
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 08 September 2022
Sec. Cardiovascular Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.921204
This article is part of the Research Topic Metabolic Syndrome-Associated Cardiovascular Diseases: From targets to therapeutic drugs View all 4 articles
 Yuqi Zhu1
Yuqi Zhu1 Gang Li1*
Gang Li1* Jari A. Laukkanen2,3,4
Jari A. Laukkanen2,3,4 Xing Song1
Xing Song1 Jing Zhang1
Jing Zhang1 Linping Wei1
Linping Wei1 Xinrui Chen1
Xinrui Chen1 Yufeng Li1
Yufeng Li1 Cheng Liu1
Cheng Liu1Background: Previous studies have shown that metabolic syndrome (MetS) is associated with increased systemic inflammation and cardiac mortality in elderly subjects. However, information on the association of inflammation markers with cardiac adverse remodeling is limited in the elderly with MetS. Therefore, we investigated whether the inflammatory marker neutrophil/lymphocyte ratio (NLR) is associated with the cardiac adverse remodeling in Chinese elderly with MetS.
Methods: A total of 1,087 hospitalized Chinese elderly (aged ≥ 65 years) with MetS were collected retrospectively. The cross-sectional data of echocardiography and clinical parameters were compared among quartile NLR groups.
Results: In the elderly with MetS, higher quartile NLR (≥3.83) was found to be associated with male gender, older age, lower estimated glomerular filtration rate (eGFR), and cardiac left ventricular (LV) dilatation (all p <0.05).
Conclusion: Higher NLR is associated with male gender, older age, renal dysfunction, and cardiac adverse remodeling in Chinese elderly with MetS.
Metabolic syndrome (MetS) presents with a cluster of chronic metabolic abnormalities, which include obesity, hyperglycemia, high blood pressure (BP) hypertriglyceridemia, and decreased high-density lipoprotein cholesterol (HDL-C) (1). MetS is considered to be a disorder with chronic systemic low-grade inflammation (2). The inflammatory response plays a central role in the development and progression of MetS and its unwanted consequences, such as atherosclerotic cardiovascular diseases (3). Recent studies reported that neutrophil to lymphocyte ratio (NLR), as a marker of inflammation, was increased in the MetS. Indeed, NLR could be used as one potential marker of MetS development (4, 5). Furthermore, higher NLR was found to be associated with the incidence of the MetS components, such as hypertension and type 2 diabetes mellitus (DM) (6–8). Previous studies have demonstrated an association between higher NLR and the severity of coronary artery disease (9, 10). Moreover, NLR was reported to be a strong prognostic indicator for cardiac adverse remodeling (11), an increased risk of cardiac events, and mortality from coronary artery disease (12–15). NLR is a promising biomarker, which is an easily available measure in clinical practice. NLR measurement has a high effectiveness-cost ratio and good reliability due to its lower variability by treatments (5, 12, 16). In addition, MetS is a common clinical condition in the elderly people (17–19). Studies have shown that elderly patients with MetS were vulnerable to increased coronary atherosclerosis-related events and cardiac mortality (20–23). MetS has been found to be related to cardiac structural changes and left ventricular (LV) dysfunction in adults (24, 25). A higher level of inflammation and increased incidence of atherosclerosis-associated LV dysfunction have been discovered in the elderly with MetS (26). However, there is still limited information on whether NLR is correlated with the adverse cardiac structural remodeling in the elderly with MetS. Therefore, the aim of this study was to explore whether NLR is associated with the cardiac adverse remodeling in Chinese elderly with MetS.
At the department of endocrinology in our hospital, a total of 1,087 Chinese elderly (aged ≥ 65 years) with MetS were collected retrospectively by review of medical records from 2014 to 2020 in a cross-sectional study.
In the present study, MetS was defined as having three or more of the following abnormalities (27). (1) Fasting plasma glucose (PG) impairment (7 mmol/L > fasting PG ≥ 6.1 mmol/L), oral 75 g glucose post-loaded 2-h PG (2 hPG) impairment (11.1 mmol/L > 2 hPG ≥ 7.8 mmol/L), or confirmed type 2 DM that is under anti-diabetic treatments. Type 2 DM was diagnosed if fasting PG ≥7.0 mmol/L and/or 2 hPG ≥11.1 mmol/L, while the subject's plasma insulin level was normal or increased (28). (2) High normal BP (140 mmHg > systolic BP ≥ 130 and/or 90 mmHg > diastolic BP ≥ 85 mmHg) or hypertension with the use of medication. Hypertension was confirmed if the subjects had average systolic BP ≥ 140 mmHg and/or diastolic BP ≥90 mmHg in upper right arm after 10 min of rest and two measurements of BP. (3) Hypertriglyceridemia [fasting plasma triglyceride (TG) ≥ 1.70 mmol/L]. (4) Decreased HDL-C (fasting plasma HDL-C <1.03 mmol/L in men; HDL-C <1.29 mmol/L in women). The MetS criterion used in this study was from the National Cholesterol Education Program Adult Treatment Panel III (27). However, in our study, the patient's information on waist circumference was not available from the medical records. Thus, the waist circumference was not used in the diagnosis of MetS in this study.
Gout, which was triggered by the crystallization of monosodium urate monohydrate inside the joints, was considered when there were gouty tophus, arthralgia, and arthritis (29). Coronary heart disease was diagnosed if there were signs of myocardial infarction on 12-lead electrocardiograms (ECG), angiography showed atherosclerotic stenosis ≥50% in one of the coronary artery lumens, or a history of coronary stent implantation was confirmed. Stroke criteria were based on typical findings on computed tomograms or magnetic resonance imaging (MRI) (30). All patients were examined by color Doppler echocardiography. White blood cell (WBC) count was assessed using a Sysmex XN-1000 Analyzer (Sysmex Corporation, Kobe, Japan). The NLR was then calculated manually. The WBC and NLR were measured averagely of three times within the first week of hospitalization for each patient, respectively.
We excluded patients with the infection that included acute viral illness, autoimmune diseases, acute gout, stroke, myocardial infarction, rheumatic or degenerative cardiac valvular disease, congenital heart disease, idiopathic cardiomyopathy, atrial fibrillation, chronic obstructive pulmonary disease, leukemia, and other malignant tumors.
For men, serum creatinine (Scr) > 80 μmol/L: estimated GFR [eGFR = 141 (Scr μmol/L/88.4/0.9)−1.209 0.993age(years)]; Scr ≤ 80 μmol/L: [eGFR = 141 (Scr μmol/L/88.4/0.9)−0.411 · 0.993age(years)]. For women, Scr > 62 μmol/L, eGFR = 144 × (Scr μmol/L/88.4/0.7) −1.209 × 0.993age(years); Scr ≤ 62 μmol/L, eGFR = 144 × (Scr μmol/L/88.4/0.7) −0.329 × 0.993age(years) (31). BMI = body weight (kg)/[height (m)]2 (32, 33).
Glucose was measured by hexose enzyme colorimetry. Hemoglobin A1c (HbA1c) was determined by high-performance liquid chromatography. TG and total cholesterol were detected by enzyme colorimetry. HDL-C was measured by homogeneous enzyme colorimetry. Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald formula. High sensitivity C-reactive protein (hsCRP) was determined by immunoturbidimetry (34).
Right atrial diameter, right ventricular diameter (RVD), left atrial diameter (LAD), LV end-systolic (LVESD) and end-diastolic diameter (LVEDD), interventricular septal thickness (IVST), LV posterior wall thickness (LVPWT), and LV ejection fraction (LVEF) were measured by transthoracic two-dimensional M-mode echocardiography using GE Vivid7 with full digital color Doppler ultrasound diagnostic instrument (35).
The statistical package for social science (SPSS) 26.0 software (IBM Company, Chicago, IL, USA) was used for statistical analysis. The cutoff values of quartile NLR used in the current study were calculated by the SPSS software, according to the NLR values of the elderly subjects in the present study. The categorical data were expressed as a percentage (%) and compared by chi-square test between two groups or Fisher exact test between multiple groups. The continuous data were expressed as mean values ± standard deviation (SD). If the variables were normally distributed, they were analyzed by one-way analysis of variance (ANOVA) between multiple groups, then followed by post-hoc analysis for comparison between two groups by least significance difference (LSD) test. If the data were in non-normal distribution, it was analyzed by Kruskal–Wallis H test between multiple groups. Then, the Mann–Whitney U test was used for comparison between the two groups. Pearson correlation analysis was used for univariate analysis. Dichotomized logistic regression analysis was used for multivariate analysis. Given that WBC, neutrophil count, lymphocyte count, and hsCRP were collinear to NLR, these parameters were not included in the regression analysis. A 2-tailed value of p < 0.05 was considered to be statistically significant.
The clinical characteristics of subjects according to the quartile of NLR are shown in Table 1. Compared with the first quartile NLR (NLR <2.03) group, patient's prevalence rates of male gender, HbA1c, WBC, neutrophil count, and hsCRP were higher; while eGFR and lymphocyte count were lower in the second quartile NLR (2.03 ≤ NLR <2.82) group (All p < 0.05, Table 1). Prevalence rates of male gender and smoking, HbA1c, WBC, neutrophil count, and hsCRP were higher; diabetes and hypertension had a longer duration; while TG, HDL-C, eGFR, and lymphocyte count were lower in the second quartile NLR (2.03 ≤ NLR <2.82) group (All p < 0.05, Table 1). Prevalence rates of male gender and smoking, HbA1c, WBC, neutrophil count, and hsCRP were higher; diabetes and hypertension had a longer duration; while TG, HDL-C, eGFR, and lymphocyte count were lower in the third quartile NLR (2.82 ≤ NLR <3.83) group (All p < 0.05, Table 1); prevalence rates of male gender, smoking, drinking and gout, systolic and diastolic BP, HbA1c, WBC, neutrophil count, and hsCRP were higher; age was older; diabetes, hypertension, and gout had a longer duration; while TG, HDL-C, LDL-C, eGFR, and lymphocyte count were lower in the fourth quartile NLR (NLR ≥ 3.83) group (All p < 0.05, Table 1).
Compared with the second quartile NLR group, the prevalence rates of the male gender, neutrophil count, and hsCRP were higher; while HDL-C, LDL-C, and lymphocyte count were lower in the third quartile NLR group (All p < 0.05, Table 1); prevalence rates of the male gender, smoking and gout, systolic and diastolic BP, WBC, neutrophil count, and hsCRP were higher; age was older; gout had a longer duration; while HDL-C, LDL-C, eGFR, and lymphocyte count were lower in the fourth quartile NLR group (all p < 0.05, Table 1). Compared with the third quartile NLR group, prevalence rates of the male gender, smoking and gout, WBC, neutrophil count, and hsCRP were higher; while eGFR and lymphocyte count were lower in the fourth quartile NLR group (all p < 0.05, Table 1).
Binary correlation analysis showed that NLR was correlated positively with prevalence rates of male gender, smoking, drinking and gout, age, diabetes, hypertensive and gout duration, systolic and diastolic BP, HbA1C, WBC, neutrophil count, and hsCRP; while it was correlated reversely with TG, HDL-C, LDL-C, eGFR, and lymphocyte count (all p < 0.05, Table 1, Figure 1).
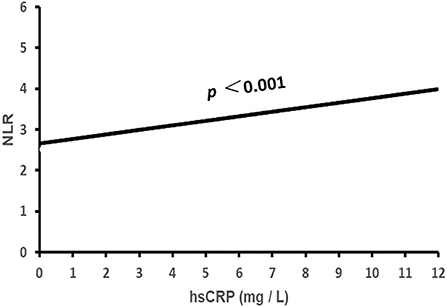
Figure 1. The association of NLR with hsCRP. NLR, neutrophil to lymphocyte ratio; hsCRP, high sensitivity C-reactive protein.
The results on the cardiac remodeling of subjects according to the quartile of NLR are shown in Table 2. Compared with first quartile NLR group, patient's LAD and LVEDD were larger in the second quartile NLR group (all p < 0.05, Table 2); RVD, LAD, LVESD, LVEDD, IVST, and LVPWT were larger in the third quartile NLR group (all p < 0.05, Table 2); and RVD, LAD, LVESD, LVEDD, IVST, and LVPWT were larger, while LVEF was lower in the fourth quartile NLR group (all p < 0.05, Table 2).
Compared with the second quartile NLR group, IVST was larger in the third quartile NLR group (all p < 0.05, Table 2); LVESD and LVEDD were larger, while LVEF was lower in the fourth quartile NLR group (all p < 0.05, Table 2); and compared with third quartile NLR group, LVESD and LVEDD were larger in the fourth quartile NLR group (all p < 0.05, Table 2).
Binary correlation analysis showed that NLR was correlated positively with RVD, LAD, LVESD LVEDD, IVST, and LVPWT, while it was correlated reversely with LVEF (all p < 0.05, Table 2).
The correlation of NLR with the subject's clinical characteristics and cardiac remodeling is shown in Table 3 and Figures 2–5. Dichotomized variable regression analysis showed that the fourth quartile NLR (≥3.83) was associated independently with male gender, older age, decreased eGFR, and cardiac LV dilatation (all p < 0.05, Table 3, Figures 2–5), after adjustment of patient's other parameters.
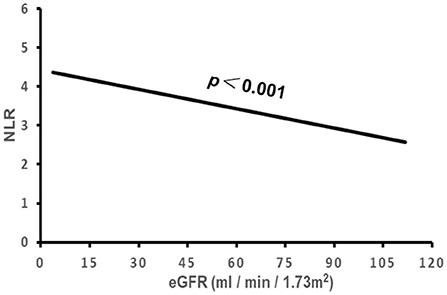
Figure 4. The association of NLR with eGFR. NLR, neutrophil to lymphocyte ratio; eGFR, estimated glomerular filtration rate.
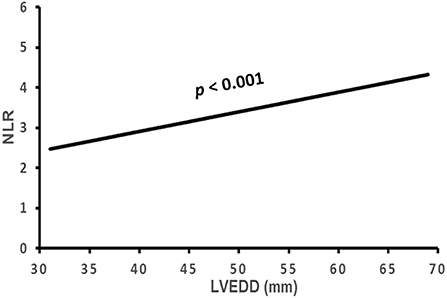
Figure 5. The association of NLR with LVEDD. NLR, neutrophil to lymphocyte ratio; LVEDD, left ventricular end-diastolic diameter.
The present study shows that higher NLR is independently associated with male gender, older age, lower eGFR, and cardiac LV remodeling in Chinese elderly with MetS.
There are some potential underlying mechanisms explaining the association between NLR and cardiac LV remodeling. As a core component of the MetS, obesity usually manifests as increased fat accumulation in visceral organs. Moreover, abdominal obesity is commonly observed in patients with MetS (36). Obesity is often associated with dyslipidemia. The prevailing consensus is that the pro-inflammatory molecules mainly derive from excessive visceral adipose tissues in patients with MetS (37). The visceral adipose tissue can secrete pro-inflammatory adipokines, leading to local and systemic inflammatory responses (38). This phenomenon is called lipotoxicity (39). With unhealthy lifestyles and increase in age, fat accumulates more in viscera, leading to increased systemic inflammatory responses (2). In the present study, we found that older age was associated with higher inflammatory marker NLR in elderly MetS. This finding is consistent with other earlier results in the elderly population (40). Our study also found that higher NLR was more commonly seen in elderly men with MetS. A higher level of inflammation in the older men may be due to unhealthy lifestyles and older age (41, 42).
Excessive adipose tissue and its secreted pro-inflammatory mediators (37) may contribute mainly to the over activation of sympathetic nervous and renin angiotensin systems (RAS) in MetS (43). This activated RAS could also be involved interactively in the aggravation of inflammatory reactions (44). Then, the higher inflammatory responses lead to increased apoptosis in glomerular endothelial cells and ensuing chronic kidney insufficiency in MetS (45). Due to adipose tissue retention-associated insulin resistance and impaired use of glucose exist in MetS, all kinds of cells are forced to use more fatty acid oxidation for their energy supply (46). However, this kind of energy supply will inevitably result in an increase in cellular oxidative stresses and inflammatory responses. Meanwhile, type 2 DM and diabetic nephropathy appear consequently (47, 48). Long-term increased inflammatory responses eventually cause glomerular endothelial injury and chronic kidney disease (49). Higher inflammatory marker NLR was observed to be associated with renal dysfunction and poor renal outcomes in adults with chronic kidney disease (50, 51). However, to our knowledge, the present study is the first to show that higher NLR is associated with lower eGFR in the elderly with MetS.
As the similar underlying mechanisms mentioned above (37), the over activated sympathetic nerve and RAS in MetS (43, 44) may also lead to increased aldosterone, water-sodium retention, cardiac overload, higher BP, resultant increased cardiomyocytic apoptosis, and myocardial fibrosis. Subsequently, this may cause cardiac remodeling and dysfunction in MetS (52, 53). Indeed, the interactive effects between aldosterone and sympathetic nerve-RAS (54) may also contribute to cardiac remodeling and dysfunction. Furthermore, as the cellular insulin resistance and impaired use of glucose exist in MetS (46), excessive cellular oxidative stresses and inflammatory responses appear sequentially in MetS (47, 48). Chronic inflammatory responses in MetS can also cause cardiomyocytic injury and cardiac adverse remodeling (55). To our knowledge, the present study is the first to show that higher inflammatory marker NLR is associated with cardiac LV remodeling in the elderly with MetS.
There were no available data on the abdominal circumference, and we only used BMI to assess body weight and did not find the association between NLR and BMI in elderly MetS. However, BMI may not accurately reflect abdominal obesity. In addition, the medical records could not include all possible obesity assessments, such as abdominal circumference, which is an indicator for MetS diagnostic criteria. The abovementioned definition of gout did not include hyperuricemia. Gout and hyperuricemia are two different entities with varying clinicopathological implications. However, we did not differentiate hyperuricemia from gout in the patient data collection. The patients diagnosed with gout were required to have hyperuricemia in this study. Therefore, the reported prevalence of gout may not reflect the exact level of gout prevalence in this population, which is a study limitation. Furthermore, the current data were based on a cross-sectional study setting. Although multivariable regression analyses were performed, there could still be some other confounding factors that contributed to the observed associations. Therefore, the results of this study still need to be further verified by prospective randomized controlled trials in the future.
Higher NLR is independently associated with male gender, older age, lower eGFR, and LV cardiac adverse remodeling in Chinese elderly with MetS. NLR may be used as a potentially cost-effective biomarker for renal and cardiac complications in MetS.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by The First Affiliated Hospital of Chongqing Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
GL contributed to the conception and design of the study. YZ organized the database. YZ, GL, XS, JZ, and CL performed the statistical analysis. YZ and GL wrote the first draft of the manuscript. JL, LW, XC, and YL: wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This study was supported by the Natural Science Research Fund of the Medical Science and Technology Research Fund of Health Bureau of Chongqing City, China (Nos. 04-2-154 and 2009-2-290) and Chongqing Science and Technology Commission in Chongqing City, China (CSTC, No. 2007BB5276).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mozaffary A, Bozorgmanesh M, Sheikholeslami F, Azizi F, Eskandari Fhadaegh F. Added value of different metabolic syndrome definitions for predicting cardiovascular disease and mortality events among elderly population: Tehran lipid and glucose study. Eur J Clin Nutr. (2014) 68:853–8. doi: 10.1038/ejcn.2014.91
2. Guarner V, Rubio-Ruiz ME. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. Interdiscip Top Gerontol. (2015) 40:99–106. doi: 10.1159/000364934
3. Nishida K, Otsu K. Inflammation and metabolic cardiomyopathy. Cardiovasc Res. (2017) 113:389–98. doi: 10.1093/cvr/cvx012
4. Buyukkaya E, Karakas MF, Karakas E, Akcay AB, Tanboga IH, Kurt M, et al. Correlation of neutrophil to lymphocyte ratio with the presence and severity of metabolic syndrome. Clin Appl Thromb Hemost. (2014) 20:159–63. doi: 10.1177/1076029612459675
5. Liu CC, Ko HJ, Liu WS, Hung CL, Hu KC, Yu LY, et al. Neutrophil-to-lymphocyte ratio as a predictive marker of metabolic syndrome. Medicine. (2019) 98:e17537. doi: 10.1097/MD.0000000000017537
6. Jhuang YH, Kao TW, Peng TC, Chen WL, Li YW, Chang PK, et al. Neutrophil to lymphocyte ratio as predictor for incident hypertension: a 9-year cohort study in Taiwan. Hypertens Res. (2019) 42:1209–14. doi: 10.1038/s41440-019-0245-3
7. Liu X, Zhang Q, Wu H, Du H, Liu L, Shi H, et al. Blood neutrophil to lymphocyte ratio as a predictor of hypertension. Am J Hypertens. (2015) 28:1339–46. doi: 10.1093/ajh/hpv034
8. Guo X, Zhang S, Zhang Q, Liu L, Wu H, Du H, et al. Neutrophil:lymphocyte ratio is positively related to type 2 diabetes in a large-scale adult population: a Tianjin chronic low-grade systemic inflammation and health cohort study. Eur J Endocrinol. (2015) 173:217–25. doi: 10.1530/EJE-15-0176
9. Chen J, Chen MH, Li S, Guo YL, Zhu CG, Xu RX, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting the severity of coronary artery disease: a Gensini score assessment. J Atheroscler Thromb. (2014) 21:1271–82. doi: 10.5551/jat.25940
10. Arbel Y, Finkelstein A, Halkin A, Birati EY, Revivo M, Zuzut M, et al. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis. (2012) 225:456–60. doi: 10.1016/j.atherosclerosis.2012.09.009
11. Borekci A, Gur M, Turkoglu C, Baykan AO, Seker T, Sahin DY, et al. Neutrophil to lymphocyte ratio predicts left ventricular remodeling in patients with ST elevation myocardial infarction after primary percutaneous coronary intervention. Korean Circ J. (2016) 46:15–22. doi: 10.4070/kcj.2016.46.1.15
12. Adamstein NH, MacFadyen JG, Rose LM, Glynn RJ, Dey AK, Libby P, et al. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. (2021) 42:896–903. doi: 10.1093/eurheartj/ehaa1034
13. Agarwal R, Aurora RG, Siswanto BB, Muliawan HS. The prognostic value of neutrophil-to-lymphocyte ratio across all stages of coronary artery disease. Coron Artery Dis. (2022) 33:137–43. doi: 10.1097/MCA.0000000000001040
14. Arbel Y, Shacham Y, Ziv-Baran T, Laufer Perl M, Finkelstein A, Halkin A, et al. Higher neutrophil/lymphocyte ratio is related to lower ejection fraction and higher long-term all-cause mortality in ST-elevation myocardial infarction patients. Can J Cardiol. (2014) 30:1177–82. doi: 10.1016/j.cjca.2014.05.010
15. Kim S, Eliot M, Koestler DC, Wu WC, Kelsey KT. Association of neutrophil-to-lymphocyte ratio with mortality and cardiovascular disease in the Jackson Heart Study and modification by the Duffy antigen variant. JAMA Cardiol. (2018) 3:455–62. doi: 10.1001/jamacardio.2018.1042
16. Scicali R, Mandraffino G, Di Pino A, Scuruchi M, Ferrara V, Squadrito G, et al. Impact of high neutrophil-to-lymphocyte ratio on the cardiovascular benefit of PCSK9 inhibitors in familial hypercholesterolemia subjects with atherosclerotic cardiovascular disease: Real-world data from two lipid units. Nutr Metab Cardiovasc Dis. (2021) 31:3401–6. doi: 10.1016/j.numecd.2021.08.034
17. Liu B, Chen G, Zhao R, Huang D, Tao L. Temporal trends in the prevalence of metabolic syndrome among middle-aged and elderly adults from 2011 to 2015 in China: the China health and retirement longitudinal study (CHARLS). BMC Public Health. (2021) 21:1045. doi: 10.1186/s12889-021-11042-x
18. Li W, Song F, Wang X, Wang L, Wang D, Yin X, et al. Prevalence of metabolic syndrome among middle-aged and elderly adults in China: current status and temporal trends. Ann Med. (2018) 50:345–53. doi: 10.1080/07853890.2018.1464202
19. Yang Q, Lin Q, Guo D, Wang H, Liu J, Zhang X, et al. Association of carotid intima media thickness with metabolic syndrome among low-income middle-aged and elderly Chinese: a population-based cross-sectional study. Front Cardiovasc Med. (2021) 8:669245. doi: 10.3389/fcvm.2021.669245
20. Li W, Song F, Wang X, Wang D, Chen D, Yue W, et al. Relationship between metabolic syndrome and its components and cardiovascular disease in middle-aged and elderly Chinese population: a national cross-sectional survey. BMJ Open. (2019) 9:e027545. doi: 10.1136/bmjopen-2018-027545
21. Ju SY, Lee JY, Kim DH. Association of metabolic syndrome and its components with all-cause and cardiovascular mortality in the elderly: a meta-analysis of prospective cohort studies. Medicine. (2017) 96:e8491. doi: 10.1097/MD.0000000000008491
22. Sun DL, Wang JH, Jiang B, Li LS, Li LS, Wu L, et al. Metabolic syndrome vs. its components for prediction of cardiovascular mortality: a cohort study in Chinese elderly adults. J Geriatr Cardiol. (2012) 9:123–9. doi: 10.3724/SP.J.1263.2012.01172
23. Wen CJ, Lee YS, Lin WY, Huang HL, Yao CA, Sung PK, et al. The metabolic syndrome increases cardiovascular mortality in Taiwanese elderly. Eur J Clin Invest. (2008) 38:469–75. doi: 10.1111/j.1365-2362.2008.01965.x
24. Burroughs Pena M, Swett K, Schneiderman N, Spevack DM, Ponce SG, Talavera GA, et al. Cardiac structure and function with and without metabolic syndrome: the Echocardiographic Study of Latinos (Echo-SOL). BMJ Open Diabetes Res Care. (2018) 6:e000484. doi: 10.1136/bmjdrc-2017-000484
25. Ayalon N, Gopal DM, Mooney DM, Simonetti JS, Grossman JR, Dwivedi A, et al. Preclinical left ventricular diastolic dysfunction in metabolic syndrome. Am J Cardiol. (2014) 114:838–42. doi: 10.1016/j.amjcard.2014.06.013
26. Suzuki T, Katz R, Jenny NS, Zakai NA, LeWinter MM, Barzilay JI, et al. Metabolic syndrome, inflammation, and incident heart failure in the elderly: the cardiovascular health study. Circ Heart Fail. (2008) 1:242–8. doi: 10.1161/CIRCHEARTFAILURE.108.785485
27. Expert Committee. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
28. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. (1998) 15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7
29. Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the national health and nutrition examination survey, 2007–2016. Arthritis Rheumatol. (2019) 71:991–9. doi: 10.1002/art.40807
30. Liu C, Li G, Laukkanen JA, Hao L, Zhao Q, Zhang J, et al. Overweight and obesity are associated with cardiac adverse structure remodeling in Chinese elderly with hypertension. Sci Rep. (2019) 9:17896. doi: 10.1038/s41598-019-54359-9
31. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. (2012) 367:20–9. doi: 10.1056/NEJMoa1114248
32. Zhao Q, Laukkanen JA, Li Q, Li G. Body mass index is associated with type 2 diabetes mellitus in Chinese elderly. Clin Interv Aging. (2017) 12:745–52. doi: 10.2147/CIA.S130014
33. Zhang J, Li G, Laukkanen JA, Liu C, Song X, Zhu Y. Low body mass is associated with reduced left ventricular mass in Chinese elderly with severe COPD. Sci Rep. (2021) 11:13074. doi: 10.1038/s41598-021-92212-0
34. Song X, Li G, Zhu Y, Laukkanen JA. Glomerular filtration dysfunction is associated with cardiac adverse remodeling in menopausal diabetic Chinese women. Clin Interv Aging. (2021) 16:603–9. doi: 10.2147/CIA.S306342
35. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imag. (2015) 16:233–70. doi: 10.1093/ehjci/jev014
36. Boren J, Williams KJ. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: a triumph of simplicity. Curr Opin Lipidol. (2016) 27:473–83. doi: 10.1097/MOL.0000000000000330
37. Reddy P, Lent-Schochet D, Ramakrishnan N, McLaughlin M, Jialal I. Metabolic syndrome is an inflammatory disorder: a conspiracy between adipose tissue and phagocytes. Clin Chim Acta. (2019) 496:35–44. doi: 10.1016/j.cca.2019.06.019
38. Nishimura S, Manabe I, Nagai R. Adipose tissue inflammation in obesity and metabolic syndrome. Discov Med. (2009) 8:55–60.
39. Zlobine I, Gopal K, Ussher JR. Lipotoxicity in obesity and diabetes-related cardiac dysfunction. Biochim Biophys Acta. (2016) 1861:1555–68. doi: 10.1016/j.bbalip.2016.02.011
40. Hashemi Moghanjoughi P, Neshat S, Rezaei A, Heshmat-Ghahdarijani K. Is the neutrophil-to-lymphocyte ratio an exceptional indicator for metabolic syndrome disease and outcomes? Endocr Pract. (2022) 28:342–8. doi: 10.1016/j.eprac.2021.11.083
41. Syauqy A, Hsu CY, Rau HH, Chao JC. Association of dietary patterns, anthropometric measurements, and metabolic parameters with C-reactive protein and neutrophil-to-lymphocyte ratio in middle-aged and older adults with metabolic syndrome in Taiwan: a cross-sectional study. Nutr J. (2018) 17:106. doi: 10.1186/s12937-018-0417-z
42. Trtica Majnaric L, Guljas S, Bosnic Z, Seric V, Wittlinger T. Neutrophil-to-lymphocyte ratio as a cardiovascular risk marker may be less efficient in women than in men. Biomolecules. (2021) 11:528. doi: 10.3390/biom11040528
43. Kamide K. Role of renin-angiotensin-aldosterone system in metabolic syndrome and obesity-related hypertension. Curr Hypertens Rev. (2014) 9:238–45. doi: 10.2174/1573402110666140812122349
44. Das UN. Renin-angiotensin-aldosterone system in insulin resistance and metabolic syndrome. J Transl Int Med. (2016) 4:66–72. doi: 10.1515/jtim-2016-0022
45. Wen J, Guo CX, Lu MG, Lu Y, Huang Y, Liu X, et al. Gender-specific association between metabolic syndrome and decreased glomerular filtration rate in elderly population. Int Urol Nephrol. (2016) 48:389–97. doi: 10.1007/s11255-015-1172-0
46. Lee SH, Park SY, Choi CS. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. (2022) 46:15–37. doi: 10.4093/dmj.2021.0280
47. Paniagua JA. Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J Diabetes. (2016) 7:483–514. doi: 10.4239/wjd.v7.i19.483
48. Carrier A. Metabolic syndrome and oxidative stress: a complex relationship. Antioxid Redox Signal. (2017) 26:429–31. doi: 10.1089/ars.2016.6929
49. Dominguez J, Wu P, Packer CS, Temm C, Kelly KJ. Lipotoxic and inflammatory phenotypes in rats with uncontrolled metabolic syndrome and nephropathy. Am J Physiol Renal Physiol. (2007) 293:F670–9. doi: 10.1152/ajprenal.00021.2007
50. Akase T, Kawamoto R, Ninomiya D, Kikuchi A, Kumagi T. Neutrophil-to-lymphocyte ratio is a predictor of renal dysfunction in Japanese patients with type 2 diabetes. Diabetes Metab Syndr. (2020) 14:481–7. doi: 10.1016/j.dsx.2020.04.029
51. Yoshitomi R, Nakayama M, Sakoh T, Fukui A, Katafuchi E, Seki M, et al. High neutrophil/lymphocyte ratio is associated with poor renal outcomes in Japanese patients with chronic kidney disease. Ren Fail. (2019) 41:238–43. doi: 10.1080/0886022X.2019.1595645
52. Purwowiyoto SL, Prawara AS. Metabolic syndrome and heart failure: mechanism and management. Med Pharm Rep. (2021) 94:15–21. doi: 10.15386/mpr-1884
53. Oosterlinck W, Herijgers P. Cardiomyocyte changes in the metabolic syndrome and implications for endogeneous protective strategies. Expert Rev Cardiovasc Ther. (2014) 12:331–43. doi: 10.1586/14779072.2014.893825
54. Zhang ZH, Yu Y, Wei SG, Felder RB. Aldosterone-induced brain MAPK signaling and sympathetic excitation are angiotensin II type-1 receptor dependent. Am J Physiol Heart Circ Physiol. (2012) 302:H742–51. doi: 10.1152/ajpheart.00856.2011
Keywords: neutrophil/lymphocyte ratio, inflammation, renal dysfunction, cardiac remodeling, metabolic syndrome, elderly, age, gender
Citation: Zhu Y, Li G, Laukkanen JA, Song X, Zhang J, Wei L, Chen X, Li Y and Liu C (2022) Higher neutrophil to lymphocyte ratio is associated with renal dysfunction and cardiac adverse remodeling in elderly with metabolic syndrome. Front. Cardiovasc. Med. 9:921204. doi: 10.3389/fcvm.2022.921204
Received: 15 April 2022; Accepted: 15 August 2022;
Published: 08 September 2022.
Edited by:
M. Faadiel Essop, Stellenbosch University, South AfricaReviewed by:
Hani Sabbour, Cleveland Clinic Abu Dhabi, United Arab EmiratesCopyright © 2022 Zhu, Li, Laukkanen, Song, Zhang, Wei, Chen, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Li, Z2FuZ2xpY3FtdUAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.