- 1Chair and Department of Cardiology, Medical University of Warsaw, Warsaw, Poland
- 2Department of Anesthesiology and Intensive Care, Medical University of Warsaw, Warsaw, Poland
- 3Department of Clinical Sciences, Maria Sklodowska-Curie Medical Academy, Warsaw, Poland
- 4Department of General, Vascular and Transplant Surgery, Medical University of Warsaw, Warsaw, Poland
- 5Department of Coronary Disease and Heart Failure, Institute of Cardiology, Jagiellonian University Medical College, Cracow, Poland
- 6John Paul II Hospital, Cracow, Poland
Patients with abdominal aortic aneurysm (AAA) have a higher risk of cardiovascular (CV) events, which seems to be associated with disturbed platelet (PLT) function. Endovascular aneurysm repair (EVAR) is an emerging, less-invasive treatment alternative to surgical AAA repair. Both platelet function abnormalities in patients with AAA and the effect of EVAR on platelet function are poorly understood. In this review, we aim to fill the gap regarding the effect of EVAR on PLT function in AAA patients by discussing PLT function disturbances in patients with AAA, PLT function changes after EVAR, evidence from clinical studies regarding PLT function before and after EVAR, and antiplatelet or and antithrombotic treatment in patients undergoing EVAR. The goal of our review is to summarize the contemporary knowledge and initiate further studies to better understand PLT function changes in patients undergoing EVAR, optimize the pharmacotherapy before and after EVAR and further improve outcomes in this group of patients.
Introduction
Abdominal aortic aneurysm (AAA) is defined as a maximal diameter of the abdominal aorta >3 cm, or as a focal dilation exceeding normal diameter of the adjacent arterial segment by 1.5-fold (1). AAA is said to affect 4–8% of men and 0.5–2% of women aged ≥ 60 years (2). The major concern in those patients is the risk of rupture. Evidence shows that AAA increases the risk of thromboembolic events (3). Patients with AAA have a higher risk of cardiovascular (CV) events including coronary and peripheral artery disease (PAD) (1, 3). The pathophysiological mechanisms behind increased risk of CV events remain not well-understood. AAA is associated with both higher fibrin turnover and thrombin generation, which could partly explain its prothrombotic effect (3, 4). The coagulation disorders may be due to the formation of an intramural thrombus within the aneurysm wall. Many studies have found an association between the volume of the aneurysm and the extent of coagulation disorders (3–5). Disturbed platelet (PLT) function seems to be an additional mechanism underlying the increased risk of CV events.
Endovascular aneurysm repair (EVAR) is an emerging, less-invasive treatment alternative to surgical repair, associated with lower mortality during the first 6 months, but comparable outcomes in the long-term follow-up (6, 7). The procedure can be performed with use of different types of anesthesia: general, local and regional. The meta-analysis of studies suggests that that mode of anesthesia may be associated with improved outcomes. In particular, local anesthesia appears to have a positive effect on outcome after emergency EVAR. However, no randomized trial data can prove that finding (8). However, the effect of EVAR on platelet function and coagulation disorders is unclear. Previously, we showed that the elimination of AAA from the circulation decreases PLT reactivity, which might be one of the benefits of EVAR (9). Other authors showed that EVAR promotes systemic inflammatory and prothrombotic response by triggering the cytokine release from the intramural thrombus of the aneurysm (3, 4, 10). Thus, the effect of EVAR on PLT function is to be elucidated. Patients after EVAR receive antagonists of the platelet P2Y12 receptors (clopidogrel). Based on the insight from CV patients, it has been suggested that low PLT reactivity during the treatment with P2Y12 antagonists is associated with bleeding events, whereas high on-treatment PLT reactivity is associated with thrombotic events (11, 12). However, the percentage of patients with low and high PLT reactivity after EVAR is unknown.
In this review, we aim to fill the gap regarding the effect of EVAR on PLT function in AAA patients by discussing (i) PLT function disturbances in patients with AAA, (ii) PLT function changes after EVAR, (iii) evidence from clinical studies regarding PLT function before and after EVAR, and (iv) antiplatelet treatment in patients undergoing EVAR. The goal of our review is to summarize the contemporary knowledge and initiate further studies to better understand PLT function changes in patients undergoing EVAR, optimize the pharmacotherapy before and after EVAR and improve outcomes in this group of patients.
Platelet function disturbances in patients with abdominal aortic aneurysm
The presence of AAA is associated with platelet function disturbances due to chronic proinflammatory response (3), including increased PLT activation and decreased PLT count (3, 13–15). The mechanism of platelet activation is shown in Figure 1.
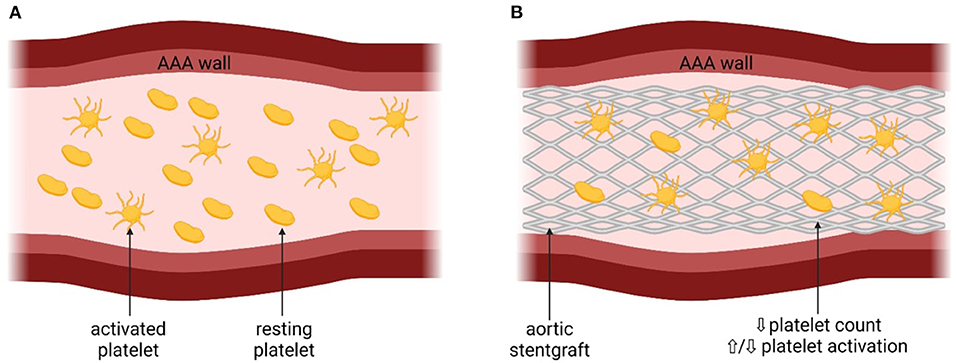
Figure 1. The mechanism of platelet activation associated with abdominal aortic aneurysm. AAA, abdominal aortic aneurysm; eoxPL, enzymatically oxidized phospholipids; sP, selectin – surface P-selectin; PF4, platelet factor 4; aIIbβ3, platelet integrin aIIbβ3; CD40L, CD40 ligand. (A) Platelets before the insertion of the stentgraft, with the presence of AAA. (B) Platelets after the insetion of the stentgraft.
Presence of AAA may lead to the disturbances in the hemodynamics in the aorta and therefore increase PLT activation (16). Indeed, in patients with AAA, increased PLT activation was observed, reflected by higher concentrations of soluble P-selectin and glycocalicin, compared to control patients (with symptomatic carotid artery stenoses or after AAA repair) (13–15). The prothrombotic state associated with increased PLT activation may increase the risk of CV complications in patients with AAA, although data derived from large-scale studies with the long-term follow-up are unavailable (3). It has also been speculated that the size of the aneurysm could influence PLT activation (5, 14). Significant positive correlation was found between the aneurysm size and the extent of PLT activation both in patients with and without Marfan disease, defined as dilation-dependent platelet activation (5, 14). Another prothrombotic mechanism in the development of AAA-associated CV complications has also been proposed, based on the enzymatically oxidized phospholipids (eoxPL) (17). In a murine model and in harvested human AAA samples from six male, it was showed that AAA per se did not cause PLT activation, but rather the procoagulant PLTs exposing eoxPL regulate AAA development through interactions with clotting factors (17). EoxPL were found in thrombus and AAA wall and delivered a procoagulant surface for binding and activation of clotting factors, promoting AAA development (17) (Figure 1). On the other hand, the presence of eoxPL somewhere else than at the AAA site diverted coagulation factors from the lesion and prevented aneurysm development (17). This highlights the complex influence of eoxPL on AAA development, showing that the modulation of the delicate balance between bleeding and thrombosis within the vessel wall or circulation could either induce or prevent the development of AAA (17).
PLT inhibitors such as acetylsalicylic acid (ASA) or P2Y12 receptor antagonist (clopidogrel) have an important role in reducing AAA progression, however their impact on the risk of AAA rupture is not yet well-established (18, 19). In one murine model clopidogrel has been shown to decrease the expression of the inflammatory cytokines, inhibit changes in AAA expansion and suppress the degradation of elastic lamina (18). The histopathological findings revealed a significant reduction in the macrophage infiltration and decreased release of matrix metalloproteinases (MMP). Overall, the treatment with clopidogrel caused a significant−47% reduction in AAA formation, however it did not influence the risk of rupture when compared to the control group (without P2Y12 inhibitor) (18). Another murine experiment studied the effect of both ASA and clopidogrel on established aneurysms (19). The results revealed a significant reduction in mortality associated with AAA rupture (ASA 0% vs. placebo 50%; clopidogrel 0% vs. placebo 47%; P < 0.01). It was further validated during the same experiment in human subjects in the retrospective analysis of 1,578 patients (19). It was demonstrated that both ASA and clopidogrel decreased the levels of active matrix metalloproteinase-2 (MMP-2), matrix metalloproteinase-9 (MMP-9), cytokines, plasma concentrations of PLT factor 4 and components of the plasminogen activation system in mice (19). Interestingly, a different murine study showed that the use of frequent platelet infusions had an anti-inflammatory effect on AAA development and reduced its formation by 52.38% (20). Furthermore, platelet infusion was found to decrease the inflammatory response by reducing the levels of MMP-2, MMP-9 and elastic lamina destruction, which contributed to slower aortic expansion. (20) Despite the use of ASA, some level of PLT activity associated with AAA remains unchanged (21). This fact is partially explained by a recently-discovered mechanism of regulating biochemical PLT activation associated with a membrane olfactory receptor 2L13 (OR2L13). Transcriptomic profiling of PLT from patients and mice with AAA showed that OR2L13 are involved in regulating PLT activation and AAA size progression by mediators of the aortic remodeling (21). In patients with AAA, both increased expression of OR2L13 and an upregulation of their signal transduction pathway was observed. A molecule which activated OR2L13 was identified–it limited AAA growth and platelet activation, therefore being a potential antiplatelet therapeutic agent (21).
Regarding low PLT count observed in patients with AAA, it was shown that activated PLTs which did not aggregate at the site of an aneurysm were subsequently removed from the circulation by the reticuloendothelial system (13). Consequently, about 10% of asymptomatic AAA patients had PLTs count below the normal range, and the mean platelet count was lower than in control patients with carotid artery stenoses (15). Based on observation from aggregometry- and flow-cytometry- based methods, platelet activation and formation of platelet-rich thrombi is accompanied by a decrease in platelet count, since platelet aggregates are no longer detected as single events following activation by an agonist (22, 23). Hence, decrease in PLT count in patients with AAA suggest that there is increased platelet activation and destruction, most likely within the aneurysm sac.
Platelet function changes after EVAR
EVAR was shown to have a strong impact both on PLT count and activity (3, 5, 13). Generally, a postimplantation inflammatory response is common after EVAR (24). However, there are controversies in the literature regarding the magnitude and direction of these changes (3, 5, 9, 25). Generally, there is consensus that PLT count decreases shortly after EVAR. However, evidence regarding the effect of EVAR on platelet activation remains contradictory. Changes in PLT count and function after EVAR are shown in Figure 2.
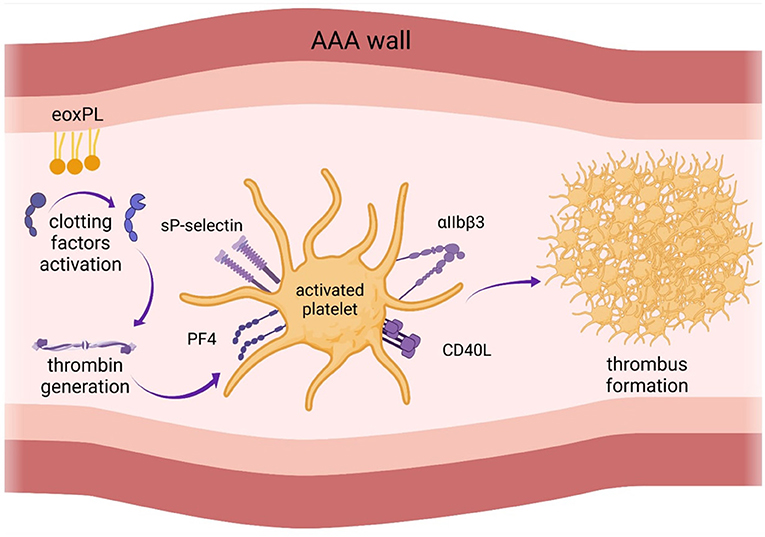
Figure 2. Changes in platelet function after endovascular aortic aneurysm repair (EVAR). There is consensus that platelet count decreases shortly after EVAR. However, evidence regarding the effect of EVAR on platelet activation remains contradictory. AAA, abdominal aortic aneurysm.
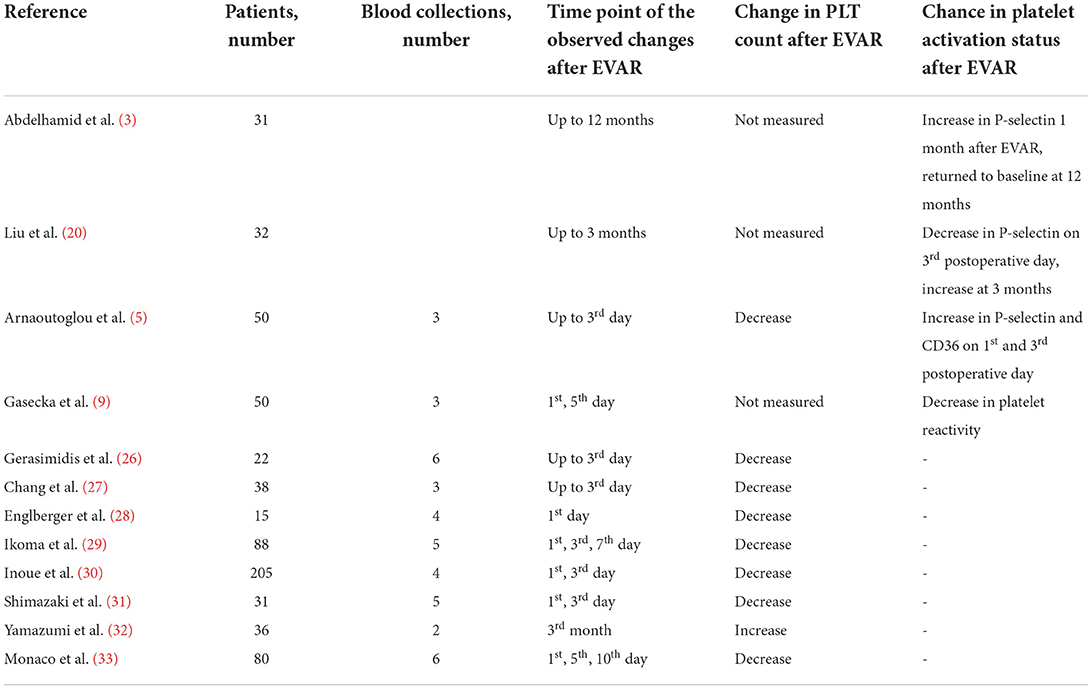
Several studies have been conducted to assess changes in PLT count and platelet activation markers after EVAR, including soluble and membrane-exposed CD62p (P-selectin), CD36 (type 2 scavenger receptor for low-density lipoproteins), CD40 ligand, platelet factor 4, and glycoprotein IIb-IIIa. The results of hitherto conducted clinical studies are summarized in Table 1.
One of the studies assessed long-term influence of EVAR on PLT activity. The authors found that the prothrombotic diathesis normalized 12 months after EVAR procedure (3). However, it also demonstrated that the level of soluble P-selectin, a biomarker of platelet activation, was higher for 1- 6 months post-EVAR, compared to the measurement before EVAR. It decreased to the baseline level at 12 months, although the median value was still significantly higher (3). In contrast, our recent study showed that PLT reactivity decreased within 24 h after branched-EVAR compared to the measurements before the procedure (9). These discrepancies might be caused by the method of PLT function assessment and different time-points of blood collection. Most of the previous studies analyzed PLT activation indirectly by measuring the number of substances released into the bloodstream (for instance P-selectin), which are sensitive to proteolysis (34). Therefore, PLT activation based on P-selectin exposure may not reflect the original platelet activation status. In our study, a more direct method of measurement, platelet impedance-based aggregometry, was used. Aggregometry specifically measures PLT activation in response to different agonists (35) and is considered the most reliable bedside platelet function tes, with well-defined cut-off values (10). However, aggregometry also does not entirely mimic the process of platelet activation in vivo, since it measures platelet response to exogenous and soluble agonists (for instance thrombin receptor-activating peptide 6 instead of thrombin) (36). Hence, results from platelet function tests in vitro should be interpreted with caution and re-evaluated with newer generation tests. Whole blood-based perfusion systems, such as total-thrombus analyzing system which allows to monitor the process of thrombus formation under arterial shear rate (37), might be a more reliable tool to assess platelet function and may help to explain the previous discrepancies. We showed that the elimination of AAA from the circulation decreases PLT reactivity already at day 1 after EVAR and further at day 3 (9). One study demonstrated that the extent of PLT activation was associated with the type of the endograft material. Polyester grafts activated PLTs to a larger extent than polytetrafluoroethylene (5). Furthermore, inflammatory response seems stronger when using stent grafts made of synthetic polyester textile than with polytetrafluoroethylene. The length of hospital stay was longer for the patients treated with polyester stent grafts (24). Usage of contrast media also influenced PLTs activity, causing endothelial injury and increased PLT activation (13). No association between the magnitude of PLT activation and 30-day mortality after EVAR was observed (5). More studies with multiple blood collections are needed to investigate the course of PLT activation after EVAR and whether these short-term changes in PLT activation significantly influence long-term clinical outcomes including mortality.
PLT count was measured in numerous studies as an indirect index of platelet activation and consumption (5, 26–33). It has been suggested that the surgery itself is associated with platelet activation and leads to decreased PLT count, similarly as described in vitro (22, 23). Many studies showed a significant decrease in PLT 1-3 days following EVAR, and return to the baseline on the 7th-10th day (5, 26–33). In conclusion, all the studies demonstrated decreased PLT count for at least 3 days after EVAR. Due to the differences in study designs and time-points of blood collection, it cannot be established when the PLT count returns to baseline or even increases following EVAR.
Currently, there are no studies showing the impact of platelet count or function following EVAR on cardiovascular outcomes including myocardial infarction and/or limb thrombosis. In our study, we showed that pre-operative platelet reactivity (measured by using ASPI, TRAP and ADP tests) was a predictor of bleeding complications (9). Nevertheless, platelet depletion (reduction >60%) following EVAR was associated with post-operative bleeding and increased mortality in one study (38). On the other hand, Inoue et al. demonstrated that the platelet count of patients with malignant type-2-endoleaks on 7th day following the operation was lower than that of patients with completed EVAR or with benign type-2-endoleaks (39). The authors also showed that lower platelet count on 7th day following the operation could serve as a risk factor for AAA enlargement among patients with type-2-endoleaks (39).
Antiplatelet treatment in patients undergoing EVAR
Treatment before EVAR
In case of smaller AAAs (<5.5 cm diameter), the conservative management along with regular imaging tests is advised (40). Currently, no medical therapy has been proven to decrease the expansion rate of AAA (41, 42).
According to the Guidelines on Management of Abdominal Aorto-Iliac Artery Aneurysms, pharmacological management in patients with AAA is in line with the treatment of patients with PAD (40). Regarding very-high CV risk, these patients should receive antiplatelet therapy, lipid lowering agents (if low-density cholesterol exceeds 2.5 mmol/L) and antihypertensives (if blood pressure is above 140 mm Hg) for the secondary prevention (40). Patients receiving statins or antihypertensive agents had 20–25% better 5-year survival rate, compared with patients not receiving such therapies (43).
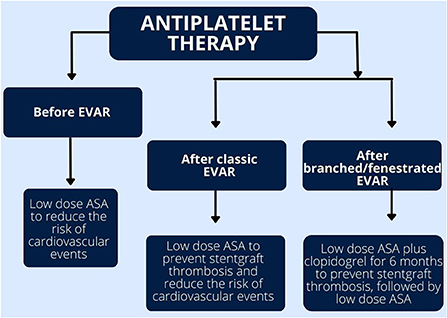
ASA was showed to decrease the risk of CV events in patients with PAD, including AAA (44, 45). A retrospective study of 12 485 patients with AAA showed a 24% reduction of 5-year mortality in patients who received antiplatelet therapy (ASA, clopidogrel and dipyridamole), compared to patients without antiplatelet medications (43). However, analyzed separately, only ASA was associated with lower mortality, other antiplatelet drugs did not affect outcomes (43). Benefits of ASA in the secondary prevention of CVD have also been demonstrated in meta-analyses of clinical trials (44–46). Hence, ASA has received class I recommendation in patients with AAA. Similarly to patients with PAD, patients with AAA should receive clopidogrel as an alternative if aspirin is contradicted (44). Antiplatelet monotherapy does not increase the risk of perioperative bleeding and should not be discontinued prior to surgery (47). If patients with AAA have indication for dual antiplatelet therapy (DAPT), such as a history of PCI with stent implantation, DAPT should be administered (40, 48). If possible, EVAR should be delayed until DAPT cessation, especially in patients at high bleeding risk (40).
Currently, there is few data to support the use of newer antiplatelet drugs, such as prasugrel and ticagrelor in patients with AAA. However, the superiority of prasugrel and ticagrelor over clopidogrel in patients with coronary artery disease makes them an interesting alternative therapeutic option also in AAA patients and requires further studies.
Treatment after EVAR
Postoperative management should focus both on the prevention of postoperative complications, such as graft thrombosis and peripheral embolization, and secondary prevention of CV events. Antiplatelet therapy can accomplish both. It has been shown that platelet activation, adhesion and aggregation plays major role in a stent-graft thrombosis (49). Hence, ASA is the basal treatment after EVAR. Six-month-long DAPT consisting of ASA and clopidogrel is recommended after fenestrated or branched EVAR (48, 50). Antiplatelet therapy also decreases the risk of PAD progression, common among patients with AAA (43). There is evidence that antiplatelet therapy significantly reduces the risk of vascular occlusion in patients undergoing peripheral angioplasty as well (50). Therefore, post-operative antiplatelet therapy is essential. Concurrently, antiplatelet therapy does not increase the risk of bleeding or endoleak into the aneurysmal sac (51). After 6 months, ASA monotherapy is the best evidence-based strategy, not only does it prevent graft thrombosis, but also reduces the aneurysm sac growth, compared to other antiplatelet or anticoagulation therapy (52). Extrapolating data derived from patients undergoing PCI to EVAR patients, the long-term low-dose ASA is as effective as the high-dose (52, 53). However, the optimal antiplatelet therapy regimens after EVAR are awaiting further investigation.
Currently there are no randomized controlled trials comparing the efficacy and safety of different antiplatelet drugs after EVAR. The choice of treatment is based on local experience of each clinical center (50, 54). Similarly, the best therapeutic strategy after EVAR in patients with indications for oral anticoagulation is yet to be established. Figure 3 summarized the recommended antiplatelet treatment regimens after EVAR.
Discussion
Increased PLT activation seems to be one of the mechanisms underlying the increased risk of CV events in patients with AAA. Furthermore, it was shown to increase the risk of AAA rapture. Therefore, targeting the PLT activation could serve as a potential therapeutic goal in patients with AAA. However, despite the use of antiplatelets agents such as ASA some, residual level of PLTs activity remains unchanged, what might be associated with AAA progression (21). On the other hand, potent antiplatelets regimens such as DAPT were shown to be demonstrated with increased bleeding risk, as incidence rates for all major bleeding events were higher in dual regimens than in mono- therapy (55).
The increased PLT activation which is present in patients with AAA despite the use of antiplatelet agents can be partially explained by PLT activation associated with a membrane olfactory receptor 2L13 (OR2L13) (21). The increased expression of OR2L13 receptor in patients with AAA and the upregulation of their signal transduction pathway was observed. A molecule which activated OR2L13 was identified and was also proved to limited AAA growth and platelet activation (21). As mentioned before, AAA per se did not cause PLT activation, but rather the procoagulant PLTs exposing eoxPL regulated AAA development through interactions with clotting factors. Those molecular mechanisms of PLT activation are not now addressed in standard antiplatelet therapy. Therefore, those molecular mechanisms could serve in future as a therapeutic targets of action for new generation of antiplatelets regimens.
It was also demonstrated that there are many other factors which can influence the PLT activation following EVAR such as graft material or the use of contrast media. Polyester grafts activated PLTs to a larger extent than polytetrafluoroethylene (5). Furthermore, the length of hospital stay was longer for the patients treated with polyester stent grafts (24). Hence, the changes of materials which are currently used could also alter the PLT activation and influence the clinical outcomes after EVAR.
Antiplatelet therapy is the cornerstone of pharmacological treatment both before and after EVAR. However, the best treatment options, tailored to the specific mechanism and degree of platelet activation in individual patients are yet to be established. To optimize the outcome of endovascular AAA repair the treatment of underlying cardiovascular disease should be continued post-operatively what includes antihypertensive therapy, lipid modifying therapy and antiplatelets (40). The accurate PLT function changes in patients undergoing EVAR require investigation, especially with new, targeted antiplatelet therapies. This could further optimize the pharmacotherapy before and after this procedure to improve the clinical outcomes in this group of patients.
Author contributions
AGa: conceptualization. AB, AI, and AGe: writing—original draft preparation. KF, TJ, KJ, and MG: writing—review and editing. AGa and AS: methodology and supervision. AS: formal analysis. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
Figures have been created with BioRender.com, licensed version purchased by AGa.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ullery B, Hallett R, Fleischmann D. Epidemiology and contemporary management of abdominal aortic aneurysms. Abdom Radiol. (2018) 43:1032–43. doi: 10.1007/s00261-017-1450-7
2. Ashton HA, Gao L, Kim LG, Druce PS, Thompson SG, Scott RA. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. (2007) 94:696–701. doi: 10.1002/bjs.5780
3. Abdelhamid MF, Davies RS, Adam DJ, Vohra RK, Bradbury AW. Changes in thrombin generation, fibrinolysis, platelet and endothelial cell activity, and inflammation following endovascular abdominal aortic aneurysm repair. J Vasc Surg. (2012) 55:41–6. doi: 10.1016/j.jvs.2011.07.094
4. Kapetanios DM, Karkos CD, Papazoglou KO. Changes in circulating markers of coagulation and fibrinolysis after EVAR. Int Angiol. (2018) 37:444–50. doi: 10.23736/S0392-9590.18.04046-4
5. Arnaoutoglou E, Kouvelos G, Papa N, Karamoutsios A, Bouris V, Vartholomatos G, et al. Platelet activation after endovascular repair of abdominal aortic aneurysm. Vascular. (2016) 24:287–94. doi: 10.1177/1708538115596911
6. Salata K, Hussain MA, de Mestral C, Greco E, Aljabri BA, Mamdani M, et al. Comparison of outcomes in elective endovascular aortic repair vs open surgical repair of abdominal aortic aneurysms. JAMA Netw Open. (2019) 2:e196578. doi: 10.1001/jamanetworkopen.2019.6578
7. Antoniou GA, Antoniou SA, Torella F. Endovascular vs. open repair for abdominal aortic aneurysm: systematic review and meta-analysis of updated peri-operative and long-term data of randomised controlled trials. Eur J Vasc Endovasc Surg. (2020) 59:385-97. doi: 10.1016/j.ejvs.2019.11.030
8. Armstrong RA, Squire Y, Rogers C, Hinchliffe R, Mouton R. Type of anesthesia for endovascular abdominal aortic aneurysm repair. J Cardiothorac Vasc Anesth. (2019) 33:462–71. doi: 10.1053/j.jvca.2018.09.018
9. Gasecka A, Zawadka M, Burban A, Idzik A, Gelo A, Graczyńska A et al. Pre-operative platelet reactivity is a strong, independent predictor of bleeding complications after branched endovascular thoracoabdominal aortic aneurysm repair. Platelets. (2022) 33:577–85. doi: 10.1080/09537104.2021.1961708
10. Arnaoutoglou E, Kouvelos G, Papa N, Kallinteri A, Milionis H, Koulouras V, et al. Prospective evaluation of post-implantation inflammatory response after EVAR for AAA: influence on patients' 30 day outcome. Eur J Vasc Endovasc Surg. (2015) 49:175–83. doi: 10.1016/j.ejvs.2014.12.006
11. Siller-Matula JM, Trenk D, Schrör K, Gawaz M, Kristensen SD, Storey RF, et al. How to improve the concept of individualised antiplatelet therapy with P2Y12receptor inhibitors - Is an algorithm the answer? Thromb Haemost. (2015) 113:37–52. doi: 10.1160/TH14-03-0238
12. Janssen PWA, Ten Berg JM. Platelet function testing and tailored antiplatelet therapy. J Cardiovasc Transl Res. (2013) 6:316–28. doi: 10.1007/s12265-013-9458-z
13. Davies RS, Abdelhamid M, Wall ML, Vohra RK, Bradbury AW, Adam DJ. Coagulation, fibrinolysis, and platelet activation in patients undergoing open and endovascular repair of abdominal aortic aneurysm. J Vasc Surg. (2011) 54:865–78. doi: 10.1016/j.jvs.2011.04.010
14. Touat Z, Lepage L, Ollivier V, Nataf P, Hvass U, Lebreuche J. Dilation-dependent activation of platelets and prothrombin in human thoracic ascending aortic aneurysm. Arterioscler Thromb Vasc Biol. (2008) 28:940–6. doi: 10.1161/ATVBAHA.107.158576
15. Milne AA, Adam DJ, Murphy WG. Effects of asymptomatic abdominal aortic aneurysm on the soluble coagulation system, platelet count and platelet activation. Eur J Vasc Endovasc Surg. (1999) 17:434–7. doi: 10.1053/ejvs.1998.0790
16. Hansen KB, Arzani A, Shadden SC. Mechanical platelet activation potential in abdominal aortic aneurysms. J Biomech Eng. (2015) 137:041005. doi: 10.1115/1.4029580
17. Allen-Redpath K, Aldrovandi M, Lauder SN, Gketsopoulou A, Tyrell VJ, Slatter DA. Phospholipid membranes drive abdominal aortic aneurysm development through stimulating coagulation factor activity. Proc Natl Acad Sci U S A. (2019) 116:8038–47. doi: 10.1073/pnas.1814409116
18. Liu O, Jia L, Liu X, Wang Y, Wang X, et al. Clopidogrel, a platelet P2Y12 receptor inhibitor, reduces vascular inflammation and angiotensin ii induced-abdominal aortic aneurysm progression. PLoS ONE. (2012) 7:e51707. doi: 10.1371/journal.pone.0051707
19. Owens AP, Edwards TL, Antoniak S, Geddings JE, Jahangir E, Wei W, et al. Platelet inhibitors reduce rupture in a mouse model of established abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. (2015) 35:2032–41. doi: 10.1161/ATVBAHA.115.305537
20. Liu X, Chen X, Xu C, Lou J, Weng Y, Tang L. Platelet protects angiotensin II-driven abdominal aortic aneurysm formation through inhibition of inflammation. Exp Gerontol. (2022) 159:111703. doi: 10.1016/j.exger.2022.111703
21. Morrell CN, Mix D, Aggarwal A, Bhandari R, Godwin M, Owens P, et al. Platelet olfactory receptor activation limits platelet reactivity and growth of aortic aneurysms. J Clin Invest. (2022) 132:e152373. doi: 10.1172/JCI152373
22. Koltai K, Kesmarky G, Feher G, Tibold A, Toth K. Platelet aggregometry testing: molecular mechanisms, techniques and clinical implications. Int J Mol Sci. (2017) 18:1803. doi: 10.3390/ijms18081803
23. Cuyper I, Meinders M, Vijver E, de Korte D, Porcelijn L, de Haas M, et al. A novel flow cytometry–based platelet aggregation assay. Blood. (2013) 121:e70–80. doi: 10.1182/blood-2012-06-437723
24. Sartipy F, Lindström D, Gillgren P, Ternhag A. The impact of stent graft material on the inflammatory response after EVAR. Vasc Endovascular. (2015) 49:79–83. doi: 10.1177/1538574415595209
25. Aho PS, Niemi T, Piilonen A, Laasila L, Renkonen R, Lepantalo M. Interplay between coagulation and Inflammation In open and endovascular abdominal aortic aneurysm repair – Impact of Intra-aneurysmal thrombus. Scand J Surg. (2007) 96:229–35. doi: 10.1177/145749690709600308
26. Gerasimidis T, Sfyroeras G, Trellopoulos G, Skoura L, Papazaglou K, Konstantinidis K et al. Impact of endograft material on the inflammatory response after elective endovascular abdominal aortic aneurysm repair. Angiology. (2005) 6:743–53. doi: 10.1177/000331970505600612
27. Chang C, Chuter T, Niemann C, Shlipak MG, Cohen MJ, Reilly LM, et al. Systemic inflammation, coagulopathy, and acute renal insufficiency following endovascular thoracoabdominal aortic aneurysm repair. J Vasc Surg. (2009) 49:1140–6. doi: 10.1016/j.jvs.2008.11.102
28. Englberger L, Savolainen H, Jandus P, Widmer M, Do DD, Haeberli A, et al. Activated coagulation during open and endovascular abdominal aortic aneurysm repair. J Vasc Surg. (2006) 43:1124–9. doi: 10.1016/j.jvs.2006.02.029
29. Ikoma A, Nakai M, Sato M, Sato HS, Takeuchi H, Tanaka F et al. Changes in inflammatory, coagulopathic, and fibrinolytic responses after endovascular repair of an abdominal aortic aneurysm: relationship between fibrinogen degradation product levels and endoleaks. Jpn J Radiol. (2014) 32:347–55. doi: 10.1007/s11604-014-0314-0
30. Inoue K, Furuyama T, Kurose S, Yoshino S, Nakayma K, Yamashita S, et al. Platelet count recovery after endovascular aneurysm repair for abdominal aortic aneurysm. Ann Vasc Dis. (2021) 14:11–8. doi: 10.3400/avd.oa.20-00030
31. Shimazaki T, Ishimaru S, Kawaguchi, Yokoi Y, Watanebe J. Blood coagulation and fibrinolytic response after endovascular stent grafting of thoracic aorta. J Vasc Surg. (2003) 37:1213–8. doi: 10.1016/S0741-5214(02)75323-8
32. Yamazumi K, Ojiro M, Okumura H, Aikou T. An activated state of blood coagulation and fibrinolysis in patients with abdominal aortic aneurysm. Am J Surg. (1998) 175:297–301. doi: 10.1016/s0002-9610(98)00014-2
33. Monaco M, Tomasso L, Stassano P, Smimmo R, Di Amicis V, Pantaleo A et al. Impact of blood coagulation and fibrinolytic system changes on early and mid term clinical outcome in patients undergoing stent endografting surgery. Interact Cardiovasc Thorac Surg. (2006) 5:724–9. doi: 10.1510/icvts.2006.136507
34. Ferroni P, Pulcinelli FM, Lenti L, Gazzaniga PP. Is soluble P-selectin determination a more reliable marker of in vivo platelet activation than CD62P flow cytometric analysis? Thromb Haemost. (1999) 81:472–3.
35. Lindahl TL, Ramström S. Methods for evaluation of platelet function. Transfus Apher Sci. (2009) 41:121–5. doi: 10.1016/j.transci.2009.07.015
36. Gasecka A, Böing AN, Filipiak KJ, Nieuwland R. Platelet extracellular vesicles as biomarkers for arterial thrombosis. Platelets. (2017) 28:228–34. doi: 10.1080/09537104.2016.1254174
37. Gasecka A, Banaszkiewicz M, Nieuwland R, van der Pol E, Najji N, Mutwil H, et al. Prostacyclin analogues inhibit platelet reactivity, extracellular vesicle release and thrombus formation in patients with pulmonary arterial hypertension. J Clin Med. (2021)10:1024. doi: 10.3390/jcm10051024
38. Pini R, Faggioli G, Gallitto E, Mascoli C, Fenelli C, et al. Platelet depletion after thoraco-abdominal aortic aneurysm endovascular repair is associated with clinically relevant hemorrhagic complications. Ann Vasc Surg. (2022) 79:106–13. doi: 10.1016/j.avsg.2021.08.036
39. Inoue K, Furuyama T, Kurose S, Yoshino S, Nakayama K, et al. Platelets reflect the fate of type II endoleak after endovascular aneurysm repair. J Vasc Surg. (2020) 72:541–8.e1 doi: 10.1016/j.jvs.2019.09.062
40. Wanhainen A, Verzini F, Herzeele I, Allaire E, Bown M, Cohnert T, et al. Clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. (2019) 57:8–93. doi: 10.1016/j.ejvs.2018.09.020
41. Kokje V, Hamming J, Lindeman J. Pharmaceutical management of small abdominal aortic aneurysms: a systematic review of the clinical evidence. Eur J Vasc Endovasc Surgery. (2015) 50:702–13. doi: 10.1016/j.ejvs.2015.08.010
42. Rughani G, Robertson L, Clark M. Medical treatment for small abdominal aortic aneurysms. Cochrane Database of Syst Rev. (2012). doi: 10.1002/14651858.CD009536.pub2
43. Bahia SS, Vidal-Diez A, Seshasai SRK, Shpitser I, Brownrigg JR, Patteson BO, et al. Cardiovascular risk prevention and all-cause mortality in primary care patients with an abdominal aortic aneurysm. Br J Surg. (2016) 103:1626–33. doi: 10.1002/bjs.10269
44. Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European society for vascular surgery (ESVS). Eur J Vasc Endovasc Surg. (2018) 55:305–68. doi: 10.1016/j.ejvs.2017.07.018
45. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European association for cardiovascular prevention and rehabilitation (EACPR). Eur Heart J. (2016) 37:2315–81. doi: 10.1093/eurheartj/ehw106
46. Graham I, Atar D, Borch-Johnsen K, Boysen G, Burrell G, Cifkova R, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J. (2007) 28:2375–414. doi: 10.1093/eurheartj/ehm316
47. Chajkof E, Dalman R, Eskandari M, Jackson BM, Lee WA, Mansour MA, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. (2018) 67:2–77. doi: 10.1016/j.jvs.2017.10.044
48. Harder S, Klinkhardt U, Alvarez J. Avoidance of bleeding during surgery in patients receiving anticoagulant and/or antiplatelet therapy. Clin Pharmacokinet. (2004) 43:963–81. doi: 10.2165/00003088-200443140-00002
49. Valgimigli M, Bueno H, Byrne RA, Collet J-P, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European. Eur Heart J. (2017) 39:213–60. doi: 10.1093/eurheartj/ehx419
50. Saratzis A, Saratzis N, Melas N, Kiskinis D. Pharmacotherapy before and after EVAR. Curr Vasc Pharmacol. (2008) 6:240–9. doi: 10.2174/157016108785909689
51. Alvarez F, Llaneza J, Franco F, Zanabili A, Rico J, Perez M et al. Effect of antiplatelet therapy on aneurysmal sac expansion associated with type II endoleaks after endovascular aneurysm repair. J Vasc Surg. (2017) 66:396–406. doi: 10.1016/j.jvs.2016.11.032
52. He R, Zhang L, Zhou T, Yuan W, Liu Y, Fu W, et al. Safety and necessity of antiplatelet therapy on patients underwent endovascular aortic repair with both stanford type b aortic dissection and coronary heart disease. Chin Med J. (2017) 130:2321–5. doi: 10.4103/0366-6999.215330
53. Sillesen H. What does ‘best medical therapy' really mean? Eur J Endovasc Surg. (2008) 35:139–44. doi: 10.1016/j.ejvs.2007.10.003
54. Cassar K, Bachoo P, Brittenden J. The role of platelets in peripheral vascular disease. Eur J Endovasc Surg. (2003) 25:6–15. doi: 10.1053/ejvs.2002.1795
Keywords: abdominal aortic aneurysm, endovascular AAA repair, EVAR, platelets, hemostasis, coagulation
Citation: Burban A, Idzik A, Gelo A, Filipiak KJ, Jakimowicz T, Jama K, Grabowski M, Gasecka A and Siniarski A (2022) Platelet function changes in patients undergoing endovascular aortic aneurysm repair: Review of the literature. Front. Cardiovasc. Med. 9:927995. doi: 10.3389/fcvm.2022.927995
Received: 25 April 2022; Accepted: 25 July 2022;
Published: 12 August 2022.
Edited by:
Konstantinos Spanos, University of Thessaly, GreeceReviewed by:
Frank Davis, University of Michigan, United StatesBhama Ramkhelawon, New York University, United States
Copyright © 2022 Burban, Idzik, Gelo, Filipiak, Jakimowicz, Jama, Grabowski, Gasecka and Siniarski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aleksander Siniarski, YWxla3NhbmRlcnNpbmlhcnNraUBnbWFpbC5jb20=
 Anna Burban
Anna Burban Aleksandra Idzik
Aleksandra Idzik Agata Gelo2
Agata Gelo2 Katarzyna Jama
Katarzyna Jama