker (150 mg for every 3 months), reduced the incidence of nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death (6). Colchicine is an anti-inflammatory drug for treating gout, familial Mediterranean fever, and pericarditis. It has shown promising efficacy in atherosclerotic heart disease. The Colchicine Cardiovascular Outcomes Trial (COLCOT) found that 0.5 mg of colchicine daily significantly lowered the risk of ischemic cardiovascular events in patients who suffered a myocardial infarction within 30 days (7). Subsequently, the Low-Dose Colchicine 2 trial (LoDoCo2), an RCT that involved 5,522 patients with chronic coronary disease, revealed that 0.5 mg/day of colchicine significantly lowered the risk of cardiovascular events (8). The evidence of these anti-inflammatory therapies suggests an approach to treating atherosclerotic disease besides lipid-lowering. Recently, a position paper of the European Society of Cardiology stated that, given the strong association among inflammation, lipids, and atherosclerosis, it would be helpful to assess the inflammatory response to lipid-lowering interventions, thereby establishing the optimal dose and type of lipid-lowering therapy for cardiovascular prevention (11).
The results of NMA showed that simvastatin 40 mg/day appeared to be the best for lowering CRP among the included statin therapies. Simvastatin is a lipophilic statin and an inactive prodrug hydrolyzed in the liver to its major active β-hydroxy acid metabolite (87). Compared with other lipid-lowering agents, simvastatin might be superior in reducing the risk of major adverse cardiovascular events in hypertriglyceridemic patients (88). Consistent with our findings, Mitra et al. supported the notion that lipophilic statins (such as simvastatin) at high-intensity dosage could significantly decrease inflammatory factor TNF-α (89). In contrast, Neda et al. tested the orientation of ligands (statins) and phosphorylcholine (the standard ligand of CRP) at the CRP active site using Molecular Operating Environment software (18). The docking experiments showed that rosuvastatin had the most robust interaction with CRP, followed by fluvastatin, pitavastatin, atorvastatin, pravastatin, simvastatin, and lovastatin. However, in addition to directly acting on CRP, statins reduce inflammation via ICAM-1 and VCAM-1 (17), and the evidence from in silico studies requires experimental studies for support. In terms of dosage, according to the 2013 ACC/AHA Guideline (90), 40 mg/day is the maximum recommended dose of simvastatin because of the risk of rhabdomyolysis at higher doses, although it is classified as moderate-intensity statin therapy. Similar to our results, a higher intensity dosage is more likely to have better anti-inflammatory effects (89). In addition, high-dose statins (e.g., simvastatin 40 mg/day and atorvastatin 80 mg/day) are associated with the most significant benefits of secondary prevention in patients with ischemic stroke or transient ischemic attack (91).
We performed subgroup analyses to determine the heterogeneity. In the hs-CRP, non-ACS, <12-month duration, and clear measurement method subgroups, simvastatin 40 mg/day appeared to be the best strategy for CRP-lowering, consistent with the NMA. In the ≥12-month duration subgroup, atorvastatin 80 mg/day was most likely to be the best. The evidence from the ≥12-month duration subgroup is more clinically meaningful because statins are long-term drugs. In the CRP subgroup, atorvastatin 80 mg/day was most likely to be the best for reducing CRP levels, while there were only three studies, which made the results unpersuasive. Conversely, there were no significant differences in dyslipidemia and ACS subgroups. Previous research showed a significant difference between statins and placebo in ACS (76). This discrepancy might be caused by the limited number and heterogeneity of included trials.
Our study has some advantages. First, we performed an NMA of RCTs, which could compare multiple treatments and enable us to synthesize data with direct and indirect evidence (30). Compared to previous meta-analyses (76, 77), this study could incorporate all available data to assess interventions more accurately (70). Second, the results were highly consistent between the direct meta-analysis and NMA and the NMA and NMA subgroups, suggesting a stable result. Finally, our study provides the ranking possibilities of different statins, which can help clinicians make choices when faced with elevated CRP levels in patients with CHD.
Although we strictly followed the PRISMA extension statement for NMA, there are some limitations. First, it would be more clinically meaningful if we included a subgroup of baseline CRP levels; however, this is challenging because these studies chose participants according to disease rather than CRP level. Second, there was significant heterogeneity among included studies. To resolve the heterogeneity, we used a random-effects model, which may have influenced differences in study design and trial populations, as well as statistical heterogeneity in some of our results (70). In addition, we conducted subgroup analyses in terms of the measurement method of CRP, the population, or the treatment duration. A leave-one-out influence analysis was performed to test the robustness of the pooled results. The results of this study can still be considered credible. Third, owing to the limited number of trials, we could not include all statin therapies recommended by the guidelines (90) and did not differentiate among statins from various brands, which might lead to errors; nevertheless, this study covered statin prescriptions commonly used in clinical, and all statins are approved, commercially available drugs. Fourth, serum CRP levels are influenced by treatment duration, while the traditional meta-analysis and NMA cannot elucidate the changing effects over time (92). We thereby excluded the studies with treatment <8 weeks and performed a subgroup analysis of treatment duration to partly solve the problem. Finally, our study only included patients with dyslipidemia or CHD, whereas statins are used more widely; however, this also reduced the clinical heterogeneity.
Statins reduce serum CRP levels in patients with dyslipidemia or CHD. Simvastatin 40 mg/day might be the most effective therapy, and atorvastatin 80 mg/day showed the best long-term effect. This study provides a reference for choosing statin therapy based on LDL-C and CRP levels.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
HX, JQJ, and JZ designed this study. JZ and XW searched the databases and wrote the original draft. WT and TXW screened the publications and extracted data. RL and JDJ specified the data. JZ and LS performed the analysis. XW and HX provided methodological guidance. TW and ZZ normalized the figures and tables. All authors reviewed the manuscript.
The study was supported by the Chinese Academy of Chinese Medical Sciences Innovation Fund (CACMS Innovation Fund, CI2021A00917), Chinese Academy of Traditional Chinese Medicine Science and Technology Major Achievement Guidance Project (ZZ13-ZD-03), and Central Public Welfare Research Institutes of China Academy of Chinese Medical Sciences (ZZ13-YQ-017-C1).
The authors would like to thank the National Clinical Research Center for Chinese Medicine Cardiology for supporting the study.
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.936817/full#supplementary-material
1. Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. (2017) 23:479–97. doi: 10.4158/EP171764.GL
2. Kopin L, Lowenstein C. Dyslipidemia. Ann Intern Med. (2017) 167:ITC81–ITC96. doi: 10.7326/AITC201712050
3. Hoogeveen RC, Ballantyne CM. Residual cardiovascular risk at low LDL: Remnants, Lipoprotein(a), and Inflammation. Clin Chem. (2021) 67:143–53. doi: 10.1093/clinchem/hvaa252
4. Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han X, et al. Research progress on the relationship between atherosclerosis and inflammation. Biomolecules. (2018) 8:80. doi: 10.3390/biom8030080
5. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. (1999) 340:115–26. doi: 10.1056/NEJM199901143400207
6. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914
7. Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. (2019) 381:2497–505. doi: 10.1056/NEJMoa1912388
8. Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. (2020) 383:1838–47. doi: 10.1056/NEJMoa2021372
9. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2020) 41:111–88. doi: 10.1093/eurheartj/ehz455
10. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. (2017) 120:229–43. doi: 10.1161/CIRCRESAHA.116.308537
11. Tuñón J, Badimón L, Bochaton-Piallat ML, Cariou B, Daemen MJ, Egido J, et al. Identifying the anti-inflammatory response to lipid lowering therapy: a position paper from the working group on atherosclerosis and vascular biology of the European Society of Cardiology. Cardiovasc Res. (2019) 115:10–9. doi: 10.1093/cvr/cvy293
12. Pathak A, Agrawal A. Evolution of C-reactive protein. Front Immunol. (2019) 10:943. doi: 10.3389/fimmu.2019.00943
13. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APHA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task force on clinical Practice Guidelines. J Am Coll Cardiol. (2019) 73:3168–209. doi: 10.1016/j.jacc.2018.11.002
14. Golia E, Limongelli G, Natale F, Fimiani F, Maddaloni V, Pariggiano I, et al. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep. (2014) 16:435. doi: 10.1007/s11883-014-0435-z
15. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. (2003) 107:499–511. doi: 10.1161/01.CIR.0000052939.59093.45
16. Machado V, Botelho J, Escalda C, Hussain SB, Luthra S, Mascarenhas P, et al. Serum C-reactive protein and periodontitis: a systematic review and meta-analysis. Front Immunol. (2021) 12:706432. doi: 10.3389/fimmu.2021.706432
17. Satny M, Hubacek JA, Vrablik M. Statins and inflammation. Curr Atheroscler Rep. (2021) 23:80. doi: 10.1007/s11883-021-00977-6
18. Shakour N, Ruscica M, Hadizadeh F, Cirtori C, Banach M, Jamialahmadi T, et al. Statins and C-reactive protein: in silico evidence on direct interaction. Arch Med Sci. (2020) 16:1432–9. doi: 10.5114/aoms.2020.100304
19. Guo S, Wang R, Yang Z, Li K, Wang Q. Effects of atorvastatin on serum lipids, serum inflammation and plaque morphology in patients with stable atherosclerotic plaques. Exp Ther Med. (2012) 4:1069–74. doi: 10.3892/etm.2012.722
20. van Wissen S, Trip MD, Smilde TJ, de Graaf J, Stalenhoef AF, Kastelein JJ. Differential hs-CRP reduction in patients with familial hypercholesterolemia treated with aggressive or conventional statin therapy. Atherosclerosis. (2002) 165:361–6. doi: 10.1016/S0021-9150(02)00280-0
21. Moutzouri E, Liberopoulos E, Mikhailidis DP, Kostapanos MS, Kei AA, Milionis H, et al. Comparison of the effects of simvastatin vs. rosuvastatin vs. simvastatin/ezetimibe on parameters of insulin resistance. Int J Clin Pract. (2011) 65:1141–8. doi: 10.1111/j.1742-1241.2011.02779.x
22. Hong YJ, Jeong MH, Hachinohe D, Ahmed K, Choi YH, Cho SH, et al. Comparison of effects of rosuvastatin and atorvastatin on plaque regression in Korean patients with untreated intermediate coronary stenosis. Circ J. (2011) 75:398–406. doi: 10.1253/circj.CJ-10-0658
23. Milionis HJ, Kakafika AI, Tsouli SG, Athyros VG, Bairaktari ET, Seferiadis KI, et al. Effects of statin treatment on uric acid homeostasis in patients with primary hyperlipidemia. Am Heart J. (2004) 148:635–40. doi: 10.1016/j.ahj.2004.04.005
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
25. P.T. HJ, James T, Jacqueline C, Miranda C, Tianjing L, J. PM, et al. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, Ltd. (2019).
26. McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A, Collaboration DESD. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Statistical Methods Med Res. (2020) 29:2520–37. doi: 10.1177/0962280219889080
27. Wang X, Yang Q, Liao Q, Li M, Zhang P, Santos HO, et al. Effects of intermittent fasting diets on plasma concentrations of inflammatory biomarkers: a systematic review and meta-analysis of randomized controlled trials. Nutrition. (2020) 79–80:110974. doi: 10.1016/j.nut.2020.110974
28. Chiavaroli L, Lee D, Ahmed A, Cheung A, Khan TA, Blanco S, et al. Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: systematic review and meta-analysis of randomised controlled trials. Bmj. (2021) 374:n1651. doi: 10.1136/bmj.n1651
29. Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care. (1990) 6:5–30. doi: 10.1017/S0266462300008916
30. Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res. (2008) 17:279–301. doi: 10.1177/0962280207080643
31. Jansen JP, Crawford B, Bergman G, Stam W. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health. (2008) 11:956–64. doi: 10.1111/j.1524-4733.2008.00347.x
32. Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Gr Stat. (1998) 7:434–55. doi: 10.1080/10618600.1998.10474787
33. van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. (2012) 3:285–99. doi: 10.1002/jrsm.1054
34. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. (2010) 29:932–44. doi: 10.1002/sim.3767
35. Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. (2005) 111:3481–8. doi: 10.1161/CIRCULATIONAHA.105.537878
36. Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. Arbiter: arterial biology for the investigation of the treatment effects of reducing cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation. (2002) 106:2055–60. doi: 10.1161/01.CIR.0000034508.55617.65
37. Tonkin AM, Blankenberg S, Kirby A, Zeller T, Colquhoun DM, Funke-Kaiser A, et al. Biomarkers in stable coronary heart disease, their modulation and cardiovascular risk: the lipid biomarker study. Int J Cardiol. (2015) 201:499–507. doi: 10.1016/j.ijcard.2015.07.080
38. Yu CM, Zhang Q, Lam L, Lin H, Kong SL, Chan W, et al. Comparison of intensive and low-dose atorvastatin therapy in the reduction of carotid intimal-medial thickness in patients with coronary heart disease. Heart. (2007) 93:933–9. doi: 10.1136/hrt.2006.102848
39. Ran D, Nie HJ, Gao YL, Deng SB, Du JL, Liu YJ, et al. A randomized, controlled comparison of different intensive lipid-lowering therapies in Chinese patients with Non-St-Elevation Acute Coronary Syndrome (Nste-Acs): ezetimibe and rosuvastatin versus high-dose rosuvastatin. Int J Cardiol. (2017) 235:49–55. doi: 10.1016/j.ijcard.2017.02.099
40. Guo J, Zhang WZ, Zhao Q, Wo JS, Cai SL. Study on the effect of different doses of rosuvastatin on ventricular remodeling in patients with acute coronary syndrome after emergency percutaneous coronary intervention. Eur Rev Med Pharmacol Sci. (2017) 21:4457–63. https://www.europeanreview.org/article/13522
41. Qu HY, Xiao YW, Jiang GH, Wang ZY, Zhang Y, Zhang M. Effect of atorvastatin versus rosuvastatin on levels of serum lipids, inflammatory markers and adiponectin in patients with hypercholesterolemia. Pharm Res. (2009) 26:958–64. doi: 10.1007/s11095-008-9798-6
42. Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (Japan-Acs [Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome] Study). J Am Coll Cardiol. (2009) 54:293–302. doi: 10.1016/j.jacc.2009.04.033
43. Lim JW, Jeong HS, Hong SJ, Kim HJ, Kim YC, Kang BG, et al. Effects of lowest-dose vs. highest-dose pitavastatin on coronary neointimal hyperplasia at 12-month follow-up in type 2 diabetic patients with non-ST elevation acute coronary syndrome: an optical coherence tomography analysis. Heart Vessels. (2019) 34:62–73. doi: 10.1007/s00380-018-1227-0
44. Komukai K, Kubo T, Kitabata H, Matsuo Y, Ozaki Y, Takarada S, et al. Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: the easy-fit study. J Am Coll Cardiol. (2014) 64:2207–17. doi: 10.1016/j.jacc.2014.08.045
45. Chan KC, Chou HH, Huang CN, Chou MC. Atorvastatin administration after percutaneous coronary intervention in patients with coronary artery disease and normal lipid profiles: impact on plasma adiponectin level. Clin Cardiol. (2008) 31:253–8. doi: 10.1002/clc.20181
46. Koh KK, Son JW, Ahn JY, Jin DK, Kim HS, Choi YM, et al. Vascular effects of diet and statin in hypercholesterolemic patients. Int J Cardiol. (2004) 95:185–91. doi: 10.1016/j.ijcard.2003.05.018
47. Koh KK, Quon MJ, Han SH, Lee Y, Ahn JY, Kim SJ, et al. Simvastatin improves flow-mediated dilation but reduces adiponectin levels and insulin sensitivity in hypercholesterolemic patients. Diabetes Care. (2008) 31:776–82. doi: 10.2337/dc07-2199
48. Koh KK, Quon MJ, Sakuma I, Lee Y, Lim S, Han SH, et al. Effects of simvastatin therapy on circulating adipocytokines in patients with hypercholesterolemia. Int J Cardiol. (2011) 146:434–7. doi: 10.1016/j.ijcard.2010.10.103
49. Koh KK, Oh PC, Sakuma I, Kim EY, Lee Y, Hayashi T, et al. Vascular and metabolic effects of ezetimibe combined with simvastatin in patients with hypercholesterolemia. Int J Cardiol. (2015) 199:126–31. doi: 10.1016/j.ijcard.2015.07.016
50. Koh KK, Oh PC, Sakuma I, Lee Y, Han SH, Shin EK. Rosuvastatin dose-dependently improves flow-mediated dilation, but reduces adiponectin levels and insulin sensitivity in hypercholesterolemic patients. Int J Cardiol. (2016) 223:488–93. doi: 10.1016/j.ijcard.2016.08.051
51. Kanadaşi M, Cayli M, Demirtaş M, Inal T, Demir M, Koç M, et al. The effect of early statin treatment on inflammation and cardiac events in acute coronary syndrome patients with low-density lipoprotein cholesterol. Heart Vessels. (2006) 21:291–7. doi: 10.1007/s00380-005-0901-1
52. Nakagomi A, Shibui T, Kohashi K, Kosugi M, Kusama Y, Atarashi H, et al. Differential effects of atorvastatin and pitavastatin on inflammation, insulin resistance, and the carotid intima-media thickness in patients with dyslipidemia. J Atheroscler Thromb. (2015) 22:1158–71. doi: 10.5551/jat.29520
53. Kitagawa K, Hosomi N, Nagai Y, Kagimura T, Ohtsuki T, Origasa H, et al. Reduction in high-sensitivity C-reactive protein levels in patients with ischemic stroke by statin treatment: Hs-CRP sub-study in J-STARS. J Atheroscler Thromb. (2017) 24:1039–47. doi: 10.5551/jat.39354
54. Chen Q, Shang X, Yuan M, Liang L, Zhong X. Effect of atorvastatin on serum omentin-1 in patients with coronary artery disease. Coron Artery Dis. (2017) 28:44–51. doi: 10.1097/MCA.0000000000000435
55. Werida R, Khairat I, Khedr NF. Effect of atorvastatin versus rosuvastatin on inflammatory biomarkers and lv function in type 2 diabetic patients with dyslipidemia. Biomed Pharmacother. (2021) 135:111179. doi: 10.1016/j.biopha.2020.111179
56. Krysiak R, Gdula-Dymek A, Okopien B. Effect of simvastatin and fenofibrate on cytokine release and systemic inflammation in type 2 diabetes mellitus with mixed dyslipidemia. Am J Cardiol. (2011) 107:1010–8.e1. doi: 10.1016/j.amjcard.2010.11.023
57. Krysiak R, Okopien B. The effect of ezetimibe and simvastatin on monocyte cytokine release in patients with isolated hypercholesterolemia. J Cardiovasc Pharmacol. (2011) 57:505–12. doi: 10.1097/FJC.0b013e318211703b
58. Kinlay S, Schwartz GG, Olsson AG, Rifai N, Leslie SJ, Sasiela WJ, et al. High-dose atorvastatin enhances the decline in inflammatory markers in patients with acute coronary syndromes in the miracl study. Circulation. (2003) 108:1560–6. doi: 10.1161/01.CIR.0000091404.09558.AF
59. Park SJ, Kang SJ, Ahn JM, Chang M, Yun SC, Roh JH, et al. Effect of statin treatment on modifying plaque composition: a double-blind, randomized study. J Am Coll Cardiol. (2016) 67:1772–83. doi: 10.1016/j.jacc.2016.02.014
60. Tani S, Takahashi A, Nagao K, Hirayama A. Contribution of apolipoprotein A-I to the reduction in high-sensitivity C-reactive protein levels by different statins: comparative study of pitavastatin and atorvastatin. Heart Vessels. (2015) 30:762–70. doi: 10.1007/s00380-014-0554-z
61. Nicholls SJ, Brewer HB, Kastelein JJ, Krueger KA, Wang MD, Shao M, et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. JAMA. (2011) 306:2099–109. doi: 10.1001/jama.2011.1649
62. Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. (2011) 365:2078–87. doi: 10.1056/NEJMoa1110874
63. Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. (2004) 291:1071–80. doi: 10.1001/jama.291.9.1071
64. Nozue T, Yamamoto S, Tohyama S, Fukui K, Umezawa S, Onishi Y, et al. Effects of statins on serum n-3 to n-6 polyunsaturated fatty acid ratios in patients with coronary artery disease. J Cardiovasc Pharmacol Ther. (2013) 18:320–6. doi: 10.1177/1074248412473202
65. Zhang X, Wang H, Liu S, Gong P, Lin J, Lu J, et al. Intensive-dose atorvastatin regimen halts progression of atherosclerotic plaques in new-onset unstable angina with borderline vulnerable plaque lesions. J Cardiovasc Pharmacol Ther. (2013) 18:119–25. doi: 10.1177/1074248412465792
66. Zamani B, Saatlo BB, Naghavi-Behzad M, Taqizadeh-Jahed M, Alikhah H, Abbasnezhad M. Effects of high versus low-dose atorvastatin on high sensitive C-reactive protein in acute coronary syndrome. Niger Med J. (2014) 55:490–4. doi: 10.4103/0300-1652.144704
67. Zhao Z, Geng J, Ge ZM, Wang W, Zhang Y, Kang WQ. Efficacy and safety of atorvastatin during early hospitalization in elderly patients with unstable angina. Clin Exp Pharmacol Physiol. (2009) 36:554–8. doi: 10.1111/j.1440-1681.2008.05110.x
68. Peters SA, Lind L, Palmer MK, Grobbee DE, Crouse JR III, O'Leary DH, et al. Increased age, high body mass index and low HDL-C levels are related to an echolucent carotid intima-media: the METEOR study. J Intern Med. (2012) 272:257–66. doi: 10.1111/j.1365-2796.2011.02505.x
69. Peng M, Dong H, Jiang X, Che W, Zou Y, Zhang Y, et al. A randomized unblinded trial to compare effects of intensive versus conventional lipid-lowering therapy in patients undergoing renal artery stenting. J Cardiol. (2019) 74:443–50. doi: 10.1016/j.jjcc.2019.04.010
70. Wang X, Li J, Ju J, Fan Y, Xu H. Effect of different types and dosages of statins on plasma lipoprotein(a) levels: a network meta-analysis. Pharmacol Res. (2021) 163:105275. doi: 10.1016/j.phrs.2020.105275
71. Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc. (2006) 101:447–59. doi: 10.1198/016214505000001302
72. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. (2008) 359:2195–207. doi: 10.1056/NEJMoa0807646
73. Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. (2016) 374:2021–31. doi: 10.1056/NEJMoa1600176
74. Kandelouei T, Abbasifard M, Imani D, Aslani S, Razi B, Fasihi M, et al. Effect of statins on serum level of Hs-CRP and CRP in patients with cardiovascular diseases: a systematic review and meta-analysis of randomized controlled trials. Mediators Inflamm. (2022) 2022:8732360. doi: 10.1155/2022/8732360
75. Alikiaii B, Heidari Z, Bagherniya M, Askari G, Sathyapalan T, Sahebkar A. The effect of statins on C-reactive protein in stroke patients: a systematic review of clinical trials. Mediators Inflamm. (2021) 2021:7104934. doi: 10.1155/2021/7104934
76. Milajerdi A, Larijani B, Esmaillzadeh A. Statins influence biomarkers of low grade inflammation in apparently healthy people or patients with chronic diseases: a systematic review and meta-analysis of randomized clinical trials. Cytokine. (2019) 123:154752. doi: 10.1016/j.cyto.2019.154752
77. Jia J, Zhang L, Wang L, Ji C, Xia R, Yang Y. A systematic review and meta-analysis on the efficacy of statins in the treatment of atherosclerosis. Ann Palliat Med. (2021) 10:6793–803. doi: 10.21037/apm-21-1243
78. Tuñón J, Bäck M, Badimón L, Bochaton-Piallat ML, Cariou B, Daemen MJ, et al. Interplay between hypercholesterolaemia and inflammation in atherosclerosis: translating experimental targets into clinical practice. Eur J Prev Cardiol. (2018) 25:948–55. doi: 10.1177/2047487318773384
79. Labos C, Brophy JM, Smith GD, Sniderman AD, Thanassoulis G. Evaluation of the pleiotropic effects of statins: a reanalysis of the randomized trial evidence using egger regression-brief report. Arterioscler Thromb Vasc Biol. (2018) 38:262–5. doi: 10.1161/ATVBAHA.117.310052
80. Hartwig FP, Borges MC, Lawlor DA., Letter by Hartwig, et al. regarding article, “Evaluation of the pleiotropic effects of statins: a reanalysis of the randomized trial evidence using egger regression”. Arterioscler Thromb Vasc Biol. (2018) 38:e85–6. doi: 10.1161/ATVBAHA.118.310897
81. Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) investigators. Circulation. (1998) 98:839–44. doi: 10.1161/01.CIR.98.9.839
82. Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. (2001) 344:1959–65. doi: 10.1056/NEJM200106283442601
83. Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. (2001) 286:64–70. doi: 10.1001/jama.286.1.64
84. Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cífková R, Cosentino F, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. (2015) 241:507–32. doi: 10.1016/j.atherosclerosis.2015.05.007
85. Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. (2002) 347:1557–65. doi: 10.1056/NEJMoa021993
86. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. (2000) 342:836–43. doi: 10.1056/NEJM200003233421202
87. Plosker GL, McTavish D. Simvastatin. A reappraisal of its pharmacology and therapeutic efficacy in hypercholesterolaemia. Drugs. (1995) 50:334–63. doi: 10.2165/00003495-199550020-00009
88. Qi YY, Yan L, Wang ZM, Wang X, Meng H, Li WB, et al. Comparative efficacy of pharmacological agents on reducing the risk of major adverse cardiovascular events in the hypertriglyceridemia population: a network meta-analysis. Diabetol Metab Syndr. (2021) 13:15. doi: 10.1186/s13098-021-00626-7
89. Abbasifard M, Kandelouei T, Aslani S, Razi B, Imani D, Fasihi M, et al. Effect of statins on the plasma/serum levels of inflammatory markers in patients with cardiovascular disease; a systematic review and meta-analysis of randomized clinical trials. Inflammopharmacology. (2022) 30:369–83. doi: 10.1007/s10787-022-00926-y
90. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2014) 63:2889–934. doi: 10.1016/j.jacc.2013.11.002
91. Tramacere I, Boncoraglio GB, Banzi R, Del Giovane C, Kwag KH, Squizzato A, et al. Comparison of statins for secondary prevention in patients with ischemic stroke or transient ischemic attack: a systematic review and network meta-analysis. BMC Med. (2019) 17:67. doi: 10.1186/s12916-019-1298-5
Keywords: statin, C-reactive protein, coronary heart disease, dyslipidemia, network meta-analysis
Citation: Zhang J, Wang X, Tian W, Wang T, Jia J, Lai R, Wang T, Zhang Z, Song L, Ju J and Xu H (2022) The effect of various types and doses of statins on C-reactive protein levels in patients with dyslipidemia or coronary heart disease: A systematic review and network meta-analysis. Front. Cardiovasc. Med. 9:936817. doi: 10.3389/fcvm.2022.936817
Received: 05 May 2022; Accepted: 04 July 2022;
Published: 27 July 2022.
Edited by:
Mark Slevin, Manchester Metropolitan University, United KingdomReviewed by:
Natalia G. Vallianou, Evaggelismos General Hospital, GreeceCopyright © 2022 Zhang, Wang, Tian, Wang, Jia, Lai, Wang, Zhang, Song, Ju and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianqing Ju, anVqaWFucWluZ0AxNjMuY29t; Hao Xu, eHVoYW90Y21AaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.