- 1Department of Cardiology, Beijing Jishuitan Hospital, Beijing, China
- 2Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Department of Magnetic Resonance Imaging, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: Prior studies have shown that myocardial fibrosis can be detected by late gadolinium enhancement (LGE) of cardiac magnetic resonance (CMR) and might be associated with higher mortality risk in hypertrophic cardiomyopathy (HCM). The objective of this study was to examine the prognostic utility of CMR in patients with hypertrophic obstructive cardiomyopathy (HOCM) undergoing alcohol septal ablation (ASA).
Materials and methods: We conducted a retrospective study which consisted of 183 consecutive patients with symptomatic drug-refractory HOCM who underwent CMR for assessment of myocardial fibrosis before ASA. The cardiovascular disease related survival was evaluated according to LGE-CMR status.
Results: The cohort comprised 74 (40.4%) women with a mean age of 51 ± 8 years. Preoperative myocardial fibrosis was detected in 148 (80.9%) patients. After a median of 6 years (range 2–11 years) follow-up, adverse clinical events occurred in 14 (7.7%) patients. Multivariate-adjusted Cox regression analyses revealed that age [hazard ratio (HR) 1.142 (1.059–1.230), p = 0.001] and LGE [HR 1.170 (1.074–1.275), p < 0.001] were independent predictors of cardiovascular mortality during follow-up.
Conclusion: Preoperative myocardial fibrosis measured by LGE-CMR was an independent predictor of increased adverse clinical outcomes in patients with HOCM undergoing ASA and could be used for the pre-operative evaluation of risk stratification and long-term prognosis after ASA in these patients.
Introduction
Hypertrophic cardiomyopathy (HCM) is the common genetic inherited heart disease with a prevalence of about 0.2% (1). Dynamic left ventricular outflow tract (LVOT) obstruction is an important pathophysiologic phenomenon, with significant impact on symptoms such as dyspnea, angina, and prognosis of the obstructive HCM (HOCM). For those with drug-refractory symptoms, alcohol septal ablation (ASA) was introduced as an alternative therapeutic option to relieve LVOT obstruction, which is associated with a lasting clinical efficacy and long-term follow-up results (2). However, there are still a subgroup of patients with unsatisfactory responses after ASA such as having symptoms and residual LVOT obstruction (3, 4).
The areas of myocardial fibrosis are thought to constitute the substrate for life-threatening arrhythmia and adverse cardiac remodeling in HCM (5). Myocardial fibrosis as measured by late gadolinium enhancement (LGE) of cardiac magnetic resonance (CMR) is directly proportional to the extent of myocardial fibrosis which has been proved by histopathological studies (6). LGE is also related to HCM related adverse events, including progressive heart failure and sudden death (7). Little is known with respect to the impact of myocardial fibrosis before ASA on the clinical outcome after ASA. Additionally, whether ASA is also effective in patients with extensive septal scarring on CMR remains unknown (8).
The purpose of our study was to examine the impact of LGE on the clinical outcomes after ASA at long-term follow-up. We also assessed whether the amount of LGE can serve as a promising tool for the risk stratification in patients with HOCM undergoing ASA.
Materials and methods
Study population
This was an observational retrospective study approved by the institutional review committee of Fuwai Hospital, China. Between September 2005 and December 2014, 183 secutive patients with HCM were recruited for ASA to our center. The diagnosis of HCM were based on typical clinical and echocardiographic characteristics with unexplained ventricular myocardial hypertrophy occurring in the absence of any other accountable cardiac or systemic disease (2, 9). The indication for ASA was HOCM with New York Heart Association (NYHA) class III/IV even the optimal drug therapy, recurrent exercise-induced pre-syncope or syncope and a resting LVOT gradient of >50 mm Hg or >100 mm Hg during provocation (9, 10). All patients wrote the informed consent.
Alcohol septal ablation procedure
The ASA technique has been previously described and was performed by the interventional cardiologists (11). Before the procedure began, a temporary pacemaker was placed in all patients. With the help of the contrast echocardiography, the septal arterial branch supplying the target septal area was identified. 1–3 mL of ethanol was injected into the artery supplying the culprit septal segments and the balloon was removed 5 min after the last alcohol injection (12). A successful procedure was defined as a reduction in the LVOT pressure gradient ≥ 50% of baseline (13). If the operator was not satisfied with the result, the whole procedure could be repeated in another septal branch.
Cardiac magnetic resonance examination
All patients underwent the CMR examinations before the ASA procedure after enrolled in the Fuwai Hospital. The CMR examinations were performed on a 1.5T MRI machine (Siemens Medical Solutions, Erlangen, Germany) with a steady-state, free-precession breath-hold cines in 3 long-axis planes and sequential 10 mm short-axis slices (14, 15). LGE images were acquired 10–20 min after administration of 0.2 mL/Kg of gadolinium-DTPA (Magnevist, Schering; Berlin, Germany) using inversion-recovery sequences using a segmented phase-sensitive inversion recovery (PSIR) spoiled gradient echo sequence (16, 17). LGE-CMR imaging was acquired in short-axis views covering the LV from the mitral annular plane to the apex with 6 mm slice thickness and 1.6 mm gaps. Typical imaging parameters were: repetition time (TR) = 8 ms, echo time (TE) = 3.6 ms. Field of view (FOV) = 380 × 320 mm2, matrix = 256 × 162, temporal resolution = 40 ms. The inversion time was adjusted to optimally null signal from normal myocardium typically between 250 and 350 ms (17).
All CMR images were analyzed by two experienced radiologic technicians with commercially available software (Medis Medical Imaging Systems, Leiden, Netherlands). LV volume and mass measurements were acquired when the endocardial and epicardial contours were manually drawn in cardiac end-systole and diastole phase. LV mass was calculated by multiplying the volume of the myocardium calculated at end-diastole by the specific gravity of the myocardium (1.05 g/ml) (18). LV volume and mass measurements were indexed to body surface area. To measure the amount of LGE, all short-axis LV slices from base to apex were inspected to ensure an area of completely nulled myocardium. The mean signal intensity (and SD) of normal myocardium was calculated, and a threshold ≥ 6 SDs exceeding the mean was used to define areas of LGE (19, 20). To define LGE areas 6 SD threshold was used and the extent of LGE was expressed as of proportion of total LV mass (%LGE) (17).
Study endpoints and definitions
The primary end points were defined as cardiovascular death, which included sudden cardiac death (SCD)/aborted SCD, heart failure-related death and stroke-related death (21). SCD/aborted SCD was a composite endpoint, which comprised of SCD, successful resuscitation after cardiac arrest as well as appropriate ICD work for ventricular fibrillation (VF) or ventricular tachycardia with haemodynamic instability (22). Chronic heart failure was diagnosed according to the symptoms such as shortness of breath at rest or during exertion and physical signs of fluid retention such as ankle swelling according to the New York Heart Association (NYHA) functional classification (21). The results of survival outcome of patients after ASA were obtained by the actual medical records.
Statistical analysis
Continuous data were expressed as mean ± SD and categorical data were describe as median (interquartile range), or n (%), respectively. The different characteristics between groups were compared using the methods of unpaired Student t-tests, chi-squared tests, or Fisher exact test when appropriate. We stratified study participants according to their myocardial fibrosis burden into patients with myocardial fibrosis above (LGE ≥ 7.7% of LV mass) and below (LGE < 7.7% of LV mass) the median of the entire cohort. Cumulative survival curves were performed with the Kaplan–Meier method. Multivariate Cox proportional hazard regression models were used to identify whether there was an association between LGE and the long-term outcomes, and the models were corrected for age, sex, and body surface area and other clinical parameters such as the LVOT gradient before ASA, LV mass, preoperative LGE. We used the receiver operating characteristic (ROC) curve to evaluate the accuracy of LGE for the prediction of adverse outcomes. The Youden index was generated to determine the optimal cut-off value of LGE. All statistical tests were 2-tailed, and p-values were statistically significant at <0.05 using the SPSS statistical software, version 22.0 (SPSS Inc., Chicago, IL, United States).
Results
Baseline clinical and cardiac magnetic resonance characteristics
For this study, a total of 183 patients with HOCM underwent ASA with the CMR evaluation pre-ablation. The clinical and CMR characteristics at baseline were summarized in Table 1. The mean age was 51 ± 8 years, and 40.4% of patients were female. The mean LGE before ASA for the entire study population was 13.7 ± 9.5 g. Compared with the patients with lower LGE group, subjects in the higher LGE group showed more severe of NYHA class (85.7 vs. 72.8%, p = 0.014) and mitral regurgitation (MR, 53.9 vs. 33.0%, p = 0.022). There were no significant differences between groups regarding the history of hypertension, diabetes, and atrial fibrillation.

Table 1. Comparison of baseline clinical characteristics in patients with myocardial fibrosis classified by the median LGE (% of LV mass).
Assessment of parameters by cardiac magnetic resonance
At the pre-ablation CMR, the higher LGE group showed higher left atrial diameter (41.9 ± 7.3 vs. 39.7 ± 7.3 mm, p = 0.034), septal thickness (23.9 ± 5.0 vs. 22.4 ± 4.5 mm, p = 0.035), and LVESV (36.2 ± 12.4 vs. 31.9 ± 10.8 ml, p = 0.014). The LV mass (195.8 ± 46.1 vs. 156.9 ± 43.7 g) and LV mass index (112.4 ± 24.2 vs. 87.8 ± 21.0 g/m2) were significantly greater in the higher LGE group than the lower group (both p < 0.001). LGE was present in 80.9% of HCM patients (Figure 1). The higher LGE group had higher LGE mass (21.1 ± 5.9 g vs. 6.4 ± 6.1 g, p < 0.001) and the extent of LGE (10.8 ± 2.4% vs. 3.7 ± 3.1%, p < 0.001).

Figure 1. Representative short-axis late gadolinium enhancement (LGE) images in a 35-year-old male with obstructive hypertrophic cardiomyopathy showing diffuse hyperenhancement (white arrows) in the ventricular septum with 11.3% LGE compared to the LV mass.
Alcohol septal ablation procedural characteristics
The volumes of the alcohol during ASA were similar between the groups (2.1 ± 0.5 vs. 1.9 ± 0.6 ml, p = 0.171). After ASA, 32 (17.7) patients needed the temporary pacemaker implantation due to complete heart block. There was no significant difference regarding the complete heart block between the two groups (16.7 vs. 18.7%, p = 0.873). The LVOT gradient pre-ASA (101.4 ± 17.8 vs. 88.9 ± 18.2 mm Hg, p < 0.001) and post-ablation gradient (35.2 ± 16.2 vs. 18.4 ± 11.8 mmHg, p < 0.001) were both higher in the higher LGE group compared with the lower group. Compared to the higher LGE group, the LVOT gradient experienced the larger decrease in the lower LGE group (70.5 ± 12.9 vs. 66.3 ± 12.2 mmHg, p = 0.024). The procedural mortality was 0%. In the follow-up, only three patients needed permanent pacemaker implantation because of complete heart block.
Long-term outcome at follow-up
The median time of follow-up was 6 years (range 2–11 years). Fourteen (7.7%) patients died of cardiovascular-related diseases. Eleven patients (6%) died of SCD. Three patients (1.7%) died of stroke. The comparison of patients who experienced cardiovascular-related death with the rest of the cohort is provided in Table 2. Compared with the survival patients, the patients who died were older and have more severe mitral regurgitation. The patients who died showed higher LVOT gradient (114.2 ± 10.8 vs. 93.6 ± 18.7 mmHg, p < 0.001) and post-ablation gradient (42.4 ± 10.3 vs. 25.5 ± 16.2 mmHg, P < 0.001). Furthermore, the septal thickness (27.3 ± 8.2 vs. 22.8 ± 4.3 mm, p = 0.001) and LV mass index (137.1 ± 23.7 vs. 96.9 ± 23.5 g/m2, p < 0.001) were higher in the group who died than the survival patient. The group who died showed higher LGE mass (25.5 ± 5.3 vs. 12.7 ± 9.1 g, p < 0.001) and more extent of LGE (11.2 ± 2.9 vs. 6.9 ± 4.5%, p < 0.001).

Table 2. Comparison of clinical and CMR characteristics of patients with cardiovascular-related mortality with the remainder of the cohort after ASA.
As shown in Figure 2, compared with the lower LGE group, the higher LGE group showed more worse clinical events including the SCD (5.5 vs. 0.5%, p = 0.005), heart failure (33.0 vs. 9.8%, p < 0.001) and repeat ASA/septal myectomy (4.9 vs. 1.6%, p = 0.07). Kaplan–Meier survival analysis showed a significant increase in cardiovascular events (log rank = 6.399, p = 0.011, Figure 3). All univariate significant parameters as well as LVM and LGE were inserted into a Cox Proportional Hazards Model. Thereby, multivariate-adjusted Cox regression analyses revealed that age [HR 1.142 (1.059–1.230), p = 0.001] and LGE [HR 1.170 (1.074–1.275), p < 0.001] were independent predictors of cardiovascular mortality during follow-up (Table 3). In addition, age [HR 1.129 (1.039–1.226), p = 0.004] and LGE [HR 1.189 (1.073–1.317), p = 0.001] were also independent predictors of SCD events in HCM patients undergoing ASA during follow-up (Table 4) (As shown in Figure 4, the ROC analysis indicated that LGE had reasonable accuracy for prediction of adverse outcomes as well as SCD events). The cut-off values of LGE of estimated 6-year cardiovascular mortality and SCD were 7% of LV mass (sensitivity 100% and specificity 46.8%, ROC area 0.774, 95% CI 0.707–0.833, p < 0.001) and 10% of LV mass (sensitivity 72.73% and specificity 76.16%, ROC area 0.793, 95% CI 0.727–0.849, p < 0.001) respectively.
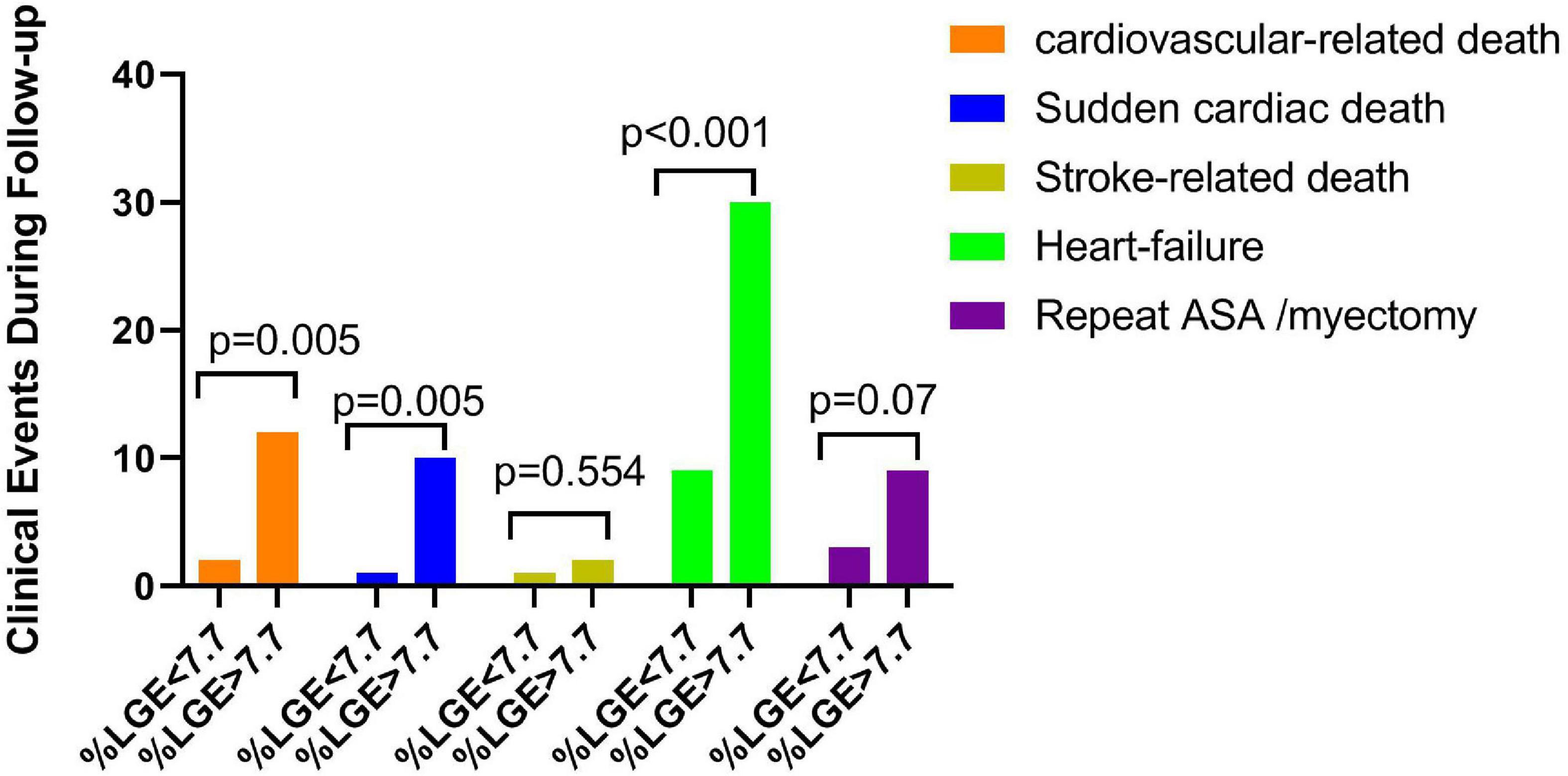
Figure 2. Postoperative adverse clinical events, including cardiovascular death, sudden cardiac death, stroke-related death, heart failure, and repeat ASA/myectomy, were categorized by the median late gadolinium enhancement (LGE) (% of LV mass).

Figure 3. Kaplan–Meier curves describing freedom from cardiovascular mortality events in patients undergoing alcohol septal ablation with an extent of LGE < 7.7% and ≥7.7% of LV mass.

Table 3. Univariate and multivariate predictor models for the composite endpoint of cardiovascular-related death in HCM patients undergoing ASA.

Table 4. Univariate and multivariate predictor models for SCD events in HCM patients undergoing ASA.

Figure 4. ROC curves of %LGE for estimated 6-year cardiovascular mortality (A) and SCD events (B) after alcohol septal ablation in patients with obstructive HCM.
Discussion
In this study, we assessed the impact of myocardial fibrosis measured by CMR on ASA outcomes in patients with HOCM. Our results suggested that those who presented with higher amount of LGE had greater incidence of long-term cardiovascular events compared with those with lower LGE group. Second, after multivariate adjustment, the degree of LGE was an independent risk factor predicting the long-term outcomes, confirming that the LGE is an available important marker of prognosis after ASA, thus more scientific follow-up plans and monitoring during out-hospital could be made to prevent from cardiac complications after ASA for those with higher amounts of LGE.
Late gadolinium enhancement has been related to adverse clinical outcomes in the progression of hypertrophic cardiomyopathy (19). We extend here those findings to patients with HCM undergoing ASA treatment. Our study is the first study to date to evaluate the prognostic value of LGE-CMR on perioperative and long-term survival after ASA for obstructive HCM. We demonstrated that preoperative LGE was the independent predictor of cardiovascular mortality after ASA on multivariate analyses. Considering that the majority of cardiovascular deaths in our study were sudden death, the presence of LGE increased the risk of sudden cardiac death in patients with HOCM after ASA, which was consistent with Rigopoulos et al. result (12). The mechanism of arrhythmias in HCM after ASA remains unclear. The areas of LGE in HCM had been proved to exhibit both depolarization and repolarization abnormalities which may trigger malignant ventricular arrhythmia. More extensive myocardial fibrosis was associated with a higher burden and prolonged bursts of ventricular tachyarrhythmia, which may cause the occurrence of SCD (15, 23, 24).
Previous studies have demonstrated there was a very low incidence of ventricular arrhythmias and sudden cardiac death after alcohol septal ablation (12, 25). In our study, sudden death occurred in 11 (6%) patients with an annual mortality rate of 0.89 per 100 patient-years and cardiovascular death occurred in 14 patients with an annual mortality ratio of 1.13 per 100 patient-years which was similar to veselka et al. results (26) that the event rate of sudden death and cardiovascular related mortality events was 0.98 and 1.16 per 100 patient-years respectively. The lower occurrence of significant ventricular arrhythmias or SCD may be related to the improved haemodynamic conditions and the regression of LV mass in the long-term after ASA. LVOT obstruction is also regarded as a clinical risk factor for sudden cardiac death (27). Ommen et al. demonstrated that the reduction of LVOT gradient contributed to the better survival from sudden cardiac death compared with patients with HOCM who only received medical drugs (28). The possible mechanism would include the reduction of arrhythmogenic substrate production and normalization of left ventricular pressure after relieving the obstruction of LVOT in patients undergoing ASA treatment.
Alcohol septal ablation has been shown to reduce LVOT gradient and alleviate symptoms safely and effectively in symptomatic patients with HOCM (29). However, whether myocardial fibrosis has an effect on the LVOT gradient reduction and long-term prognosis is still unclear. In our study, we showed that high amounts of LGE by CMR were associated with more severe HCM phenotype and LV remodeling including higher LV mass index and lower EF. Moreover, the group with higher LGE had greater baseline LVOT gradient as well as residual gradient after ASA, which was consistent with the recent findings that the LGE + group had higher residual LVOT gradient than the LGE-group (30). Jensen et al. also demonstrated that the baseline and residual LVOT gradients were both higher in HCM patients with more pronounced hypertrophy (31). Myocardial fibrosis is the process of excessive deposition of extracellular matrix proteins secondary to the hypertrophy in response to the pressure overload and may be a determinant of disease progression and clinical prognosis (16, 20, 32).
Several published studies have shown the factors associated with unfavorable outcomes undergoing ASA. Sorajja et al. showed that residual LVOT gradients after ASA were associated with reduced survival (2). Jensen et al. found that severe septal hypertrophy before ASA was the marker of poor outcome after ASA (31). Veselka et al demonstrated that early postdischarge LVOT gradient ≥30 mm Hg and baseline septum thickness were the independent predictors of cardiovascular events in the patients after ASA (33). In our study, when the LGE was not included in the multivariate model, the baseline LVOT gradient and LVM were independent predictors of cardiovascular mortality, however, after the LGE was added to the model, age and LGE before ASA were the independent predictors of mortality events. Because the amount of LGE was an important marker of increased risk for SCD and the development of heart failure (7), identification of LGE may provide additional information and proof for those patients with higher LGE who may benefit from implanted cardioverter-defibrillator (ICD) therapy after receiving ASA treatment (34).
Although the residual LVOT gradient and the severity of preoperative MR were higher in patients with the worse clinical outcome, the parameters were not predictors of outcome on multiple regression analysis, which was consistent with Chang et al. results (3). The univariate association of residual LVOT gradient and outcome may be attribute to the need for a larger infarct in certain cases with a large culprit septal area to achieve successful results. Furthermore, in accordance with Faber et al.’s results (4), the parameter of peak creatine kinase as the indicator of infarct size was not related to ASA outcome in the multivariate analysis. The reason may be explained that the larger infarct size was associated with the larger decrease of septal mass in the short term and the long term outcome may be attributed to the reduction of LVOT gradient and the concomitant decrease in LV wall stress (35). Myocardial fibrosis may lead to the increase of LV wall stress and portend susceptibility for progression to heart failure as well as risk for arrhythmic sudden death.
Clinical implications
Currently, the clinical evaluation of patients with HCM when receiving ASA treatment is based mainly the symptoms and LVOT gradient. Because myocardial fibrosis progresses slowly with the LV mass in HCM, which is also correlated with the poor clinical outcomes such as heart failure and the future implantation of ICD (19), our findings proved that myocardial fibrosis also predicts mortality in patients after ASA, suggesting the LGE–CMR could potentially be useful for selecting the operative approach for reducing the risk of mortality. Patients with >7% amount of LGE, indicating lower survival and higher risk of heart failure and residual LVOT gradient could be carefully monitored to prevent from cardiovascular events. Moreover, the higher risk of sudden cardiac death in HCM patients with higher extent of LGE indicates that they could potentially benefit from ICD device to improve long-term survival (22).
Limitation
Firstly, this was a retrospective, single-center study with a modest number of events. There is potential for over-fitting in statistical analysis. Furthermore, because the age range of the patients was about 43–59 years old in our study, whether the LGE affects the prognosis of patients with HCM after ASA in other age groups (such as adolescents and elderly) will be tested in the next study. Secondly, we did not enroll the patients who received ICD treatment before ASA because of the contraindication of CMR examination. Moreover, there were lower incidence of ICD implantation for the patients with higher risk of SCD after ASA because of misgivings about potential complications and cost in Fuwai Hospital, which was consistent with other results in our cohort (36) as well as other cohort in China (29). The potential for selection bias may exist in our study. However, the results obtained from this kind of HCM population may reflect the natural disease course to a certain extent. Thirdly, genetic data were not routinely performed. The relationship of genetic data to the clinical outcome in patients after ASA may be studied in the future study. Fourthly, at present, there is no general consensus on the protocols of LGE examination in HCM. The protocol of the LGE examination by CMR commonly used in Fuwai Hospital in this study may be different with other institutions. The possibility that different protocols introduce bias in LGE quantification cannot be ruled out. Finally, our study focused only the survival data, whereas did not perform to follow-up the patients’ functional status and CMR-related improvement, which may be considered in the future study.
Conclusion
Our study demonstrates that the preoperative LGE by CMR can predict the long-term cardiovascular mortality in patients undergoing ASA. Therefore, the evaluation of LGE pre-ablation could act as a new marker for risk stratification in patients with HCM undergoing ASA.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Committee of Fuwai Hospital, China. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YC and SQ conceived and designed the manuscript. YC analyzed the data and wrote the manuscript. JY, YZ, XZ, and WL edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The present study was supported by the National Natural Science Foundation of China (No. 81700330) and Beijing Municipal Excellent Talents Foundation (2016000021469G176) and Beijing Jishuitan Hospital Nova Program XKXX2018 (XKXX201802).
Acknowledgments
We thank Medis Medical Imaging System and Qmass software (Netherlands) for the support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. (2013) 381:242–55. doi: 10.1016/S0140-6736(12)60397-3
2. Sorajja P, Ommen SR, Holmes DJ, Dearani JA, Rihal CS, Gersh BJ, et al. Survival after alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation. (2012) 126:2374–80. doi: 10.1161/CIRCULATIONAHA.111.076257
3. Chang SM, Lakkis NM, Franklin J, Spencer WR, Nagueh SF. Predictors of outcome after alcohol septal ablation therapy in patients with hypertrophic obstructive cardiomyopathy. Circulation. (2004) 109:824–7. doi: 10.1161/01.CIR.0000117089.99918.5A
4. Faber L, Welge D, Fassbender D, Schmidt HK, Horstkotte D, Seggewiss H. One-year follow-up of percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy in 312 patients: Predictors of hemodynamic and clinical response. Clin Res Cardiol. (2007) 96:864–73. doi: 10.1007/s00392-007-0578-9
5. Wang J, Bravo L, Zhang J, Liu W, Wan K, Sun J, et al. Radiomics analysis derived from LGE-MRI predict sudden cardiac death in participants with hypertrophic cardiomyopathy. Front Cardiovasc Med. (2021) 8:766287. doi: 10.3389/fcvm.2021.766287
6. Kwon DH, Smedira NG, Rodriguez ER, Tan C, Setser R, Thamilarasan M, et al. Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: Correlation with histopathology and prevalence of ventricular tachycardia. J Am Coll Cardiol. (2009) 54:242–9. doi: 10.1016/j.jacc.2009.04.026
7. Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. (2014) 130:484–95. doi: 10.1161/CIRCULATIONAHA.113.007094
8. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: The task force for the diagnosis and management of hypertrophic cardiomyopathy of the European society of cardiology (ESC). Eur Heart J. (2014) 35:2733–79. doi: 10.1093/eurheartj/ehu284
9. Jensen MK, Almaas VM, Jacobsson L, Hansen PR, Havndrup O, Aakhus S, et al. Long-term outcome of percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: A Scandinavian multicenter study. Circ Cardiovasc Interv. (2011) 4:256–65. doi: 10.1161/CIRCINTERVENTIONS.110.959718
10. Batzner A, Pfeiffer B, Neugebauer A, Aicha D, Blank C, Seggewiss H. Survival after alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. (2018) 72:3087–94. doi: 10.1016/j.jacc.2018.09.064
11. Gietzen FH, Leuner CJ, Raute-Kreinsen U, Dellmann A, Hegselmann J, Strunk-Mueller C, et al. Acute and long-term results after transcoronary ablation of septal hypertrophy (TASH). Catheter interventional treatment for hypertrophic obstructive cardiomyopathy. Eur Heart J. (1999) 20:1342–54. doi: 10.1053/euhj.1999.1520
12. Rigopoulos AG, Daci S, Pfeiffer B, Papadopoulou K, Neugebauer A, Seggewiss H. Low occurrence of ventricular arrhythmias after alcohol septal ablation in high-risk patients with hypertrophic obstructive cardiomyopathy. Clin Res Cardiol. (2016) 105:953–61. doi: 10.1007/s00392-016-1005-x
13. Lu M, Du H, Gao Z, Song L, Cheng H, Zhang Y, et al. Predictors of outcome after alcohol septal ablation for hypertrophic obstructive cardiomyopathy: An echocardiography and cardiovascular magnetic resonance imaging study. Circ Cardiovasc Interv. (2016) 9:e002675. doi: 10.1161/CIRCINTERVENTIONS.115.002675
14. Maron MS, Appelbaum E, Harrigan CJ, Buros J, Gibson CM, Hanna C, et al. Clinical profile and significance of delayed enhancement in hypertrophic cardiomyopathy. Circ Heart Fail. (2008) 1:184–91. doi: 10.1161/CIRCHEARTFAILURE.108.768119
15. Weissler-Snir A, Hindieh W, Spears DA, Adler A, Rakowski H, Chan RH. The relationship between the quantitative extent of late gadolinium enhancement and burden of nonsustained ventricular tachycardia in hypertrophic cardiomyopathy: A delayed contrast-enhanced magnetic resonance study. J Cardiovasc Electrophysiol. (2019) 30:651–7. doi: 10.1111/jce.13855
16. Conte MR, Bongioanni S, Chiribiri A, Leuzzi S, Lardone E, Di Donna P, et al. Late gadolinium enhancement on cardiac magnetic resonance and phenotypic expression in hypertrophic cardiomyopathy. Am Heart J. (2011) 161:1073–7. doi: 10.1016/j.ahj.2011.03.022
17. Chen X, Zhao T, Lu M, Yin G, Xiangli W, Jiang S, et al. The relationship between electrocardiographic changes and CMR features in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. (2014) 30:55–63. doi: 10.1007/s10554-014-0416-x
18. Rudolph A, Abdel-Aty H, Bohl S, Boye P, Zagrosek A, Dietz R, et al. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol. (2009) 53:284–91. doi: 10.1016/j.jacc.2008.08.064
19. Habib M, Adler A, Fardfini K, Hoss S, Hanneman K, Rowin EJ, et al. Progression of myocardial fibrosis in hypertrophic cardiomyopathy: A cardiac magnetic resonance study. JACC Cardiovasc Imaging. (2021) 14:947–58. doi: 10.1016/j.jcmg.2020.09.037
20. Olivotto I, Maron BJ, Appelbaum E, Harrigan CJ, Salton C, Gibson CM, et al. Spectrum and clinical significance of systolic function and myocardial fibrosis assessed by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol. (2010) 106:261–7. doi: 10.1016/j.amjcard.2010.03.020
21. Zhu L, Wang J, Wang Y, Jia L, Sun K, Wang H, et al. Plasma uric acid as a prognostic marker in patients with hypertrophic cardiomyopathy. Can J Cardiol. (2015) 31:1252–8. doi: 10.1016/j.cjca.2015.02.018
22. Liebregts M, Faber L, Jensen MK, Vriesendorp PA, Hansen PR, Seggewiss H, et al. Validation of the HCM Risk-SCD model in patients with hypertrophic cardiomyopathy following alcohol septal ablation. Europace. (2018) 20:f198–203. doi: 10.1093/europace/eux251
23. Freitas P, Ferreira AM, Arteaga-Fernandez E, de Oliveira AM, Mesquita J, Abecasis J, et al. The amount of late gadolinium enhancement outperforms current guideline-recommended criteria in the identification of patients with hypertrophic cardiomyopathy at risk of sudden cardiac death. J Cardiovasc Magn Reson. (2019) 21:50. doi: 10.1186/s12968-019-0561-4
24. Prinz C, Schwarz M, Ilic I, Laser KT, Lehmann R, Prinz EM, et al. Myocardial fibrosis severity on cardiac magnetic resonance imaging predicts sustained arrhythmic events in hypertrophic cardiomyopathy. Can J Cardiol. (2013) 29:358–63. doi: 10.1016/j.cjca.2012.05.004
25. Jensen MK, Prinz C, Horstkotte D, van Buuren F, Bitter T, Faber L, et al. Alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy: Low incidence of sudden cardiac death and reduced risk profile. Heart. (2013) 99:1012–7. doi: 10.1136/heartjnl-2012-303339
26. Veselka J, Krejèí J, Tomašov P, Zemánek D. Long-term survival after alcohol septal ablation for hypertrophic obstructive cardiomyopathy: A comparison with general population. Eur Heart J. (2014) 35:2040–5. doi: 10.1093/eurheartj/eht495
27. Elliott PM, Gimeno JR, Tomé MT, Shah J, Ward D, Thaman R, et al. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur Heart J. (2006) 27:1933–41. doi: 10.1093/eurheartj/ehl041
28. Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. (2005) 46:470–6. doi: 10.1016/j.jacc.2005.02.090
29. Wang Y, Zhao HW, Wang CF, Meng QK, Cui CS, Zhang XJ, et al. Gender disparities in clinical outcome after alcohol septal ablation for hypertrophic obstructive cardiomyopathy in the Chinese han population: A cohort study. Heart Lung Circ. (2020) 29:1856–64. doi: 10.1016/j.hlc.2020.04.014
30. Polaková E, Liebregts M, Marková N, Adla T, Kara B, Ten BJ, et al. Effectiveness of alcohol septal ablation for hypertrophic obstructive cardiomyopathy in patients with late gadolinium enhancement on cardiac magnetic resonance. Int J Cardiol. (2020) 319:101–5. doi: 10.1016/j.ijcard.2020.06.049
31. Jensen MK, Jacobsson L, Almaas V, van Buuren F, Hansen PR, Hansen TF, et al. Influence of septal thickness on the clinical outcome after alcohol septal alation in hypertrophic cardiomyopathy. Circ Cardiovasc Interv. (2016) 9:e003214. doi: 10.1161/CIRCINTERVENTIONS.115.003214
32. Chen YZ, Qiao SB, Hu FH, Yuan JS, Yang WX, Cui JG, et al. Left ventricular remodeling and fibrosis: Sex differences and relationship with diastolic function in hypertrophic cardiomyopathy. Eur J Radiol. (2015) 84:1487–92. doi: 10.1016/j.ejrad.2015.04.026
33. Veselka J, Tomašov P, Januška J, Krejèí J, Adlová R. Obstruction after alcohol septal ablation is associated with cardiovascular mortality events. Heart. (2016) 102:1793–6. doi: 10.1136/heartjnl-2016-309699
34. Maron BJ, Spirito P, Shen WK, Haas TS, Formisano F, Link MS, et al. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA. (2007) 298:405–12. doi: 10.1001/jama.298.4.405
35. Aqel RA, Hage FG, Zohgbi GJ, Tabereaux PB, Lawson D, Heo J, et al. Serial evaluations of myocardial infarct size after alcohol septal ablation in hypertrophic cardiomyopathy and effects of the changes on clinical status and left ventricular outflow pressure gradients. Am J Cardiol. (2008) 101:1328–33. doi: 10.1016/j.amjcard.2007.12.042
Keywords: hypertrophic cardiomyopathy, alcohol septal ablation, myocardial fibrosis, outcome, late gadolinium enhancement (LGE) MRI
Citation: Chen Y, Zhao X, Yuan J, Zhang Y, Liu W and Qiao S (2022) Preoperative myocardial fibrosis is associated with worse survival after alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy: A delayed enhanced cardiac magnetic resonance study. Front. Cardiovasc. Med. 9:924804. doi: 10.3389/fcvm.2022.924804
Received: 20 April 2022; Accepted: 20 July 2022;
Published: 11 August 2022.
Edited by:
Federico Migliore, University of Padua, ItalyReviewed by:
Ferenc Imre Suhai, Semmelweis University, HungaryWolfgang Rottbauer, Ulm University Medical Center, Germany
Copyright © 2022 Chen, Zhao, Yuan, Zhang, Liu and Qiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youzhou Chen, Y2hlbnlvdXpob3UxOTg1QDE2My5jb20=; Wei Liu, eGlzaDY5Nzhqc3RAc2luYS5jb20=; Shubin Qiao, cXNiZndAc2luYS5jb20=
 Youzhou Chen
Youzhou Chen Xingshan Zhao1
Xingshan Zhao1