
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 12 August 2022
Sec. Heart Valve Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.922353
This article is part of the Research Topic Heart Valve Biomechanics and Mechanobiology View all 9 articles
Bicuspid aortic valve (BAV), which affects up to 2% of the general population, results from the abnormal fusion of the cusps of the aortic valve. Patients with BAV are at a higher risk for developing aortic dilatation, a condition known as bicuspid aortopathy, which is associated with potentially life-threatening sequelae such as aortic dissection and aortic rupture. Although BAV biomechanics have been shown to contribute to aortopathy, their precise impact is yet to be delineated. Herein, we present the latest literature related to BAV biomechanics. We present the most recent definitions and classifications for BAV. We also summarize the current evidence pertaining to the mechanisms that drive bicuspid aortopathy. We highlight how aberrant flow patterns can contribute to the development of aortic dilatation. Finally, we discuss the role cardiac magnetic resonance imaging can have in assessing and managing patient with BAV and bicuspid aortopathy.
Bicuspid aortic valve (BAV) is the most common type of congenital heart disease (CHD), affecting 0.5–2% of the general population (1–3). BAV results from the fusion of the cusps of the aortic valve, which is normally a tri-leaflet valve that facilitates the flow of oxygenated blood from the left ventricle into the aorta. Different fusion patterns of the three leaflets have been identified to lead to a bicuspid morphology. Various classifications have also been proposed to describe BAV, including Sievers, Schaefer, and Michelena (4–6). The pathophysiology of BAV is not well understood, but genetics are thought to have a role (7–9). Nevertheless, different fusion patterns of BAV have been shown to result in aberrant blood flow dynamics through the aortic valve (10). This dysregulation in the blood flow can contribute to dilatation of the aorta, a condition known as bicuspid aortopathy (10). Bicuspid aortopathy can predispose patients to aortic dissection, which is associated with high rates of morbidity and mortality (11). Given these important clinical implications, various groups have aimed to better understand the pathophysiology of BAV and bicuspid aortopathy. Cardiovascular societies have also suggested standardizing the definition and classification for BAV. Moreover, biomedical engineers are striving to elucidate the biomechanics of BAV and bicuspid aortopathy. Their findings, coupled with innovative multi-modality imaging techniques, have tremendously helped improve our knowledge of the natural history of BAV and bicuspid aortopathy. Herein, we review the latest literature on BAV and bicuspid aortopathy as it pertains to clinical concepts, biomechanics, and non-invasive imaging. We also summarize the literature pertaining to BAV and bicuspid aortopathy biomechanics. Finally, we explore whether multimodality imaging techniques can be used to better inform clinical decision-making algorithms for patients with a BAV.
Obtaining an accurate estimate of the incidence of BAV in the adult population is not trivial. Most data stems from autopsy series. In the largest autopsy series of 21,417 consecutive autopsies, Larson and colleagues identified 293 (1.37%) patients with BAV (12). Pauperico et al. studied 2,000 cadaveric aortic valves and found only 13 (0.65%) of them to be bicuspid (13). Roberts (14) and Datta (15) also used autopsy series to determine the prevalence of BAV. Three studies have also aimed to determine the prevalence of BAV in alive patients (16–18). Basso et al. screened 817 primary school children using transthoracic echocardiography (TTE) and found 0.5% of the population to have BAV, where there was a higher incidence in males when compared to females (0.75 vs. 0.24%) (16). Tutar and colleagues studied 1,075 newborns to determine the prevalence of BAV in neonates (17). They also used TTE and found BAV to be present in 0.46% of live births, where 0.71% of male neonates had BAV compared to 0.19% of females (17). Sillesen et al. performed a cross-sectional, population-based study on all newborns born in Copenhagen (18). In total, 25,556 underwent TTE and BAV was found in 196 newborns, where they noted a 2:1 ratio for males to females (18). Other studies have also demonstrated BAV to be more common in males, suggesting a potential genetic predisposition for this CHD (9, 19). With respect to non-syndromic BAV, it has been found to be associated with multifactorial inheritance, low penetrance, and variable phenotypes (20). It has been postulated that reduced number of X chromosome genes that evade inactivation could explain the higher frequency of BAV in men (20). Various studies have also shown BAV to be common in XO Turner syndrome where more than 30% of patients with Turner syndrome have BAV (1, 21). The relative prevalence of BAV in men and women is important since different studies have found that clinical outcomes can be heavily influenced by sex (19, 22–24).
There is strong evidence supporting an underlying genetic predisposition for acquiring BAV, where it has been found to cluster in families (25–27). Variance component methodology and modeling have established the heritability of BAV to be up to 89%, suggesting an almost exclusive genetic cause (8, 28–30). There is also mounting clinical data from familial studies that have shown BAV to have an autosomal dominant pattern of inheritance with reduced penetrance and variable expressivity (31). Moreover, BAV is associated with aortic coarctation, patent ductus arteriosus, and anomalies in proximal coronary artery anatomy (9, 32). Nevertheless, the specific genes and genetic abnormalities that result in BAV are yet to be defined. Indeed, a host of genes, including NOTCH 1, FBN1, and TGFβ1/2, with divergent inheritance pattern can also contribute to the development of BAV (31, 33–39). NOTCH1 is a transmembrane receptor that plays a role in valvulogenesis and extracellular matrix (ECM) modeling, and its loss of function leads to the development of BAV (40). However, mutations in NOTCH1 account for <5% of BAV cases, with most occurring sporadically with no known heritable thoracic aortic aneurysm gene identified (6).
Moreover, as noted above, although BAV is usually present in isolation, it is linked with other genetic syndromes, such as Andersen syndrome, Turner syndrome, William Beuren, Bosley-Salih-Alorainy, Tetralogy of Fallot, hypoplastic left heart syndrome, and Athabascan Brainstem Dysgenesis syndrome (33, 41). BAV may also be present in patients with connective tissue diseases including familial Type A aortic dissection, Marfan syndrome, Loeys-Dietz syndrome, and vascular Ehlers-Danlos syndrome (41). Indeed, there is evidence showing that, in patients with Marfan syndrome, the prevalence of BAV was more than four times higher than what has been shown in the general population (16, 42, 43). Table 1, which is adapted from Giusti and colleagues (44) summarizes the genes that have been implicated in the BAV patient population. Germane to this manuscript, there is also literature supporting the notion that BAV can be non-syndromic, where functional and hemodynamic factors can modulate valve phenotype and morphology during development leading to either a tricuspid aortic valve or a BAV and its various types, respectively (45–48).

Table 1. Summary of the genes that have been implicated in the BAV patient population [adapted from Giusti et al. (44)].
One of the challenges with BAV literature and research has been the heterogeneity of definitions and classifications that have been used. Further compounding this complexity, assigned nomenclature has been based on pathology specimens and images obtained from echocardiography, CT scans, and cardiac magnetic resonance (CMR) imaging. Since the 1970's, 11 published definitions have been proposed to describe BAV fusion patterns (49). Using pathology specimens, Roberts was the first to employ “anterior-posterior” and “right-left” terminology (14). Bradenburg used echocardiography images and a clock-face nomenclature (50), while Angelini used autopsy specimens and based the classification on the presence of a raphe and employed anterior-posterior/right-left cusps terminology (51). Sabet et al. also used the presence or absence of raphe on 534 cadaveric specimens to define BAV (52). Probably the most commonly used nomenclature and classification for BAV is the one suggested by Sievers and Schmidtke (4) (Figure 1). Using autopsy specimens from 304 cadavers, they established the “type” of BAV based on the presence of raphe: Type 0: no raphe; type 1: 1 raphe; and type 2: 2 raphes. Other definitions and classifications have been proposed by Schaefer (5), Kang (53), Michelena (6), Jilaihawi (54), Sun (55), and Murphy (56). Table 2 summarizes these definitions and classifications. The diverse heterogeneity in BAV nomenclature has been confusing. Some of the proposed categories for BAV have also had limited clinical application. To address these challenges, an international consensus statement on BAV nomenclature and classification was prepared and published in 2021 (49). This statement, which has been endorsed by major cardiovascular societies, recognizes three types of bicuspid valves. First, the fused type that has either a right-left cusp fusion, or right-non-coronary cusp fusion, or left-non-coronary cusp fusion phenotype. Second, the 2-sinus type, which has latero-lateral and antero-posterior phenotypes. Third, is the partial-fusion type. Based on this categorization, BAV right to left cusp fusion (R-L) is the most prevalent (70-80%), followed by right to non-coronary cusp fusion (R-N) (20–30%), and least commonly, left to non-coronary cusp fusion (L-N) (3–6%). The consensus statement also emphasizes that the presence of raphe and the symmetry of the fused type phenotypes are critical aspects to describe. Using a standardized nomenclature should help in simplifying BAV literature. Clinical application and correlation of the various types of BAV can also be better conveyed.
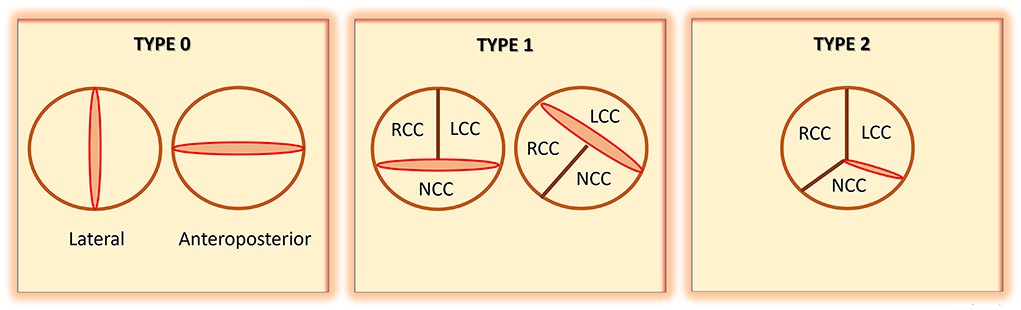
Figure 1. Schematic representation of bicuspid aortic valve (BAV), as defined by Sievers and Schmidtke [modified from Sievers et al. (4)]. RCC, right coronary cusp; LCC, left coronary cusp; NCC, non-coronary cusp.

Table 2. A summary of the definitions and classifications that have been used in the BAV literature [adapted from Michelena et al. (49)].
The clinical implications of BAV and bicuspid aortopathy are noteworthy. BAV is associated with aortic valve insufficiency (AI), aortic valve stenosis (AS), infective endocarditis, aortic dilatation, and aortic dissection (48). While AI is present in approximately 30% of BAV patients, AS is much more common. Furthermore, over half of patients with BAV develop aortic dilatation and this may progress to aneurysm, dissection, and rupture, which are frequently lethal complications (49). Although BAV can cause morbidity and mortality through valvular disease and bicuspid aortopathy, the overall survival for patients with a BAV is similar to that of the non-BAV population (57). Thus, if surgical intervention is provided at the appropriate time, BAV patients should benefit from identical survival rates to non-BAV patients. Salient to clinical management, bicuspid aortopathy can have varied presentations, where dilatation may occur in the aortic root, the ascending aorta, the proximal aortic arch, or any combination of these sites (58). The tubular ascending aorta is the most common segment affected in bicuspid aortopathy, where 60–70% of aneurysms occur at this site (59). When aortopathy occurs at the level of the aortic root, the sinuses of Valsalva are predominantly affected (59). There is evidence suggesting that dilatation of this segment is associated with more rapidly progressive aortopathy (60–62). In contrast, non-BAV aortic aneurysms, such as degenerative aneurysms, tend to begin in the mid ascending aorta and progress distally and proximally, while aneurysms secondary to connective tissue diseases are limited to the aortic root (59).
The clinical manifestation of BAV-associated complications is heterogenous, spanning from some patients developing deadly aortic dilatation and subsequent dissection, and others suffering no symptoms at all (49). Various studies have quantified the risk over time of developing an aneurysm in the ascending aorta in BAV patients. These studies have found that 20–30% of BAV patients have aortic dilatation during a follow-up of 9–25 years (6, 57, 63). Critically, studies have found that the risk of acquiring aortic dilatation was 80 times higher in BAV patients compared to the general population (6). Furthermore, recent studies have found that the rate of aortic dissection in patients with BAV 15 years after aortic valve replacement (AVR) was 0.55%, which is not significantly higher than that for patients with tricuspid aortic valve (0.41%) (63–65). Studies have also aimed at better understanding the difference between aortic aneurysms in patients with and without a BAV. Patients with BAV presented at a smaller aortic diameter; their aortic aneurysms grew more rapidly; and a higher number of BAV patients required surgical treatment at a significantly younger age compared to non-BAV patients with an aortic aneurysm (66). The same study found that BAV patients who had aortic valve stenosis and aortic dilatation were at an increased risk for aortic dissection, rupture, or death before surgery compared to patients with normally functioning BAV (66). It is also important to emphasize that the absence of valvular dysfunction does not lessen the risk of aortic dissection in BAV patients (67). Conversely, BAV patients who also have aortic stenosis or aortic regurgitation are at an increased risk of aortic dissection and rupture (68). Interestingly, BAV patients who also have aortic regurgitation are more prone to aortic dissection (69).
It is important to summarize the contemporary clinical outcomes for patients with BAV, which have been extensively covered by Michelena and colleagues from the International BAV Consortium (6). Excellent overall survival rates have been found for patients with BAV in community, population-based studies, while outcomes are poorer in referral center patients who have required aortic valve replacement. Heart failure is uncommon in patients with BAV, and aortic stenosis is a more common indication for surgery compared to aortic insufficiency. Development of aortic aneurysm (aortic diameter >45 mm) occurs in 25–45% of patients over prolonged periods of follow-up, but aortic dissection is a rare event (~1%) outside of tertiary referral center populations, where it is more common (~10%) (6).
Collectively, the diversity in BAV and bicuspid aortopathy complicates management as interventions must coincide with risk of developing aortic complications, which is challenging to determine in a clinically heterogenous population. Ideally, non-invasive biomarkers that have high prognostic sensitivity and specificity should be established to accurately predict the natural course of BAV-associated complications (70). Such biomarkers, which do not require invasive approaches to obtain them, can then be used to intervene on the appropriate patient at the most optimal time to prevent serious complications (71–73).
BAV is associated with an increase in aortic wall shear stress (WSS) (74–76), the tangential shear force that blood flow exerts on a vessel wall, and thus potentially expression of aortopathy. Four-dimensional flow magnetic resonance imaging (4-D flow MRI) can be used to assess for WSS, the aortic valve, and the thoracic aorta (77). Utilizing novel imaging techniques such as 4D flow MRI presents an opportunity to characterize and risk stratify patients with BAV who are at risk of severe aortic complications (78). The mechanism underlying bicuspid aortopathy is a widely debated subject, oscillating between genetic predisposition to hemodynamic causes. Like BAV, NOTCH 1 is the gene that has been implicated in bicuspid aortopathy (48). A study of first-degree relatives of BAV patients with tricuspid valves (TAV) were found to have altered aortic shape and hemodynamics despite the absence of valvular disease or aortic dilatation. These findings suggest the presence of an unidentified genetic component of BAV aortopathy that is independent of bicuspid valve pathology (79). Bicuspid aortopathy frequently affects the ascending aorta and rarely the descending aorta, and these regions have distinct embryological origins (30). Smooth muscle cells derived from the ascending aorta of BAV patients also demonstrate impaired contractility, further suggesting that bicuspid aortopathy may develop from a genetic defect that results in abnormal differentiation (80).
In contrast, the hemodynamic theory of bicuspid aortopathy postulates that BAV cusp fusion leads to subclinical stenosis resulting in abnormal hemodynamics and increased WSS, ultimately eliciting aortic remodeling (81). In a tricuspid aortic valve (TAV) the systolic velocity jet is unidirectional and does not abnormally impinge on the aortic wall. The abnormal hemodynamics observed in BAV are determined by the phenotype of valve cusp fusion (Figure 2A). The fusion pattern of the cusps and their opening angle, which is a quantifier of the restricted cusp motion, influences aortic root shape eccentricity, with a larger sinus located at the non-fused cusp (82), and aortic aneurysm growth rate, respectively (47). Alterations in ventricular outflow hemodynamics leads to varying levels of WSS at certain regions of the aorta as detected by 4D flow MRI (Figure 2B). For example, A R-L BAV velocity jet is directed anteriorly with greater axial WSS at the aortic root, while a R-N BAV jet is directed toward the posterior aorta with greater circumferential WSS at the mid-distal aorta (30, 83) (Figure 2C).
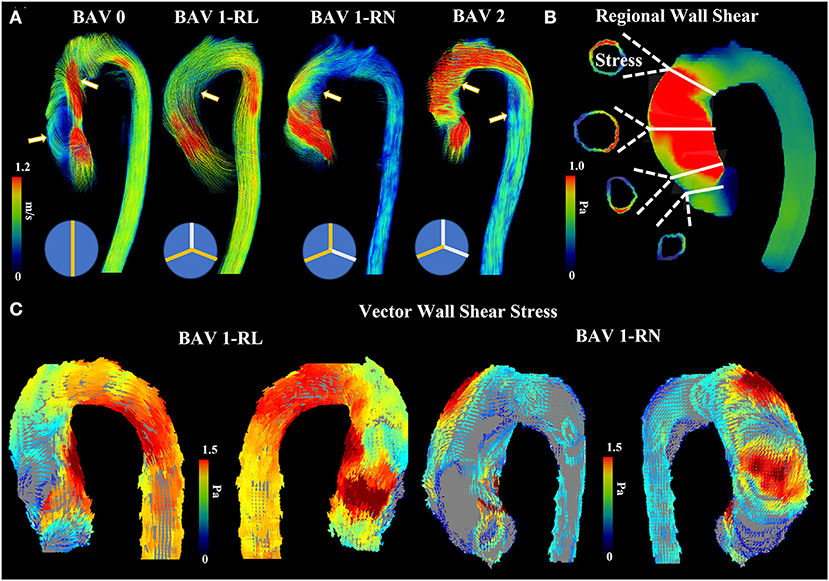
Figure 2. Abnormal bicuspid aortic valve (BAV) hemodynamics. (A) shows four patients with different BAV phenotype. Arrows point to regions where helical and abnormal flow patterns can be observed. (B) shows an example of regional wall shear stress in the ascending aorta. Four landmark locations are illustrated: left ventricular outflow tract, sinus of Valsalva, mid-ascending aorta, and distal ascending aorta. (C) shows anterior and posterior view from vectorial wall shear stress in RL and RN patients.
These conflicting theories have made management strategies difficult, where practices vary among cardiac surgeons with respect to the timing of intervention and the extent of a reparative operation (84). In BAV patients with a higher genetic predisposition to aortopathy, such as those with NOTCH1 mutations, prophylactic surgical resection of the aorta may be warranted to prevent serious complications. However, in cases of BAV where aortopathy is presumed to be secondary to altered biomechanics and blood flow dynamics to a BAV, conservative management and/or aortic valve replacement (AVR) may be the preferred approach (85). Strategies to identify which patients would benefit from surgery is critical since an operation is not without risks. Ascending aorta replacement grafts are stiff with altered geometry, which can lead to downstream descending aorta distention and aneurysm (86). Presently, prophylactic surgery for aortic dilatation is guided by aortic diameter and presence of risk factors that would predispose one to aortic complications (85). With a spectrum of clinical presentations it is difficult to ascertain whether this is justified, as up to 59% of BAV aortas dissect below the surgical threshold diameter of 55 mm, while large aortic aneurysms can be stable for years (30). The underlying etiology of BAV aortopathy is likely a result of complex interplay that is beyond only hemodynamic alterations or simply a genetically predisposed aorta, thus aortic diameter alone is not a sufficient determinant of which BAV patients need surgery (71).
Numerous factors affect rate of aortic dilatation in BAV. Different studies have shown that aortic wall shear stress (WSS) is a major factor associated with ascending aorta growth (49, 81, 87). Normally, physiologic WSS decreases with age (88). In BAV, the skewed orifice jet and displaced flow results in specific patterns of elevated systolic WSS. BAV patients have abnormal right-handed helical flow that impinges on the greater curvature of the ascending aorta resulting in increased WSS (89). The jet skewness, velocity, and shear stress overloads have demonstrated to coincide with the aortic wall region prone to dilatation in a way that is specific to the cusp fusion phenotype. As previously discussed, R-L BAV exhibits increased WSS at the root and proximal ascending aorta greater curvature, and this promotes root and mid-ascending aorta dilatation that presents as a type 2 aortopathy (90, 91). In comparison, a R-N BAV velocity jet is directed toward the posterior distal aortic wall promoting arch dilatation as a type 3 or sometimes type 1 aortopathy (92, 93). This suggests a mechanistic link between BAV fusion morphology and aortopathy expression.
Elasticity and recoil are crucial functions of the aorta during systole and diastole. These functions are facilitated by the extracellular matrix (ECM) components and architecture of the medial layer of the aorta. The ascending aorta of BAV patients have been found to have altered biomechanical properties and ECM protein dysregulation due to elevated WSS forces, which contributes to the progression of aortopathy (76, 94–96). Two major structural ECM proteins are elastin and collagen, and their content is altered in BAV (97). One mouse model demonstrated that deficiencies in elastogenesis cause ECM disorganization, inflammation, and aortic valve disease (97, 98). Moreover, BAV aortas demonstrate perturbed elastin metabolism, which manifests with increased medial elastin degradation and decreased elasticity, resulting in increased stiffness (76, 99). The aneurysmal aorta of BAV patients shows increased collagen-related strength, stiffness, and alignment with overall decreased wall thickness, which contributes to its susceptibility to aneurysmal dilatation (100). The decrease in wall thickness may be directly related to collagen deposition due to elastin fragmentation. From a mechanical point of view, due to higher stiffness and strength of collagen compared to elastin, a lower degree of wall thickness is sufficient for achieving the same structural behavior. Indeed, the elastin in these areas is less abundant, less aligned, and contains greater distances between layers. This is associated with lower delamination strength and an increased risk of dissection (85). Furthermore, a recent study by Deveja et al., found that intact wall and layer-specific failure stretch and stress was significantly higher in ascending thoracic aorta aneurysm (ATAA) of BAV patients when compared to non-aneurysmal aortas and ATAA of tricuspid aortic valve patients (101). Interestingly, their findings suggest that BAV-ATAA is not associated with an increased susceptibility to dissection initiation (101). Moreover, biaxial load testing has shown that aneurysmal growth may be driven by greater elastic energy (defined as the area under the stress-strain curve) in the proximal ascending aorta of BAV patients compared to those with a tricuspid valve (102). However, a loss of actin in the inner media and increased intimal thickness due to flow-induced stress has also been found in both groups of patients, suggesting that hemodynamics may not play as large of a role in the development of aortopathy (103). Adding further complexity, in addition to WSS on the aortic wall layers, oscillatory shear stress (OSS) has also been found to be elevated in BAV (40). OSS is related to WSS as it demarcates areas where the direction of the shear stress changes multiple times in a small area. This adversely impacts the endothelium since its role is to return to homeostasis in response to non-physiologic flow. Indeed, the endothelium is healthier when subjected to laminar flow with lower OSS and mid-range WSS (104).
Moreover, WSS can also contribute to aortopathy through its impact on aortic wall stiffness. There is evidence showing that increased circumferential stiffness and elastic fiber thinning in areas of the BAV aortic wall is affected by high WSS (75). Aortic wall stiffness is also known to be associated with ATAAs and cardiovascular events and may thus be a marker of aortopathy progression (94). However, it is not yet clear whether aortic stiffness is due to a primary genetic defect or the result of a secondary adaptation to BAV flow disturbances (105). Nevertheless, both BAV and tricuspid valve ATAAs demonstrate increased aortic stiffness that does not differ between BAV and tricuspid aortic valve patients. Our group has observed that aortic stiffness may be more linear in the BAV population, possibly resulting in stiffer behavior at physiologic levels of strain and potentially less stiff than tricuspid aortic valve aortas at supra-physiologic degrees of strain (106). Nevertheless, this overlap suggests that ECM irregularities may not be specific to BAV but rather to aortic aneurysmal tissue in general (107).
Aortic mechanoreceptors detect abnormal WSS to produce different biological responses in endothelial cells and smooth muscle cells. Additionally, turbulent blood flow activates remodeling and inflammatory pathways (108). Areas of elevated WSS are also associated with increased smooth muscle cell death (109). Furthermore, premature calcification of the aortic valve is thought to be related to altered hemodynamics and over half of BAV patients older than 35 years of age will develop early onset calcific aortic valve disease that quickly progresses to aortic stenosis within 10–12 years (92). The process of calcification is thought to be related to an upregulation in pro-calcification factors caused by micromechanical forces experienced by a BAV aorta (110). Also of note, the presence of aortic stenosis or aortic insufficiency exacerbates the magnitude of WSS in BAV patients and increases the risk of dilatation (111, 112). In aortic stenosis, the enhanced WSS eventually overrides previous flow patterns associated with BAV, making BAV with severe aortic stenosis WSS patterns indistinguishable from tricuspid valves with severe aortic stenosis (113, 114).
Moreover, BAV is associated with a greater total pressure gradient along the aorta distal to the aortic valve and higher viscous energy loss compared to tricuspid valves. An increase in loss of both pressure and energy are known to be related to abnormal helical flow and aortic dilatation in BAV patients (Figure 3) (74, 78). BAV also causes increased reverse flow and reduced stasis of blood (70). Finally, regions of high WSS also contain decreased endothelial nitric oxide synthase (eNOS) expression in non-dilated BAV, suggesting that alterations in eNOS expression occur independent of aberrant hemodynamics in BAV and may have a genetic etiology (115). Interestingly, proteomic analysis of BAV ATAA tissue and NOTCH1 knockdown mice studies demonstrated that BAV aneurysmal tissue has impaired mitochondrial dynamics with attenuated fusion (116). This abnormality could be partially rescued with mitochondrial fusion activation, presenting a potential therapeutic target for BAV ATAAs (116).
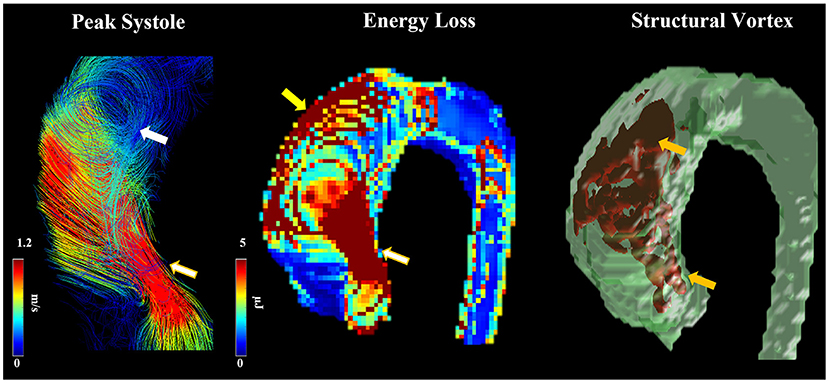
Figure 3. Abnormal helical flow and dilation. Flow patterns at peak systole were observed in a patient with RN fusion. Arrows point to regions with high helicity (white arrow) and vorticity (orange arrows), abnormal jet (white-golden arrows), elevated energy loss due to flow impingement (yellow arrow).
Four-dimensional cardiac magnetic resonance imaging (4D flow MRI) has become a central feature for assessing and following patients with BAV and bicuspid aortopathy. Images obtained from 4D flow MRI are both quantitative and qualitative and can be used in patients with known BAV and those who have had a surgical intervention to replace their BAV (117, 118). 4D flow MRI can reveal the relation between aortic flow patterns and regional WSS (119). As magnitude of WSS correlates with pathologic remodeling and aortopathy, 4D flow MRI WSS measurements provide an important risk-stratifying clinical tool that can identify aortic regions at risk of dilatation and devastating complications (120). The first step to developing this prognostic tool is creating a reference atlas of normal physiologic WSS patterns (Figure 4) (73). Generating individualized heat maps that identify abnormal WSS patterns can guide resection strategies and improve outcomes in patients with BAV (72). Moreover, WSS may also be used to follow post-operative changes after surgical restoration of normal anatomy in BAV aortopathy. Indeed, WSS patterns have been shown to improve post-aortic valve replacement in BAV patients with aortic insufficiency (117). However, methods for quantifying WSS must be generalizable to all patients with BAV (81). Gordon and colleagues have developed a standardized and reproducible 4D flow MRI workflow with prospective application to WSS and other BAV-related hemodynamic measurements (121). Although WSS can be a promising marker of aortopathy, one study found that, at 3 years follow-up of BAV patients, aortic wall areas with high WSS had no significant anatomical remodeling (122). This suggests that although WSS alterations may precede aortopathy and contribute to its progression, this is a very slow process that probably occurs over years. Moreover, 4D flow MRI WSS has been shown to be likely underestimated due to limited temporal and spatial resolution and thus may need complementary techniques for evaluating bicuspid aortopathy (123). Such data is critical when managing patients with BAV and bicuspid aortopathy as the timing of surgical resection should be contextualized against the relative growth rate of the aneurysm.
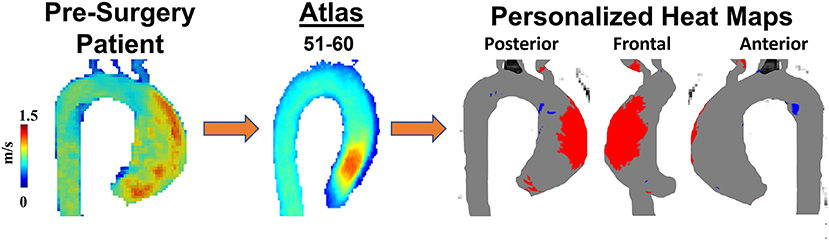
Figure 4. Personalized heat maps. A 60-year-old man with BAV Type 1 RL phenotype was scanned to obtain 4D flow velocities prior to surgical planning. Patient's velocity field was compared with an age and sex-match atlas allowing to identify abnormal regions (high wall shear stress in red, low wall shear stress in blue) of wall shear stress using heat maps.
Other alterations in aorta hemodynamics observed in BAV may be applied in conjunction with WSS to stratify risk in surgical BAV patients. Dilated R-N BAV aortas demonstrate increased flow displacement and overall flow angle at the sino-tubular junction that is associated with a wider distal ascending aorta diameter (124). Flow displacement measures the displacement of systolic flow from the centerline of the aorta and is normalized by aortic diameter (125). It can be easily derived from 2-dimensional phase contrast data to quantify aortic flow eccentricity. It is also a metric that is easier to measure than WSS (81). Dilated BAV aortas experience elevated flow skewness and eccentricity, retrograde flow, and right-handed helical flow, which may contribute to progression of aortic enlargement (126). Moreover, although aortic root flow eccentricities may not be necessarily altered in aneurysmal bicuspid aortas compared to tricuspid aortas, flow eccentricities have been shown to correlate with WSS and blood helicity, which are known to contribute to aortopathy (95). Finally, a BAV MRI pulsatile flow circulation model has been used to quantify outflow jet eccentricity (10). The model has demonstrated that asymmetric BAV outflow jets are directed at the aortic wall facing the smaller leaflet, and these regions may be at increased risk of aortic events (10). Cardiac magnetic resonance imaging can also be used to determine aortic pulse wave velocity (PWV). PWV can be used to measure aortic wall stiffness, where higher PWV has been found in BAV patients and been associated with aortic dilatation (105, 127). In contrast, Singh et al. found that PWV was not elevated in patients with bicuspid aortopathy (128). Despite these conflicting results, aortic stiffness is known to be predictive of aortic dilatation in the Marfan patient population (129). Future studies may better delineate whether aortic stiffness can be a valid predictor of aortopathy in patients with BAV. Overall, these patterns in altered hemodynamics may further compound WSS and correspond to an increased risk of aortopathy.
Additional imaging strategies can be used to complement 4D flow MRI to provide a more comprehensive assessment of BAV and improve risk stratification of patients. For example, echocardiography speckle-tracking imaging identifies early abnormalities in aorta elasticity (130). Left ventricular myocardial strain analysis correlates to myocardial remodeling and may help inform decision making in BAV patients (112), while computational fluid dynamics provide more precise WSS measurements (94). In turn, WSS of the BAV leaflets may be measured by fluid structure interaction (FSI) (131). The advantage of FSI is that it provides stresses in the aortic wall (in addition to WSS), where such a calculation for WSS may be more accurate because the wall is modeled as elastic and not simply as a rigid boundary. FSI also provides measurements of vessel structural stress, which correlates with aortic media degeneration (132). Machine learning has also been employed to classify BAV aortas at risk of dilatation using 4D flow MRI parameters (133). Machine learning uses computer systems that can “learn” from datasets by using statistical models and algorithms to identify patterns in the data. Such a strategy may facilitate more accurate and efficient predictive models and streamline prognostication for BAV patients. It is important to emphasize that a complete imaging-based assessment of the heart and aorta should be performed for patients with BAV. Valve function may be assessed in detail using echocardiography and cardiac MR (56), whereas 4D flow MRI and CT scans can be done to generate accurate and informative images of the aorta in BAV patients (134).
These studies collectively confirm the safety, feasibility, and potential clinical applicability of 4D flow MRI in assessing and following BAV patients. They also show some of the limitations associated with this imaging modality, which are amplified by the heterogenous nature of the BAV patient population. Future studies should include larger number of healthy controls and BAV patients to map normal physiological hemodynamics and identify BAV patients at risk of deadly aortic complications. Indeed, a personalized medicine approach to BAV aortopathy clinical decision-making involves detecting flow derangements with 4D flow MRI and other imaging modalities; assessing biomechanical wall properties; measuring the levels of circulating biomarkers of wall remodeling and endothelial dysfunction; and identifying genetic alterations. Such parameters can be used to help determine which, if any, regions of the aorta need to be resected; guide in the timing and extent of any potential surgical interventions; and provide prognostic insight into the clinical course of aortopathy.
Bicuspid aortic valve is a congenital heart disease that affects up to 2% of the general population. In addition to placing those patients at a higher risk for valvular dysfunction, BAV predisposes patients to developing bicuspid aortopathy. While some factors have been identified, our knowledge of the potential genetic causes for BAV and bicuspid aortopathy remains incomplete. Moreover, our understanding of the altered hemodynamics and biomechanics that preside in this patient population continues to evolve. Such an understanding has been augmented by novel, non-invasive multimodality imaging techniques. Collectively, these advances have been translated to clinical practice, where care providers and surgeons are now able to base their management plans on more accurate evidence. This is an exciting area that seamlessly blends principles in biomedical engineering with advanced imaging techniques and precise treatment strategies that can be personalized. Mathematical modeling, machine learning, and other non-invasive biomarkers can herald the next generation of impactful innovations for patients with BAV and bicuspid aortopathy.
AF and JG: contributed to conception and design of this paper, acquisition and interpretation of information, drafting and critically revising manuscript for intellectual content, and has approved the final version of manuscript. MK, ED, and PF: contributed to drafting and critically revising manuscript for intellectual content and has approved the final version of manuscript. All authors contributed to the article and approved the submitted version.
JG received funding from the University of Calgary URGC SEM #1054341 and start-up funds. The Natural Sciences and Engineering Research Council of Canada/Conseil de recherche en sciences naturelles et en génie du Canada RGPIN-2020-04549 and DGECR-2020-00204.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Masri A, Svensson LG, Griffin BP, Desai MY. Contemporary natural history of bicuspid aortic valve disease: a systematic review. Heart (British Cardiac Society). (2017) 103:1323–30. doi: 10.1136/heartjnl-2016-309916
2. Coffey S, Cairns BJ, Iung B. The modern epidemiology of heart valve disease. Heart (British Cardiac Society). (2016) 102:75–85. doi: 10.1136/heartjnl-2014-307020
3. Abdulkareem N, Smelt J, Jahangiri M. Bicuspid aortic valve aortopathy: genetics, pathophysiology and medical therapy. Interact Cardiovasc Thorac Surg. (2013) 17:554–9. doi: 10.1093/icvts/ivt196
4. Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. (2007) 133:1226–33. doi: 10.1016/j.jtcvs.2007.01.039
5. Schaefer BM, Lewin MB, Stout KK, Gill E, Prueitt A, Byers PH, et al. The bicuspid aortic valve: an integrated phenotypic classification of leaflet morphology and aortic root shape. Heart (British Cardiac Society). (2008) 94:1634–8. doi: 10.1136/hrt.2007.132092
6. Michelena HI, Prakash SK, Della Corte A, Bissell MM, Anavekar N, Mathieu P, et al. Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation. (2014) 129:2691–704. doi: 10.1161/CIRCULATIONAHA.113.007851
7. Loscalzo ML, Goh DL, Loeys B, Kent KC, Spevak PJ, Dietz HC. Familial thoracic aortic dilation and bicommissural aortic valve: a prospective analysis of natural history and inheritance. Am J Med Genet A. (2007) 143a:1960–7. doi: 10.1002/ajmg.a.31872
8. Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol. (2004) 44:138–43. doi: 10.1016/j.jacc.2004.03.050
9. Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. (2010) 55:2789–800. doi: 10.1016/j.jacc.2009.12.068
10. Hattori K, Nakama N, Takada J, Nishimura G, Moriwaki R, Kawasaki E, et al. Bicuspid aortic valve morphology and aortic valvular outflow jets: an experimental analysis using an MRI-compatible pulsatile flow circulation system. Sci Rep. (2021) 11:2066. doi: 10.1038/s41598-021-81845-w
11. Wojnarski CM, Svensson LG, Roselli EE, Idrees JJ, Lowry AM, Ehrlinger J, et al. Aortic dissection in patients with bicuspid aortic valve-associated aneurysms. Ann Thorac Surg. (2015) 100:1666–74. doi: 10.1016/j.athoracsur.2015.04.126
12. Larson EW, Edwards WD. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol. (1984) 53:849–55.
13. Pauperio HM, Azevedo AC, Ferreira CS. The aortic valve with two leaflets–a study in 2,000 autopsies. Cardiol Young. (1999) 9:488–98. doi: 10.1017/S1047951100005400
14. Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol. (1970) 26:72–83.
15. Datta BN, Bhusnurmath B, Khattri HN, Sapru RP, Bidwai PS, Wahi PL. Anatomically isolated aortic valve disease. Morphologic study of 100 cases at autopsy. Jpn Heart J. (1988) 29:661–70.
16. Basso C, Boschello M, Perrone C, Mecenero A, Cera A, Bicego D, et al. An echocardiographic survey of primary school children for bicuspid aortic valve. Am J Cardiol. (2004) 93:661–3. doi: 10.1016/j.amjcard.2003.11.031
17. Tutar E, Ekici F, Atalay S, Nacar N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am Heart J. (2005) 150:513–5. doi: 10.1016/j.ahj.2004.10.036
18. Sillesen AS, Vøgg O, Pihl C, Raja AA, Sundberg K, Vedel C, et al. Prevalence of bicuspid aortic valve and associated aortopathy in newborns in Copenhagen, Denmark. JAMA. (2021) 325:561–7. doi: 10.1001/jama.2020.27205
19. Kong WK, Regeer MV, Ng AC, McCormack L, Poh KK, Yeo TC, et al. Sex Differences in phenotypes of bicuspid aortic valve and aortopathy: insights from a large multicenter, international registry. Circ Cardiovasc Imaging. (2017) 10:e005155. doi: 10.1161/CIRCIMAGING.116.005155
20. Prakash SK, Bondy CA, Maslen CL, Silberbach M, Lin AE, Perrone L, et al. Autosomal and X chromosome structural variants are associated with congenital heart defects in Turner syndrome: The NHLBI GenTAC registry. Am J Med Genet A. (2016) 170:3157–64. doi: 10.1002/ajmg.a.37953
21. Miller MJ, Geffner ME, Lippe BM, Itami RM, Kaplan SA, DiSessa TG, et al. Echocardiography reveals a high incidence of bicuspid aortic valve in Turner syndrome. J Pediatr. (1983) 102:47–50. doi: 10.1016/S0022-3476(83)80284-4
22. Michelena HI, Mankad SV. Sex Differences in bicuspid aortic valve adults. Circ Cardiovasc Imaging. (2017) 10:e006123. doi: 10.1161/JAHA.116.004211
23. Vignac M, Björck HM, Olsson C, Eriksson MJ, Jouven X, Michos ED, et al. Sex differences in aortopathy and valve diseases among patients undergoing cardiac surgery. Ann Thorac Surg. (2022). doi: 10.1016/j.athoracsur.2022.02.040. [Epub ahead of print].
24. Granath C, Mohamed SA, Olsson C, Grattan M, Mertens L, Franco-Cereceda A, et al. Valve disease and aortopathy associations of bicuspid aortic valve phenotypes differ between men and women. Open Heart. (2021) 8:e001857. doi: 10.1136/openhrt-2021-001857
25. Glick BN, Roberts WC. Congenitally bicuspid aortic valve in multiple family members. Am J Cardiol. (1994) 73:400–4.
26. Clementi M, Notari L, Borghi A, Tenconi R. Familial congenital bicuspid aortic valve: a disorder of uncertain inheritance. Am J Med Genet. (1996) 62:336–8. doi: 10.1002/(SICI)1096-8628(19960424)62:4<336::AID-AJMG2=3.0.CO;2-P
27. Huntington K, Hunter AG, Chan KL, A. prospective study to assess the frequency of familial clustering of congenital bicuspid aortic valve. J Am Coll Cardiol. (1997) 30:1809–12. doi: 10.1016/S0735-1097(97)00372-0
28. Lewin MB, McBride KL, Pignatelli R, Fernbach S, Combes A, Menesses A, et al. Echocardiographic evaluation of asymptomatic parental and sibling cardiovascular anomalies associated with congenital left ventricular outflow tract lesions. Pediatrics. (2004) 114:691–6. doi: 10.1542/peds.2003-0782-L
29. Freeze SL, Landis BJ, Ware SM, Helm BM. Bicuspid aortic valve: a review with recommendations for genetic counseling. J Genet Couns. (2016) 25:1171–8. doi: 10.1007/s10897-016-0002-6
30. Lo Presti F, Guzzardi DG, Bancone C, Fedak PWM, Della Corte A. The science of BAV aortopathy. Prog Cardiovasc Dis. (2020) 63:465–74. doi: 10.1016/j.pcad.2020.06.009
31. Laforest B, Nemer M. Genetic insights into bicuspid aortic valve formation. Cardiol Res Pract. (2012) 2012:180297. doi: 10.1155/2012/180297
32. Fedak PWM, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation. (2002) 106:900–4. doi: 10.1161/01.CIR.0000027905.26586.E8
33. Debiec RM, Hamby SE, Jones PD, Safwan K, Sosin M, Hetherington SL, et al. Contribution of NOTCH1 genetic variants to bicuspid aortic valve and other congenital lesions. Heart. (2022) 108:1114–20. doi: 10.1136/heartjnl-2021-320428
34. Kerstjens-Frederikse WS, van de Laar IM, Vos YJ, Verhagen JM, Berger RM, Lichtenbelt KD, et al. Cardiovascular malformations caused by NOTCH1 mutations do not keep left: data on 428 probands with left-sided CHD and their families. Genet Med. (2016) 18:914–23. doi: 10.1038/gim.2015.193
35. Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, et al. Mutations in NOTCH1 cause aortic valve disease. Nature. (2005) 437:270–4. doi: 10.1038/nature03940
36. High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet. (2008) 9:49–61. doi: 10.1038/nrg2279
37. Niessen K, Karsan A. Notch signaling in cardiac development. Circ Res. (2008) 102:1169–81. doi: 10.1161/CIRCRESAHA.108.174318
38. Wang Y, Fang Y, Lu P, Wu B, Zhou B NOTCH. Signaling in aortic valve development and calcific aortic valve disease. Front Cardiovasc Med. (2021) 8:682298. doi: 10.3389/fcvm.2021.682298
39. Soto-Navarrete MT, López-Unzu M, Durán AC, Fernández B. Embryonic development of bicuspid aortic valves. Prog Cardiovasc Dis. (2020) 63:407–18. doi: 10.1016/j.pcad.2020.06.008
40. Godby RC, Munjal C, Opoka AM, Smith JM, Yutzey KE, Narmoneva DA, et al. Cross talk between NOTCH signaling and biomechanics in human aortic valve disease pathogenesis. J Cardiovasc Dev Dis. (2014) 1:237–56. doi: 10.3390/jcdd1030237
41. Duran AC, Frescura C, Sans-Coma V, Angelini A, Basso C, Thiene G. Bicuspid aortic valves in hearts with other congenital heart disease. J Heart Valve Dis. (1995) 4:581–90.
42. Nistri S, Basso C, Marzari C, Mormino P, Thiene G. Frequency of bicuspid aortic valve in young male conscripts by echocardiogram. Am J Cardiol. (2005) 96:718–21. doi: 10.1016/j.amjcard.2005.04.051
43. Nistri S, Porciani MC, Attanasio M, Abbate R, Gensini GF, Pepe G. Association of Marfan syndrome and bicuspid aortic valve: frequency and outcome. Int J Cardiol. (2012) 155:324–5. doi: 10.1016/j.ijcard.2011.12.009
44. Giusti B, Sticchi E, De Cario R, Magi A, Nistri S, Pepe G. Genetic bases of bicuspid aortic valve: the contribution of traditional and high-throughput sequencing approaches on research and diagnosis. Front Physiol. (2017) 8:612. doi: 10.3389/fphys.2017.00612
45. Nistri S, Grande-Allen J, Noale M, Basso C, Siviero P, Maggi S, et al. Aortic elasticity and size in bicuspid aortic valve syndrome. Eur Heart J. (2008) 29:472–9. doi: 10.1093/eurheartj/ehm528
46. Conti CA, Della Corte A, Votta E, Del Viscovo L, Bancone C, De Santo LS, et al. Biomechanical implications of the congenital bicuspid aortic valve: a finite element study of aortic root function from in vivo data. J Thorac Cardiovasc Surg. (2010) 140:890–6. doi: 10.1016/j.jtcvs.2010.01.016
47. Della Corte A, Bancone C, Conti CA, Votta E, Redaelli A, Del Viscovo L, et al. Restricted cusp motion in right-left type of bicuspid aortic valves: a new risk marker for aortopathy. J Thorac Cardiovasc Surg. (2012) 144:360–9. doi: 10.1016/j.jtcvs.2011.10.014
48. Michelena HI, Della Corte A, Prakash SK, Milewicz DM, Evangelista A, Enriquez-Sarano M. Bicuspid aortic valve aortopathy in adults: Incidence, etiology, and clinical significance. Int J Cardiol. (2015) 201:400–7. doi: 10.1016/j.ijcard.2015.08.106
49. Michelena HI, Della Corte A, Evangelista A, Maleszewski JJ, Edwards WD, Roman MJ, et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. J Thorac Cardiovasc Surg. (2021) 162:e383–414. doi: 10.1016/j.jtcvs.2021.06.019
50. Brandenburg RO, Tajik AJ, Edwards WD, Reeder GS, Shub C, Seward, Seward JB Accuracy of 2-dimensional echocardiographic diagnosis of congenitally bicuspid aortic valve: echocardiographic-anatomic correlation in 115 patients. J Am Coll Cardiol. (1983) 51:1469–73.
51. Angelini A, Ho SY, Anderson RH, Devine WA, Zuberbuhler JR, Becker AE, et al. The morphology of the normal aortic valve as compared with the aortic valve having two leaflets. J Thorac Cardiovasc Surg. (1989) 98:362–7. doi: 10.1016/S0022-5223(19)34382-X
52. Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc. (1999) 74:14–26. doi: 10.4065/74.1.14
53. Kang JW, Song HG, Yang DH, Baek S, Kim DH, Song JM, et al. Association between bicuspid aortic valve phenotype and patterns of valvular dysfunction and bicuspid aortopathy: comprehensive evaluation using MDCT and echocardiography. JACC Cardiovascular imaging. (2013) 6:150–61. doi: 10.1016/j.jcmg.2012.11.007
54. Jilaihawi H, Chen M, Webb J, Himbert D, Ruiz CE, Rodés-Cabau J, et al. A bicuspid aortic valve imaging classification for the TAVR era. JACC Cardiovascular imaging. (2016) 9:1145–58. doi: 10.1016/j.jcmg.2015.12.022
55. Sun BJ, Lee S, Jang JY, Kwon O, Bae JS, Lee JH, et al. Performance of a simplified dichotomous phenotypic classification of bicuspid aortic valve to predict type of valvulopathy and combined aortopathy. J Am Soc Echocardiogr. (2017) 30:1152–61. doi: 10.1016/j.echo.2017.08.002
56. Murphy IG, Collins J, Powell A, Markl M, McCarthy P, Malaisrie SC, et al. Comprehensive 4-stage categorization of bicuspid aortic valve leaflet morphology by cardiac MRI in 386 patients. Int J Cardiovasc Imaging. (2017) 33:1213–21. doi: 10.1007/s10554-017-1107-1
57. Michelena HI, Desjardins VA, Avierinos JF, Russo A, Nkomo VT, Sundt TM, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation. (2008) 117:2776–84. doi: 10.1161/CIRCULATIONAHA.107.740878
58. Fazel SS, Mallidi HR, Lee RS, Sheehan MP, Liang D, Fleischman D, et al. The aortopathy of bicuspid aortic valve disease has distinctive patterns and usually involves the transverse aortic arch. J Thorac Cardiovasc Surg. (2008) 135:901–7. doi: 10.1016/j.jtcvs.2008.01.022
59. Della Corte A, Bancone C, Buonocore M, Dialetto G, Covino FE, Manduca S, et al. Pattern of ascending aortic dimensions predicts the growth rate of the aorta in patients with bicuspid aortic valve. JACC Cardiovascular imaging. (2013) 6:1301–10. doi: 10.1016/j.jcmg.2013.07.009
60. Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg. (2012) 42:832–7. doi: 10.1093/ejcts/ezv259
61. Rylski B, Desai ND, Bavaria JE, Vallabhajosyula P, Moser W, Pochettino A, et al. Aortic valve morphology determines the presentation and surgical approach to acute type A aortic dissection. Ann Thoracic Surg. (2014) 97:1991–6. doi: 10.1016/j.athoracsur.2013.12.090
62. Etz CD, von Aspern K, Hoyer A, Girrbach FF, Leontyev S, Bakhtiary F, et al. Acute type A aortic dissection: characteristics and outcomes comparing patients with bicuspid versus tricuspid aortic valve. Eur J Cardiothorac Surg. (2015) 48:142–50. doi: 10.1093/ejcts/ezu388
63. Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, et al. Outcomes in adults with bicuspid aortic valves. Jama. (2008) 300:1317–25. doi: 10.1001/jama.300.11.1317
64. Itagaki S, Chikwe JP, Chiang YP, Egorova NN, Adams DH. Long-term risk for aortic complications after aortic valve replacement in patients with bicuspid aortic valve versus marfan syndrome. J Am Coll Cardiol. (2015) 65:2363–9. doi: 10.1016/j.jacc.2015.03.575
65. Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, et al. Incidence of aortic complications in patients with bicuspid aortic valves. Jama. (2011) 306:1104–12. doi: 10.1001/jama.2011.1286
66. Davies RR, Kaple RK, Mandapati D, Gallo A, Botta DM, Elefteriades JA, et al. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg. (2007) 83:1338–44. doi: 10.1016/j.athoracsur.2006.10.074
67. Hahn RT, Roman MJ, Mogtader AH, Devereux RB. Association of aortic dilation with regurgitant, stenotic and functionally normal bicuspid aortic valves. J Am Coll Cardiol. (1992) 19:283–8. doi: 10.1016/0735-1097(92)90479-7
68. Keane MG, Wiegers SE, Plappert T, Pochettino A, Bavaria JE, Sutton MG. Bicuspid aortic valves are associated with aortic dilatation out of proportion to coexistent valvular lesions. Circulation. (2000) 102:Iii35–9. doi: 10.1161/01.cir.102
69. Girdauskas E, Rouman M, Disha K, Espinoza A, Misfeld M, Borger MA, et al. Aortic dissection after previous aortic valve replacement for bicuspid aortic valve disease. J Am Coll Cardiol. (2015) 66:1409–11. doi: 10.1016/j.jacc.2015.07.022
70. Geeraert P, Jamalidinan F, Burns F, Jarvis K, Bristow MS, Lydell C, et al. Hemodynamic assessment in bicuspid aortic valve disease and aortic dilation: new insights from voxel-by-voxel analysis of reverse flow, stasis, and energetics. Front Bioeng Biotechnol. (2021) 9:725113. doi: 10.3389/fbioe.2021.725113
71. Fatehi Hassanabad A, Feindel CM, Verma S, Fedak PWM. Evolving surgical approaches to bicuspid aortic valve associated aortopathy. Front Cardiovasc Med. (2019) 6:19. doi: 10.3389/fcvm.2019.00019
72. Fatehi Hassanabad A, Garcia J, Verma S, White JA, Fedak PWM. Utilizing wall shear stress as a clinical biomarker for bicuspid valve-associated aortopathy. Curr Opin Cardiol. (2019) 34:124–31. doi: 10.1097/HCO.0000000000000601
73. Fatehi Hassanabad A, Barker AJ, Guzzardi D, Markl M, Malaisrie C, McCarthy PM, et al. Evolution of precision medicine and surgical strategies for bicuspid aortic valve-associated aortopathy. Front Physiol. (2017) 8:475. doi: 10.3389/fphys.2017.00475
74. Geeraert P, Jamalidinan F, Fatehi Hassanabad A, Sojoudi A, Bristow M, Lydell C, et al. Bicuspid aortic valve disease is associated with abnormal wall shear stress, viscous energy loss, and pressure drop within the ascending thoracic aorta: A cross-sectional study. Medicine (Baltimore). (2021) 100:e26518. doi: 10.1097/MD.0000000000026518
75. Bollache E, Guzzardi DG, Sattari S, Olsen KE, Di Martino ES, Malaisrie SC, et al. Aortic valve-mediated wall shear stress is heterogeneous and predicts regional aortic elastic fiber thinning in bicuspid aortic valve-associated aortopathy. J Thorac Cardiovasc Surg. (2018) 156:2112–20.e2. doi: 10.1016/j.jtcvs.2018.05.095
76. Guzzardi DG, Barker AJ, van Ooij P, Malaisrie SC, Puthumana JJ, Belke DD, et al. Valve-related hemodynamics mediate human bicuspid aortopathy: insights from wall shear stress mapping. J Am Coll Cardiol. (2015) 66:892–900. doi: 10.1016/j.jacc.2015.06.1310
77. Jamalidinan F, Hassanabad AF, François CJ, Garcia J. Four-dimensional-flow magnetic resonance imaging of the aortic valve and thoracic aorta. Radiol Clin North Am. (2020) 58:753–63. doi: 10.1016/j.rcl.2020.02.008
78. Fatehi Hassanabad A, Burns F, Bristow MS, Lydell C, Howarth AG, Heydari B, et al. Pressure drop mapping using 4D flow MRI in patients with bicuspid aortic valve disease: A novel marker of valvular obstruction. Magn Reson Imaging. (2020) 65:175–82. doi: 10.1016/j.mri.2019.11.011
79. Schnell S, Smith DA, Barker AJ, Entezari P, Honarmand AR, Carr ML, et al. Altered aortic shape in bicuspid aortic valve relatives influences blood flow patterns. Eur Heart J Cardiovasc Imaging. (2016) 17:1239–47. doi: 10.1093/ehjci/jew149
80. Guzzardi DG, Verma S, Fedak PW. Bicuspid aortic valve aortopathy: mechanistic and clinical insights from recent studies. Curr Opin Cardiol. (2017) 32:111–6. doi: 10.1097/HCO.0000000000000359
81. Edlin J, Youssefi P, Bilkhu R, Figueroa CA, Morgan R, Nowell J, et al. Haemodynamic assessment of bicuspid aortic valve aortopathy: a systematic review of the current literature. Eur J Cardiothorac Surg. (2019) 55:610–7. doi: 10.1093/ejcts/ezy312
82. Stefek HA, Lin KH, Rigsby CK, Michelena HI, Aouad P, Barker AJ, et al. Eccentric enlargement of the aortic sinuses in pediatric and adult patients with bicuspid aortic valves: a cardiac MRI study. Pediatr Cardiol. (2020) 41:350–60. doi: 10.1007/s00246-019-02264-3
83. Hope MD, Hope TA, Meadows AK, Ordovas KG, Urbania TH, Alley MT, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology. (2010) 255:53–61. doi: 10.1148/radiol.09091437
84. Verma S, Yanagawa B, Kalra S, Ruel M, Peterson MD, Yamashita MH, et al. Knowledge, attitudes, and practice patterns in surgical management of bicuspid aortopathy: a survey of 100 cardiac surgeons. J Thorac Cardiovasc Surg. (2013) 146:1033–40.e4. doi: 10.1016/j.jtcvs.2013.06.037
85. Borger MA, Fedak PWM, Stephens EH, Gleason TG, Girdauskas E, Ikonomidis JS, et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: Full online-only version. J Thorac Cardiovasc Surg. (2018) 156:e41–74. doi: 10.1016/j.jtcvs.2017.10.161
86. Palumbo MC, Rong LQ, Kim J, Navid P, Sultana R, Butcher J, et al. Prosthetic aortic graft replacement of the ascending thoracic aorta alters biomechanics of the native descending aorta as assessed by transthoracic echocardiography. PLoS ONE. (2020) 15:e0230208. doi: 10.1371/journal.pone.0230208
87. Soulat G, Scott MB, Allen BD, Avery R, Bonow RO, Malaisrie SC, et al. Association of regional wall shear stress and progressive ascending aorta dilation in bicuspid aortic valve. JACC Cardiovascular imaging. (2022) 15:33–42. doi: 10.1016/j.jcmg.2021.06.020
88. Scott MB, Huh H, van Ooij P, Chen V, Herrera B, Elbaz M, et al. Impact of age, sex, and global function on normal aortic hemodynamics. Magnetic Res Med. (2020) 84:2088–102. doi: 10.1002/mrm.28250
89. Kimura N, Nakamura M, Komiya K, Nishi S, Yamaguchi A, Tanaka O, et al. Patient-specific assessment of hemodynamics by computational fluid dynamics in patients with bicuspid aortopathy. J Thorac Cardiovasc Surg. (2017) 153:S52–S62.e3. doi: 10.1016/j.jtcvs.2016.12.033
90. McNally A, Madan A, Sucosky P. Morphotype-dependent flow characteristics in bicuspid aortic valve ascending aortas: a benchtop particle image velocimetry study. Front Physiol. (2017) 8:44. doi: 10.3389/fphys.2017.00044
91. Mahadevia R, Barker AJ, Schnell S, Entezari P, Kansal P, Fedak PW, et al. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation. (2014) 129:673–82. doi: 10.1161/CIRCULATIONAHA.113.003026
92. Kazik HB, Kandail HS, LaDisa JF, Lincoln J. Molecular and mechanical mechanisms of calcification pathology induced by bicuspid aortic valve abnormalities. Front. Cardiovasc. Med. (2021) 8:677977. doi: 10.3389/fcvm.2021.677977
93. van Ooij P, Potters WV, Collins J, Carr M, Carr J, Malaisrie SC, et al. Characterization of abnormal wall shear stress using 4D flow MRI in human bicuspid aortopathy. Ann Biomed Eng. (2015) 43:1385–97. doi: 10.1007/s10439-014-1092-7
94. Salmasi MY, Pirola S, Sasidharan S, Fisichella SM, Redaelli A, Jarral OA, et al. High wall shear stress can predict wall degradation in ascending aortic aneurysms: an integrated biomechanics study. Front Bioeng Biotechnol. (2021) 9:750656. doi: 10.3389/fbioe.2021.750656
95. Jayendiran R, Campisi S, Viallon M, Croisille P, Avril S. Hemodynamics alteration in patient-specific dilated ascending thoracic aortas with tricuspid and bicuspid aortic valves. J Biomech. (2020) 110:109954. doi: 10.1016/j.jbiomech.2020.109954
96. Pasta S, Phillippi JA, Gleason TG, Vorp DA. Effect of aneurysm on the mechanical dissection properties of the human ascending thoracic aorta. J Thorac Cardiovasc Surg. (2012) 143:460–7. doi: 10.1016/j.jtcvs.2011.07.058
97. Angel PM, Narmoneva DA, Sewell-Loftin MK, Munjal C, Dupuis L, Landis BJ, et al. Proteomic alterations associated with biomechanical dysfunction are early processes in the Emilin1 deficient mouse model of aortic valve disease. Ann Biomed Eng. (2017) 45:2548–62. doi: 10.1007/s10439-017-1899-0
98. Munjal C, Opoka AM, Osinska H, James JF, Bressan GM, Hinton RB. TGF-β mediates early angiogenesis and latent fibrosis in an Emilin1-deficient mouse model of aortic valve disease. Dis Model Mech. (2014) 7:987–96. doi: 10.1242/dmm.015255
99. Chung JC, Wong E, Tang M, Eliathamby D, Forbes TL, Butany J, et al. Biomechanics of aortic dissection: a comparison of aortas associated with bicuspid and tricuspid aortic valves. J Am Heart Assoc. (2020) 9:e016715. doi: 10.1161/JAHA.120.016715
100. Forsell C, Björck HM, Eriksson P, Franco-Cereceda A, Gasser TC. Biomechanical properties of the thoracic aneurysmal wall: differences between bicuspid aortic valve and tricuspid aortic valve patients. Ann Thorac Surg. (2014) 98:65–71. doi: 10.1016/j.athoracsur.2014.04.042
101. Deveja RP, Iliopoulos DC, Kritharis EP, Angouras DC, Sfyris D, Papadodima SA, et al. Effect of aneurysm and bicuspid aortic valve on layer-specific ascending aorta mechanics. Ann Thorac Surg. (2018) 106:1692–701. doi: 10.1016/j.athoracsur.2018.05.071
102. Durbak E, Tarraf S, Gillespie C, Germano E, Cikach F, Blackstone E, et al. Ex vivo biaxial load testing analysis of aortic biomechanics demonstrates variation in elastic energy distribution across the aortic zone zero. J Thorac Cardiovasc Surg. (2021). doi: 10.1016/j.jtcvs.2021.09.071. [Epub ahead of print].
103. Grewal N, Girdauskas E, DeRuiter M, Goumans MJ, Poelmann RE, Klautz RJM, et al. The role of hemodynamics in bicuspid aortopathy: a histopathologic study. Cardiovasc Pathol. (2019) 41:29–37. doi: 10.1016/j.carpath.2019.03.002
104. Meng H, Tutino VM, Xiang J, Siddiqui A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis AJNR. Am J Neuroradiol. (2014) 35:1254–62. doi: 10.3174/ajnr.A3558
105. Rumman RK, Slorach C, Hui W, Lopez C, Larios G, Fan S, et al. Regional vascular changes and aortic dilatation in pediatric patients with bicuspid aortic valve. Can J Cardiol. (2022) 38:688–94. doi: 10.1016/j.cjca.2022.01.020
106. Sigaeva T, Sattari S, Polzer S, Appoo JJ, Di Martino ES. Biomechanical properties of ascending aortic aneurysms: quantification of inter- and intra-patient variability. J Biomech. (2021) 125:110542. doi: 10.1016/j.jbiomech.2021.110542
107. Pascaner AF, Houriez-Gombaud-Saintonge S, Craiem D, Gencer U, Casciaro ME, Charpentier E, et al. Comprehensive assessment of local and regional aortic stiffness in patients with tricuspid or bicuspid aortic valve aortopathy using magnetic resonance imaging. Int J Cardiol. (2021) 326:206–12. doi: 10.1016/j.ijcard.2020.11.046
108. Antequera-González B, Martínez-Micaelo N, Alegret JM. Bicuspid aortic valve and endothelial dysfunction: current evidence and potential therapeutic targets. Front Physiol. (2020) 11:1015. doi: 10.3389/fphys.2020.01015
109. Maredia A, Guzzardi D, Aleinati M, Iqbal F, Khaira A, Madhu A, et al. Aorta-specific DNA methylation patterns in cell-free DNA from patients with bicuspid aortic valve-associated aortopathy. Clin Epigenetics. (2021) 13:147. doi: 10.1186/s13148-021-01137-y
110. Jiang Y, Chen J, Wei F, Wang Y, Chen S, Li G, et al. Micromechanical force promotes aortic valvular calcification. J Thorac Cardiovasc Surg. (2021). doi: 10.1016/j.jtcvs.2021.08.014
111. Farag ES, van Ooij P, Planken RN, Dukker KCP, de Heer F, Bouma BJ, et al. Aortic valve stenosis and aortic diameters determine the extent of increased wall shear stress in bicuspid aortic valve disease. JMRI. (2018) 48:522–30. doi: 10.1002/jmri.25956
112. Stefek HA, Berhane H, Robinson JD, Reilly B, Ruh A, Markl M, et al. Comprehensive MR analysis of cardiac function, aortic hemodynamics and left ventricular strain in pediatric cohort with isolated bicuspid aortic valve. Pediatr Cardiol. (2019) 40:1450–9. doi: 10.1007/s00246-019-02157-5
113. van Ooij P, Markl M, Collins JD, Carr JC, Rigsby C, Bonow RO, et al. Aortic valve stenosis alters expression of regional aortic wall shear stress: new insights from a 4-dimensional flow magnetic resonance imaging study of 571 subjects. J Am Heart Assoc. (2017) 6:e005959. doi: 10.1161/JAHA.117.005959
114. Shan Y, Li J, Wang Y, Wu B, Barker AJ, Markl M, et al. Aortic stenosis exacerbates flow aberrations related to the bicuspid aortic valve fusion pattern and the aortopathy phenotype. Eur J Cardiothorac Surg. (2019) 55:534–42. doi: 10.1093/ejcts/ezy308
115. Gauer S, Balint B, Kollmann C, Federspiel JM, Henn D, Bandner-Risch D, et al. Dysregulation of endothelial nitric oxide synthase does not depend on hemodynamic alterations in bicuspid aortic valve aortopathy. J Am Heart Assoc. (2020) 9:e016471. doi: 10.1161/JAHA.120.016471
116. Abudupataer M, Zhu S, Yan S, Xu K, Zhang J, Luo S, et al. Aorta smooth muscle-on-a-chip reveals impaired mitochondrial dynamics as a therapeutic target for aortic aneurysm in bicuspid aortic valve disease. eLife. (2021) 10:e69310. doi: 10.7554/eLife.69310
117. Lenz A, Petersen J, Riedel C, Weinrich JM, Kooijman H, Schoennagel BP, et al. 4D flow cardiovascular magnetic resonance for monitoring of aortic valve repair in bicuspid aortic valve disease. J Cardiovasc Magn Reson. (2020) 22:29. doi: 10.1186/s12968-020-00608-0
118. Garcia J, Barker AJ, Markl M. The role of imaging of flow patterns by 4D flow MRI in aortic stenosis. JACC Cardiovascular imaging. (2019) 12:252–66. doi: 10.1016/j.jcmg.2018.10.034
119. Rodríguez-Palomares JF, Dux-Santoy L, Guala A, Kale R, Maldonado G, Teixidó-Turà G, et al. Aortic flow patterns and wall shear stress maps by 4D-flow cardiovascular magnetic resonance in the assessment of aortic dilatation in bicuspid aortic valve disease. J Cardiovasc Magn Reson. (2018) 20:28. doi: 10.1186/s12968-018-0451-1
120. Bollache E, Fedak PWM, Markl M, Barker AJ. On the 'cusp' of clinical feasibility: aortic wall shear stress derived non-invasively with 4D flow MRI. J Thorac Dis. (2019) 11:E96–e7. doi: 10.21037/jtd.2019.06.54
121. Gordon DZ, Abbasi MA, Lee J, Sarnari R, Sojoudi A, Wei Q, et al. Four-dimensional flow magnetic resonance imaging quantification of blood flow in bicuspid aortic valve. J Thorac Imaging. (2020) 35:383–8. doi: 10.1097/RTI.0000000000000535
122. Piatti F, Sturla F, Bissell MM, Pirola S, Lombardi M, Nesteruk I, et al. 4D Flow analysis of BAV-related fluid-dynamic alterations: evidences of wall shear stress alterations in absence of clinically-relevant aortic anatomical remodeling. Front Physiol. (2017) 8:441. doi: 10.3389/fphys.2017.00441
123. Cosentino F, Scardulla F, D'Acquisto L, Agnese V, Gentile G, Raffa G, et al. Computational modeling of bicuspid aortopathy: towards personalized risk strategies. J Mol Cell Cardiol. (2019) 131:122–31. doi: 10.1016/j.yjmcc.2019.04.026
124. Raghav V, Barker AJ, Mangiameli D, Mirabella L, Markl M, Yoganathan AP. Valve mediated hemodynamics and their association with distal ascending aortic diameter in bicuspid aortic valve subjects. JMRI. (2018) 47:246–54. doi: 10.1002/jmri.25719
125. Sigovan M, Hope MD, Dyverfeldt P, Saloner D. Comparison of four-dimensional flow parameters for quantification of flow eccentricity in the ascending aorta. JMRI. (2011) 34:1226–30. doi: 10.1002/jmri.22800
126. Oliveira D, Rosa SA, Tiago J, Ferreira RC, Agapito AF, Sequeira A. Bicuspid aortic valve aortopathies: an hemodynamics characterization in dilated aortas. Comput Methods Biomech Biomed Engin. (2019) 22:815–26. doi: 10.1080/10255842.2019.1597860
127. Boonyasirinant T, Rajiah P, Flamm SD. Abnormal aortic stiffness in patients with bicuspid aortic valve: phenotypic variation determined by magnetic resonance imaging. Int J Cardiovasc Imaging. (2019) 35:133–41. doi: 10.1007/s10554-018-1433-y
128. Singh A, Horsfield MA, Bekele S, Greenwood JP, Dawson DK, Berry C, et al. Aortic stiffness in aortic stenosis assessed by cardiovascular MRI: a comparison between bicuspid and tricuspid valves. Eur Radiol. (2019) 29:2340–9. doi: 10.1007/s00330-018-5775-6
129. Nollen GJ, Groenink M, Tijssen JG, Van Der Wall EE, Mulder BJ. Aortic stiffness and diameter predict progressive aortic dilatation in patients with Marfan syndrome. Eur Heart J. (2004) 25:1146–52. doi: 10.1016/j.ehj.2004.04.033
130. Aquila I, Frati G, Sciarretta S, Dellegrottaglie S, Torella D, Torella M. New imaging techniques project the cellular and molecular alterations underlying bicuspid aortic valve development. J Mol Cell Cardiol. (2019) 129:197–207. doi: 10.1016/j.yjmcc.2019.02.015
131. Emendi M, Sturla F, Ghosh RP, Bianchi M, Piatti F, Pluchinotta FR, et al. Patient-specific bicuspid aortic valve biomechanics: a magnetic resonance imaging integrated fluid-structure interaction approach. Ann Biomed Eng. (2021) 49:627–41. doi: 10.1007/s10439-020-02571-4
132. Li F, Wang S, Gao Q, Chen X, Yin G, Yu C, et al. Vessel structural stress mediates aortic media degeneration in bicuspid aortopathy: New insights based on patient-specific fluid-structure interaction analysis. J Biomech. (2021) 129:110805. doi: 10.1016/j.jbiomech.2021.110805
133. Franco P, Sotelo J, Guala A, Dux-Santoy L, Evangelista A, Rodríguez-Palomares J, et al. Identification of hemodynamic biomarkers for bicuspid aortic valve induced aortic dilation using machine learning. Comput Biol Med. (2022) 141:105147. doi: 10.1016/j.compbiomed.2021.105147
Keywords: bicuspid aortic valve, bicuspid aortopathy, biomechanics, BAV-mediated hemodynamics, 4D flow MRI
Citation: Fatehi Hassanabad A, King MA, Di Martino E, Fedak PWM and Garcia J (2022) Clinical implications of the biomechanics of bicuspid aortic valve and bicuspid aortopathy. Front. Cardiovasc. Med. 9:922353. doi: 10.3389/fcvm.2022.922353
Received: 17 April 2022; Accepted: 25 July 2022;
Published: 12 August 2022.
Edited by:
Marie Billaud, Brigham and Women's Hospital and Harvard Medical School, United StatesReviewed by:
Anna Malashicheva, Institute of Cytology, RussiaCopyright © 2022 Fatehi Hassanabad, King, Di Martino, Fedak and Garcia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julio Garcia, anVsaW8uZ2FyY2lhZmxvcmVzQHVjYWxnYXJ5LmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.