- 1Cardiology Unit, Department of Clinical and Molecular Medicine, School of Medicine and Psychology, Sant'Andrea Hospital, Sapienza University of Rome, Rome, Italy
- 2Division of Cardiology, Department of Medical and Surgical Science, Magna Graecia University, Catanzaro, Italy
- 3IRCCS Neuromed, Pozzilli (IS), Italy
- 4Cardiology Unit, Belcolle Hospital, ASL Viterbo, Viterbo, Italy
Background: Among several potential mechanisms, mitochondrial dysfunction has been proposed to be involved in the pathogenesis of coronary artery disease (CAD). A mitochondrial complex I deficiency severely impairs cardiovascular health and contributes to CAD development. Previous evidence highlighted a key role of NDUFC2, a subunit of complex I, deficiency in the increased occurrence of renal and cerebrovascular damage in an animal model of hypertension, and of juvenile ischemic stroke occurrence in humans. Furthermore, a significant decrease of NDUFC2 mRNA was detected in peripheral blood mononuclear cells from patients experiencing acute coronary syndrome (ACS). The T allele at NDUFC2/rs23117379 variant is known to associate with reduced gene expression and mitochondrial dysfunction.
Objective: In the present study we tested the impact of the T/C NDUFC2/rs23117379 variant on occurrence of ACS in a prospective cohort of CAD patients (n = 260).
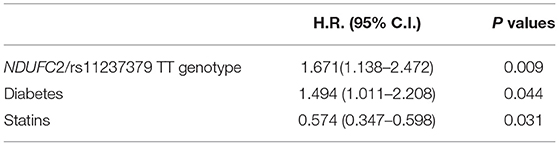
Results: Hypertension, smoking habit, diabetes and hypercholesterolemia were present in a large proportion of patients. Non-ST-elevation myocardial infarction (NSTEMI) represented the most frequent type of ACS (44%, n = 115), followed by ST-elevation myocardial infarction (STEMI) (34%, n = 88) and unstable angina (22%, n = 57). The alleles/genotypes distribution for T/C at NDUFC2/rs23117379 revealed that the TT genotype was associated with a trend toward the development of ACS at an earlier age (TT 61 ± 12, CT 65 ± 12 and CC 66 ± 11 years; p = 0.051 after adjustment for gender, hypertension, smoking habit, diabetes and hypercholesterolemia) and with a significant predictive role for ACS recurrence (hazard ratio [HR]1.671; 95% confidence interval [CI], 1.138–2.472; p = 0.009).
Conclusions: Our findings are consistent with a deleterious effect of NDUFC2 deficiency on acute coronary events predisposition and further support a role of the NDUFC2/rs23117379 variant as a genetic cardiovascular risk factor.
Introduction
Coronary artery disease (CAD) represents the most common cause of cardiovascular (CV) death worldwide. Prevention and treatment of traditional CV risk factors, such as hypertension, dyslipidemia, diabetes, and smoking, represent the most relevant strategy to fight the occurrence of CVD (1). Genetic factors are also known to play a contributory role (2).
Over the last few years, several efforts have been made to identify molecular mechanisms involved in the development and progression of atherosclerotic plaques and in triggering acute inflammatory processes potentially contributing to plaque instability. Among others, a role of mitochondrial dysfunction in the development of atherosclerosis has been shown (3–6). Of note, mitochondrial complex I deficiency was detected in patients with acute coronary syndromes (ACS), with a consequent significant increase of reactive oxygen species (ROS) levels, reduced adenosine triphosphate (ATP) levels and a higher degree of mitochondrial structural damage and dysfunction (7–9).
In this context, the NDUFC2 (NADH dehydrogenase [ubiquinone] 1 subunit) has emerged as a key fundamental subunit of mitochondrial complex I that is needed for the appropriate assembly and activity of the complex. In-vitro, NDUFC2 disruption in vascular cells alters mitochondrial complex I assembly and activity, reducing mitochondrial membrane potential and ATP levels and increasing ROS production and inflammation (7, 10). In-vivo, Ndufc2 silencing may contribute to cause increased cerebral and renal vascular damage in an animal model of hypertension (11). In humans, NDUFC2 messenger ribonucleic acid (mRNA) level was significantly down-regulated, along with the expression of antioxidant molecules such as uncoupling protein 2 (UCP2) and superoxide dismutases 1 and 2 (SOD1, SOD2), at the time of ACS occurrence (7). Moreover, the T allele at NDUFC2/rs11237379 variant, which is associated with a significant reduction of gene expression (10), is accompanied by increased occurrence of early-onset ischemic stroke through a recessive mode of transmission (11). The NDUFC2/rs11237379 variant is commonly represented in the general population (12).
The present study was performed to evaluate (1) the impact of the carrier status of the T vs. C allele at NDUFC2/rs11237379 variant on the occurrence of a first ACS episode, and (2) the long-term prognostic impact of T vs C allele on the incidence of recurrent ACS episodes in a prospective cohort of Caucasian CAD patients.
Methods
This was a single-center prospective study that included patients affected by CAD admitted and followed at the Unit of Cardiology, S.Andrea Hospital in Rome, Italy, between April 2009 and May 2016.
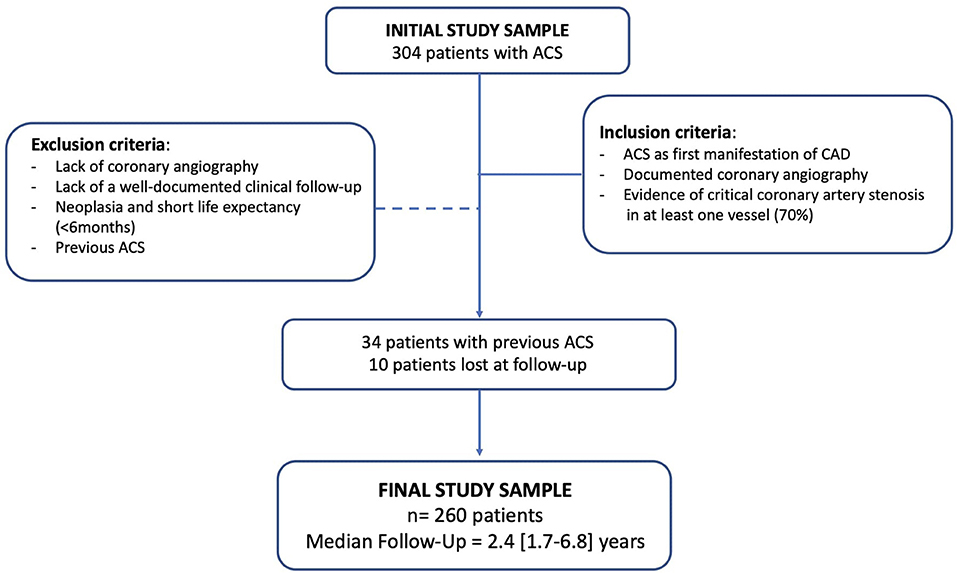
The inclusion criteria were ACS episodes [unstable angina, non-ST-segment elevation MI (NSTEMI), ST-segment elevation MI (STEMI)] as first manifestation of CAD, diagnosed based on standard criteria (13, 14); evidence of critical coronary artery stenosis in at least one vessel (70%), as documented by coronary angiography; availability of a well-documented clinical follow-up after the first ACS episode. Exclusion criteria were lack of coronary angiography, of a well-documented clinical follow-up, of availability of most of the parameters selected for statistical analysis; patients with neoplasia and short life expectancy (<6 months).
Out of 304 originally enrolled patients, 260 individuals had their hospitalization for a first ACS episode at our institution and were subsequently followed. The remaining 44 patients had already experienced a first ACS episode before admission at our hospital (n = 34) or were lost at follow-up (n = 10). A total of 260 CAD patients were finally included in the analysis (Figure 1).
The following parameters were recorded for each patient: demographic data, presence/absence of hypertension, hypercholesterolemia, diabetes, tobacco use, prescribed medications, data on cardiac geometry and function, type of coronary revascularization following the first ACS episode.
Hypertension was diagnosed based on the World Health Organization/International Society of Hypertension criteria and if subjects were routinely receiving antihypertensive therapy (15, 16). Hypercholesterolemia was defined by a total cholesterol blood level higher than 220 mg/dl or routinely use of lipid lowering drugs. Type 1 and 2 diabetes mellitus were diagnosed according to the American Diabetes Association (ADA) Guidelines and/or if subjects were receiving antidiabetic therapy (17, 18). Smoking habit was also recorded (i.e., smokers were considered former only if they had stopped smoking >2 months before entering the study). Left ventricle (LV) internal diameters, wall thickness, LV systolic and diastolic function were measured according to the guidelines of the American Society of Echocardiography and European Association of Cardiovascular Imaging (19).
At the time of recruitment, each patient, after providing informed written consent, underwent a venous blood sample drawing for assessment of the carrier status of the T/C allele at NDUFC2/rs11237379. The study was approved by the Ethical Committee of the S. Andrea Hospital (1916_09).
Following the first ACS episode, clinical follow-up of each patient was well documented. Patients performed periodic check-up visits with a frequency of 1–2 per year. Follow-up data of all patients were obtained through review of ambulatory visits, phone calls and careful revision of medical records. Thus, a detailed and complete cardiac history, with available information regarding re-hospitalizations, new revascularization procedures and medical treatments administered over the years, was available for all patients included in the study.
Genomic DNA was extracted by a commercially available kit (Qiagen, Milan, Italy). The NDUFC2/rs11237379 variant was characterized by a previously reported procedure (11). In particular, the real-time polymerase chain reaction (RT-PCR) was performed by a TaqMan technology assay (Life Technologies) using the ViiA 7 Real-Time PCR System (Applied Biosystem, Foster City, CA, USA).
Statistical Analysis
Data analysis regarding sample characteristics was performed with SPSS software package (version 25.0, SPSS Inc, Chicago, Illinois, USA). Continuous variables are expressed as mean standard deviation; categorical variables are expressed with the corresponding frequencies and percentages.
Differences between groups were analyzed either by t-test or 1-way analysis of variance (ANOVA) if groups were more than two. The Bonferroni post-hoc least significant difference test was performed to complete the analysis for multiple comparisons. To evaluate whether the NDUFC2/rs11237379 variant was independently associated with a younger age at first ACS presentation, a covaried 1-way ANOVA was performed considering age, gender, hypertension, diabetes mellitus, dyslipidemia, and smoking habit as covariates and considering the variant as an independent value. Chi-square test was used for categorical variables. Genotype frequencies were evaluated and Hardy–Weinberg equilibrium (HWE) was tested using Pearson's Chi-square test. The assumption of both dominant (score of 0 for CC genotype, 1 for CT and TT genotypes) and recessive (score of 0 for combined CC and CT genotypes, 1 for TT genotype) models for genotype analysis was considered.
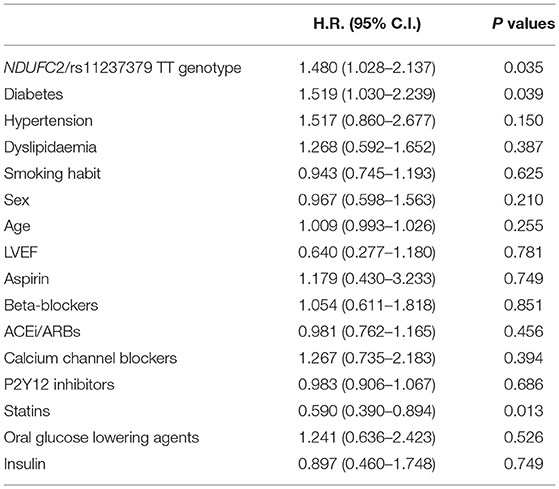
At univariate analysis by Cox regression model, the following predictors were considered: NDUFC2/rs11237379 TT genotype carrier status, sex, age at first event, diabetes, hypercholesterolemia, hypertension, treatment with beta-blocker, acetylsalicylic acid, calcium channel blocker, angiotensin-converting enzyme inhibitor (ACEI), AT1 receptor blocker (ARB), other antiplatelet drugs, statin.
Multivariate models were selected using a forward stepwise regression method based on optimization of the Akaike Information Criterion, finally selecting the following covariates: NDUFC2/rs11237379 TT genotype status, diabetes, treatment with statin.
A value of P < 0.05 was chosen as the cut-off level to declare statistical significance in all analyses.
Results
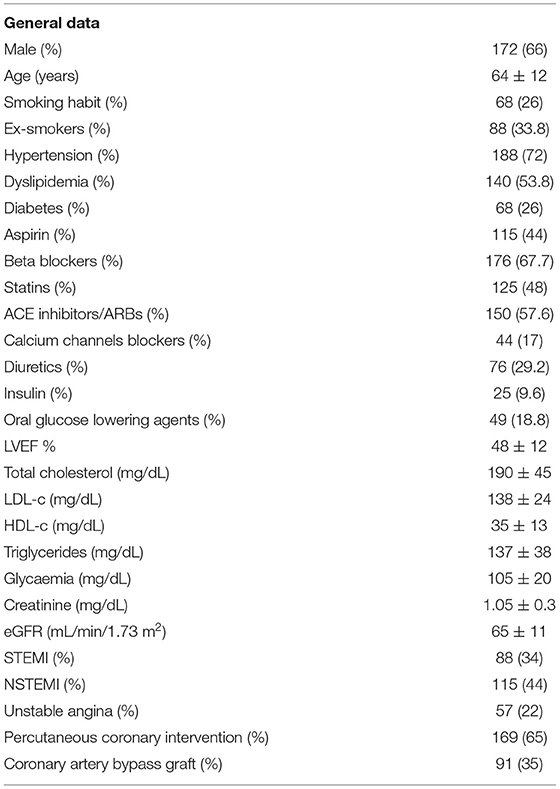
Table 1 shows the main clinical characteristics of the study cohort and the risk factors distribution at the time of the first ACS episode. Male patients represented 66% of the cohort (172 subjects) with a mean age of 64 ± 12 years. A large percentage of patients were hypertensive (71.9%, n = 187), either smokers or ex-smokers (66%, n = 172), affected by diabetes (26%, n = 68) and by hypercholesterolemia (53.8%, n = 140). NSTEMI represented the most frequent type of ACS (44%, n = 115), followed by STEMI (34%, n = 88) and unstable angina (22%, n = 57).
Regarding the T/C alleles distribution at NDUFC2/rs11237379 variant, 45.4% of the enrolled subjects were carrier of the C allele and 54.6% carried the T allele. The frequency of the observed genotypes was 22.3% for CC genotype (n = 58), 46.5% for CT genotype (n = 121) and 31.2% for TT genotype (n = 81); chi-square test: p > 0.05 respecting the HWE.
There were no significant differences among genotype groups regarding gender (p = 0.064), prevalence of hypertension (p = 0.908), smoking habit (p = 0.075), diabetes (p = 0.268) and hypercholesterolemia (p = 0.145) (Table 2). In addition, no significant difference was detected between the groups regarding the number of diseased coronary arteries (p = 0.645), the type of performed coronary revascularization (p = 0.274) and the type of implanted coronary stents (p = 0.185) (Table 2).
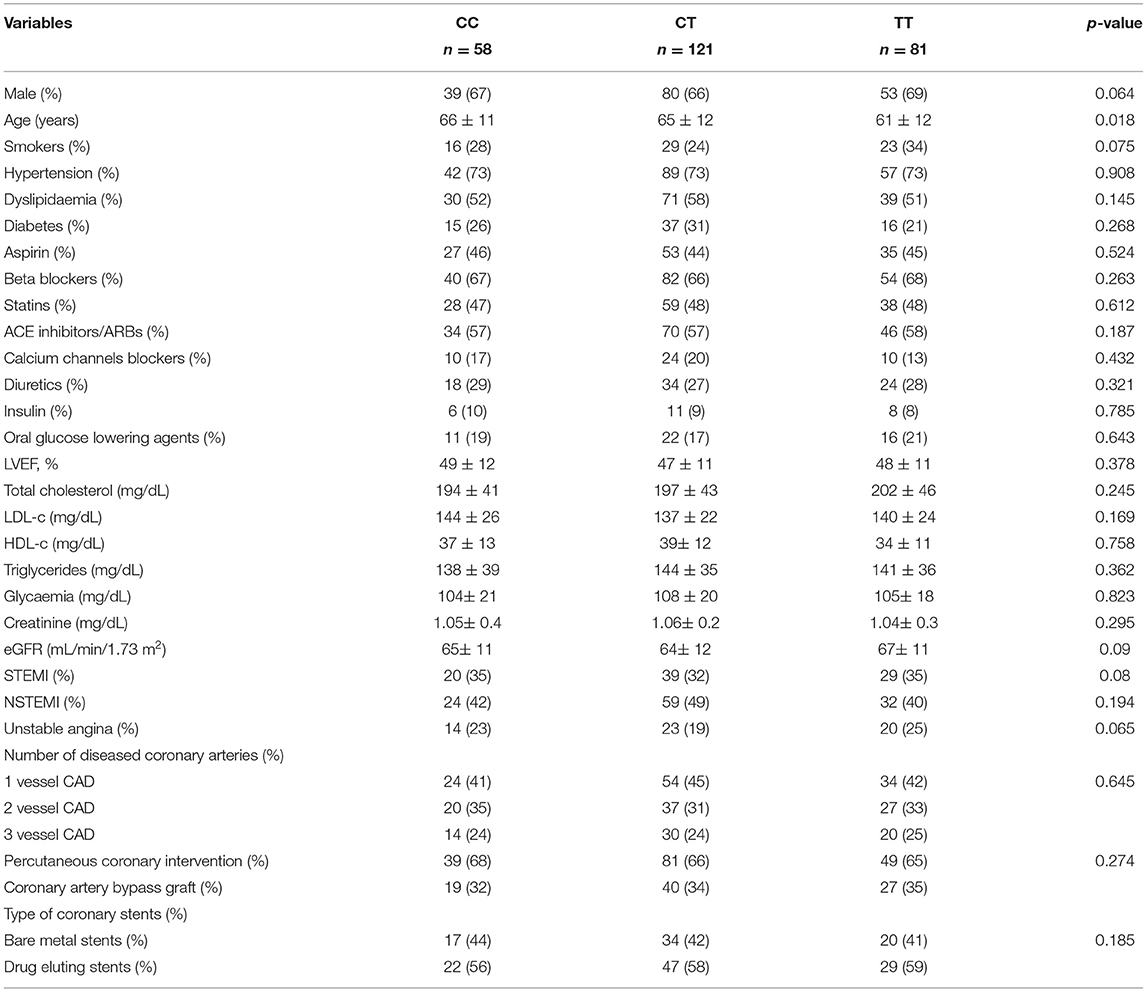
Table 2. Main clinical variables of the study sample according to NDUFC2/rs11237379 genotype at the time of a first ACS occurrence.
The carrier status of the TT genotype was associated with a younger age at the first ACS event compared to subjects carrying the CT and CC genotypes (61 ± 12, 65 ± 12 and 66 ± 11 years, respectively; p = 0.018). After adjustment for gender, hypertension, smoking habit, diabetes and hypercholesterolemia, we observed a trend toward statistical significance (p = 0.051).
The median follow-up time was 2.4 years (interquartile range 1.7–6.8 years). During the follow-up period, recurrent ACS episodes were experienced by 25 patients in the CC genotype group (43.1%), 53 subjects in the CT genotype group (43.8%) and 47 in the TT genotype group (58%). Among the 125 patients who experienced recurrent ACS, 75 were treated with PCI (60%) and 50 with CABG (40%), without significant differences among groups (p = 0.655).
Presence of the T allele associated with increased occurrence of recurrent ACS by a recessive mode of transmission (odds ratio [OR] 2.7, 95% confidence interval [CI] 1.39–5.26; p = 0.003).
At univariate analysis, the TT genotype carrier status and diabetes showed a significant impact on recurrent ACS (Table 3), maintaining a significant predictive role also at multivariate Cox analysis (Table 4). The treatment with statins was confirmed as a significant protective factor toward ACS recurrence over the follow-up time (Tables 3, 4).
Discussion
The major goals of the present study were to investigate (1) the impact of the carrier status of the T vs. C allele at NDUFC2/rs11237379 variant on the occurrence of a first ACS episode and (2) the prognostic impact of T vs. C allele on the occurrence of new coronary events (unstable angina, NSTEMI, STEMI) in CAD patients. The results of the study demonstrate that ACS tended to occur at a younger age in patients carrying the TT genotype and, most importantly, that carrier status for the TT genotype was a significantly independent risk factor for ACS recurrence in an Italian cohort of CAD patients. The frequency of the TT genotype was 29% in our cohort, consistent with previous reports (11, 12). Notably, the T allele at this variant is known to associate with reduced NDUFC2 expression (10, 11).
As previously shown, the downregulation of NDUFC2 is responsible of an impairment of the complex I assembly leading to a reduction of its activity, decreased ATP synthesis and increased ROS generation (10, 11). In turn, the accumulation of ROS is known to promote mitochondrial protein and DNA damage with subsequent alterations of mitochondrial structure and function and further increase of ROS levels. At the vascular level, the accumulation of ROS causes a reduction of nitric oxide bioavailability, which compromises vascular relaxation and favors the atherosclerosis development (20, 21). The latter underlies the decrease of coronary and cerebral blood flow ultimately producing organ and tissue ischemia. More specifically, NDUFC2 downregulation in vascular cells is reported to cause deleterious effects on cell viability, impairing angiogenesis, and stimulating the release of molecules involved in atherogenesis and plaque instability (10, 22).
NDUFC2 downregulation was first discovered as a contributory mechanism to cerebral and renal vascular damage upon a high-salt Japanese-style diet (JD) in the animal model of stroke-prone spontaneously hypertensive rat (SHRSP) (11). The same renal and cerebrovascular injuries developed in the animal model of JD-fed spontaneously hypertensive stroke-resistant rat (SHR-SR) once subjected to a heterozygous deletion of Ndufc2 (11).
In humans, the T allele variant at NDUFC2/rs11237379 was associated with increased occurrence of early-onset ischemic stroke by a recessive mode of transmission (11). The functional significance of rs11237379 was documented by its direct relationship with gene expression level, with the T allele being significantly associated with reduced gene expression (11). Consistently, another study documented that the reduced gene expression associated to the TT genotype led to increased oxidative stress and significant ultrastructural impairment of mitochondrial morphology with a loss of internal cristae, particularly after the exposure to stress stimuli such as high-NaCl concentration or LPS (10).
Furthermore, NDUFC2 mRNA level was significantly downregulated, along with a higher degree of mitochondrial structural damage and dysfunction, in ACS compared to stable angina patients (7). In-vitro, NDUFC2 silencing favored the endothelial expression of tumor necrosis factor α (TNFα), intercellular adhesion molecule 1 (ICAM), vascular cell adhesion molecule 1 (VCAM), matrix metallopeptidase 9 (MMP9) and CD40 ligand (CD40L) (7), all markers of inflammation, atherogenesis and plaque instability (23).
The results of the present study strongly suggest that the carrier status of the TT genotype at the NDUFC2/rs11237379 is an independent predictor of risk for ACS.
The clinical characteristics of our population were comparable with those of previously reported CAD patients' cohorts, with a higher prevalence of common risk factors for CAD, namely hypertension, hypercholesterolemia, and diabetes. The latter turned out to be a significant risk factor for recurrence of ACS in our study sample, whereas both hypertension and dyslipidemia were not (likely because of the ongoing medical treatments). Regarding the impact of therapy, the use of statins was confirmed as a protective strategy for secondary prevention of ACS (24).
Based on the previous evidence and the current results, the knowledge of the carrier status for the TT genotype at NDUFC2/rs11237379 may contribute to identify a more complete CV risk profile of affected patients and may provide a straightforward indication for more aggressive treatment strategies as well as for more frequent clinical monitoring, potentially improving the management of patients with CV risk factors and history of CV diseases.
The principal limitations of our study include the relatively small sample size, the single center design, the inclusion of only Caucasian patients and the lack of a control group represented by CAD patients without ACS.
Conclusions
In a prospective cohort of Italian CAD patients, the TT NDUFC2/rs11237379 genotype appears to predispose to earlier ACS occurrence in carrier subjects. Remarkably, patients experiencing ACS have a significantly increased risk to develop new coronary events. In this regard, the carrier status for the TT genotype may represent a novel genetic CV risk factor. Overall, our findings support the role of NDUFC2 deficiency, and of the consequent complex I-dependent mitochondrial dysfunction, as a relevant contributory mechanism to the development of endothelial and vascular damage and of atherothrombotic events ending with ACS.
Further studies are certainly needed to confirm and strengthen the current findings and to support a potential indication for screening the allele/genotype configuration at the NDUFC2/rs11237379 variant in CAD patients in the attempt to improve both clinical and therapeutic management.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical Committee of the S. Andrea Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
GG and SM selected the patients and performed the statistical analysis. GG wrote the manuscript draft. MC, RS, FB, and SM performed the genetic analysis. SB selected the patients and performed the follow-up. MV and CA revised the manuscript. SR conceived the work, revised the manuscript, and provided the funding. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the Italian Ministry of Health, by a University Sapienza grant (project number RM1181641BF8C865), by Progetto PRIN 2017 (from the Italian Ministry of Instruction, University and Research, no. 2017PZY5K7).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Visseren F, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. ESC National Cardiac Societies, ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. (2021) 42:3227–337. doi: 10.1093/eurheartj/ehab484
2. McPherson R, Tybjaerg-Hansen A. Genetics of coronary artery disease. Circ Res. (2016) 118:564–78. doi: 10.1161/CIRCRESAHA.115.306566
3. Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. (2007) 100:460–73. doi: 10.1161/01.RES.0000258450.44413.96
4. Bisaccia G, Ricci F, Gallina S, Di Baldassarre A, Ghinassi B. Mitochondrial dysfunction and heart disease: critical appraisal of an overlooked association. Int J Mol Sci. (2021) 22:614. doi: 10.3390/ijms22020614
5. Ramachandra C, Hernandez-Resendiz S, Crespo-Avilan GE, Lin YH, Hausenloy DJ. Mitochondria in acute myocardial infarction and cardioprotection. EBioMedicine. (2020) 57:102884. doi: 10.1016/j.ebiom.2020.102884
6. Peoples JN, Saraf A, Ghazal N, Pham TT, Kwong JQ. Mitochondrial dysfunction and oxidative stress in heart disease. Exp Mol Med. (2019) 51:1–13. doi: 10.1038/s12276-019-0355-7
7. Raffa S, Chin X, Stanzione R, Forte M, Bianchi F, Cotugno M, et al. The reduction of NDUFC2 expression is associated with mitochondrial impairment in circulating mononuclear cells of patients with acute coronary syndrome. Int J Cardiol. (2019) 286:127–33. doi: 10.1016/j.ijcard.2019.02.027
8. Gershoni M, Levin L, Ovadia O, Toiw Y, Shani N, Dadon S, et al. Disrupting mitochondrial-nuclear coevolution affects OXPHOS complex I integrity and impacts human health. Genome Biol Evol. (2014) 6, 2665–80. doi: 10.1093/gbe/evu208
9. Mimaki M, Wang X, McKenzie M, Thorburn DR, Ryan MT. Understanding mitochondrial complex I assembly in health and disease. Biochim Biophys Acta. (2012) 1817:851–62. doi: 10.1016/j.bbabio.2011.08.010
10. Raffa S, Scrofani C, Valente S, Micaloni A, Forte M, Bianchi F, et al. In vitro characterization of mitochondrial function and structure in rat and human cells with a deficiency of the NADH: ubiquinone oxidoreductase Ndufc2 subunit. Hum Mol Genet. (2017) 26:4541–55. doi: 10.1093/hmg/ddx333
11. Rubattu S, Di Castro S, Schulz H, Geurts AM, Cotugno M, Bianchi F, et al. Ndufc2 Gene inhibition is associated with mitochondrial dysfunction and increased stroke susceptibility in an animal model of complex human disease. J Am Heart Assoc. (2016) 5:e002701. doi: 10.1161/JAHA.115.002701
12. Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, et al. 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature. (2015) 526:68–74. doi: 10.1038/nature15393
13. Thygesen K Alpert JS Jaffe AS Simoons ML Chaitman BR White HD Writing Writing Group on the Joint ESC/ACCF/AHA/WHF and and Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Euro Heart J. (2012) 33:2551–2567. doi: 10.1093/eurheartj/ehs184
14. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. and Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Global Heart. (2018) 13: 305–38. doi: 10.1161/CIR.0000000000000617
15. Neal B, MacMahon S. The World Health Organization-International Society of Hypertension Blood Pressure Lowering Treatment Trialists' Collaboration: prospective collaborative overviews of major randomized trials of blood pressure-lowering treatments. Curr Hypert Rep. (1999) 1:346–56. doi: 10.1007/s11906-999-0045-2
16. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. (2020) 38:982–1004. doi: 10.1097/HJH.0000000000002453
17. American Diabetes Association. Standards of medical care in diabetes−2012. Diabetes Care. (2012) 35 Suppl 1(Suppl 1):S11–S63. doi: 10.2337/dc12-s011
18. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. (2018) 41(Suppl 1):S13–S27. doi: 10.2337/dc18-S002
19. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Chamber Quantification Writing Group, American Society of Echocardiography's Guidelines and Standards Committee, and European Association of Echocardiography Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. (2005) 18:1440–1463. doi: 10.1016/j.echo.2005.10.005
20. Chen Q, Wang Q, Zhu J, Xiao Q, Zhang L. Reactive oxygen species: key regulators in vascular health and diseases. Br J Pharmacol. (2018) 175:1279–92. doi: 10.1111/bph.13828
21. Gallo G, Volpe M, Savoia C. Endothelial dysfunction in hypertension: current concepts and clinical implications. Front Med. (2022) 8:798958. doi: 10.3389/fmed.2021.798958
22. Forte M, Bianchi F, Cotugno M, Marchitti S, De Falco E, Raffa S, et al. Pharmacological restoration of autophagy reduces hypertension-related stroke occurrence. Autophagy. (2020) 16:1468–81. doi: 10.1080/15548627.2019.1687215
23. Lutgens E, Lievens D, Beckers L, Donners M, Daemen M. CD40 and its ligand in atherosclerosis. Trends Cardiovasc Med. (2007) 17:118–23. doi: 10.1016/j.tcm.2007.02.004
Keywords: acute coronary syndrome, genetic, NDUFC2, complex I, mitochondrial dysfunction
Citation: Gallo G, Migliarino S, Cotugno M, Stanzione R, Burocchi S, Bianchi F, Marchitti S, Autore C, Volpe M and Rubattu S (2022) Impact of a NDUFC2 Variant on the Occurrence of Acute Coronary Syndromes. Front. Cardiovasc. Med. 9:921244. doi: 10.3389/fcvm.2022.921244
Received: 15 April 2022; Accepted: 05 May 2022;
Published: 31 May 2022.
Edited by:
Michele Ciccarelli, University of Salerno, ItalyReviewed by:
Marco Di Maio, University of Salerno, ItalyMarco Matteo Ciccone, University of Bari Aldo Moro, Italy
Copyright © 2022 Gallo, Migliarino, Cotugno, Stanzione, Burocchi, Bianchi, Marchitti, Autore, Volpe and Rubattu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Speranza Rubattu, c3BlcmFuemFkb25hdGVsbGEucnViYXR0dUB1bmlyb21hMS5pdA==
†These authors have contributed equally to this work
 Giovanna Gallo
Giovanna Gallo Serena Migliarino2†
Serena Migliarino2† Rosita Stanzione
Rosita Stanzione Simone Burocchi
Simone Burocchi Franca Bianchi
Franca Bianchi Simona Marchitti
Simona Marchitti Camillo Autore
Camillo Autore Massimo Volpe
Massimo Volpe