
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 06 September 2022
Sec. Cardiac Rhythmology
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.934664
This article is part of the Research Topic Diagnosis, Monitoring, and Treatment of Heart Rhythm: New Insights and Novel Computational Methods View all 30 articles
 Wenqiang Han1†
Wenqiang Han1† Yan Liu1†
Yan Liu1† Rina Sha1
Rina Sha1 Huiyu Liu1
Huiyu Liu1 Aihua Liu1
Aihua Liu1 Kellina Maduray1
Kellina Maduray1 Junye Ge1
Junye Ge1 Chuanzhen Ma1
Chuanzhen Ma1 Jingquan Zhong1,2*
Jingquan Zhong1,2*Background: At present, catheter ablation is an effective method for rhythm control in patients with atrial fibrillation (AF). However, AF recurrence is an inevitable problem after catheter ablation. To identify patients who are prone to relapse, we developed a predictive model that allows clinicians to closely monitor these patients and treat them with different personalized treatment plans.
Materials and methods: A total of 1,065 patients who underwent AF catheter ablation between January 2015 and December 2018 were consecutively included in this study, which examines the results of a 2-year follow-up. Patients with AF were divided into development cohort and validation cohort. Univariate and multivariate analyses were carried out on the potential risk factors. Specific risk factors were used to draw the nomogram according to the above results. Finally, we verified the performance of our model compared with CHADS2 and CHA2DS2-Vasc scores by receiver operating characteristic (ROC) curve and calibration curve and plotted the decision analysis curve (DAC).
Results: A total of 316 patients experienced AF recurrence. After univariate and multivariate analyses, AF history (H), age (A), snoring (S), body mass index (BMI) (B), anteroposterior diameter of left atrial (LA) (L), and persistent AF (P) were included in our prediction model. Our model showed a better performance compared with CHADS2 and CHA2DS2-Vasc scores, and the area under ROC curve (95%CI) was 0.7668 (0.7298–0.8037) vs. 0.6225 (0.5783–0.6666) and 0.6267 (0.5836–0.6717).
Conclusion: We established a nomogram (HASBLP score) for predicting AF recurrence after the first catheter ablation at a 2-year follow-up, which can be used as a tool to guide future follow-up of patients. However, its usefulness needs further validation.
Atrial fibrillation (AF) is the most common arrhythmia in adults worldwide (1). AF is associated with substantial morbidity and mortality, placing a significant burden on patients, families, and healthcare systems. The estimated prevalence of AF in adults is between 2% and 4% (2), and it will continue to rise due to the lengthening of life expectancy and improvement of screening methods (3–5). Catheter ablation of AF has been recommended by several important guidelines as an effective rhythm control strategy (1, 6), since it reduces hospitalization rate and improves the quality of life; however, its most significant disadvantage is recurrence. Recurrence of AF would not only affect enthusiasm for catheter ablation in patients with AF but also bring some potential risks.
According to several studies, both individuals with and without an AF recurrence have a different chance of developing thromboembolism (7–10). Nevertheless, AF recurrence is usually asymptomatic (11), causing an unawareness of the episode in a considerable number of patients. Therefore, the continued use of oral anticoagulation in patients with AF after catheter ablation remains controversial (12). The objective world needs a prediction model to predict the probability of AF recurrence to guide the follow-up after AF catheter ablation. At the same time, a recurrence prediction model could also assist in screening patients undergoing catheter ablation. Several predictors of arrhythmia recurrence, including left atrial (LA) size, LA fibrosis, non-paroxysmal AF, hypertension, and sleep apnea syndrome, had been proposed in previous studies (13). CHADS2 and CHA2DS2-Vasc scores have been shown to predict the recurrence of AF to some extent (14); however, as a prediction model, the result does not seem ideal.
In this study, we attempted to develop a predictive model for recurrence after the first catheter ablation in patients with AF by following and reviewing clinical data from those with AF and compared our predictive model with the CHADS2 and CHA2DS2-Vasc score models.
We aimed to establish a prediction model with the outcome of 2-year follow-up of patients with AF after catheter ablation. This study was based on data from a prospective observational study (Chinese Clinical Trial Registry: ChiCTR-OCH-14004674) of patients who underwent ablation at our center. The primary endpoint of this study was AF recurrence, defined as symptomatic or documented AF, atrial flutter, or atrial tachycardia >30s after a 3-month blanking period after the first catheter ablation.
All patients who underwent AF catheter ablation between January 2015 and December 2018 were consecutively included in this study unless they met any of the following exclusion criteria: (1) patients with a previous history of catheter ablation; (2) patients with < 24 months of follow-up; or (3) patients who underwent cardiac surgery during the follow-up period. Prior to catheter ablation, coronary computed tomography (CTA) or transesophageal echocardiography was performed to rule out cardiac thrombosis.
Age, gender, course of AF, type of AF, history of related diseases, LA size, and left ventricular ejection fraction (LVEF) were collected before the procedure, and AF history (years) was found based on medical records or according to patient-reported time of first documented AF. The types of AF were divided into paroxysmal AF and persistent AF (e.g., long-standing persistent AF). LA size was represented by its anteroposterior diameter measured by echocardiography. Patients with heart failure were defined as ≥ class 2 (classification of NYHA heart function) according to the admission diagnosis.
Oral anticoagulant (OAC; warfarin, rivaroxaban, or dabigatran) was used at least 3 months after catheter ablation. All patients had a follow-up of at least 24 months after the procedure. Documented AF was evaluated by electrocardiography (ECG) and a 24-h Holter monitoring at the first, third, and sixth months and every 6 months thereafter. If the patient did not show up for a scheduled follow-up, our office contacted them telephonically to recommend 24-h Holter monitoring at the local hospital and collect information on recurrence. Time and outcome of primary events were recorded during follow-up.
Data analysis was performed using IBM SPSS Statistic 25 and R,1 and the significance level was set at p < 0.05. Two-thirds of all patients were taken as development cohort and one-third of patients as validation cohort by random sampling. The rank sum test was used for numerical variables with non-normal distribution, independent t-test random was used for numerical variables with normal distribution, and categorical variables were tested by chi-square test (χ2 test). Univariate analysis was performed using the abovementioned methods and variables with p < 0.05 were included in the subsequent logistic regression. Iteratively reweighted least squares (IWLS) were used to fit the logistic regression model based on development cohort data (model 1); then, according to the results of logistic regression, the variables with p < 0.05 were selected to form model 2.
Nomogram was constructed in accordance with the results of model 2. A nomogram is valuable because it converts anticipated probabilities into points on a scale of 0–100 in a user-friendly graphic interface (15). The total number of points accumulated by various factors corresponds to a patient’s expected likelihood (16, 17). The point system ranks effect estimates irrespective of statistical significance, and it is modified by the existence of other factors.
The total score of the nomogram was the sum of the corresponding score assigned to each risk factor, which corresponds to the recurrence risk.
Receiver operating characteristic (ROC) and calibration curves were plotted using development cohort data and validation cohort data, respectively. Subsequently, a decision curve analysis (DCA) diagram was drawn from development cohort data to guide clinical decision-making. Risk factors included in CHADS2 and CHA2DS2-Vasc scores were used to form model CHADS2 and CHA2DS2-Vasc. Using development cohort data, ROC curves for the CHADS2 and CHA2DS2-Vasc models were created, and the area under the curve (AUC) was calculated.
As shown in Table 1, a total of 1,065 patients (no recurrence: recurrence = 749:316) were included in this study; the development cohort consisted of 710 patients (no recurrence: recurrence = 490:220), while the validation cohort consisted of 355 patients (no recurrence: recurrence = 259:96). Non-normally distributed continuous data were presented as median (Q1, Q3), normally distributed data were presented as mean ± standard (SD), and categorical variables were presented as percentages. Finally, after univariate analysis, age (p < 0.01), body mass index (BMI; p < 0.01), AF history (p < 0.01), snoring (p < 0.01), hypertension (p = 0.04), coronary heart disease (p < 0.01), diabetes (p = 0.01), heart failure (p < 0.01), valve diseases (p = 0.02), cardiomyopathy (p = 0.03), persistent AF (p < 0.01), and the anteroposterior diameter of the LA (p < 0.01) were found to be statistically significant with AF recurrence.
The abovementioned statistically significant risk factors were used to build model 1 (recurrence∼age + snoring + BMI + AF history + hypertension + coronary heart disease + diabetes + heart failure + valve diseases + cardiomyopathy + persistent AF + LA).
According to the results of logistic regression, the variables with p < 0.05 were selected to form model 2 (recurrence∼age + snoring + BMI + AF history + persistent AF + LA) to facilitate the clinical application, age was divided into five segments (< 40 years, 40–49 years, 50–59 years, 60–69 years, and ≥ 70 years), LA anteroposterior diameter was divided into four segments (<35 mm, 35–39.99 mm, 40–44.99 mm, and ≥ 45 mm), and BMI was divided into four segments according to the Chinese standard (< 24, 24–26.99, 27–29.99, and ≥ 30). In addition, the result of model 2, which we termed the HASBLP score (AF history, age, snoring, BMI, LA, and persistent AF), was used to plot the nomogram (Figure 1).

Figure 1. Atrial fibrillation (AF) recurrence nomogram. The nomogram was developed in the development cohort. The total score of the nomogram was the sum of the corresponding score assigned to each risk factor, and the total score corresponds to the recurrence risk.
The ROC curve of model 1, model HASBLP, and model CHADS2 and CHA2DS2-Vasc in the development cohort data is shown in Figure 2A, and their AUCs, shown in Table 2, were 0.7766 (95%CI, 0.7397–0.8135), 0.7668 (95%CI, 0.7298–0.8037), 0.6225 (95%CI, 0.5783–0.6666), and 0.6267 (95%CI, 0.5836–0.6717), respectively. Based on this result, we found that the CHADS2 and CHA2DS2-Vasc scores predict AF recurrence with suboptimal results, and the HASBLP score was better able to predict AF recurrence. The ROC curves of model 1 and HASBLP score with validation cohort data are shown in Figure 2B; calibration curves of model 1 and HASBLP score with development and validation cohort data are shown in Figures 3A–D. The analysis of DCA showed that the recurrence probability of patients was in the range of about 5 to 80%, and this model has the highest accuracy and net benefit in clinical application (Figure 4).
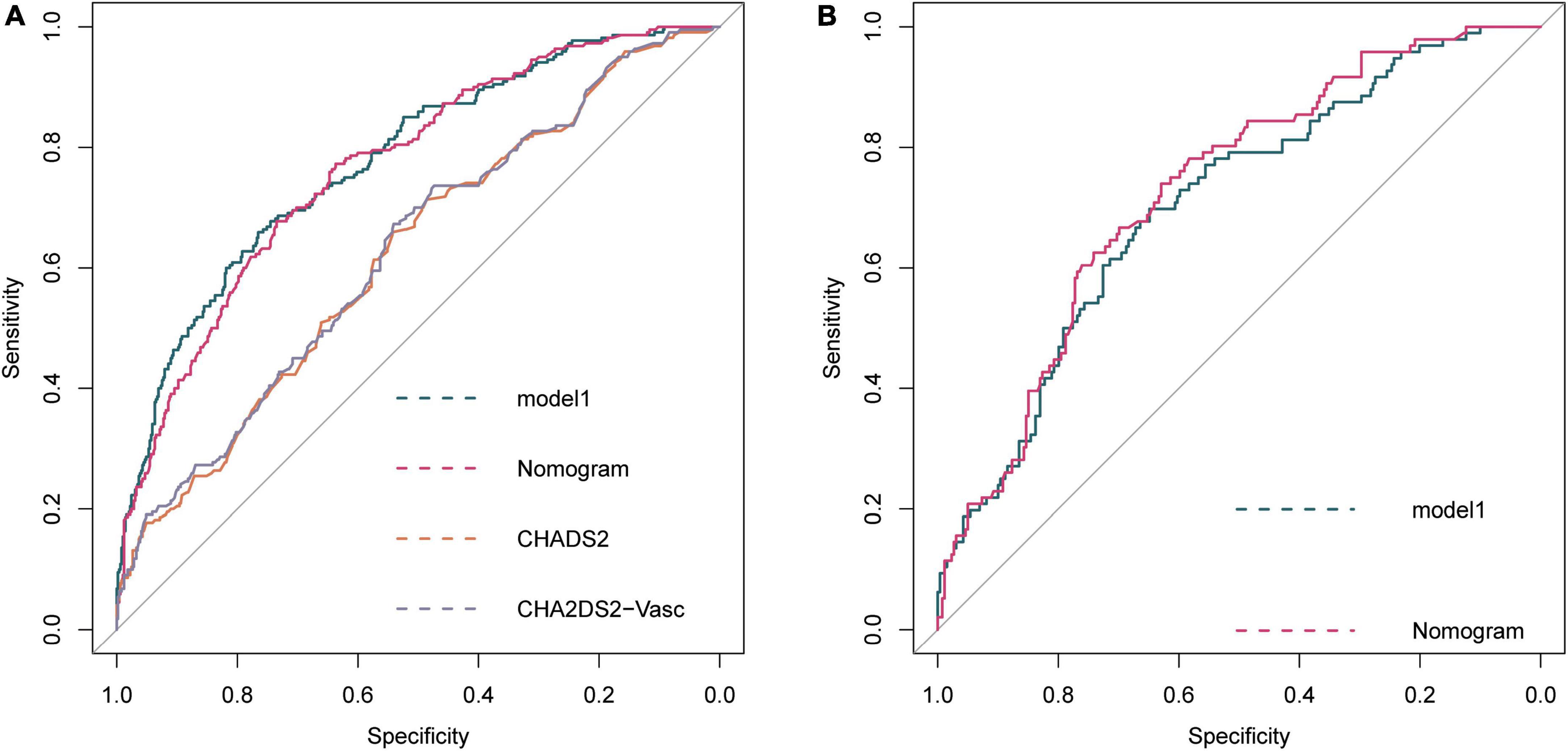
Figure 2. Receiver operating characteristic (ROC) curve of prediction model. (A) Development cohort; (B) Validation cohort. Model 1: Recurrence∼ age + snoring + BMI + AF history + hypertension + coronary heart disease + diabetes + heart failure + valve diseases + cardiomyopathy + persistent AF + LA. HASBLP: Recurrence∼age + snoring + BMI + AF history + persistent AF + LA. CHADS2: Recurrence∼heart failure + hypertension + age + diabetes + stroke. CHA2DS2-Vasc: Recurrence∼heart failure + hypertension + age + diabetes + stroke + vascular disease + female.
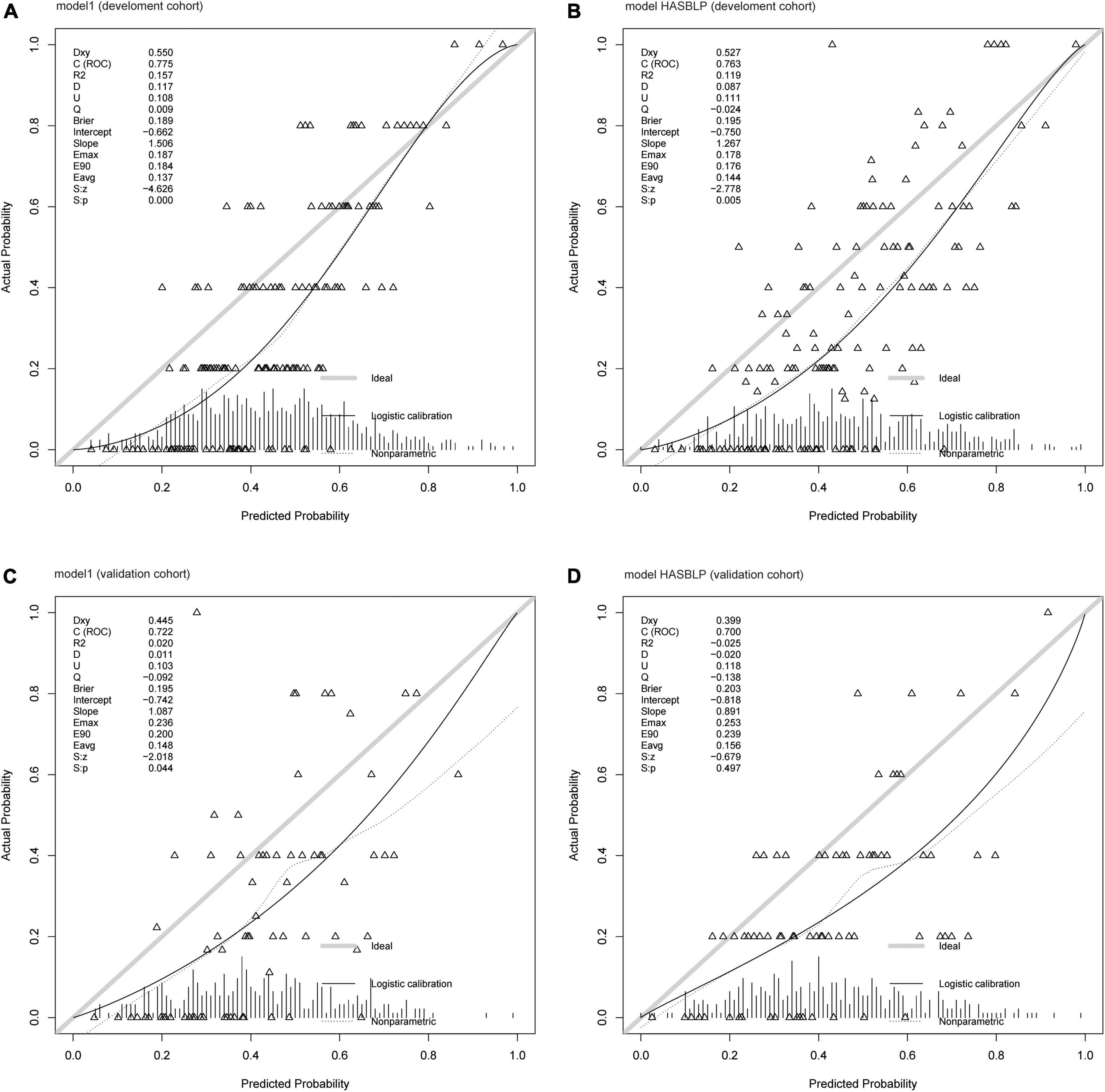
Figure 3. Calibration curve of prediction models. (A) Model 1 (development cohort); (B) model HASBLP (development cohort); (C) model 1 (validation cohort); and (D) model HASBLP (validation cohort).
Using clinical data and follow-up results of patients with AF in our center, we constructed a prediction model to predict AF recurrence after the first catheter ablation, which showed better performance compared with CHADS2 and CHA2DS2-Vasc scores. Several other scores, such as DR-FLASH (AUC 0.801) (18), CAAP-AF (AUC 0.691) (19), ATLAS (AUC 0.750) (20), APPLE (AUC 0.634) (21), and MB-LATER (AUC 0.782) (22), have shown good predictive effectiveness in their respective studies. However, there are differences in the overall variables included in our study compared with these studies, so it is difficult to compare them directly.
It is reported that age is the most common risk factor for AF recurrence in several trials and other prediction models (20, 21, 23, 24), which indicated that younger patients with AF may have a lower risk of recurrence following catheter ablation and thereby may be more suitable for the procedure. Being overweight or obese not only promotes the development of AF but also increases the risk of recurrence after catheter ablation (25–28). This may be associated with an increase in epicardial adipose tissue, which is a source of pro-inflammatory adipocytokines, leading to microvascular dysfunction and myocardial fibrosis (29). Inflammation has been proven to affect the occurrence of AF through multiple pathways (30). Obesity was also accompanied by other cardiovascular disease risk factors, such as hypertension, diabetes, and sleep apnea syndrome (31, 32), so weight loss could not only reduce the AF load (33) but also reduce AF recurrence after catheter ablation (34). Other studies have mentioned a history of AF as a risk factor for recurrence of AF after catheter ablation (35, 36); this may be due to the changes in the atrial matrix caused by risk factors over time. Jens Cosedis Nielsen’s trial proved that the efficacy of catheter ablation in patients with paroxysmal AF is better than that of patients with persistent AF (37). Although catheter ablation is effective for patients with persistent AF, the risk of recurrence is higher than that of patients with paroxysmal AF. Age, AF burden, obesity, smoking, renal insufficiency, and other cardiovascular risk factors promote atrial remodeling (38). While atrial enlargement is more likely a result of multiple factors, it often reflects atrial fibrosis. A study on MRI evaluation of atrial fibrosis and AF recurrence suggested that atrial fibrosis may be an independent risk factor for recurrence of AF after catheter ablation (39). In our study, the LA anteroposterior diameter measured by echocardiography represented the atrial size, which was slightly less accurate than the LA volume measured by CT or echocardiography. Still, it could increase the applicability of the model. Previous studies have shown that snoring is related to sleep apnea (SA) (40, 41). While snoring does not represent SA, habitual snoring is often a form of SA (42). Obstructive sleep apnea syndrome (OSAS) could promote the occurrence and progress of cardiovascular diseases, such as hypertension and arrhythmia (40, 41). A meta-analysis had shown OSAS could promote AF recurrence (43), and continuous positive airway pressure ventilation had a positive effect on preventing AF recurrence, which may be related to the correction of hypoxemia during sleep (44). In addition, a recent study showed that a healthy sleep pattern is associated with lower risks of AF and bradyarrhythmia, independent of traditional risk factors (45). An AF patient with snoring may be comorbid with OSAS or hypoxemia (44); however, some patients rarely get a proper diagnosis and treatment for a variety of reasons. Therefore, in our prediction model, OSAS was replaced by snoring. Snoring during sleep may be inaccurate and ambiguous compared with OSAS, but snoring as a common phenomenon is more practical in our opinion.
The present model might guide patients with AF to correct reversible risk factors after catheter ablation, such as weight loss, improvement of hypoxemia during sleep, and drug intervention for the process of cardiac fibrosis. It proposes that early treatment with catheter ablation not only allows for better symptom control but may also reduce the probability of AF recurrence. With the exploration of recurrence risk factors and the prediction models, we could screen patients who intend to undergo catheter ablation. For patients with a high risk of recurrence, catheter ablation should be carefully examined.
In addition, a long-term use of OAC or cessation of OAC after 3 months post-ablation remains controversial (46, 47). In our previous study, we concluded that cessation of OAC in non-recurrent AF may be reasonable; however, cessation appeared unsafe in recurrent AF with a high thromboembolism risk (10). With the help of the prediction model, patients at a high risk of recurrence could be identified after catheter ablation, allowing us to monitor these patients closely and encourage them to continue OAC.
There are several other limitations to our study. Due to following up with 24-h Holter ECG only, it might miss some patients with asymptomatic recurrence, which was inevitable in our study. This was a single-center study and the sample size should be expanded for more robust conclusions. Our prediction model needs to be verified by multicenter research. In this model, two variables (LA size and snoring) may be questioned for inaccuracies.
This study established a model (HASBLP score) for predicting AF recurrence after the first catheter ablation, which can be used as a tool to guide patients’ follow-up. Compared with CHADS2 and CHA2DS2-Vasc scores, this model showed a better performance in predicting AF recurrence. However, its role requires further validation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Qilu Hospital of Shandong University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
WH and YL: conceptualization, methodology, writing—original draft, writing—review and editing, investigation, and visualization. RS, HL, AL, KM, JG, and CM: investigation and resources. JZ: conceptualization, writing—review and editing, supervision, and funding acquisition. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (81970282), the Natural Science Foundation of Shandong Province (ZR2021MH126), and the Qingdao Key Health Discipline Development Fund. The funding sources had no roles in the design and conduct of the study; collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
We thank Keen Yang and the team of ultrasound department at Shenzhen People’s Hospital for their support and help.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS). Eur Heart J. (2021) 42:373–498.
2. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. (2019) 139:e56–528.
3. Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. (2013) 112:1142–7. doi: 10.1016/j.amjcard.2013.05.063
4. Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. (2013) 34:2746–51. doi: 10.1093/eurheartj/eht280
5. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res. (2017) 120:1501–17. doi: 10.1161/CIRCRESAHA.117.309732
6. Writing Group Members, January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Heart Rhythm. (2019) 16:e66–93. doi: 10.1016/j.hrthm.2019.01.024
7. Sjalander S, Holmqvist F, Smith JG, Platonov PG, Kesek M, Svensson PJ, et al. Assessment of use vs discontinuation of oral anticoagulation after pulmonary vein isolation in patients with atrial fibrillation. JAMA Cardiol. (2017) 2:146–52. doi: 10.1001/jamacardio.2016.4179
8. Bunch TJ, May HT, Bair TL, Weiss JP, Crandall BG, Osborn JS, et al. Atrial fibrillation ablation patients have long-term stroke rates similar to patients without atrial fibrillation regardless of CHADS2 score. Heart Rhythm. (2013) 10:1272–7. doi: 10.1016/j.hrthm.2013.07.002
9. Gallo C, Battaglia A, Anselmino M, Bianchi F, Grossi S, Nangeroni G, et al. Long-term events following atrial fibrillation rate control or transcatheter ablation: a multicenter observational study. J Cardiovasc Med (Hagerstown). (2016) 17:187–93. doi: 10.2459/JCM.0000000000000311
10. Rong B, Han W, Lin M, Hao L, Zhang K, Chen T, et al. Thromboembolic risk of cessation of oral anticoagulation post catheter ablation in patients with and without atrial fibrillation recurrence. Am J Cardiol. (2020) 137:55–62. doi: 10.1016/j.amjcard.2020.09.036
11. Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. (1994) 89:224–7. doi: 10.1161/01.CIR.89.1.224
12. Chew D, Piccini JP. Long-term oral anticoagulant after catheter ablation for atrial fibrillation. Europace. (2021) 23:1157–65. doi: 10.1093/europace/euaa365
13. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehaa612
14. Letsas KP, Efremidis M, Giannopoulos G, Deftereos S, Lioni L, Korantzopoulos P, et al. CHADS2 and CHA2DS2-VASc scores as predictors of left atrial ablation outcomes for paroxysmal atrial fibrillation. Europace. (2014) 16:202–7. doi: 10.1093/europace/eut210
15. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. (2008) 26:1364–70. doi: 10.1200/JCO.2007.12.9791
16. Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ Jr, Dotan ZA, DiBlasio CJ, et al. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol. (2005) 23:7005–12. doi: 10.1200/JCO.2005.01.867
17. Diblasio CJ, Kattan MW. Use of nomograms to predict the risk of disease recurrence after definitive local therapy for prostate cancer. Urology. (2003) 62(Suppl. 1):9–18. doi: 10.1016/j.urology.2003.09.029
18. Kosiuk J, Dinov B, Kornej J, Acou WJ, Schönbauer R, Fiedler L, et al. Prospective, multicenter validation of a clinical risk score for left atrial arrhythmogenic substrate based on voltage analysis: DR-FLASH score. Heart Rhythm. (2015) 12:2207–12. doi: 10.1016/j.hrthm.2015.07.003
19. Winkle RA, Jarman JW, Mead RH, Engel G, Kong MH, Fleming W, et al. Predicting atrial fibrillation ablation outcome: the CAAP-AF score. Heart Rhythm. (2016) 13:2119–25. doi: 10.1016/j.hrthm.2016.07.018
20. Mesquita J, Ferreira AM, Cavaco D, Moscoso Costa F, Carmo P, Marques H, et al. Development and validation of a risk score for predicting atrial fibrillation recurrence after a first catheter ablation procedure – ATLAS score. Europace. (2018) 20:f428–35. doi: 10.1093/europace/eux265
21. Kornej J, Hindricks G, Shoemaker MB, Husser D, Arya A, Sommer P, et al. The APPLE score: a novel and simple score for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation. Clin Res Cardiol. (2015) 104:871–6. doi: 10.1007/s00392-015-0856-x
22. Mujović N, Marinković M, Marković N, Shantsila A, Lip GY, Potpara TS. Prediction of very late arrhythmia recurrence after radiofrequency catheter ablation of atrial fibrillation: the MB-LATER clinical score. Sci Rep. (2017) 7:40828. doi: 10.1038/srep40828
23. Prystowsky EN, Padanilam BJ, Fogel RI. Treatment of atrial fibrillation. JAMA. (2015) 314:278–88. doi: 10.1001/jama.2015.7505
24. Jud FN, Obeid S, Duru F, Haegeli LM. A novel score in the prediction of rhythm outcome after ablation of atrial fibrillation: the SUCCESS score. Anatol J Cardiol. (2019) 21:142–9. doi: 10.14744/AnatolJCardiol.2018.76570
25. Wang TJ, Parise H, Levy D, D’Agostino RB Sr, Wolf PA, Vasan RS, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. (2004) 292:2471–7. doi: 10.1001/jama.292.20.2471
26. Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study). J Am Coll Cardiol. (2010) 55:2319–27. doi: 10.1016/j.jacc.2010.02.029
27. Needleman M, Calkins H. The role of obesity and sleep apnea in atrial fibrillation. Curr Opin Cardiol. (2011) 26:40–5. doi: 10.1097/HCO.0b013e328341398e
28. Nalliah CJ, Sanders P, Kottkamp H, Kalman JM. The role of obesity in atrial fibrillation. Eur Heart J. (2016) 37:1565–72. doi: 10.1093/eurheartj/ehv486
29. Wong CX, Ganesan AN, Selvanayagam JB. Epicardial fat and atrial fibrillation: current evidence, potential mechanisms, clinical implications, and future directions. Eur Heart J. (2017) 38:1294–302. doi: 10.1093/eurheartj/ehw045
30. Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. (2015) 12:230–43. doi: 10.1038/nrcardio.2015.2
31. Conen D, Tedrow UB, Koplan BA, Glynn RJ, Buring JE, Albert CM. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation. (2009) 119:2146–52. doi: 10.1161/CIRCULATIONAHA.108.830042
32. Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. (2007) 49:565–71. doi: 10.1016/j.jacc.2006.08.060
33. Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. (2013) 310:2050–60. doi: 10.1001/jama.2013.280521
34. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. (2014) 64:2222–31. doi: 10.1016/j.jacc.2014.09.028
35. Canpolat U, Aytemir K, Yorgun H, Sahiner L, Kaya EB, Oto A. A proposal for a new scoring system in the prediction of catheter ablation outcomes: promising results from the Turkish cryoablation registry. Int J Cardiol. (2013) 169:201–6. doi: 10.1016/j.ijcard.2013.08.097
36. Liang J, Liang H, Deng J, Wang X, Wang X, Wu L. [Clinical study on lymph node metastasis regularity in 1456 patients with gastric cancer]. Chin J Gastrointest Surg. (2018) 21:1154–60.
37. Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. (2012) 367:1587–95. doi: 10.1056/NEJMoa1113566
38. Kirchhof P, Calkins H. Catheter ablation in patients with persistent atrial fibrillation. Eur Heart J. (2017) 38:20–6. doi: 10.1093/eurheartj/ehw260
39. Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. (2014) 311:498–506. doi: 10.1001/jama.2014.3
40. Fava C, Montagnana M, Favaloro EJ, Guidi GC, Lippi G. Obstructive sleep apnea syndrome and cardiovascular diseases. Semin Thromb Hemost. (2011) 37:280–97. doi: 10.1055/s-0031-1273092
41. Stansbury RC, Strollo PJ. Clinical manifestations of sleep apnea. J Thorac Dis. (2015) 7:E298–310.
42. Lin GM, Colangelo LA, Lloyd-Jones DM, Redline S, Yeboah J, Heckbert SR, et al. Association of sleep apnea and snoring with incident atrial fibrillation in the multi-ethnic study of atherosclerosis. Am J Epidemiol. (2015) 182:49–57. doi: 10.1093/aje/kwv004
43. Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol. (2011) 108:47–51. doi: 10.1016/j.amjcard.2011.02.343
44. Linz D, McEvoy RD, Cowie MR, Somers VK, Nattel S, Lévy P, et al. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. (2018) 3:532–40. doi: 10.1001/jamacardio.2018.0095
45. Li X, Zhou T, Ma H, Huang T, Gao X, Manson JE, et al. Healthy sleep patterns and risk of incident arrhythmias. J Am Coll Cardiol. (2021) 78:1197–207. doi: 10.1016/j.jacc.2021.07.023
46. Themistoclakis S, Corrado A, Marchlinski FE, Jais P, Zado E, Rossillo A, et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol. (2010) 55:735–43.
Keywords: atrial fibrillation, catheter ablation, recurrence, prediction model, nomogram
Citation: Han W, Liu Y, Sha R, Liu H, Liu A, Maduray K, Ge J, Ma C and Zhong J (2022) A prediction model of atrial fibrillation recurrence after first catheter ablation by a nomogram: HASBLP score. Front. Cardiovasc. Med. 9:934664. doi: 10.3389/fcvm.2022.934664
Received: 02 May 2022; Accepted: 10 August 2022;
Published: 06 September 2022.
Edited by:
Haibo Ni, University of California, Davis, United StatesReviewed by:
Tchavdar Shalganov, National Heart Hospital, BulgariaCopyright © 2022 Han, Liu, Sha, Liu, Liu, Maduray, Ge, Ma and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingquan Zhong, MTg1NjAwODY1OTdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.