
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 16 August 2022
Sec. Coronary Artery Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.922497
 Mingdi Li1
Mingdi Li1 Iris Wenyu Zhou1
Iris Wenyu Zhou1 Janine Trevillyan2
Janine Trevillyan2 Anna C. Hearps3
Anna C. Hearps3 Anthony Lin Zhang1
Anthony Lin Zhang1 Anthony Jaworowski1,3,4*
Anthony Jaworowski1,3,4*Inflammation drives cardiovascular disease (CVD) in individuals with underlying chronic inflammatory diseases, including People with HIV (PWH), independently of dyslipidemia. Adjunctive treatments that lower inflammation may be useful to lower CVD risk in such populations. There is very little data on the efficacy of Chinese herbal medicine (CHM) in reducing inflammation in PWH to address its potential in reducing this CVD risk factor, therefore we evaluated its impact on inflammatory biomarkers relevant to CVD risk in the general population. Six English and Chinese databases were searched for studies investigating CHM’s effects on inflammatory biomarkers relevant to CVD from respective inceptions to February 2022. A systematic review and meta-analysis of randomized controlled trials (RCTs) were conducted and the most-frequently prescribed herbs were identified. Thirty-eight RCTs involving 4,047 participants were included. Greater than or equal to 50% of included studies had a low risk of bias in five domains (random sequence generation, detection, attrition, reporting and other bias) and 97% had a high risk of performance bias. CHM provided significant additive effects on attenuating relevant inflammatory indices including hs-CRP (SMD −2.05, 95% CI −2.55 to −1.54), IL-6 (SMD −1.14, 95% CI −1.63 to −0.66) and TNF-α levels (SMD −0.88, 95% CI −1.35 to −0.41), but no significant effects on hs-CRP were found between CHM and placebo when co-treating with Western drugs (MD 0.04, 95% CI −1.66 to 1.74). No severe adverse events were reported in CHM groups. The two most prevalent herbs present in formulae demonstrating reduction of at least one inflammatory biomarker were Dan shen (Salviae Miltiorrhizae Radix et Rhizoma) and Huang qi (Astragali Radix). CHM, in combination with standard anti-inflammatory medications, may depress inflammation and reduce the risk of inflammatory conditions such as CVD. Rigorously-conducted trials and adequate reporting are needed to provide more robust evidence supporting the use of CHM to reduce CVD risk in people with underlying chronic inflammation such as PWH.
People with HIV (PWH) have an approximately twofold increased risk of cardiovascular disease (CVD) which is independent of traditional risk factors and is a major cause of morbidity and mortality in this population (1). CVD is an inflammatory disease (2) where underlying chronic inflammatory conditions are associated with heightened risk. Increased CVD risk in PWH who do not have elevated risk due to traditional risk factors is thought to be due to chronic inflammation and immune activation that persist despite virologic control with antiretroviral therapy. Persistent inflammation and innate immune activation in PWH have been suggested to result from a combination of residual human immunodeficiency virus (HIV) replication, reactivation of latent viruses such as cytomegalovirus and increased translocation of bacterial products from the gut (3). That chronic inflammation contributes significantly to increased CVD risk in PWH is evidenced by observations that high levels of inflammatory factors including hs-CRP and IL-6 are independently associated with increased risk of a cardiovascular event in PWH on effective antiretroviral therapy (4). Understanding how to reduce CVD risk due to these factors in PWH will inform strategies for other chronic conditions such as rheumatoid arthritis and cancer.
Inflammation activates monocytes and vascular endothelial cells, promoting monocyte migration into coronary arteries and initiating atherosclerotic plaque formation and progression (4). There is considerable research associating markers of monocyte activation and CVD in PWH (5) and our previous research has identified an atherosclerotic phenotype of monocytes from PWH (6) strengthening the association and suggesting plausible mechanisms for how activated monocytes may promote atherosclerosis. Decreasing inflammation in PWH receiving antiretroviral therapy may reduce the risk of age-related, non-acquired immunodeficiency syndrome (AIDS) comorbidities including CVD and alleviate excess morbidity and mortality in this population. A number of different strategies have been trialed to lower inflammation in PWH using medications aimed at targeting microbial translocation [e.g., sevelamer (3)], inhibiting innate immune signaling (7) or inhibiting cytomegalovirus (CMV) replication (8), however, many have shown limited or inconsistent efficacy in reducing HIV-related inflammation (9).
Chinese herbal medicine (CHM) as a major component of traditional Chinese medicine, is a fully institutionalized part of Chinese health care and is widely used with western medicine (9). CHM refers to both herbal formulas and single herbs: formulas contain a combination of two or more herbs prepared based on Chinese medicine theory; single herbs may be derived from plant, animal, or mineral sources and traceable in the Chinese pharmacopeia (10). In a retrospective, community-based study, it was reported that PWH who have consistently used CHM have improved lipid profiles in their blood and reduced risk of CVD (11). However, it is not known whether CHM can improve inflammatory outcomes and markers of innate immune activation, which can drive CVD in PWH independently of dyslipidemia.
As there are insufficient studies to date on the effect of CHM in reducing inflammatory biomarkers relevant to CVD in PWH, we conducted a meta-analysis of studies addressing this research question in the general population with CVD to inform future studies on PWH and provide data which may be of relevance to the potential use of CHM in other inflammatory conditions where CVD risk may be driven by factors apart from traditional risk factors.
This systematic review was conducted following the requirements of the Cochrane Handbook (12). It is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 statement (13).
Six databases (PubMed, EMBASE, Cochrane CENTRAL, CINAHL, AMED, and CNKI) were searched for peer-reviewed full-text randomized controlled trials (RCTs) published regardless of language of publication from their respective inceptions to February 2022 by using CHM, traditional Chinese medicine, CVD, inflammation/anti-inflammatory and their MeSH terms and synonyms as keywords. The results were exported into, and managed by, EndNote. The reference lists of relevant trials and reviews were screened for additional studies. The list of keywords and sample search strategies are provided in Supplementary Table 1.
Articles retrieved for evaluation were assessed by two independent reviewers. One reviewer screened the title and abstract and two independent reviewers screened the full-text of potential records for eligibility. Any disagreement was discussed between the two reviewers. If an agreement could not be reached, discrepancies were discussed and resolved with a third party.
All searched articles were screened and evaluated according to the following inclusion and exclusion criteria: articles were considered for inclusion if they (1) were RCTs, (2) recruited individuals with cardiovascular conditions (including arterial occlusive diseases, hypertension, peripheral vascular diseases, myocardial ischemia, cardiac arrest or heart arrest, CVD, ischemic heart diseases, coronary artery diseases, and atherosclerosis), (3) contained CHM in any oral forms (such as decoction and capsule) as treatment, (4) included placebo or no treatment as a control intervention and (5) assessed inflammatory biomarkers as one of the outcome measures. Trials evaluating the additive effects of CHM (i.e., CHM plus co-intervention versus co-intervention alone) were also included. Eligible co-interventions considered were western medicine (WM) drugs (for CVD and/or inflammation), lifestyle changes (e.g., dietary and exercise), and routine treatment (for CVD and/or inflammation). Routine treatment refers to three situations: (1) where only the categories of the drugs were provided (e.g., beta-blockers) but the publication did not specify drug name and/or dosage; (2) routine care such as oxygen supplementation were included in the intervention; or (3) where the authors claimed without specifying that the control group received routine/standard treatment.
Studies were excluded if they were not RCTs or recruited participants with atrial fibrillation, cerebrovascular conditions, secondary CVD (e.g., renal hypertension, pulmonary heart disease, sepsis with intraperitoneal hypertension), post-surgical conditions, underlying disease and complications (e.g., diabetes mellitus, cancers, renal diseases, cerebrovascular conditions, pulmonary conditions, periodontitis, retinal vein occlusion, erectile dysfunction, hypertensive eyeground hemorrhage, mental conditions, dementia, cardiac arrhythmia, liver cirrhosis, and hyperuricemia), or acute/emergency conditions (e.g., angina pectoris and heart failure). Studies including participants aged <18 years, or who were pregnant, were also excluded. Studies employing ineligible CHM treatment (including external application or injection), provided insufficient information on formula name/ingredients, or used inconsistent formulae in the CHM group (i.e., modified or chose formulae and varied ingredient dosage based on symptoms among participants) were excluded. Trials were also excluded if they assessed the combination of CHM and other Chinese medicine treatment (including cupping, acupuncture, electro-acupuncture, scraping, acupoint injection, bath, external CHM application etc.), employed an ineligible control group (e.g., CHM and acupuncture), failed to clearly identify the co-interventions, or assessed comparisons which could not identify the clinical effects of CHM (e.g., CHM + WM vs. placebo).
All extracted data were entered into a predesigned data collection form which included details about article bibliography profile (author name, published year, language, and setting), participants (diagnostic criteria of CVD, sample size, gender, and age), interventions (number of included comparisons and duration) and outcome measures. Information about control interventions and herbal ingredients, forms and administration of treatment intervention were entered into a separate table. In the present study, outcome measures evaluated were the inflammatory biomarkers high sensitivity c-reactive protein (hs-CRP), interleukin (IL)-1, IL-6, IL-8, IL-10, monocyte chemotactic protein-1 (MCP-1), and tumor necrosis factor (TNF)-α and endothelial activation markers soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular cell adhesion molecule-1 (sVCAM-1). The most commonly studied herbs in the included studies with positive results were summarized descriptively. Where herbal ingredients were not provided in a paper, the formula names were checked in Zhong Yi Fang Ji Da Ci Dian (14) for detailed ingredients.
The quality of included RCTs was assessed against the risk of bias (e.g., selection, performance, attrition, detection, reporting, and other biases) according to the risk of bias tool from the Cochrane Handbook (12) independently by two reviewers. Any disagreement was resolved by discussion between the two reviewers or, if required, with a third party.
The extracted quantitative data from published studies were analyzed by RevMan 5.3 (15). Continuous data were weighted by the Mean Differences (MD) with 95% confidence intervals (CI). Standardized mean difference (SMD) was used in place of MD when different scales/units were used in more than one study for the same outcome measures. Heterogeneity was assessed statistically using I2. To minimize potential heterogeneity, random effects were applied where I2 was >50% (12). Descriptive synthesis was also used to aid in data presentation when appropriate. Sensitivity analysis and publication bias were also considered where applicable. Leave-one-out sensitivity analysis and publication bias were performed where >10 studies were included. Publication bias was examined by visual inspection of funnel plots and quantified by Egger’s regression and Begg and Mazumdar’s rank correlation tests which were calculated by Meta-Essentials, version 1.4 (16).
A total of 28,642 records were identified after applying the keywords in the database search and 65 records were identified through other sources. Thirty eight records met the selection criteria of this review. All of them were included in the meta-analysis (17–53), except one study which did not report the units of any assessed outcomes (54). Figure 1 illustrates the selection process of included RCTs.
All included RCTs were conducted in mainland China. Except for one paper published in English (51), all studies were published in Chinese. A total of 4,047 participants were recruited in the 38 included RCTs. One RCT (19) provided no information on the gender or age of the participants. four RCTs provided both sex and age information for all participants, but did not report data of each group (18, 25, 27, 54). Three trials investigated combined conditions: essential hypertension with carotid atherosclerosis (44), hypertension with hyperlipidemia (42) and hypertension with atherosclerosis (45). Eighteen trials recruited participants with various high blood pressure conditions, including hypertension (19, 20, 22, 34, 36, 38, 39, 41, 43, 50, 53), prehypertension (46), essential hypertension (30, 33, 35, 47), elderly patients with isolated systolic hypertension (28) and hypertension with dizziness (48); eight studies investigated atherosclerosis or cervical atherosclerosis (18, 23, 24, 26, 31, 40, 49, 52); six investigated coronary heart disease (17, 32, 37, 51, 54) and coronary heart disease with hyperlipemia (21); and three investigated coronary artery disease (25, 27, 29). The diagnostic criteria of all the involved CVD were provided in each paper accordingly (Table 1).
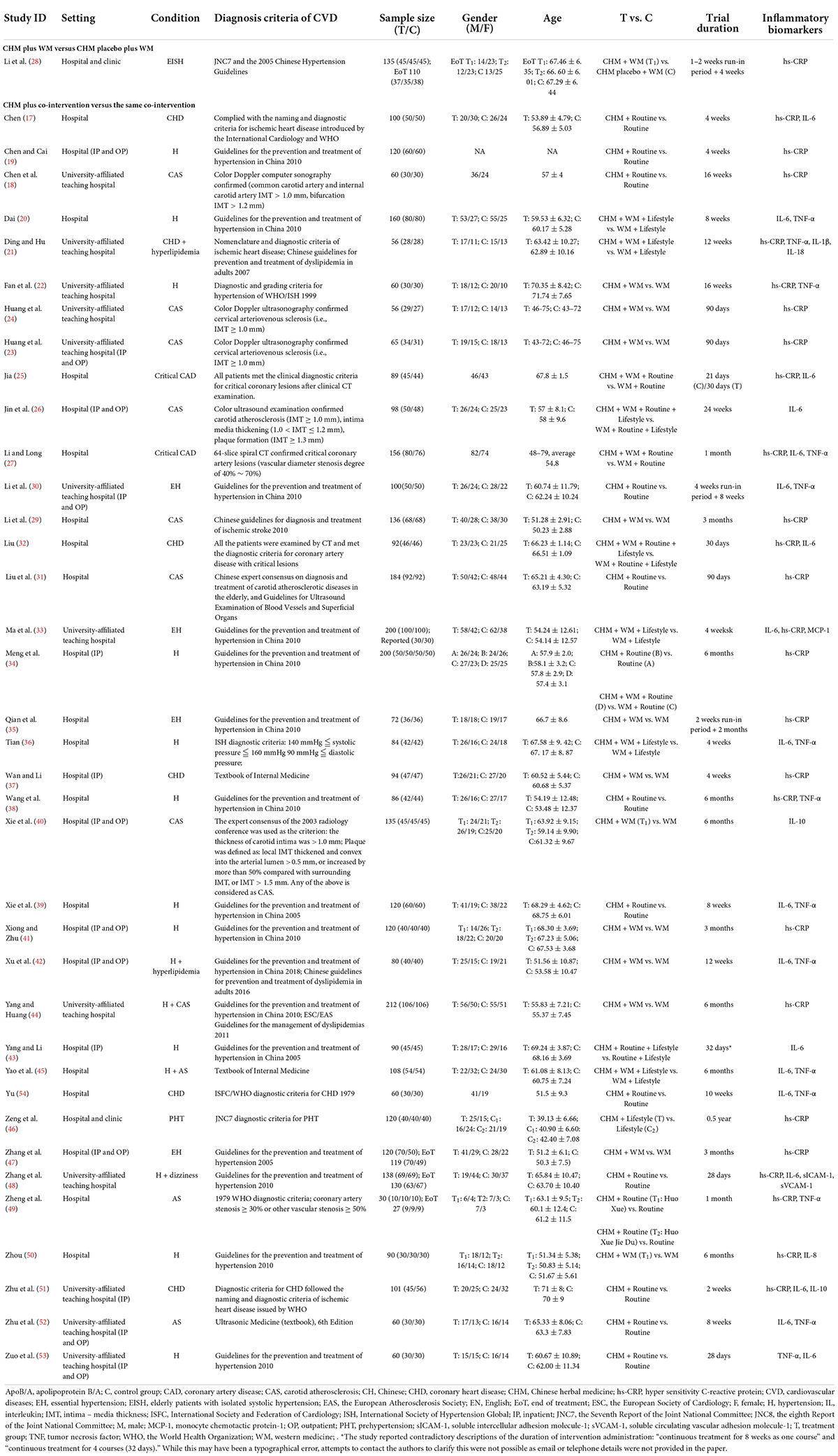
Table 1. Characteristics of included randomized controlled trials of Chinese herbal medicine on cardiovascular conditions.
Most of the included RCTs were composed of two arms, however, two studies containing more than two arms and making two comparisons were also included (34, 49) bringing the total number of comparisons in this review to 40. In these 38 studies, one study (34) formed two comparisons with different co-interventions; one compared CHM plus routine treatment with routine treatment alone, while the other compared CHM plus WM plus routine treatment versus WM plus routine treatment; one study (49) formed two comparisons with different CHM interventions: i.e., either Huo Xue or Huo Xue Jie Du plus routine treatment compared with routine treatment alone; the other co-interventions involved in the remaining 35 studies were WM (22–24, 29, 35, 37, 40–42, 44, 47, 50), routine treatment (17–19, 30, 31, 38, 39, 48, 51–54), lifestyle changes (46), WM + routine treatment (25, 27), WM + lifestyle changes (20, 21, 33, 36, 45), routine treatment + lifestyle changes (43), WM + routine treatment + lifestyle changes (26, 32). Despite the duration of treatment and control intervention being different in one trial (25) (21 days for the control and 30 days for the treatment), the treatment duration of all the groups in the remaining studies were the same and varied between 4 weeks to 6 months and no study reported follow-up after treatment. The characteristics of included RCTs are summarized in Table 1 and details of interventions are provided in Supplementary Table 2.
Greater than or equal to 50% of included studies had low risk of bias in the domains of random sequence generation, detection, attrition, reporting and other bias and 97% had a high risk of performance bias.
Randomization of participants in all of the included studies were stated to be performed via random sequence generation. However, two studies performed quasi-random (alternation) (19, 43) (high risk), 17 studies fail to specify the randomization method (17, 18, 23, 24, 27, 29–32, 34, 35, 39, 40, 42, 44, 53, 54) (unclear risk); and the rest (19 studies) provided appropriate randomization methods (e.g., random number table method) (20–22, 25, 26, 28, 33, 36–38, 41, 45–52). None of the included studies provided adequate information on how randomized numbers were allocated to participants and achieved an unclear risk of bias on the allocation concealment.
One study (28) used a placebo to achieve blinding of participants and personnel (low risk), whereas the remaining studies, irrespective of whether they stated the study to be blinded or not, could not achieve blinding due to different forms of interventions used in two groups (e.g., decoction versus tablet, capsule versus tablet) (high risk).
None of the included RCTs provided sufficient information on the blinding of outcome assessors. However, as all the included outcome measures in this review are laboratory results, the outcome measurements were not likely to be influenced by lack of blinding. Thus, they have low risk of detection bias.
High attrition bias was noticed in two studies: one study (31) performed per-protocol analysis of treatment intervention and stated that it excluded participants who experienced severe adverse events; the reported participant number (69/69) given in the Methods section of one study (48) was not equal to that presented in the Results section (63/67) which indicated that outcome data were missing in both intervention groups (6 versus 2, 8.69% versus 2.89%), but reasons were not clearly reported to be able to assess if they were balanced across groups. Additionally, unclear risk of bias was noticed in 17 studies due to poor statements describing drop out numbers and blurred descriptions of whether they performed pre-protocol analysis methods (21, 25, 26, 32, 34, 36–40, 44–46, 49, 51–53).
Trial protocol was not identified in any of the included studies. After comparing the described outcomes in the “Materials and methods” section to those reported in the “Results” section, five studies failed to report findings for all the outcome measures described in “Materials and methods” section (35, 40, 46, 47, 54) (high risk); five studies reported all findings described in the “Materials and methods” section but added other outcomes in Results section (17, 19, 28, 49, 51) (unclear risk); and the rest of the RCTs provide consistent information in those two sections (low risk).
“Other bias” was assessed from two aspects: authors’ description of relevant clinical and demographic baseline parameters and relevant funding information. The authors from one study (18) did not provide a clear statement of baseline values (unclear risk); while the remaining studies claimed to have matched control and treatment groups at baseline: 31 studies stated the p-value > 0.05 for assessed parameters (17, 19–26, 28–34, 36–46, 49, 50, 52, 54) and two studies reported statistical baseline comparison data in tables (35, 53) and four did not report relevant statistical data (27, 47, 48, 51). Twelve studies did not provide sufficient information on funding (17, 23, 25, 27, 31, 32, 36, 39, 43, 47, 53, 54) (unclear risk) and the rest were funded by government programs or organizations.
The risk of bias results are summarized and presented in Figures 2, 3.
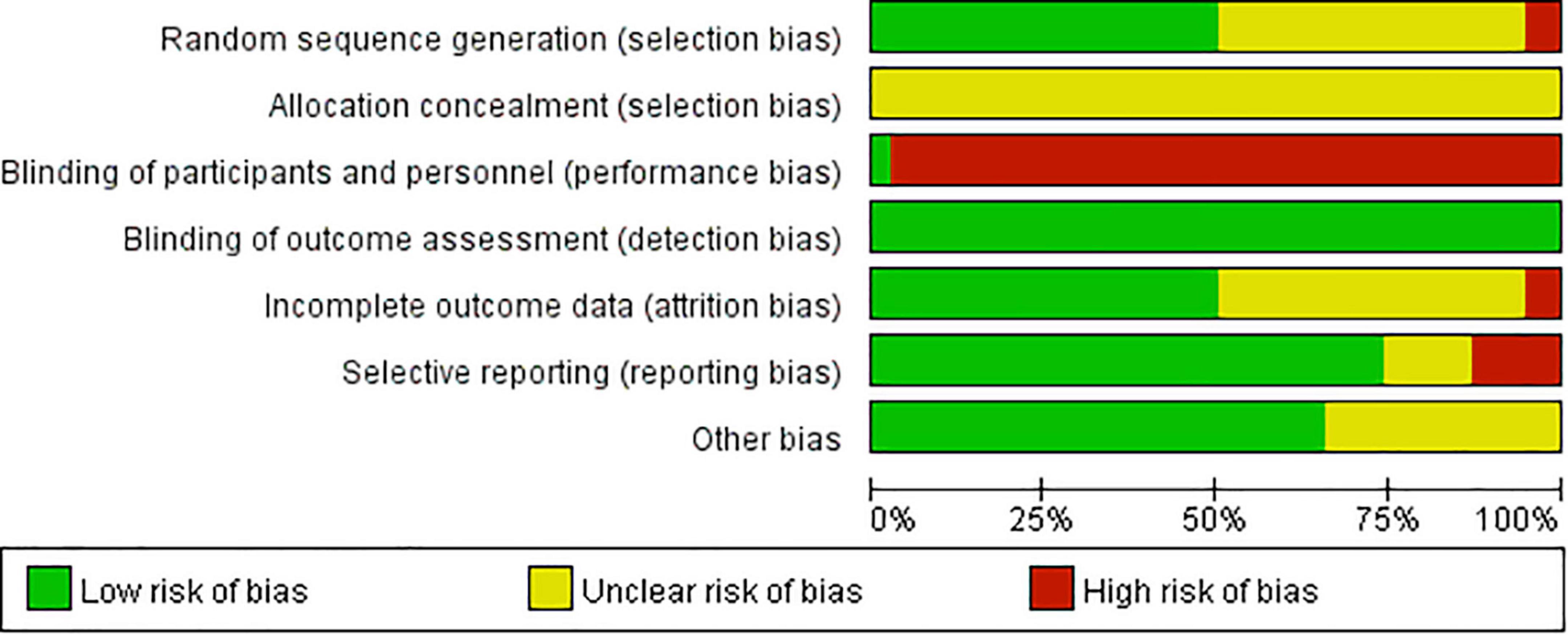
Figure 2. Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
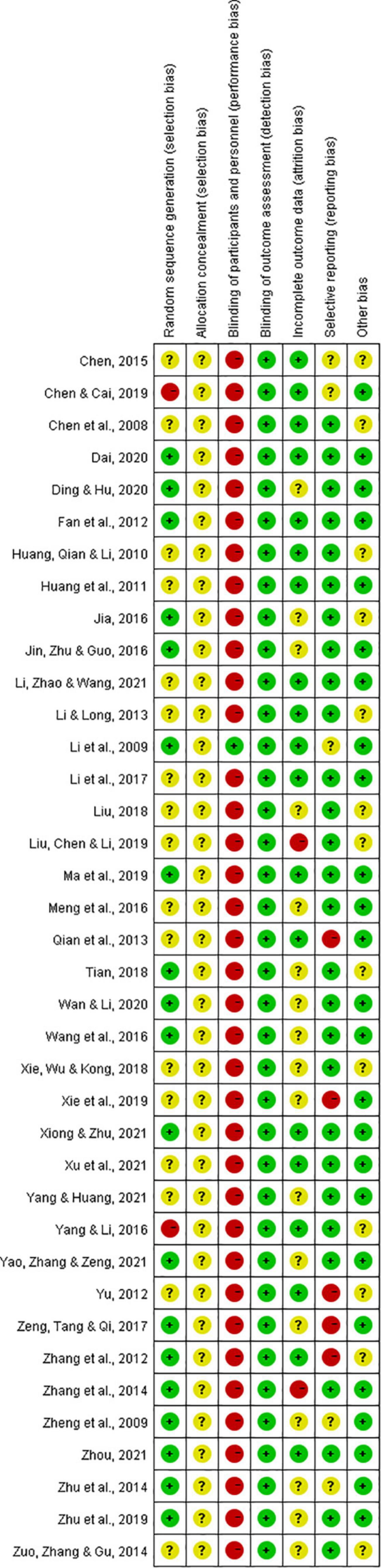
Figure 3. Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
None of the included studies compared CHM with no treatment. One study (28) employed a placebo control and compared CHM plus WM with CHM placebo plus WM, whereas all of the other included studies compared CHM plus co-intervention to the same co-intervention to investigate the additive effects of CHM on the intervention. Two outcomes from four studies (32, 40, 49, 51) had improperly matched baseline values of the parameters measured and did not address this problem in data analyses (i.e., they are not reporting changes from baseline between control and treatment arms) (Supplementary Table 3), thus they were not included in the meta-analysis. All the included data were categorized by interventions and sub-grouped by outcome measures.
One study (28) compared Jiang ya capsule plus WM with CHM placebo plus WM and reported no significant differences in hs-CRP levels after a 4-week intervention period (MD 0.04, 95% CI −1.66 to 1.74).
Meta-analysis results indicated significant additive effects of CHM on reducing hs-CRP (SMD −2.05, 95% CI −2.55 to −1.54, I2 = 96%, p < 0.00001) (17–19, 21–25, 27, 29, 31–35, 37, 38, 41, 43, 44, 46–50), IL-6 (SMD −1.14, 95% CI −1.63 to −0.66, I2 = 95%, p < 0.00001) (17, 20, 25–27, 30, 33, 36, 42, 43, 45, 48, 51–53) and TNF-α levels (SMD −0.88, 95% CI −1.35 to −0.41, I2 = 92%, p = 0.0002) (20–22, 27, 30, 36, 38, 39, 42, 45, 49, 52, 53) when combined with other interventions (Supplementary Figures 1–3). Table 2 summarizes the meta-analysis results for hs-CRP, IL-6 and TNF-α in CHM plus co-intervention groups (before and after treatment), in co-intervention groups (before and after treatment), and between two groups (at the baseline and at the end of treatment). Detailed statistical analysis data of Table 2 are provided in Supplementary Figures 4–12. Sensitivity analysis indicated that no single study significantly altered the direction or magnitude of the pooled estimates.
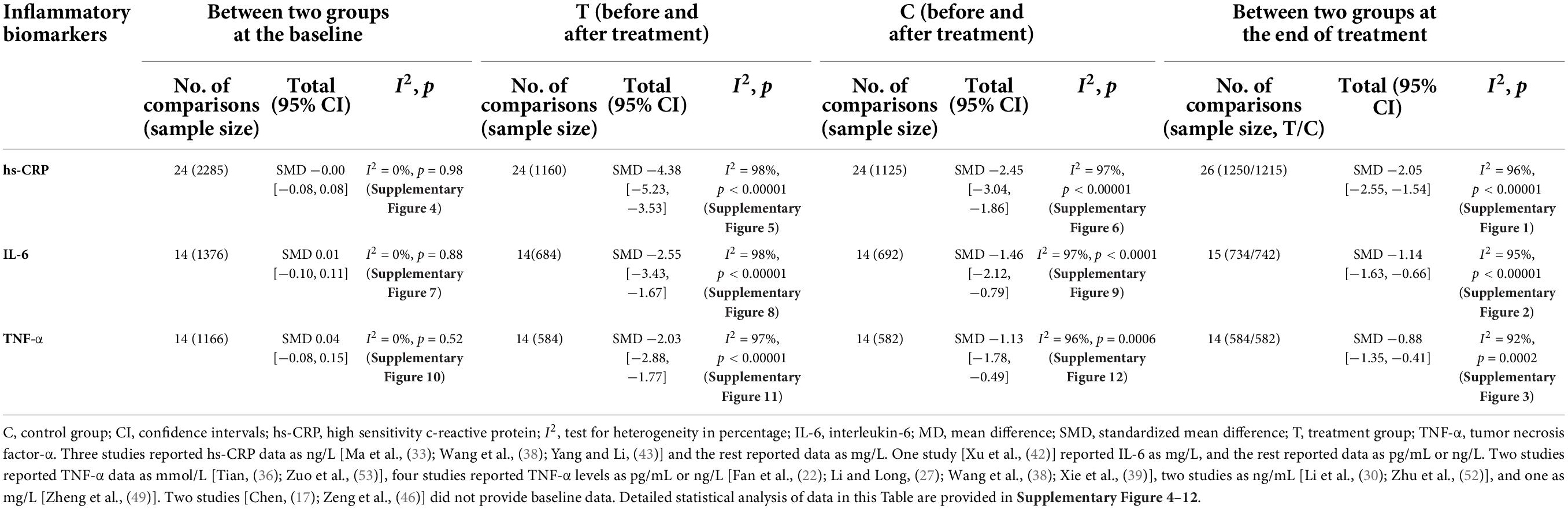
Table 2. Hs-CRP, IL-6, and TNF-α in Chinese herbal medicine plus co-intervention groups and the same co-intervention groups.
Seven other inflammatory biomarkers were assessed in five separate studies.
One study (33) compared levels of MCP-1 following treatment with CHM (Ban Xia Bai Zhu Tian Ma Tang) plus co-intervention (WM + lifestyle) versus co-intervention alone. After 4-weeks intervention, MCP-1 levels in the treatment group were significantly lower than that in the control group (MD −31.32, 95% CI −55.15 to −7.49).
One study (21) assessed levels of IL-1β and IL-18 with a 12-weeks intervention period and results showed significant reduction of IL-1β (MD −0.17, 95% CI −0.24 to −0.10) and IL-18 levels (MD −27.89, 95% CI −43.04 to −12.74) in the combined group (Shu Gan Qing Zhi Fang + WM + lifestyle changes) when compared to the control group (WM + lifestyle).
One study (48) assessed sICAM-1 and sVCAM-1 (markers of endothelial activation) by comparing CHM (Shu Nao Xin Di Wan) plus routine treatment to routine treatment. The results showed that CHM had a significant additive effect of reducing both soluble ICAM-1 levels (MD −40.03, 95% CI −76.72 to −3.34) and soluble VCAM-1 levels (MD −0.55, 95% CI −1.04 to −0.06) after a 28-day intervention.
One study (50) assessed IL-8 levels by comparing CHM (Tong Mai Jie Du Fang) plus WM (nifedipine extended-release tablets and atorvastatin calcium tablets) to WM and after 6-month intervention IL-8 level reduced significantly (MD −6.36, 95% CI −7.78 to −4.94) in the co-treatment group.
Two studies assessed IL-10 levels, reporting different results: one study (40) reported an increase in IL-10 levels with an increase significantly higher in the CHM (Shen Qi Mai Xin Tong Capsule) plus WM group compared to WM alone (MD 14.37, 95% CI 5.01 to 23.73); whereas the other study (51) reported a reduction in IL-10 levels where the reduction was greater for the control group (routine treatment) compared to the co-treatment group (Lian Dou Qing Mai Recipe plus routine treatment) (MD 0.17, 95% CI 0.10 to 0.24).
Similar to the meta-analysis, subgroup analyses of covariates (co-intervention, condition and trial duration) identified significant differences. Subgroup analysis of co-interventions showed that CHM significantly reduced hs-CRP when comparing different co-interventions, conditions and trial duration, and IL-6 and TNF-α when comparing different trial duration (Table 3). Detailed statistical subgroup analysis of hs-CRP, IL-6 and TNF-α based on different co-interventions is presented in Supplementary Figures 13–15, based on different conditions in Supplementary Figures 16–18, and based on different trial duration in Supplementary Figures 19–21.
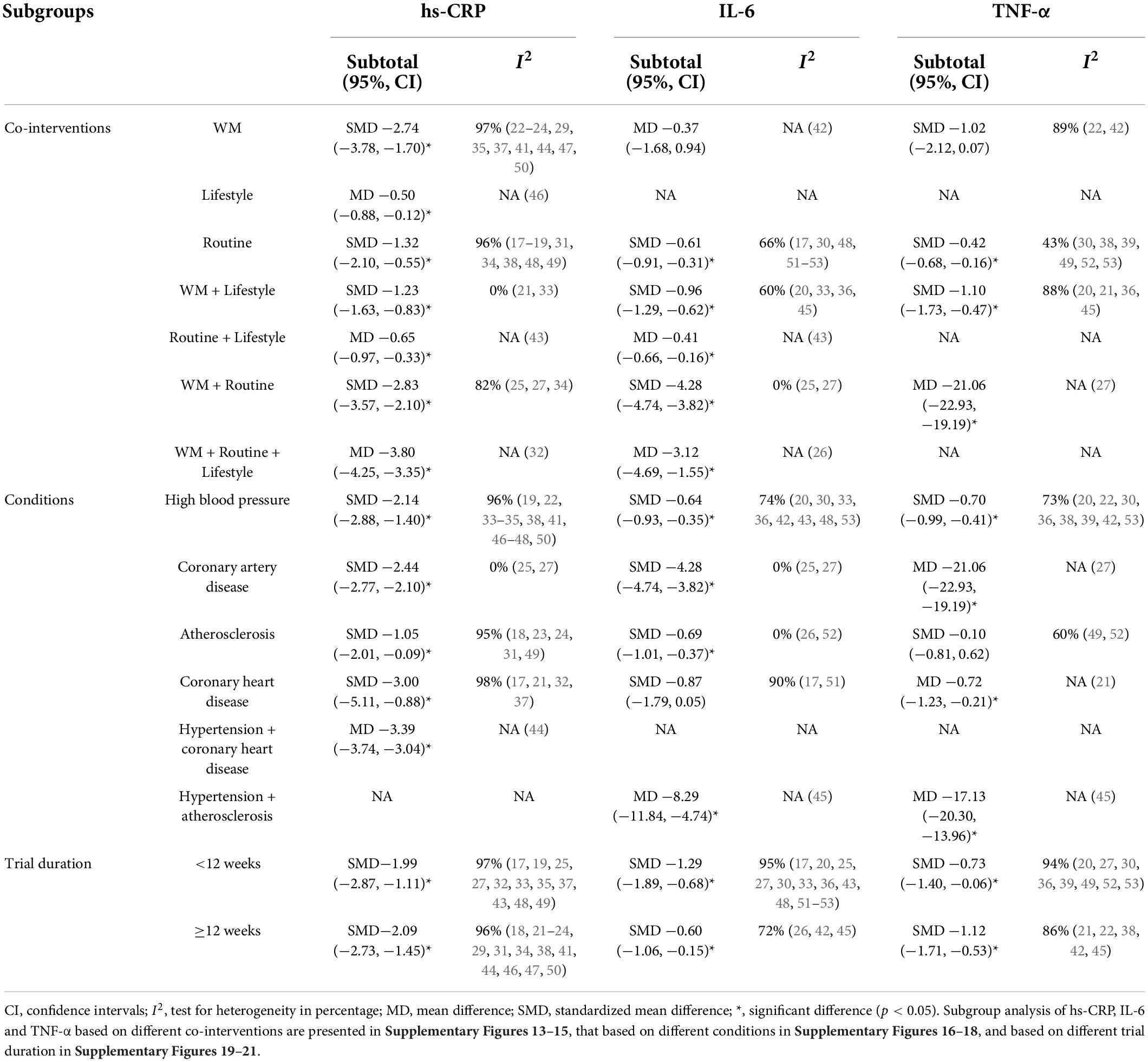
Table 3. Subgroup analysis of hs-CRP, IL-6, and TNF-α of Chinese herbal medicine plus co-intervention group and the same co-intervention groups.
One study (29) compared CHM (Hua Ban Fang) plus WM (atorvastatin calcium tablets and aspirin enteric sol sac) after a 3-month intervention, and the co-treatment group showed a significantly lower adverse event rate than the WM group (χ2 = 8.84, p < 0.01). Sixteen of the included studies assessed safety and reported no significant difference between the groups, with no vital adverse events in the treatment group (18, 19, 22, 26, 28, 30, 33, 42–47, 49, 50, 52). Details are listed in Supplementary Table 4.
Visual inspection of funnel plots revealed potential asymmetry for hs-CRP, IL-6 and TNF-α (Supplementary Figure 22), however, after further quantifying the funnel plots using Egger’s and Begg’s tests, a significant influence from small studies occurred in hs-CRP (Egger’s: p = 0.001, Begg’s: p = 0.001) and IL-6 (Egger’s: p = 0.020, Begg’s: p = 0.255), but not in TNF-α (Egger’s: p = 0.761, Begg’s: p = 0.784).
Among the 37 studies included for meta-analyses, 35 studies reported lowering effects of CHM for at least one inflammatory biomarker. One study employed a single herb (Da huang, Rhei Radix et Rhizoma) as CHM intervention (54). The ingredients of formulae were not provided in four publications (34, 44, 47, 48) and could not be identified from Zhong Yi Fang Ji Da Ci Dian (14). The most frequently used herbs present within the remaining 32 formulas were identified and the top 3 were Dan shen (Salviae Miltiorrhizae Radix et Rhizoma, n = 16), Huang qi (Astragali Radix, n = 12) and Gan cao (Glycyrrhizae Radix et Rhizoma, n = 10), followed by Dang gui (Angelicae Sinensis Radix, n = 9), Chuan xiong (Chuanxiong Rhizoma, n = 8), Bai zhu (Atractylodis Macrocephalae Rhizoma, n = 7), Niu xi (Achyranthis Bidentatae Radix, n = 7) and Tian ma (Gastrodiae Rhizoma, n = 7).
To the best of our knowledge, this is the first meta-analysis that has sought to provide an assessment of the clinical effects of CHM, alone and in combination with other treatments, on inflammatory biomarkers in individuals with underlying CVD. Findings from 38 included studies (totaling 4,047 participants) revealed that CHM may significantly decrease plasma levels of inflammatory biomarkers relevant to CVD risk (hs-CRP, IL-6, and TNF-α) as well as the monocyte chemokine MCP-1, and markers of vascular endothelial cell activation, sICAM-1 and sVCAM-1. In contrast, the analysis did not reveal any clinical effects of CHM in combination with a standard intervention on the levels of the anti-inflammatory cytokine IL-10, based on data from two included studies. Categorical subgroup analysis identified substantial between-group differences for covariates, suggesting that the hs-CRP attenuating effect did not alter among different covariates (co-interventions, conditions, and trial durations), whereas the anti-inflammatory effects related to IL-6 and TNF-α may vary depending on co-interventions and conditions. Most included formulae for meta-analyses were identified to have lowering effects on at least one inflammatory biomarker, except those from two studies (40, 49). Dan shen (Salviae Miltiorrhizae Radix et Rhizoma), Huang qi (Astragali Radix), and Gan cao (Glycyrrhizae Radix et Rhizoma) were determined to be the most frequently used herbs in the included formulae. The safety analysis indicated that CHM formulae were safe to use as an add-on therapy to WM, routine treatment and lifestyle changes in CVD patients.
Cardiovascular disease is a leading cause of death in PWH (55) and rates of CVD appear to be increased in PWH compared to age-matched, HIV-uninfected individuals (56). Traditional assessments for CVD risk, including lipid profiles (i.e., total cholesterol and high-density lipoprotein cholesterol), present in a comparable way irrespective of HIV status (55). Together with the observation that increased CVD risk in PWH is independent of traditional risk factors [reviewed in (5)], they may not be the most appropriate primary outcomes to investigate mechanisms of altered risks of CVD in PWH. In contrast, generalized inflammation biomarkers are predictive of CVD events in PWH (57). The characteristics of untreated HIV infection include increases in the levels of inflammatory biomarkers (e.g., IL-6 and hs-CRP) and adhesion molecule expression relevant to atherosclerotic plaque formation. IL-6 and hs-CRP were also strongly associated with the development of AIDS events and opportunistic diseases (57, 58). These factors were also determined to be important in the CVD pathogenesis. Inflammatory biomarkers and thrombosis have the potential to improve CVD risk stratification beyond traditional and HIV-specific factors (59). Additionally, low physical functional status in PWH was associated with significantly higher IL-6 and TNF-α levels (56). Thus, we considered that inflammatory biomarkers are suitable to evaluate CHM as an adjunctive CVD prevention strategy for individuals with HIV infection and may be useful to explain the potential effects of CHM on improvement of quality of life in future studies.
Inflammation plays a crucial role in the pathophysiology of CVD, yet it is not known whether CHM can improve inflammatory outcomes and markers of innate immune activation relevant to CVD. We initially intended to evaluate this in PWH, since there is considerable evidence that residual inflammation and innate immune activation contributes to CVD in these individuals independently of traditional risk factors such as hypercholesterolemia. However, preliminary surveys revealed that there was an absence of studies on the anti-inflammatory effects of CHM in PWH to enable a systematic review or meta-analyses to be performed to address this question. Most current meta-analyses either focused on CHM for the treatment of AIDS (60) or investigated the effects of CHM with or without co-interventions on CVD management, but their favorable outcomes were blood lipid profiles (61), quality of life (62) or other specific parameters of the investigated conditions (e.g., blood pressure) (63), and not inflammatory biomarkers. The available studies were not sufficient to conduct a systematic review: only two RCTs and one parallel controlled study were returned after a comprehensive search of English and Chinese databases. Even though the number of relevant studies were limited, the results were suggestive of potential therapeutic effects. Wu et al. (64) lead a university-affiliated-hospital-based RCT conducted in Fujian China. Seventy individuals with AIDS with lung infection were treated with either WM alone (Cephalosporin antibiotics + Levofloxacin + Fluconazole) or in combination with CHM (Ai Fei Yi Hao). After 14-days of intervention, results indicated a significant decrease in hs-CRP in the co-treatment group (MD −8.64, 95% CI −15.06 to −2.22). Hs-CRP was also assessed by a hospital-based parallel controlled study (65) conducted in Zhengzhou, China, in which 102 individuals with AIDS with lung infection were allocated to a routine treatment group (anti-viral drug regimen and anti-microbial therapies) and a CHM (Fu Zheng Qing Fei Tang) plus routine treatment group. A significant additive effect of CHM on lowering hs-CRP was reported compared to the routine treatment group (MD −1.17, 95% CI −1.91 to −0.43), but this paper failed to report the intervention period. These results are consistent with the findings of the present meta-analysis of CHM intervention’s additive effects on hs-CRP levels in HIV-uninfected individuals despite the differences in co-intervention regimes, conditions and trial durations. Additionally, one study (66) evaluated the effects of CHM on IL-6 levels with reference to placebo control (n = 70) by a university-affiliated-hospital-based RCT conducted in Guangxi, China. Two formulas were provided to participants in the CHM group (n = 140) based on their Chinese medicine differential diagnosis: Shen Ling Fu Zheng Capsules for “Qi and Blood deficiency syndrome,” Qing Du Capsules for “damp-heat syndrome.” CHM significantly reduced serum IL-6 levels compared to placebo after an 18-month intervention (MD −2.62, 95% CI −4.86 to −0.38). Similar results were seen in our meta-regression of IL-6 levels. Our finding also bring attention to the possible categorical difference triggered by co-intervention regimes and types of CVD. The present systematic review is, to our knowledge, the first meta-analysis that sought to provide an assessment on clinical effects of CHM (with or without co-interventions) on inflammatory biomarkers among cardiovascular condition populations.
There is comparatively little data on whether reduction of inflammatory biomarkers following therapeutic interventions correlates with improved outcomes with respect to risk and progression of CVD, especially in PWH. However, given the association between higher levels of inflammatory biomarkers and the risk of CVD it is reasonable to assume that treatments that reduce inflammation will be beneficial in this regard. However, it is important that direct evidence for this is obtained by examining how reduction in inflammation impacts more robust measures of coronary artery disease, including surrogate measures of atherosclerosis such as carotid artery intima-media thickness and pulse wave velocity, direct measures of atherosclerotic disease such as imaging of plaques, and cardiovascular events.
Among the 32 formulas with clear identified ingredients, Dan shen (Salviae Miltiorrhizae Radix et Rhizoma), Huang qi (Astragali Radix) and Gan cao (Glycyrrhizae Radix et Rhizoma) were the most frequently used herbs in the included formulas with anti-inflammatory effects. Dan shen is the root of Salvia miltiorrhiza Bge. (10). The extract of Dan shen and its main constituents (i.e., salvianolic acid B, tanshinone IIA and protocatechuic acid) showed attenuating effects on the expression of VCAM-1 and ICAM-1, inhibition on the release of sVCAM-1 and sICAM-1 in human umbilical vein endothelial cells, and strong downregulating properties on TNF-α induced expression of CD40 (67). Huang qi, is derived from the root of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao and Astragalus membranaceus (Fisch.) Bge. (10). One of its major bioactive constituents, astragalosides have been reported to induce T cell activation, regulate T cell balance, enhance CD45 phosphatase activity, inhibit pro-inflammatory cytokines and the NFκB pathway (68). Gan cao, also known as licorice, originates from Glycyrrhiza uralensis Fisch., Glycyrrhiza inflata Bat. and Glycyrrhiza glabra L. (10). Both the bioactive compounds present in, and metabolites of, Gan cao have shown anti-inflammatory properties: glycyrrhizin, a bioactive compound, interacts with the lipid bilayer of RAW 264.7 cells to attenuate inflammatory responses in macrophages (69); β-glycyrrhetinic acid, the major metabolite of glycyrrhizin (70), can reduce inflammatory responses by inhibiting glucocorticoid metabolism (71). In the context of PWH, some constituents from Dan shen and Gan cao have been reported to have effects in vitro on HIV infection. Lithospermic acid and lithospermic acid B are two selective HIV-1 integrase inhibitors present in Dan shen which strongly suppressed the acute HIV-1 infection of H9 cells (72). Lithospermic acid is also a non-covalent competitive inhibitor of nucleic acid binding to HIV nucleocapsid making it a potential representative of a new class of HIV inhibitor (73). Gan cao was identified to be one of the most frequent clinically used herbs in PWH with hyperlipidemia (31.58%) in a population-based study conducted in Taiwan (11). Glycyrrhizic acid from Gan cao inhibits the replication of HIV (74); glycocoumarin and lico-pyranocoumarin inhibit giant cell formation in HIV-infected cell cultures (71, 75). These two herbs may be worth exploring further to discover more options on driving CVD in PWH of dyslipidemia.
I2 is a measure of the extent of heterogeneity (12). The I2 of the three main outcomes were 96% (hs-CPR), 95% (IL-6) and 92% (TNF-α), which indicated considerable heterogeneity. Subgroup analysis was applied to further explore heterogeneity. After subgroup analysis of the included studies based on the co-interventions and conditions, several had an I2 below 50% (indicating less than substantial heterogeneity): TNF-α of the routine treatment subgroup (43%), hs-CRP of the WM plus lifestyle subgroup (0%), IL-6 of the WM plus routine treatment subgroup (0%), hs-CRP and IL-6 of the coronary artery disease subgroup (0 and 0%), IL-6 of the atherosclerosis subgroup (0%). Subgroup analysis of trial duration did not make a noticeable difference to I2. The statistical heterogeneity showed variation in intervention effects in these included studies. This review aimed to take a broader perspective in the intervention effects of CHM on inflammatory biomarkers in CVD. Therefore, clinical variation may possibly lead to the high heterogeneity: (1) CVD included diverse diseases. Due to the limited number of included studies, the categorization of condition subgroup analysis was not very specific and the inflammatory drivers of these diseases may be different; (2) similar to that observed with condition, even though subgroup analysis of the co-interventions reduced the I2 to a certain level, the diversity of the control groups could contribute to the high heterogeneity; (3) distinctiveness of the CHM interventions should also be considered. The herbal ingredients of each formula are different. These adhesion interventions may not have the same intervention effects in the same way in every included studies.
The quality of methodology plays an essential role in the strength of evidence: pre-registered protocols, rigorous study design (including application of suitable placebo controls) and adherence to adequate reporting will improve the credibility of findings. None of the included studies performed protocol registration before trial conduct. A weakness of the data set used in the present meta-analysis is that only one study (28) introduced a placebo. The blinding of participants and personnel could be easily compromised due to the low application rate of placebo controls. The remaining RCTs were almost universally not blinded, increasing the risk of bias significantly. Although a perfect imitation of a CHM formula to use as placebo is difficult to devise due to its special color, taste and smell, two main types of placebo composition in the WHO-registered trials of CHM have been commonly employed: 63% of placebo control formulas used excipients (coloring and favoring agents, excluding CHM ingredients) and 37% of them included CHM ingredients (76). Reporting of the characteristics of placebo controls is encouraged. Details such as composition, physical similarity, pharmacologically inert tests, quality control and evaluation methods are recommended. Similarly, information about CHM regimen characteristics should also be detailed, including composition (herbal ingredients), the place of origin, processing method, dosage of the herbs etc. However, four of the included studies did not even list the herbal ingredients. Additionally, information on allocation concealment and blinding of outcome assessment were absent. As the majority of the included studies were published before 2017, i.e., the publication date of CONSORT Extension for Chinese Herbal Medicine Formulas 2017 (77), future study design and reporting methods could be improved by following the CONSORT statement.
Noting the limitation of quality and quantity of the results, this meta-analysis demonstrates that a combination of CHM and other therapies administered in CVD studies may attenuate inflammatory biomarker levels, especially hs-CRP, IL-6, TNF-α, MCP-1, sICAM-1 and sVCAM-1. Dan shen, Huang qi and Gan cao were the most frequently used herbs in the included formulas with anti-inflammatory effects, which are worth exploring further in the context of CVD in settings of chronic inflammatory conditions including HIV infection. Rigorously-conducted trials and adequate reporting following published standards are needed to provide more robust findings.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
AJ, AZ, and ML conceptualized the review. ML performed the search. ML and IZ performed manuscript screening, data mining, and data analysis. AZ discussed disagreement. ML took the lead in writing the manuscript and wrote the original draft. JT, AH, AZ, and AJ provided editing and feedback on each version of the manuscript. All authors approved the final version of the manuscript accepted for publication.
This work was funded, in part, by a grant from the Opalgate Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.922497/full#supplementary-material
AIDS, acquired immunodeficiency syndrome; CHM, Chinese herbal medicine; CI, confidence interval; CVD, cardiovascular disease; HIV, human immunodeficiency virus; hs-CRP, high sensitivity c-reactive protein; sICAM-1, soluble intercellular adhesion molecule-1; IL, interleukin; MCP-1, monocyte chemotactic protein-1; MD, mean differences; PWH, people with HIV; RCT, randomized controlled trial; SMD, standardized mean difference; TNF, tumor necrosis factor; sVCAM-1, soluble vascular cell adhesion molecule 1; WM, western medicine.
1. Shah AS, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: Systematic review and meta-analysis. Circulation. (2018) 138:1100–12. doi: 10.1161/CIRCULATIONAHA.117.033369
3. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. (2013) 382:1525–33.
4. De Luca A, de Gaetano Donati K, Colafigli M, Cozzi-Lepri A, De Curtis A, Gori A, et al. The association of high-sensitivity c-reactive protein and other biomarkers with cardiovascular disease in patients treated for HIV: A nested case–control study. BMC Infect Dis. (2013) 13:414. doi: 10.1186/1471-2334-13-414
5. Jaworowski A, Hearps AC, Angelovich TA, Hoy JF. How monocytes contribute to increased risk of atherosclerosis in virologically-suppressed HIV-positive individuals receiving combination antiretroviral therapy. Front Immunol. (2019) 10:1378. doi: 10.3389/fimmu.2019.01378
6. Maisa A, Hearps AC, Angelovich TA, Pereira CF, Zhou J, Shi MD, et al. Monocytes from HIV-infected individuals show impaired cholesterol efflux and increased foam cell formation after transendothelial migration. AIDS. (2015) 29:1445–57. doi: 10.1097/QAD.0000000000000739
7. Piconi S, Parisotto S, Rizzardini G, Passerini S, Terzi R, Argenteri B, et al. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy–treated immunologic nonresponders. Blood J Am Soc Hematol. (2011) 118:3263–72. doi: 10.1182/blood-2011-01-329060
8. Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. (2011) 203:1474–83. doi: 10.1093/infdis/jir060
9. Kettelhut A, Bowman E, Funderburg NT. Immunomodulatory and Anti-Inflammatory Strategies to Reduce Comorbidity Risk in People with HIV. Curr HIV/AIDS Rep. (2020) 17:394–404. doi: 10.1007/s11904-020-00509-y
10. Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. Beijing: China Medical Science Press (2015).
11. Tsai FJ, Li TM, Cheng CF, Wu YC, Lai CH, Ho TJ, et al. Effects of Chinese herbal medicine on hyperlipidemia and the risk of cardiovascular disease in HIV-infected patients in Taiwan. J Ethnopharmacol. (2018) 219:71–80. doi: 10.1016/j.jep.2018.03.006
12. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: John Wiley & Sons (2019).
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71.
15. The Cochrane Collaboration. Review Manager (RevMan) (Version 5.3) [Computer Software]. Copenhagen: The Nordic Cochrane Centre (2014).
16. Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of meta-essentials: A free and simple tool for meta-analysis. Res Synth Methods. (2017) 8:537–53. doi: 10.1002/jrsm.1260
17. Chen P. Fifty cases with coronary atherosclerotic heart disease by the therapy of integrated Chinese and Western medicine. Henan Tradit Chin Med. (2015) 35:2057–9.
18. Chen XC, Xie YL, Wu C, Fan DL. [Effect of Yiqi Huoxue Huatan Tongluo recipe on carotid artery intima-media thickness and hypersensitivity C-reactive protein in patients with hypertension]. Liaoning J Tradit Chin Med. (2008) 35:492–3.
19. Chen ZZ, Cai SH. Observation of clinical curative effect of modified Xiaoxianxiong decoction on phlegm-heat hypertension. J Math Med. (2019) 32:742–5.
20. Dai ZB. Yanggan Yishui recipe in the treatment of grade 1 hypertension and its effect on blood pressure variability. Shaanxi J Tradit Chin Med. (2020) 41:1062–5.
21. Ding Y, Hu N. Effect of Shugan Qingzhi method on serum inflammatory factors, vascular endothelial function and cardiovascular events in patients with coronary heart disease with hyperlipidemia. J Liaoning Univ TCM. (2020) 22:176–9.
22. Fan DL, Chen XC, Yu YY, Jiang K, Gu J. Effect of Soufeng Tongluo prescription on serum levels of TNF-α and hs-CRP in elderly patients with isolated systolic hypertension. J Liaoning Univ TCM. (2012) 14:124–6.
23. Huang C, Qian HL, Li L. The clinical influence of leech with earthworm perscription to the patients of carotid atherosclerosis with blood stasis syndrome. Liaoning J Tradit Chin Med. (2010) 37:665–7.
24. Huang C, Qian HL, Li L, Mo XY. Influence of Huoxue Lishui decoction on endothelial function in Carotid atherosclerosis with blood stasis syndrome. Chin J Integr Med Cardio Cerebrovasc Dis. (2011) 9:795–7.
25. Jia FL. [The effect of traditional Chinese medicine combined with atorstatin on the prognosis and inflammatory response of blood lipids in patients with coronary heart disease with borderline coronary disease]. Guide Chin Med. (2016) 14:216–7.
26. Jin XM, Zhu K, Guo XF. Curative effect of Shenqi Roumai mixture on carotid atherosclerosis and serum IL-6 in the patients. Mod J Integr Tradit Chin West Med. (2016) 25:3886–8.
27. Li FH, Long X. Influence of traditional Chinese medicine combined with atorvastatin on prognosis, blood lipid and inflammation in borderline lesion coronary heart disease. Chin J Exp Tradit Chin Formulae. (2013) 19:305–8.
28. Li H, Zhao WM, Han YX, Liu JG, Yao MJ, Liu L, et al. Effect of a integrative medical regimen on levels of vascular endothelial function and hypersensitive C-reactive protein in elderly patients with isolated systolic hypertension. CJITWM. (2009) 29:115–9.
29. Li QH, Zhao D, Wang BL. [Effects of Hua Ban Fang combined with conventional Western medicine on carotid intimal-meso-inlaminate thickness, total plaque score, serum index and TCM symptom score in patients with carotid atherosclerotic plaque]. TCM Res. (2021) 34:28–32.
30. Li YZ, Huang H, Gu WJ, Liu N, Ji MD, Wu LL, et al. Effects of Zishen Qinggan formula on arterial function and inflammatory factors in patients with essential hypertension. J Nanjing Univ Tradit Chin Med. (2017) 33:344–8.
31. Liu CL, Chen GH, Li L. Study on the effect of compound Danshen Chuanxiong herbal formula granules on carotid atherosclerosis in elderly patients. World Latest Med Inf. (2019) 19:15–6.
32. Liu J. [Observation of prognosis and blood lipid inflammatory response of coronary heart disease treated with traditional Chinese medicine combined with atorvastatin]. Public Med Forum Mag. (2018) 22:1414–5.
33. Ma HN, Su W, Xie JJ, Shen Y. Clinical study on Banxia Baizhu Tianma decoction in the treatment of primary hypertension with syndrome of wind-phlegm invading upward. World J Integr Tradit West Med. (2019) 14:1579–83.
34. Meng LQ, Li DJ, Liang J, Luo WB. Effect of compound Danshen dripping pill combined with simvastatin on pulse wave velocity, vascular endothelial function and other indicators of patients with hypertension and carotid atherosclerosis. CJGMCM. (2016) 31:6–9.
35. Qian WD, Fang ZY, Jiang WM, Lu HT. Effect of Jiangzhi Kangyanghua mixture on high-sensitivity C-reactive protein and vascular endothelial functions of hypertension patients. Zhongguo Zhong Yao Za Zhi. (2013) 38:3583–6.
36. Tian ZX. [Effect of Qingxuan Jiangya decoction combined with Western medicine on blood pressure and blood lipid in patients with simple hypertension of Yin deficiency and Yang hyperactivity]. J Sichuan Tradit Chin Med. (2018) 36:82–5.
37. Wan L, Li JX. Study on the clinical effect of Huoxue Tongluo Decoction combined with Western medicine on coronary atherosclerotic heart blood stasis syndrome. J Practical Tradit Chin Intern Med. (2020) 34:89–92.
38. Wang HT, Liu YJ, Zhang XX, Wang YH. [Clinical observation on Pinggan Qianyang decoction in treating early and middle primary hypertension with hyperactivity of Liver Yang]. Shaanxi J Tradit Chin Med. (2016) 37:982–4.
39. Xie HJ, Wu JM, Kang XZ. Effect of Yiqi Huatan prescription in the treatment of systolic hypertension of qi-deficiency and phlegm type. Chin J Prim Med Pharm. (2018) 25:1314–7.
40. Xie LY, Huang LJ, Wu BX, Luo KR, Luo YM, Chen ZJ. [Shenqimaixintong preparation combined with simvastatin anti-atherosclerosis and its effect on serum IL-10]. Jiangxi J Tradit Chin Med. (2019) 50:33–5.
41. Xiong GY, Zhu T. [Tiao Zhi Jiang Ya Fang combined with amlodipine besylate tablets for the treatment of damp-phlegm type primary hypertension in 40 cases]. TCM Res. (2021) 34:20–4.
42. Xu XY, Xi FY, Zhang CQ, Wu XT, Wang YH, Dai P, et al. [Effect of Qian Yang Yu Yin granule on vascular elastic function in patients with Yin deficiency and Yang upsurge type hypertension and hyperlipidemia]. J Nanjing Univ Tradit Chin Med. (2021) 37:865–70.
43. Yang LL, Li J. Kidney resolve depression soup combined internal medicine foundation treatment of senile hypertension depressive state random parallel control study. J Practical Tradit Chin Intern Med. (2016) 30:57–60.
44. Yang SL, Huang LJ. Efficacy of amlodipine and atorvastatin calcium tablets combined with Xuefu Zhuyu Soft Capsule in the treatment of patients with primary hypertension and carotid atherosclerosis. World J Integr Tradit West Med (2021) 16:108–12.
45. Yao QB, Zhang B, Zeng XX. [Efficacy of Yi Qi Huo Xue Tong Mai Tang in the treatment of hypertension with atherosclerosis and its effect on blood lipids, inflammatory mediators and oxidative stress]. Chin J Intergr Med Cardio Cerebrovasc Dis. (2021) 19:3526–30.
46. Zeng J, Tang XN, Qi MX. Clinical effect of Ganlu Xiaodu micropills in treatment of prehypertension with damp-heat syndrome: An analysis of 40 cases. Hunan J Tradit Chin Med. (2017) 33:1–4.
47. Zhang KQ, Jia HL, Liu HT, Li HL. [Effect of Cizhu Tejiang capsule on atherosclerosis in patients with essential hypertension]. CJGMCM. (2012) 27:1144–5.
48. Zhang YH, Xu SX, Zhu YP, Liu XY, Zhai AS, Zhang JP. Influence of combined Shunanxin dripping pills on inflammatory factors to hypertensive vertigo. Chin Tradit Patent Med. (2014) 36:2055–9.
49. Zheng F, Zhou MX, Xu H, Chen KJ. Effects of herbs with function of activating blood circulation and detoxication on serum inflammatory markers and blood lipids in stable patients with coronary heart disease. CJTCMP. (2009) 24:1153–7.
50. Zhou YH. [Tong Mai Jie Du Fang combined with atorvastatin for the treatment of hypertension]. Jilin J Chin Med. (2021) 41:621–4.
51. Zhu H, Lu S, Su W, Gong S, Zhang Z, Li P. Effect of liandouqingmai recipe on quality of life and inflammatory reactions of patients with coronary heart disease. J Tradit Chin Med. (2014) 34:539–43. doi: 10.1016/s0254-6272(15)30059-5
52. Zhu Y, Huang H, Gu WJ, He JJ, Liu FM. Clinical effect of Luhuang granule in treatment of atherosclerosis: An analysis of 60 cases. J Anhui Univ Chin Med. (2019) 38:20–5.
53. Zuo KK, Zhang MJ, Gu N. Clinical research of Sangji mixture on the immunological and inflammatory reaction in hypertension of overabundant liver-fire with phlegm syndrome. CJTCMP. (2014) 29:1649–52.
54. Yu HY. [Intervention effect and clinical efficacy of Da huang on serum inflammatory factor levels in patients with coronary heart disease]. Strait Pharm J. (2012) 24:157–8.
55. Currier JS, Lundgren JD, Carr A, Klein D, Sabin CA, Sax PE, et al. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation. (2008) 118:e29–35.
57. Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. (2012) 7:e44454. doi: 10.1371/journal.pone.0044454
58. Rodger AJ, Fox Z, Lundgren JD, Kuller LH, Boesecke C, Gey D, et al. Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis. (2009) 200:973–83. doi: 10.1086/605447
59. Baker JV, Duprez D. Biomarkers and HIV-associated cardiovascular disease. Curr Opin HIV AIDS. (2010) 5:511–6.
60. Deng X, Jiang M, Zhao X, Liang J. Efficacy and safety of Traditional Chinese Medicine for the treatment of acquired immunodeficiency syndrome: A systematic review. J Tradit Chin Med. (2014) 34:1–9.
61. Xiong XJ, Wang PQ, Duan L, Liu W, Chu FY, Li SJ, et al. Efficacy and safety of Chinese herbal medicine Xiao Yao San in hypertension: A systematic review and meta-analysis. Phytomedicine. (2019) 61:152849. doi: 10.1016/j.phymed.2019.152849
62. Jiao HC, Ju JQ, Li YL, Ma XS, Jiang HQ, Zhao J, et al. Efficacy of Chinese herbal medicine on health-related quality of life (SF-36) in hypertensive patients: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. (2015) 23:494–504. doi: 10.1016/j.ctim.2015.04.002
63. Wang J, Xiong X, Yang G, Zhang Y, Liu Y, Zhang Y, et al. Chinese herbal medicine Qi Ju Di Huang Wan for the treatment of essential hypertension: A systematic review of randomized controlled trials. eCAM. (2013) 2013:e262685. doi: 10.1155/2013/262685
64. Wu SM, Li Q, Chen YH, Chen W, Ao W. Clinical effect of Aifei NO.1 in treatment of HIV/AIDS complicted with pulmonary infection and phlegm heat. Chin J AIDS STD. (2017) 23:996–8.
65. Wang YL, Zhao QX, Liu XH, Hou MJ, Yuan HZ. Effect of Fuzheng Qing fei decoction on PCT, CRP and CD4+ levels in patients with AIDS complicated with pulmonary infection. CJGMCM. (2020) 35:11–3.
66. Li X, Su QJ, Liang J, Deng X. Research on curative effect and safety of intervening asymptomatic HIV infection with traditional Chinese medicine. CJTCMP. (2015) 30:3520–3.
67. Stumpf C, Fan Q, Hintermann C, Raaz D, Kurfürst I, Losert S, et al. Anti-inflammatory effects of danshen on human vascular endothelial cells in culture. Am J Chin Med. (2013) 41:1065–77.
68. Qi Y, Gao F, Hou L, Wan C. Anti-inflammatory and immunostimulatory activities of astragalosides. Am J Chin Med. (2017) 45:1157–67. doi: 10.1142/S0192415X1750063X
69. Schröfelbauer B, Raffetseder J, Hauner M, Wolkerstorfer A, Ernst W, Szolar Oliver HJ. Glycyrrhizin, the main active compound in liquorice, attenuates pro-inflammatory responses by interfering with membrane-dependent receptor signalling. Biochem J. (2009) 421:473–82. doi: 10.1042/BJ20082416
70. Gumpricht E, Dahl R, Devereaux MW, Sokol RJ. Licorice compounds glycyrrhizin and 18β-glycyrrhetinic acid are potent modulators of bile acid-induced cytotoxicity in rat hepatocytes. J Biol Chem. (2005) 280:10556–63. doi: 10.1074/jbc.M411673200
71. Asl MN, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res. (2008) 22:709–24. doi: 10.1002/ptr.2362
72. Abd-Elazem IS, Chen HS, Bates RB, Huang RCC. Isolation of two highly potent and non-toxic inhibitors of human immunodeficiency virus type 1 (HIV-1) integrase from Salvia miltiorrhiza. Antivir Res. (2002) 55:91–106. doi: 10.1016/s0166-3542(02)00011-6
73. Mori M, Ciaco S, Mély Y, Karioti A. Inhibitory effect of lithospermic acid on the HIV-1 nucleocapsid protein. Molecules. (2020) 25:5434. doi: 10.3390/molecules25225434
74. Ito M, Sato A, Hirabayashi K, Tanabe F, Shigeta S, Baba M, et al. Mechanism of inhibitory effect of glycyrrhizin on replication of human immunodeficiency virus (HIV). Antivir Res. (1988) 10:289–98.
75. De Simon F, Aquino R, De Tommasi N, Mahmood N, Piacente S, Pizza C. Anti-HIV Aromatic compounds from higher plants. In: C Tringali editor. Bioactive Compounds from Natural Sources. (London: Taylor&Francis) (2001).
76. Zhang X, Tian R, Zhao C, Tang X, Lu A, Bian Z. Placebo design in WHO-registered trials of Chinese herbal medicine need improvements. BMC Compl Alternative Med. (2019) 19:299. doi: 10.1186/s12906-019-2722-2
Keywords: inflammatory biomarkers, Chinese herbal medicine, cardiovascular disease risk, people with HIV, systematic review and meta-analysis
Citation: Li M, Zhou IW, Trevillyan J, Hearps AC, Zhang AL and Jaworowski A (2022) Effects and safety of Chinese herbal medicine on inflammatory biomarkers in cardiovascular diseases: A systematic review and meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 9:922497. doi: 10.3389/fcvm.2022.922497
Received: 18 April 2022; Accepted: 25 July 2022;
Published: 16 August 2022.
Edited by:
Harry H. X. Wang, Sun Yat-sen University, ChinaReviewed by:
Nicholas Funderburg, The Ohio State University, United StatesCopyright © 2022 Li, Zhou, Trevillyan, Hearps, Zhang and Jaworowski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anthony Jaworowski, YW50aG9ueS5qYXdvcm93c2tpQHJtaXQuZWR1LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.