- 1Department of Cardiology and Vascular Medicine, Faculty of Medicine University of Padjadjaran, Bandung, Indonesia
- 2School of Medicine and Health Sciences, Atma Jaya Catholic University of Indonesia, Jakarta, Indonesia
- 3Division of Physiology, Department of Biomedical Sciences, Faculty of Medicine University of Padjadjaran, Bandung, Indonesia
- 4Faculty of Medicine University of Padjadjaran, Bandung, Indonesia
Introduction: Risk stratification in Brugada Syndrome (BrS) patients is still challenging due to the heterogeneity of clinical presentation; thus, some additional risk markers are needed. Several studies investigating the association between RVOT conduction delay sign on electrocardiography (ECG) and major arrhythmic events (MAE) in BrS patients showed inconclusive results. This meta-analysis aims to evaluate the association between RVOT conduction delay signs presented by aVR sign and large S wave in lead I, and MAE in BrS patients.
Methods: The literature search was performed using several online databases from the inception to March 16th, 2022. We included studies consisting of two main components, including ECG markers of RVOT conduction delay (aVR sign and large S wave in lead I) and MAE related to BrS (syncope/VT/VF/SCD/aborted SCD/appropriate ICD shocks)
Results: Meta-analysis of eleven cohort studies with a total of 2,575 participants showed RVOT conduction delay sign was significantly associated with MAE in BrS patients [RR = 1.87 (1.35, 2.58); p < 0.001; I2= 52%, Pheterogeneity = 0.02]. Subgroup analysis showed that aVR sign [RR = 2.00 (1.42, 2.83); p < 0.001; I2= 0%, Pheterogeneity = 0.40] and large S wave in lead I [RR = 1.74 (1.11, 2.71); p = 0.01; I2= 60%, Pheterogeneity = 0.01] were significantly associated with MAE. Summary receiver operating characteristics analysis revealed the aVR sign [AUC: 0.77 (0.73–0.80)] and large S wave in lead I [AUC: 0.69 (0.65–0.73)] were a good predictor of MAE in BrS patients.
Conclusion: RVOT conduction delay sign, presented by aVR sign and large S wave in the lead I, is significantly associated with an increased risk of MAE in BrS patients. Hence, we propose that these parameters may be useful as an additional risk stratification tool to predict MAE in BrS patients.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/#recordDetails, identifier: CRD42022321090.
Introduction
Brugada syndrome (BrS) is an inherited channelopathy that predisposes to sudden cardiac death (SCD) due to ventricular tachyarrhythmias (VTA) (1). The prevalence of BrS was 0.5 per 1,000 populations around the globe and accounted for 4 per cent of SCD (2, 3). Although BrS has a low prevalence, the risk of SCD caused by VTA in BrS patients with a structurally normal heart remains high, up to 20% (4). Shanghai et al. (5) and Sieira et al. (6) scores, which were established previously to diagnose BrS, have been used recently as stratification risk scores to predict SCD (7). The components of these two scores are type 1 Brugada electrocardiographic (ECG) pattern, syncope or unexplained cardiac arrest history, family history of SCD or confirmed BrS, and positive genetic result (5, 6). However, until now, risk stratification in BrS patients is still challenging because of a dynamic ECG pattern of Brugada, heterogeneity of clinical presentation, and unidentified clinical history.
Furthermore, in some cases, the decision of ICD implantation may be challenging, especially in BrS type I pattern on ECG with unexplained syncope (ex; syncope due to cardiac vs. non-cardiac) and without prior cardiac arrest or documented VTA. Hence, several studies investigated the association between several ECG markers and major arrhythmic events (MAE) in BrS patients to predict the likelihood of SCD and VTA events. Several meta-analyses revealed that first-degree atrioventricular block, fragmented QRS, wide QRS complex, early repolarization, especially in the inferolateral region, atrial fibrillation, Tpeak-Tend dispersion, Tpeak-Tend interval, and (Tpeak-Tend)/QTc ratio were significantly associated with a higher risk of MAE in BrS patients (8–14).
Studies by Coronel et al. (15) and Panonne et al. (16–18) also discovered the right ventricular outflow tract (RVOT) conduction delay as the origin of VTA in BrS patients. Therefore, hypothetically, RVOT conduction delay sign may predict the VTA events in BrS patients. RVOT conduction delay can be represented by two ECG findings, a positive aVR sign and a large S wave in the lead I (19). Several observational studies investigated the association between RVOT conduction delay sign on ECG and MAE in patients with BrS, but the results were equivocal (20–24). Hence, this meta-analysis aims to systematically evaluate the association between RVOT conduction delay presented by aVR sign and large S wave in the lead I with MAE in patients with BrS.
Methods
This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guideline.
Search Strategy
We conducted a systematic literature search from several databases, including Pubmed, EBSCO host, Europe PMC, and Proquest. The keyword of “(aVR sign or S wave in the lead I) AND (major arrhythmic events or syncope or ventricular tachyarrhythmia or ventricular tachycardia or ventricular fibrillation or sudden cardiac death or aborted sudden cardiac death or appropriate implantable cardioverter-defibrillator shocks) AND (Brugada syndrome)” was used. The timeframe was from the inception until March 16th, 2022. Two independent authors performed title or abstract screening and eligibility assessment of the articles. Discrepancies were resolved by discussion.
Eligibility Criteria
Studies that met the following criteria were included: prospective or retrospective cohort studies reporting ECG findings of RVOT conduction delay and MAE in BrS patients. BrS is diagnosed in patients with typical type 1 Brugada ECG patterns that occur spontaneously or drug-induced (25). There were two ECG patterns indicating RVOT conduction delay evaluated in this study, including a positive aVR sign and a large S wave in the lead I. The positive aVR sign is defined as R wave amplitude ≥0.3 mV in lead aVR or R/q ≥ 0.75 in lead aVR (20–22). While the criteria diagnosis of large S wave in the lead I is S wave amplitude ≥0.1 mV and/or S wave duration >40 ms in the lead I (22–24, 26–29). The outcome of interest in this study was MAE, including syncope, ventricular tachycardia (VT), ventricular fibrillation (VF), sudden cardiac death (SCD), aborted SCD, and appropriate implantable cardioverter-defibrillator (ICD) shocks. Syncope is defined as a patient with loss of consciousness that is expectingly caused by VTA and after excluding other possible causes, including neurally mediated syncope (23). VT and VF are defined as wide QRS complex tachycardia, originally initiated from the ventricular wall. VT and VF were recorded at the follow-up period by ECG, Holter monitoring, or implantable cardioverter-defibrillator (ICD) (23, 26). SCD is defined as death most likely caused by an arrhythmic event, and no evident extra-cardiac causes were identified (25). Moreover, studies that met one of the following criteria were removed: (1) review articles, (2) editorial/commentaries, (3) abstracts, (4) letters, (5) case reports, (6) case-control studies, (7) cross-sectional studies, and (8) studies in languages other than English.
Data Extraction and Quality Assessment
Two independent authors performed data extraction of the eligible studies using standardized extraction form for the first author, study design, location of the study, inclusion criteria, sample size, age, sex, male gender, RVOT conduction delay sign on ECG and its criteria, mean/median duration of follow up and the outcomes. Any discrepancies were resolved by discussion.
Two independent authors performed the risk of bias assessment using the Newcastle-Ottawa Scale (NOS) (30). Scoring for every article was assigned based on its degree of bias [low (included) and high (excluded)]. If a study received a total score equal to or >7, it indicated a good journal quality with a low risk of bias. Otherwise, if a study received a total score of six or less, it was determined to have a significant risk of bias and was eliminated from the study selection process. Discrepancies during the risk of bias assessment were resolved by discussion with the third reviewer.
Statistical Analysis
All statistical analysis was performed using Review Manager software version 5.4.1 (Cochrane Collaboration) and STATA software version 16. We calculated the pooled risk ratio (RR) and its 95% confidence interval using the Mantel-Haenzel formula to characterize the association between ECG findings of RVOT conduction delay and MAE in patients with BrS. The significance was obtained if the two-tailed p-value was ≤ 0.05. Inconsistency index (I2) test ranging from 0 to 100% was used to assess heterogeneity among the studies, in which I2 values >50% or Pheterogeneity < 0.05 indicate moderate to high heterogeneity (31). If high heterogeneity was found, a random-effects model was assigned to calculate the pooled RR. Otherwise, a fixed-effects model was used if low heterogeneity was noted. We performed a sensitivity analysis by excluding the study by leaving one method to detect the source of heterogeneity and obtained statistical robustness. Moreover, we performed a sub-group analysis comprising two ECG patterns of RVOT conduction delay to compare which ECG patterns were more significantly associated with MAE in BrS patients. We also performed a summary receiver operating characteristics (SROC) analysis to assess the predictive accuracy of these ECG markers in predicting MAE in BrS patients. Lastly, Begg's funnel plot and Egger's test were employed to discover publication bias and small-study effects.
Results
Characteristics of Included Studies
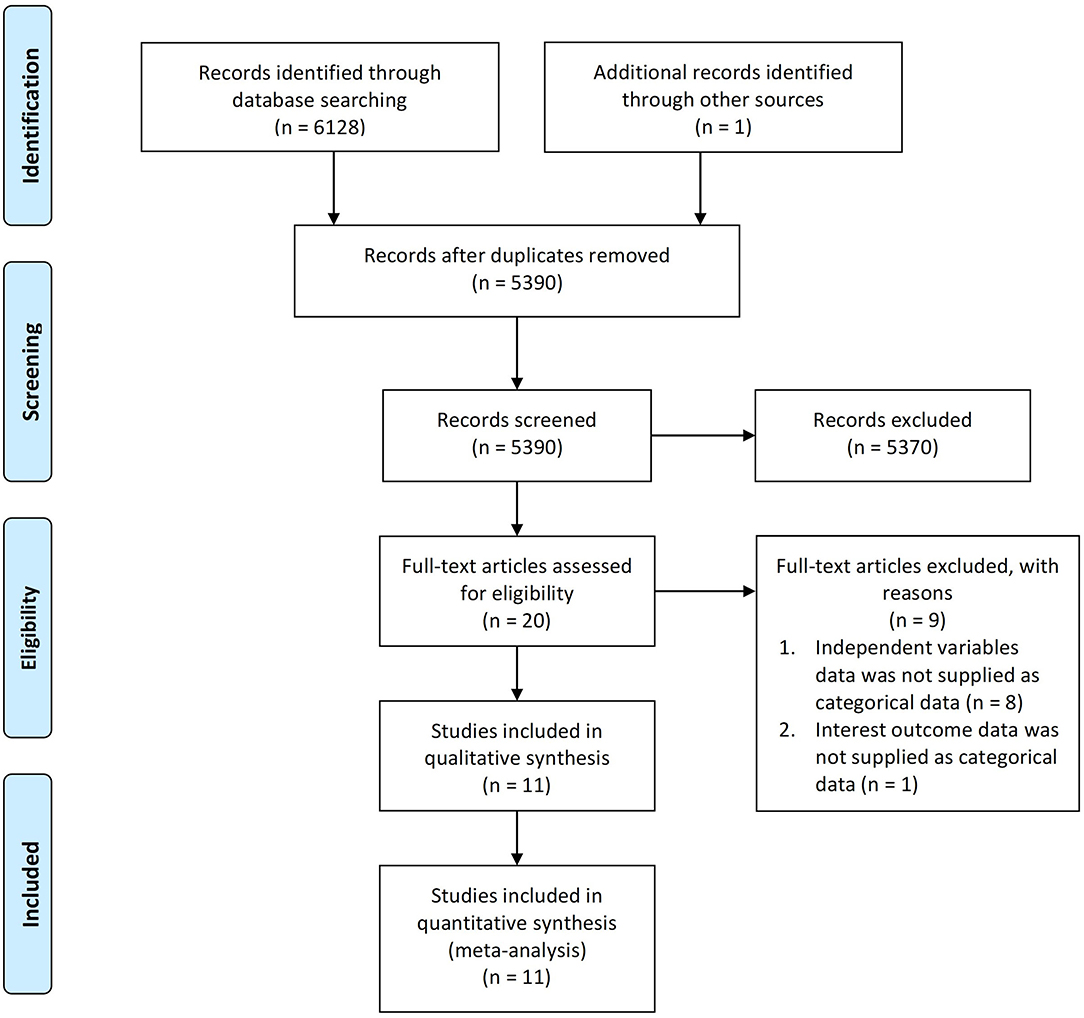
A total of 6,129 articles were collected from the literature search, one of which was discovered via the hand-searching procedure and 5,390 of which remained after duplicates were removed. Following a screening procedure for titles and abstracts, 20 suitable studies were retained. Furthermore, 9 studies were excluded based on two reasons: (1) independent variables were not supplied in categorical data (n = 8), and (2) outcomes of interests were not supplied in categorical data (n = 1). Eventually, at the end of the preliminary search, 11 cohort studies (20–24, 26–29, 32, 33) were included in the qualitative analysis. Bias risk quality assessment was performed using the New Castle Ottawa Scale (NOS), resulting in 5 studies receiving a score of 7 and 6 studies receiving a score of 8, which indicates that all included studies were good quality journals with a low risk of bias (Supplementary Table 1). Hence, these 11 cohort studies with a total of 2,575 participants were included in the quantitative analysis. The PRISMA flowchart describes the selection process as illustrated in Figure 1.
Our meta-analysis consisted of two studies (23, 29) using a prospective design, while the rest used a retrospective design (20–22, 24, 26–28, 32, 33). Most of the participants were male (77.5%), and there were two studies (20, 22) where all the subjects were male. There were two types of ECG parameters indicating RVOT conduction delay, which were investigated in these included studies, including a positive aVR sign and a large S wave in the lead I. The number of participants who had positive aVR sign and large S wave in the lead I were 16.3 and 36.3%, respectively (20–22, 26). The total MAE calculated for all participants in all included studies was 15.9%. The characteristic of included studies is described in Table 1.
Meta-Analysis of RVOT Conduction Delay Sign and Major Arrhythmic Events
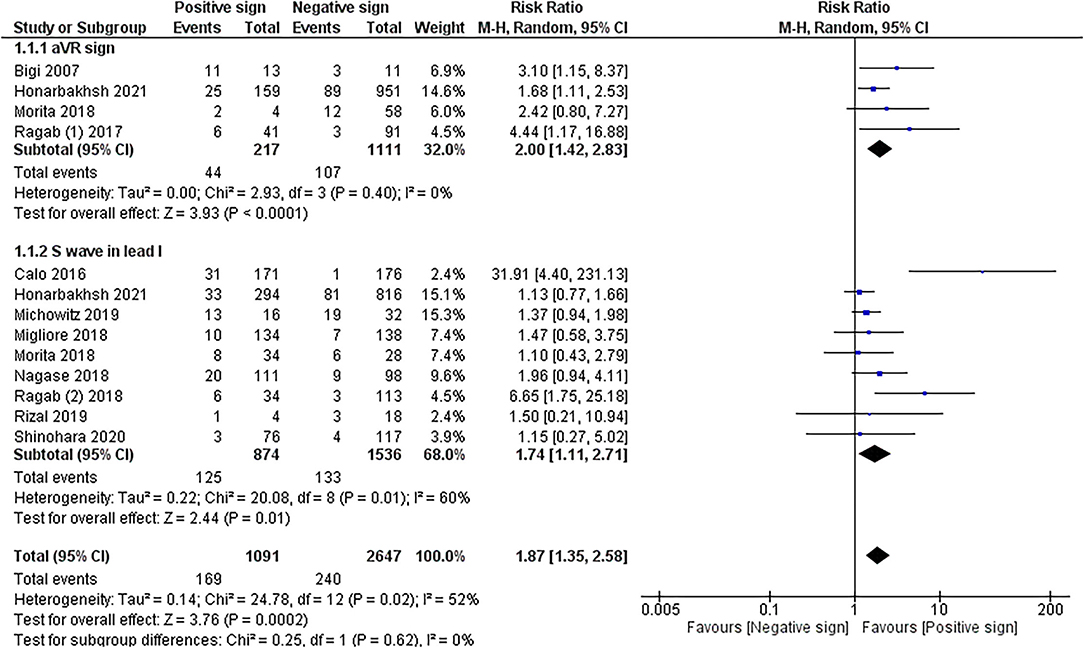
The meta-analysis in 10 cohorts studies with pooled subjects of 2,575 participants showed that RVOT conduction delay sign presented by positive aVR sign and prominent S wave in lead I combined was significantly associated with MAE in patients with BrS (RR = 1.87 (1.35, 2.58); p < 0.001; I 2= 52%, Pheterogeneity = 0.02). The meta-analysis of ECG patterns of RVOT conduction delay and MAE in patients with BrS is described in Figure 2.
Sub-group Analysis of aVR Sign and Major Arrhythmic Events
Sub-group analysis of 4 cohorts studies (20–22, 26) with pooled subjects of 1,328 participants revealed that aVR sign significantly increased the risk of MAE in BrS patients (RR = 2.00 (1.42, 2.83); p < 0.001; I2= 0%, Pheterogeneity = 0.40).
Sub-group Analysis of Large S Wave in the Lead I and Major Arrhythmic Events
Sub-group analysis of 9 cohort studies (22–24) with pooled subjects of 2,410 participants showed that a large S wave in the lead I was significantly associated with a higher risk of MAE in BrS patients [RR = 1.74 (1.11, 2.71); p = 0.01; I2= 60%, Pheterogeneity = 0.01]. Due to moderate heterogeneity, sensitivity analysis was performed by excluding the Calo et al. study resulting in reduced heterogeneity to 8%, and the association remained significant [RR = 1.39 (1.08, 1.78); p = 0.01; I 2= 8%, Pheterogeneity = 0.37].
SROC Analysis of RVOT Conduction Delay Sign in Predicting Major Arrhythmic Events
SROC analysis showed that positive aVR sign on ECG had a great diagnostic performance in predicting MAE in BrS patients [AUC: 0.77 (0.73–0.80), sensitivity: 38%, specificity: 83%, positive likelihood ratio: 2.2 (1.4, 3.4), negative likelihood ratio: 0.75 (0.50, 1.13), diagnostic odds ratio: 3.0 (1, 7)]. On the other hand, large S wave in lead I on ECG was also a good predictor of MAE in BrS patients [AUC: 0.69 (0.65–0.73), sensitivity: 57%, specificity: 69%, positive likelihood ratio: 1.85 (1.3, 2.62), negative likelihood ratio: 0.62 (0.42,0.92), diagnostic odds ratio: 2.98 (1.47, 6.07)]. SROC analysis is described in Figure 3.
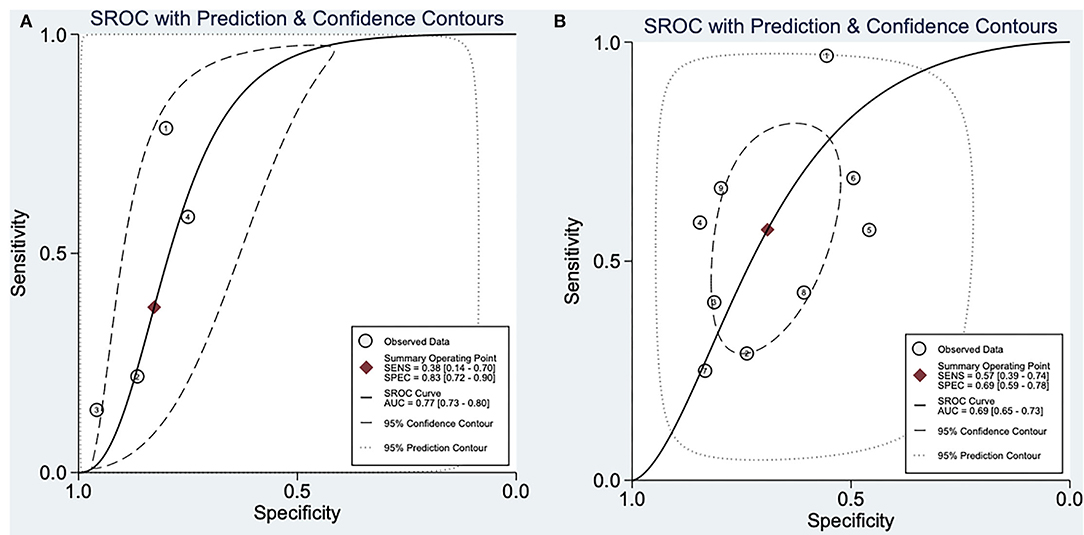
Figure 3. Summary receiver operatic characteristic analysis. (A) SROC analysis of aVR sign in predicting major arrhythmic events in BrS patients. (B) SROC analysis of large S wave in lead I in predicting major arrhythmic events in BrS patients. SROC, summary receiver operating characteristic; AUC, area under curve; SENS, sensitivity; SPEC, specificity.
Publication Bias
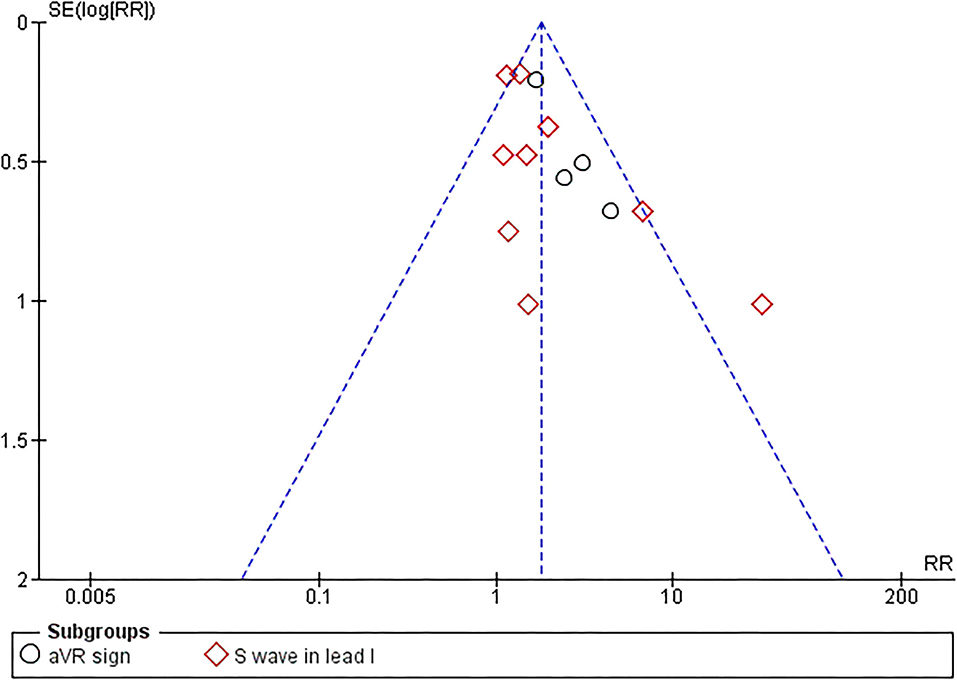
Begg's funnel plot analysis revealed qualitatively symmetrical funnel plots, suggesting no evidence of publication bias in association between RVOT conduction delay sign and MAE (Figure 4). Moreover, Egger's test showed no evidence of small-study effects for RVOT conduction delay sign and MAE (p = 0.097).
Discussion
To the best of our knowledge, this is the first meta-analysis to demonstrate the association between RVOT conduction delay on ECG and MAE in patients with BrS. There were several significant findings in our study. Firstly, the RVOT conduction delay sign on ECG was significantly associated with MAE in BrS patients. Secondly, a positive aVR sign and large S wave in the lead I were significantly associated with higher MAE risk in BrS patients.
Up until now, there are two widely accepted hypotheses related to the pathomechanism of BrS, including repolarization and depolarization abnormalities, which all originated from RVOT (17, 34). Pannone et al. performed a high-density RVOT epicardial electroanatomic mapping (EAM) analysis using an ajmaline test in BrS patients and showed the presence of high-frequency potentials (HFPs) in all patients before and after the ajmaline test, and low-frequency potentials (LFPs) in all patients after the ajmaline test. HFPs and LFPs, which were the expression of abnormal depolarization and repolarization, respectively, were correlated with RVOT conduction delay (17).
These RVOT conduction delay pathomechanism can also be elaborated in the case of widely known positive SCN5A mutation, which can increase the risk of SCD (5, 6). Another study by Pannone et al. (18) by utilizing electrocardiographic imaging (ECGI) after ajmaline administration revealed a significantly longer RVOT activation time (RVOT-AT) and RVOT recovery time (RVOT-RT) in BrS patients with positive SCN5A mutation compared to BrS patients without SCN5A mutation. According to EAM analysis combined with ajmaline administration, BrS patients with positive SCN5A mutation also had substantially higher HFP activation time (HFPat), LFPat, and LFP duration (LFPd), which concludes that BrS patients with SCN5A mutation exhibited RVOT conduction delay.
Furthermore, Pannone et al. (16) also evaluated the ECGI and EAM analyses in BrS patients with a history of aborted SCD, which showed a significantly higher RVOT-AT and lower RVOT activation-recovery interval (RVOT-ARI) in ECGI after ajmaline administration compared to patients without a history of aborted SCD. Consistently, EAM analysis confirmed that BrS patients with a history of aborted SCD had significantly higher HFPat, LFPat, and LFPd, indicating that RVOT conduction delay also plays an important role in the development of VTA in BrS patients (17). It appears that ajmaline, a sodium channel blocker agent, could unmask the covert electrical substrate that caused the RVOT conduction delay in BrS patients. Moreover, an experimental study conducted by Coronel et al. studied the heart structure of BrS patients who underwent heart implantation surgery and did not have prior structural heart disease. This study showed that the interstitial fibrotic process caused slow conduction in the RVOT area. Based on the activation mapping, this RVOT conduction delay was responsible for typical ECG changes in BrS patients and was the source of VTA (15). Therefore, based on all this evidence, identifying RVOT conduction delay signs may help predict MAE incidence in BrS patients.
A vectorcardiography (VCG) study in BrS patients showed that right terminal conduction delay (RECD) was detected in the upper right quadrant and posterior quadrant of the VCG (35). These VCG regions correspond to the aVR leads on the ECG and were anatomically opposite to the RVOT. Hence, the RVOT conduction delay produced a prominent R wave in lead aVR and a large S wave in the lead I due to its rightward direction of the depolarization wave. Although aVR sign and large S wave in lead I are good predictors of MAE, the prevalence of these ECG markers varied among BrS patients according to clinical presentation and age. Minier et al. (36) study showed that positive aVR signs commonly occurred in young BrS patients (under 17 years old) compared to middle-aged (17–59 years) and old (60 years and over) BrS patients. Moreover, Bigi et al. (20) study showed that BrS patients that previously experienced VTA were more likely to have higher R-wave amplitude or R/q ratio in lead aVR. Based on two cohort studies, positive aVR signs more frequently occurred in symptomatic patients than asymptomatic BrS patients (20, 21). In contrast, the other two cohort studies found that the incidence of positive aVR signs was not significantly different between symptomatic and asymptomatic BrS patients (37–39). On the other hand, three cohort studies found that large S wave in the lead I was not different between symptomatic and asymptomatic BrS patients (27, 37, 39). Nonetheless, Ragab et al. study revealed that large S wave in lead I was more likely occurred in symptomatic BrS patients. Thus, further cohort studies are needed to compare the impact of these ECG markers on MAE in BrS patients according to patients' age and clinical presentation.
Moderate heterogeneity was detected in the analysis of large S wave in the lead I with MAE; thereby, sensitivity analysis by excluding Calo et al. study was performed, resulting in significantly reduced heterogeneity to 8% and without altering the significance. It is due to Calo et al. study, which has a remarkably high-risk ratio (RR) compared to the other studies. The possible explanation was because the 5 per cent of BrS patients with large S wave in the lead I in Calo et al. study appeared to have an MAE on the same day or within days of their diagnosis (i.e., approximately one-third of all participants who had MAE during follow-up). It is questionable that MAE develops in such a short time after the initial diagnosis of BrS in the Calo et al. study. Nevertheless, it might be explained that all participants in this study had spontaneous type I Brugada ECG pattern and had most of the inducible VTA by electrophysiological study (EPS) compared to other included studies, which carried a high risk of MAE.
A retrospective study conducted by Pannone et al. (16) showed that several components of ECGI analysis after ajmaline induction, including RVOT-AT > 110.5 milliseconds, delta RVOT-AT > 40.3 milliseconds, RVOT-ARI < 267.5 milliseconds and delta RVOT-ARI < 18 milliseconds were good predictors of aborted SCD events in BrS patients with AUC value was 0.92, 0.86, 0.79, and 0.76, respectively. Compared to our results, the prognostic value of aVR wave sign and large S wave in lead I were inferior to RVOT-AT and delta RVOT-AT yet comparable to RVOT-ARI and delta RVOT-ARI. Although our result shows a modest AUC in both aVR and large S wave sign, we should underline the potential role of ECG to detect the RVOT conduction delay in daily practice as ECG could provide a more non-invasive characteristic, yet also still a convenient and affordable tool to be operated even in the most primary care. Hence, we may suggest the positive aVR sign and large S wave in lead I on ECG as an essential factor during risk stratification to predict MAE in BrS patients.
The positive aVR sign and large S wave in the lead I on ECG are described in Figure 5. The diagnosis criteria of aVR sign are R wave amplitude ≥ 0.3 mV in lead aVR or R/q ≥ 0.75 in lead aVR (20–22). On the other hand, the criteria for diagnosis of S wave in the lead I are S-wave amplitude ≥ 0.1 mV and/or duration >40 ms in the lead I (23, 26–29, 37).
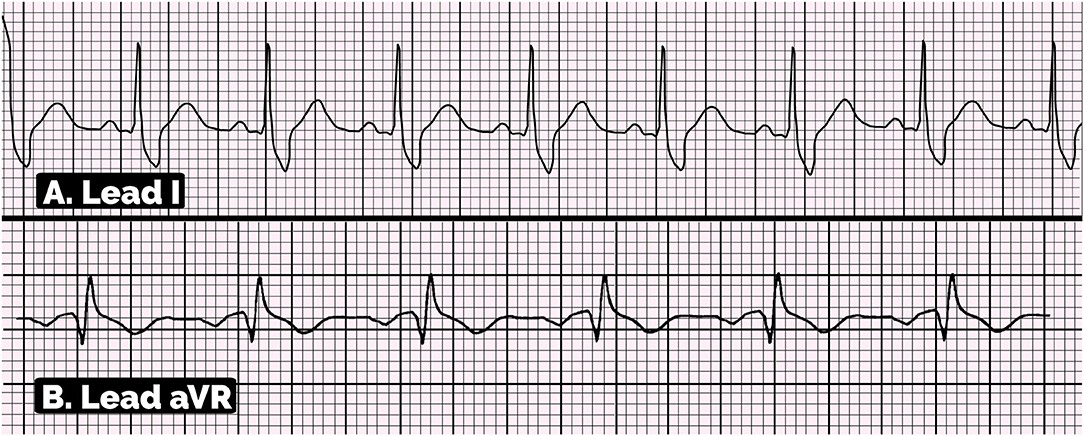
Figure 5. Electrocardiographic pattern of positive aVR sign and large S wave in lead I. (A) This ECG showed S-wave amplitude ≥0.1 mV and duration >40 ms in lead I, suggesting the large S wave in lead I. (B) This ECG showed the positive aVR sign with the amplitude of R wave in aVR lead is ≥0.3 mV and R/q ratio is ≥0.7.
This meta-analysis has several limitations. Firstly, most of the included studies were retrospective designs, increasing the risk of recall and selection bias. Secondly, several confounding factors, including ECG pattern of spontaneous type I Brugada, prior syncope, family history of SCD, presence of SCN5A gene mutation, and inducible VTA in EPS, were not adjusted in most included studies, possibly leading to an increased risk of bias. Thirdly, high heterogeneity was noted in a sub-group analysis of large S wave in the lead I, which was likely caused by differences in included participants' characteristics in the Calo et al. study. Lastly, due to the lack of studies investigating the impact of ECG parameters indicating RVOT conduction delay sign on MAE in BrS patients according to patients' age and clinical presentation, further prospective cohort studies are still needed to understand this ECG pattern in BrS patients better.
Conclusion
In conclusion, this meta-analysis shows that the RVOT conduction delay sign, including the positive aVR sign and large S wave in the lead I, is significantly associated with MAE in patients with BrS. Furthermore, the aVR sign and large S wave in lead I can be used as a potential ECG marker to predict MAE. Further prospective cohort studies are still needed to establish the association between these ECG parameters and MAE in BrS patients in the near future.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
MI conceived and designed the study. IP and MB performed study selection, data extraction, and interpreted the data. MI, IP, RP, and MB performed extensive search of relevant topics. IP and RP performed statistical analysis. MP, HG, MA, and AK performed review and extensive editing of the manuscript. All authors contributed significantly to the writing of the manuscript and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Quinta Febriani for illustrating the aVR sign and large S wave in lead I figure for this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.931622/full#supplementary-material
References
1. Sieira J, Brugada P. The definition of the Brugada syndrome. Eur Heart J. (2017) 38:3029–34. doi: 10.1093/eurheartj/ehx490
2. Vutthikraivit W, Rattanawong P, Putthapiban P, Sukhumthammarat W, Vathesatogkit P, Ngarmukos T, et al. Worldwide prevalence of Brugada Syndrome: a systematic review and meta-analysis. Acta Cardiol Sin. (2018) 34:267–77. doi: 10.6515/ACS.201805_34(3)0.20180302B
3. Sarquella-Brugada G, Campuzano O, Arbelo E, Brugada J, Brugada R Brugada syndrome: clinical and genetic findings. Genet Med Off J Am Coll Med Genet. (2016) 18:3–12. doi: 10.1038/gim.2015.35
4. Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, et al. Brugada syndrome: report of the second consensus conference: endorsed by the heart rhythm society and the European heart rhythm association. Circulation. (2005) 111:659–70. doi: 10.1161/01.CIR.0000152479.54298.51
5. Kawada S, Morita H, Antzelevitch C, Morimoto Y, Nakagawa K, Watanabe A, et al. Shanghai score system for diagnosis of Brugada syndrome: validation of the score system and system and reclassification of the patients. JACC Clin Electrophysiol. (2018) 4:724–30. doi: 10.1016/j.jacep.2018.02.009
6. Sieira J, Conte G, Ciconte G, Chierchia GB, Casado-Arroyo R, Baltogiannis G, et al. A score model to predict risk of events in patients with Brugada syndrome. Eur Heart J. (2017) 38:1756–63. doi: 10.1093/eurheartj/ehx119
7. Delise P. Risk stratification in Brugada syndrome: the challenge of the grey zone. Eur Heart J. (2021) 42:1696–7. doi: 10.1093/eurheartj/ehaa1100
8. Kewcharoen J, Rattanawong P, Kanitsoraphan C, Mekritthikrai R, Prasitlumkum N, Putthapiban P, et al. Atrial fibrillation and risk of major arrhythmic events in Brugada syndrome: a meta-analysis. Ann Noninvasive Electrocardiol Off J Int Soc Holter Noninvasive Electrocardiol Inc. (2019) 24:e12676. doi: 10.1111/anec.12676
9. Pranata R, Yonas E, Chintya V, Deka H, Raharjo SB. Association between PR Interval, First-degree atrioventricular block and major arrhythmic events in patients with Brugada syndrome - systematic review and meta-analysis. J Arrhythmia. (2019) 35:584–90. doi: 10.1002/joa3.12188
10. Pranata R, Yonas E, Vania R, Huang I. Markers of ventricular repolarization as an additional non-invasive electrocardiography parameters for predicting ventricular tachycardia/fibrillation in patients with Brugada syndrome - a systematic review and meta-analysis. Indian Pacing Electrophysiol J. (2019) 19:205–10. doi: 10.1016/j.ipej.2019.06.003
11. Georgopoulos S, Letsas KP, Liu T, Kalafateli M, Korantzopoulos P, Bürkle G, et al. A meta-analysis on the prognostic significance of inferolateral early repolarization pattern in Brugada syndrome. Europace. (2018) 20:134–9. doi: 10.1093/europace/euw394
12. Rattanawong P, Riangwiwat T, Prasitlumkum N, Limpruttidham N, Kanjanahattakij N, Chongsathidkiet P, et al. Baseline fragmented QRS increases the risk of major arrhythmic events in Brugada syndrome: systematic review and meta-analysis. Ann Noninvasive Electrocardiol Off J Int Soc Holter Noninvasive Electrocardiol Inc. (2018) 23:e12507. doi: 10.1111/anec.12507
13. Rattanawong P, Kewcharoen J, Techorueangwiwat C, Kanitsoraphan C, Mekritthikrai R, Prasitlumkum N, et al. Wide QRS complex and the risk of major arrhythmic events in Brugada syndrome patients: a systematic review and meta-analysis. J Arrhythmia. (2020) 36:143–52. doi: 10.1002/joa3.12290
14. Tse G, Gong M, Li CKH, Leung KSK, Georgopoulos S, Bazoukis G, et al. Tpeak-Tend, Tpeak-Tend/QT ratio and Tpeak-Tend dispersion for risk stratification in Brugada syndrome: a systematic review and meta-analysis. J Arrhythmia. (2018) 34:587–97. doi: 10.1002/joa3.12118
15. Coronel R, Casini S, Koopmann TT, Wilms-Schopman FJG, Verkerk AO, de Groot JR, et al. right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome. Circulation. (2005) 112:2769–77. doi: 10.1161/CIRCULATIONAHA.105.532614
16. Pannone L, Monaco C, Sorgente A, Vergara P, Calburean P, Gauthey A, et al. Ajmaline-induced abnormalities in Brugada syndrome: evaluation with ECG imaging. J Am Heart Assoc. (2022) 11:e024001. doi: 10.1161/JAHA.121.024001
17. Pannone L, Monaco C, Sorgente A, Vergara P, Calburean PA, Gauthey A, et al. High-density epicardial mapping in Brugada syndrome: depolarization and repolarization abnormalities. Heart Rhythm. (2022) 19:397–404. doi: 10.1016/j.hrthm.2021.09.032
18. Pannone L, Monaco C, Sorgente A, Vergara P, Gauthey A, Calburean PA, et al. SCN5A mutation in Brugada syndrome is associated with substrate severity detected by electrocardiographic imaging and high-density electroanatomic mapping. Heart Rhythm. (2022) 19:945–51. doi: 10.1016/j.hrthm.2022.01.034
19. Li KHC, Lee S, Yin C, Liu T, Ngarmukos T, Conte G, et al. Brugada syndrome: a comprehensive review of pathophysiological mechanisms and risk stratification strategies. Int J Cardiol Heart Vasc. (2020) 26:100468. doi: 10.1016/j.ijcha.2020.100468
20. Babai Bigi MA, Aslani A, Shahrzad S. aVR sign as a risk factor for life-threatening arrhythmic events in patients with Brugada syndrome. Heart Rhythm. (2007) 4:1009–12. doi: 10.1016/j.hrthm.2007.04.017
21. Ragab AAY, Houck CA, van der Does LJME, Lanters EAH, Burghouwt DE, Muskens AJQM, et al. Usefulness of the R-Wave sign as a predictor for ventricular tachyarrhythmia in patients with Brugada syndrome. Am J Cardiol. (2017) 120:428–34. doi: 10.1016/j.amjcard.2017.04.044
22. Morita H, Miyamoto M, Watanabe A, Tsukuda S, Morimoto Y, Kawada S, et al. Progression of electrocardiographic abnormalities associated with initial ventricular fibrillation in asymptomatic patients with Brugada syndrome. Heart Rhythm. (2018) 15:1468–74. doi: 10.1016/j.hrthm.2018.06.035
23. Calò L, Giustetto C, Martino A, Sciarra L, Cerrato N, Marziali M, et al. A new electrocardiographic marker of sudden death in Brugada syndrome: the S-Wave in lead I. J Am Coll Cardiol. (2016) 67:1427–40. doi: 10.1016/j.jacc.2016.01.024
24. Rizal A, Raharjo SB, Hanafy DA, Yuniadi Y. Predictors of appropriate shocks and ventricular arrhythmia in indonesian with Brugada syndrome. Indones J Cardiol. (2019) 40. doi: 10.30701/ijc.v40i2.767
25. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of cardiology (ESC)endorsed by: association for European paediatric and congenital cardiology (AEPC). Eur Heart J. (2015) 36:2793–867. doi: 10.1093/eurheartj/ehv316
26. Honarbakhsh S, Providencia R, Garcia -Hernandez Jorge, Martin CA, Hunter RJ, Lim WY, et al. A Primary Prevention Clinical Risk Score Model for Patients With Brugada Syndrome (BRUGADA-RISK). JACC Clin Electrophysiol. (2021) 7:210–22. doi: 10.1016/j.jacep.2020.08.032
27. Nagase S, Kamakura T, Kataoka N, Wada M, Yamagata K, Ishibashi K, et al. Low-voltage type 1 ECG is associated with fatal ventricular tachyarrhythmia in Brugada syndrome. J Am Heart Assoc Cardiovasc Cerebrovasc Dis. (2018) 7:e009713. doi: 10.1161/JAHA.118.009713
28. Michowitz Y, Milman A, Andorin A, Sarquella-Brugada G, Gonzalez Corcia MC, Gourraud JB, et al. Characterization and management of arrhythmic events in young patients with Brugada syndrome. J Am Coll Cardiol. (2019) 73:1756–65. doi: 10.1016/j.jacc.2019.01.048
29. Migliore F, Testolina M, Zorzi A, Bertaglia E, Silvano M, Leoni L, et al. First-degree atrioventricular block on basal electrocardiogram predicts future arrhythmic events in patients with Brugada syndrome: a long-term follow-up study from the Veneto region of Northeastern Italy. EP Eur. (2019) 21:322–31. doi: 10.1093/europace/euy144
30. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
31. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
32. Shinohara T, Takagi M, Kamakura T, Sekiguchi Y, Yokoyama Y, Aihara N, et al. Risk stratification in asymptomatic patients with Brugada syndrome: utility of multiple risk factor combination rather than programmed electrical stimulation. J Cardiovasc Electrophysiol. (2021) 32:507–14. doi: 10.1111/jce.14848
33. Ragab AAY, Houck CA, van der Does LJME, Lanters EAH, Muskens AJQM, de Groot NMS. Prediction of ventricular tachyarrhythmia in Brugada syndrome by right ventricular outflow tract conduction delay signs. J Cardiovasc Electrophysiol. (2018) 29:998–1003. doi: 10.1111/jce.13496
34. Meregalli PG, Wilde AAM, Tan HL. Pathophysiological mechanisms of Brugada syndrome: depolarization disorder, repolarization disorder, or more? Cardiovasc Res. (2005) 67:367–78. doi: 10.1016/j.cardiores.2005.03.005
35. Peréz-Riera AR, Ferreira Filho C, de Abreu LC, Ferreira C, Yanowitz FG, Femenia F, et al. Do patients with electrocardiographic Brugada type 1 pattern have associated right bundle branch block? a comparative vectorcardiographic study. Europace. (2012) 14:889–97. doi: 10.1093/europace/eur395
36. Minier M, Probst V, Berthome P, Tixier R, Briand J, Geoffroy O, et al. Age at diagnosis of Brugada syndrome: influence on clinical characteristics and risk of arrhythmia. Heart Rhythm. (2020) 17:743–9. doi: 10.1016/j.hrthm.2019.11.027
37. Morita H, Watanabe A, Kawada S, Miyamoto M, Morimoto Y, Nakagawa K, et al. Identification of electrocardiographic risk markers for the initial and recurrent episodes of ventricular fibrillation in patients with Brugada syndrome. J Cardiovasc Electrophysiol. (2018) 29:107–14. doi: 10.1111/jce.13349
38. Junttila MJ, Brugada P, Hong K, Lizotte E, DE Zutter M, Sarkozy A, et al. Differences in 12-lead electrocardiogram between symptomatic and asymptomatic Brugada syndrome patients. J Cardiovasc Electrophysiol. (2008) 19:380–3. doi: 10.1111/j.1540-8167.2007.01050.x
39. Sacilotto L, Scanavacca MI, Olivetti N, Lemes C, Pessente GD, Wulkan F, et al. Low rate of life-threatening events and limitations in predicting invasive and noninvasive markers of symptoms in a cohort of type 1 Brugada syndrome patients: data and insights from the GenBra registry. J Cardiovasc Electrophysiol. (2020) 31:2920–8. doi: 10.1111/jce.14732
Keywords: Brugada syndrome, RVOT conduction delay sign, aVR sign, S wave in the lead I, major arrhythmic events
Citation: Iqbal M, Putra ICS, Pranata R, Budiarso MN, Pramudyo M, Goenawan H, Akbar MR and Kartasasmita AS (2022) Electrocardiographic Markers Indicating Right Ventricular Outflow Tract Conduction Delay as a Predictor of Major Arrhythmic Events in Patients With Brugada Syndrome: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 9:931622. doi: 10.3389/fcvm.2022.931622
Received: 29 April 2022; Accepted: 26 May 2022;
Published: 17 June 2022.
Edited by:
Carlo de Asmundis, University Hospital Brussels, BelgiumReviewed by:
Luigi Pannone, University Hospital Brussels, BelgiumGiannis G. Baltogiannis, Vrije University Brussel, Belgium
Copyright © 2022 Iqbal, Putra, Pranata, Budiarso, Pramudyo, Goenawan, Akbar and Kartasasmita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Iqbal, bW9oYW1tYWRpcWJhbDE3OEBnbWFpbC5jb20=
 Mohammad Iqbal
Mohammad Iqbal Iwan Cahyo Santosa Putra
Iwan Cahyo Santosa Putra Raymond Pranata
Raymond Pranata Michael Nathaniel Budiarso
Michael Nathaniel Budiarso Miftah Pramudyo
Miftah Pramudyo Hanna Goenawan
Hanna Goenawan Mohammad Rizki Akbar1
Mohammad Rizki Akbar1 Arief Sjamsulaksan Kartasasmita
Arief Sjamsulaksan Kartasasmita