- 1Department of Biostatistics, Epidemiology and Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Seoul, South Korea
- 3Samsung Advanced Institute for Health Sciences and Technology (SAIHST), Samsung Medical Center, Sungkyunkwan University, Seoul, South Korea
- 4Institute for Biomedical Informatics, University of Pennsylvania, Philadelphia, PA, United States
- 5Genomics and Computational Biology Graduate Group, University of Pennsylvania, Philadelphia, PA, United States
- 6Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, United States
- 7Samsung Genome Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
Background: Previous studies primarily targeted the ability of polygenic risk scores (PRSs) to predict a specific disease, and only a few studies have investigated the association between genetic risk scores and cardiovascular (CV) mortality. We assessed PRSs for coronary artery disease (CAD) and type 2 diabetes (T2DM) as the predictive factors for CV mortality, independent of traditional risk factors, and further investigated the additive effect between lifestyle behavior and PRS on CV mortality.
Methods: We used genetic and phenotypic data from UK Biobank participants aged 40–69 years at baseline, collected with standardized procedures. Genome-wide PRSs were constructed using >6 million genetic variants. Cox proportional hazard models were used to analyze the relationship between PRS and CV mortality with stratification by age, sex, disease status, and lifestyle behavior.
Results: Of 377,909 UK Biobank participants having European ancestry, 3,210 (0.8%) died due to CV disease during a median follow-up of 8.9 years. CV mortality risk was significantly associated with CAD PRS [low vs. very high genetic risk groups, CAD PRS hazard ratio (HR) 2.61 (2.02–3.36)] and T2DM PRS [HR 2.08 (1.58–2.73)], respectively. These relationships remained significant even after an adjustment for a comprehensive range of demographic and clinical factors. In the very high genetic risk group, adherence to an unfavorable lifestyle was further associated with a substantially increased risk of CV mortality [favorable vs. unfavorable lifestyle with very high genetic risk for CAD PRS, HR 8.31 (5.12–13.49); T2DM PRS, HR 5.84 (3.39–10.04)]. Across all genetic risk groups, 32.1% of CV mortality was attributable to lifestyle behavior [population attributable fraction (PAF) 32.1% (95% CI 28.8–35.3%)] and 14.1% was attributable to smoking [PAF 14.1% (95% CI 12.4–15.7%)]. There was no evidence of significant interaction between PRSs and age, sex, or lifestyle behavior in predicting the risk of CV mortality.
Conclusion: PRSs for CAD or T2DM and lifestyle behaviors are the independent predictive factors for future CV mortality in the white, middle-aged population. PRS-based risk assessment could be useful to identify the individuals who need intensive behavioral or therapeutic interventions to reduce the risk of CV mortality.
Introduction
Common chronic diseases such as cardiovascular (CV) disease or diabetes represent a huge public health burden. Cardiovascular disease (CVD) is one of the leading causes of mortality worldwide; it has been estimated that CVD accounts for about 30% of all-cause mortality (1). It is well established that type 2 diabetes mellitus (T2DM) is also a major risk factor for mortality (2). Moreover, people with T2DM have a higher risk of CV mortality than people without diabetes, and risk of mortality continuously increases as glycemic levels increase, even if levels do not reach those needed for a diabetes diagnosis (3). Thus, early screening and prevention in individuals at risk of these diseases are important strategies for reducing CV morbidity and mortality.
Traditional risk factors for common chronic diseases do not typically manifest early in life, and thus, it is difficult to fully identify high-risk individuals. Polygenic risk scores (PRSs) that comprise single-nucleotide polymorphisms (SNPs) offer a means for early screening of and preventive interventions in common chronic diseases, including coronary artery disease (CAD) and diabetes (4). Many studies have demonstrated the predictive ability of PRSs to identify those with higher genetic risk of incident disease. For instance, Khera et al. suggested that PRSs measuring the cumulative genetic burden of five common diseases are well correlated with the case status (5). However, most previous studies have assessed PRS in terms of predicting specific diseases; the utility of PRS in predicting mortality has not yet been evaluated comprehensively (5–7). A few studies have shown associations between PRS and disease-specific mortality but included only a small number of SNPs (8–11). Furthermore, a mortality event is a complicated outcome in which a number of potential confounding factors are involved. The majority of previous analyses did not adjust for the comprehensive range of baseline demographic, lifestyle, and clinical factors and thus could not fairly compare inherited effects with other acquired effects for mortality. It is also uncertain if a healthy lifestyle can offset the genetic risk for CV mortality. Thus, it is important to evaluate the association between CV mortality and a PRS for CAD and T2DM calculated using millions of SNPs, to test this association for significance after more thorough adjustments, and to examine any differences among individuals grouped by their age, sex, disease status, or lifestyle behaviors.
The UK Biobank is a nationwide, prospective cohort of participants aged 40 to 69 and provides a variety of genetic, phenotypic, and health-related information (12). We primarily aimed to investigate the associations between the PRSs for CAD and T2DM, lifestyle behaviors, and CV mortality risk in middle-aged UK Biobank participants of European ancestry. We further investigated the association between PRS and CV mortality according to age, sex, and disease status.
Materials and methods
Study population
The UK Biobank recruited 502,505 participants aged 40 to 69 years between 2007 and 2010 (12). At baseline, participants provided their signed consent and completed a touchscreen questionnaire, in-person interview, and physical assessment in one of 22 assessment centers across the UK. The touchscreen questionnaires covered sociodemographic factors, lifestyle behaviors, and health-related factors, such as smoking status, alcohol frequency, eating habits, and medical history. Anthropometric measurements, including height, weight, waist circumference, and blood pressure, were measured by trained staff according to a standard protocol. During the baseline assessment visit, blood samples were collected and processed by standardized protocols (13). Only baseline assessments were used in this study. The UK Biobank was approved by the National Research Ethics Committee (17 June 2011 [RES reference 11/NW/0382]; extended on 10 May 2016 [RES reference 16/NW/0274]). This research using the UK Biobank resource was approved under the application number 33002.
Genotyping and quality control
UK Biobank samples (version 3; March 2018) were genotyped using either the Affymetrix UK BiLEVE Axiom array or the Affymetrix UK Biobank Axiom array; these include >800,000 genotyped SNPs, 95% of which are shared between the two platforms. Imputation via IMPUTE2 was carried out centrally by UK Biobank researchers using the merged 1000 Genomes Project panel and UK 10K panel (14). We used genetic data from participants identified as “white-British” ancestry based on both self-report and principal component analysis of ancestry. We excluded individuals whose reported sex did not match with that inferred from genetic data and individuals with second-degree or closer relatives also in the Biobank. After exclusions, 377,909 individuals were eligible for the genetic analyses. After imputation, variant-level quality control (QC) was carried out by filtering SNPs based on the following: (1) minor allele frequency <0.01 and (2) imputation quality score (INFO) <0.3. A total of 9,505,768 imputed autosomal SNPs passed the QC criteria.
Polygenic risk scores
To generate individual genetic risk scores, we derived PRSs based on the precalculated weights for SNPs provided by Khera et al., which were determined using LDpred from large-scale genome-wide association study (GWAS) summary statistics and downloaded from https://cvd.hugeamp.org/downloads.html (5). The PRSs for T2DM and CAD were respectively constructed based on the GWAS summary statistics from the Diabetes Genetics Replication and Meta-analysis (DIAGRAM) consortium, consisting of 6,917,436 SNPs, and the Coronary Artery Disease Genome-wide Replication and Meta-analysis plus the Coronary Artery Disease Genetics (CARDIOGRAMplusC4D) consortium, consisting of 6,630,150 SNPs (15, 16). We computed the PRSs from beta coefficients as the weighted sum of the risk alleles by applying PLINK 1.90 with the score command.
Ascertainment of mortality outcomes
All participants provided their consent for follow-up linkage to their death registration and health-related records. Date and underlying cause of death were obtained from death certificates provided by the National Health Service Central Register (Scotland) and the National Health Service Information Center (England and Wales). Mortality data for our UK Biobank dataset were available until 30 November 2016 for centers in Scotland and until 31 January 2018 for centers in England and Wales. Causes of death were classified using the 10th revision of the International Statistical Classification of Disease (ICD-10).
Ascertainment of variables
Information on smoking status, alcohol frequency, physical activity, eating habits, medical history, and medication use were collected through a touchscreen questionnaire or in-person interview during the baseline visit for the UK Biobank project. Height, weight, and waist circumference were assessed by trained medical staff at the same time. Regular physical activity was defined as participating in either moderate activity ≥5 days a week or vigorous activity ≥3 days a week. We used four lifestyle factors: current smoking, obesity, physical activity, and dietary pattern, as recommended by the strategic goals of the American Heart Association (AHA) (7, 17, 18). Participants were categorized based on the overall lifestyle scores into the following four risk subgroups: favorable (defined as having at least three healthy lifestyle factors), intermediate (having two healthy lifestyle factors), and unfavorable (having one or fewer healthy lifestyle factors). Additional definitions and details regarding lifestyle factors are provided in Supplementary Table S1.
Diagnosis of prevalent CAD or T2DM at baseline was based on the self-report in an in-person interview at enrollment or on diagnostic and procedure codes in electronic health records. Prevalent CAD was defined as a composite of angina and myocardial infarction. T2DM ascertainment was based on the self-report during an in-person interview at enrollment or an ICD-10 diagnostic code in hospitalization records. Medical history of other major comorbidities was also collected concerning dyslipidemia, hypertension, heart failure, ischemic stroke, chronic lung disease, chronic kidney disease, and cancer. We used self-reported diagnoses, hospitalization records, and first occurrence information to define prevalent comorbidities; these definitions are presented in Supplementary Table S2. The Charlson Comorbidity Index (CCI), a composite comorbidity score, was calculated based on the ICD-10 codes and was used to assess the severity of any underlying comorbidities (19). Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (20).
Statistical analysis
Continuous variables are reported as means with standard deviations and categorical variables as frequencies and proportions. Baseline characteristics were compared between genetic risk groups using the chi-square test for categorical variables and ANOVA for continuous variables. The association between PRS status and mortality was investigated using Cox proportional hazards models. As in the previous studies, we categorized study participants based on the PRS into low, intermediate, or high groups (7, 18) and further classified the top 1% of the PRS distribution as a very high-risk group in the light of the curve of cumulative incidence of prevalent disease over the PRS distribution (Supplementary Figure S1). Thus, participants were categorized into the following four risk subgroups: low (0–19th percentile), intermediate (20–79th percentile), high (80–98th percentile), and very high (99th percentile). Model 1 was adjusted for age, sex, genotyping array, and the first ten principal components (PCs) of ancestry. Model 2 was fully adjusted for the major variables associated with risk of CV mortality, such as age, sex, genotyping array, the first ten PCs, baseline blood pressure, lifestyle behavior, laboratory findings, baseline use of medications, CCI, and major comorbidities, including hypertension, dyslipidemia, ischemic stroke, heart failure, cancer, chronic liver disease, chronic lung disease, and chronic kidney disease. We also calculated a p-value for linear trends in regression based on the categorical risk groups. The population attributable fraction (PAF) was calculated to quantify the proportion of CV mortality in a population attributable to lifestyle behaviors. Stratified analyses were conducted using cutoffs and considered lifestyle behaviors, age at enrollment, sex, and the presence of CAD and T2DM at baseline. To investigate whether effect modification of the association between PRS and mortality occurred for lifestyle behaviors, age, sex, or disease status, we assessed multiplicative interactions between PRS and each of the stratification variables. In our survival analyses, individuals were censored according to the date of follow-up loss, the date of follow-up end (31 January 2018 for England and Wales; 30 November 2016 for Scotland), or the date of death. Cases with missing data were excluded from modeling (Supplementary Table S3). Log minus log plots and Schoenfeld residuals were used to assess the proportional hazard assumption. We also conducted additional sensitivity analyses. First, we repeated the analyses by substituting outcome with death related to atherosclerotic CVD (ASCVD) and death related to CAD, which we defined based on the ICD-10 code listed as the primary cause of death. Second, given the possibility of potential competing risk for death, we performed analysis after exclusion of major comorbidities, including chronic liver disease, chronic lung disease, chronic kidney disease, and cancer. All statistical analyses were performed using PLINK 1.9 and R (version 3.9.0).
Results
Population characteristics
Baseline demographic and clinical characteristics of all 377,909 participants are presented in Table 1; characteristics for each PRS group are presented in Supplementary Table S4. Men comprised 46.3% of participants, and the mean age at the study baseline was 56.5 years. Overall, participants at higher genetic risk for CAD or T2DM tended to have higher blood pressure, BMI, waist circumference, HbA1c, eGFR, CCI, and more frequent use of aspirin, anti-hypertensive agents, or lipid-lowering agents than those at low genetic risk.
Association of PRS with cardiovascular mortality
During the median follow-up of 8.9 years (interquartile range 8.3–9.5 years), 15,570 participants (4.1%) died; of those deaths, 21.1% were related to CV. The overall mortality rate was 0.95 per 1000 person-years (95% confidence interval (CI) 0.92–0.99). Compared with low genetic risk, higher genetic risk was associated with a higher risk of CV mortality during follow-up (p for trend < 0.001) (Figure 1, Supplementary Table S5). In stepwise multivariable models, very high genetic risk for either CAD or T2DM remained significantly associated with a high risk of CV mortality, although the strength of the association was attenuated (Supplementary Table S5). In the fully adjusted model, participants at very high genetic risk for CAD or T2DM had approximately 2-fold increased risk of CV mortality relative to those with the corresponding low genetic risk. Associations between PRSs and mortality were attenuated when adjusting for PRS-specific disease status at baseline (Supplementary Table S5, model 4). In all sensitivity analyses, the results remained similar, irrespective of substituting death outcome due to ASCVD (Supplementary Table S6) or CAD (Supplementary Table S7), and after excluding those participants who had major comorbidities at baseline (Supplementary Table S8).
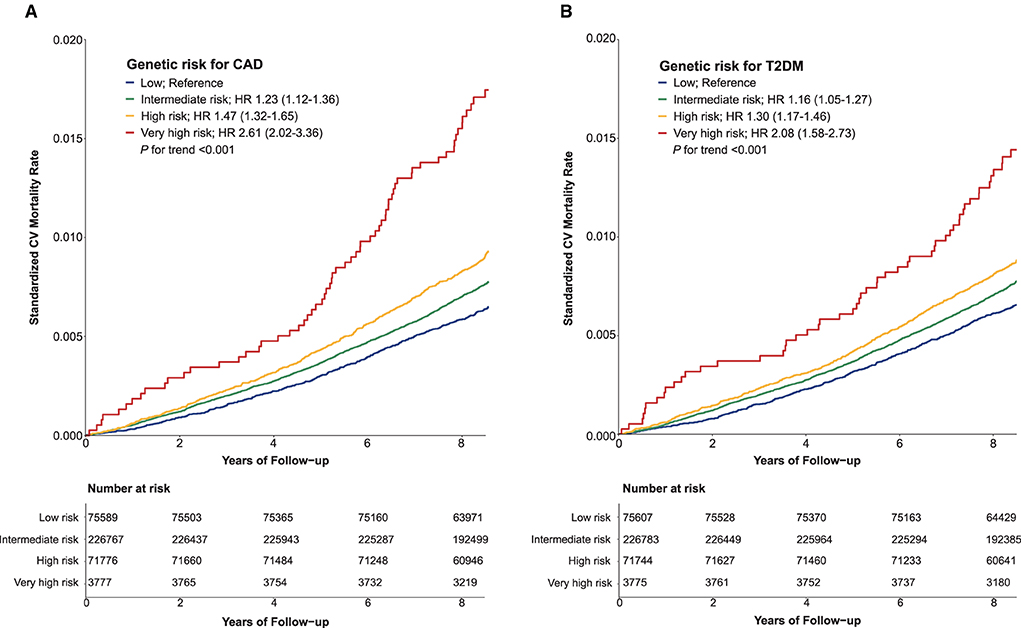
Figure 1. Standardized cardiovascular mortality rates according to categories of polygenic risk score for coronary artery disease (A) and type 2 diabetes mellitus (B). CAD, coronary artery disease; CV, cardiovascular; HR, hazard ratio; T2DM, type 2 diabetes mellitus.
PRS and lifestyle behaviors for cardiovascular mortality
Compared to a favorable lifestyle, an unfavorable lifestyle was associated with an increased risk of CV mortality in all genetic risk groups for CAD and T2DM alike (Figure 2, Supplementary Tables S9, S10, and Supplementary Figure S2). In the groups with high and very high genetic risk for CAD, adherence to an unfavorable lifestyle was respectively associated with 4.6- and 8.3-fold increased risk of CV mortality (p-value < 0.001). Similarly, in the high and very high genetic risk groups for T2DM, an unfavorable lifestyle was associated with 3.9- and 5.8-fold respective increased risk of CV mortality (p-value < 0.001). Adherence to a favorable lifestyle was associated with reduced risk of CV mortality across all genetic risk categories [unfavorable vs. favorable lifestyle group, hazard ratio (HR) 0.33 (95% CI 0.30–0.36)]. However, participants at very high genetic risk with a favorable lifestyle still featured high mortality risk [HR 2.82 for CAD and 1.59 for T2DM]. The PAF of favorable lifestyle behaviors for CV mortality was 32.1% (95% CI 28.8–35.3%), and the PAF of smoking was 14.1% (95% CI 12.4–15.7%). There were no significant interactions between PRSs and lifestyle behaviors in predicting risk of CV mortality. Among the four lifestyle habits included in this analysis, smoking with very high genetic risk for CAD and T2DM had the strongest effect on CV mortality (Supplementary Figures S3–S5).
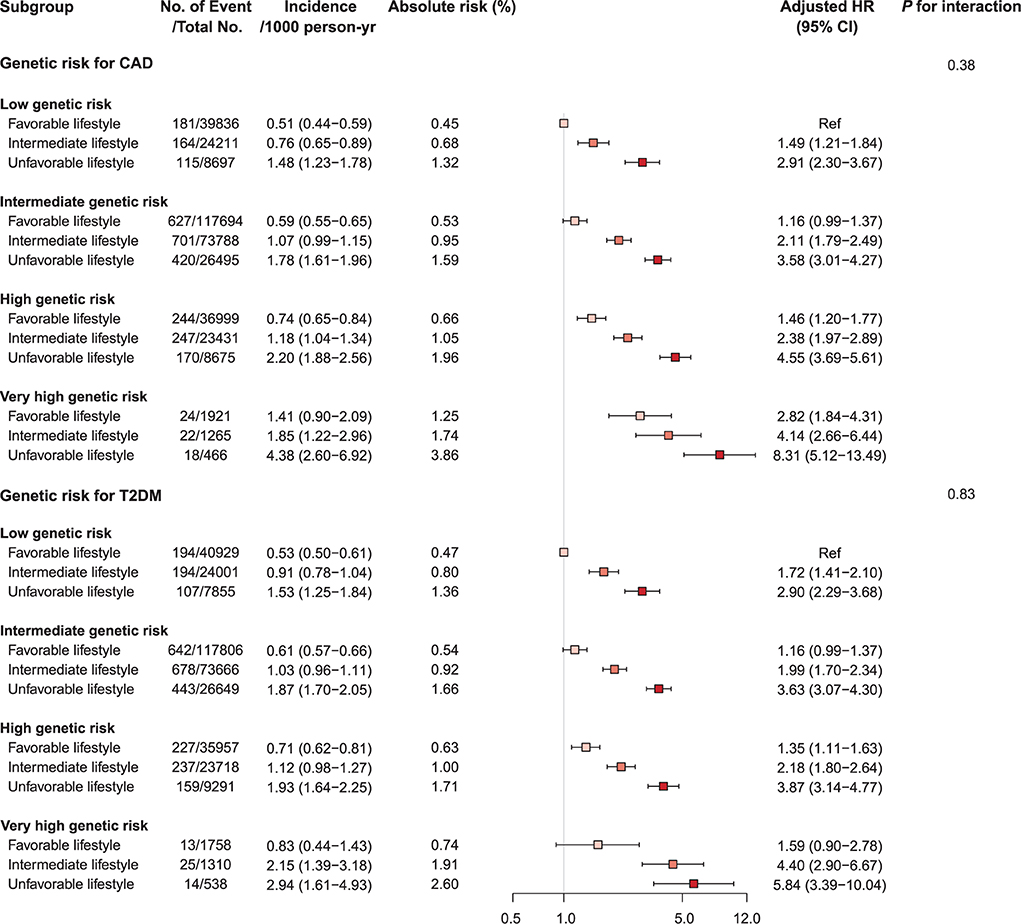
Figure 2. Forest plot of cardiovascular mortality according to genetic risk and lifestyle risk. Cox regression model was adjusted for age, sex, genotyping array, and first ten principal components of ancestry. p-values are for testing the interaction between each genetic risk category and lifestyle category. CAD, coronary artery disease; CI, confidence interval; HR, hazard ratio; T2DM, type 2 diabetes mellitus.
PRS, age, sex, and disease status for cardiovascular mortality
We investigated the interactions of PRS, age, and sex in the context of mortality risks and found that the association of mortality risk with PRS varied slightly by age and sex (Figure 3, Supplementary Tables S11, S12, and Supplementary Figure S5). Higher genetic risks of CAD and T2DM were associated with CV mortality both in the young (<55 years) and the old (≥55 years) and were associated with increased risk of CV mortality in both sexes, with generally higher risk in men. The CAD PRS was not associated with risk of CV mortality among participants with CAD at baseline, even in those having very high genetic risk [HR 1.19 (95% CI 0.63–2.24), p-value = 0.60]. By contrast, a robust association between very high genetic risk for T2DM and mortality was observed among participants with CAD or T2DM at baseline [CAD PRS, HR 2.31 (1.40–3.80); T2DM PRS, HR 2.99 (1.59–5.62), p-value < 0.001] (Figure 4, Supplementary Table S13 and Supplementary Figures S6, S7).
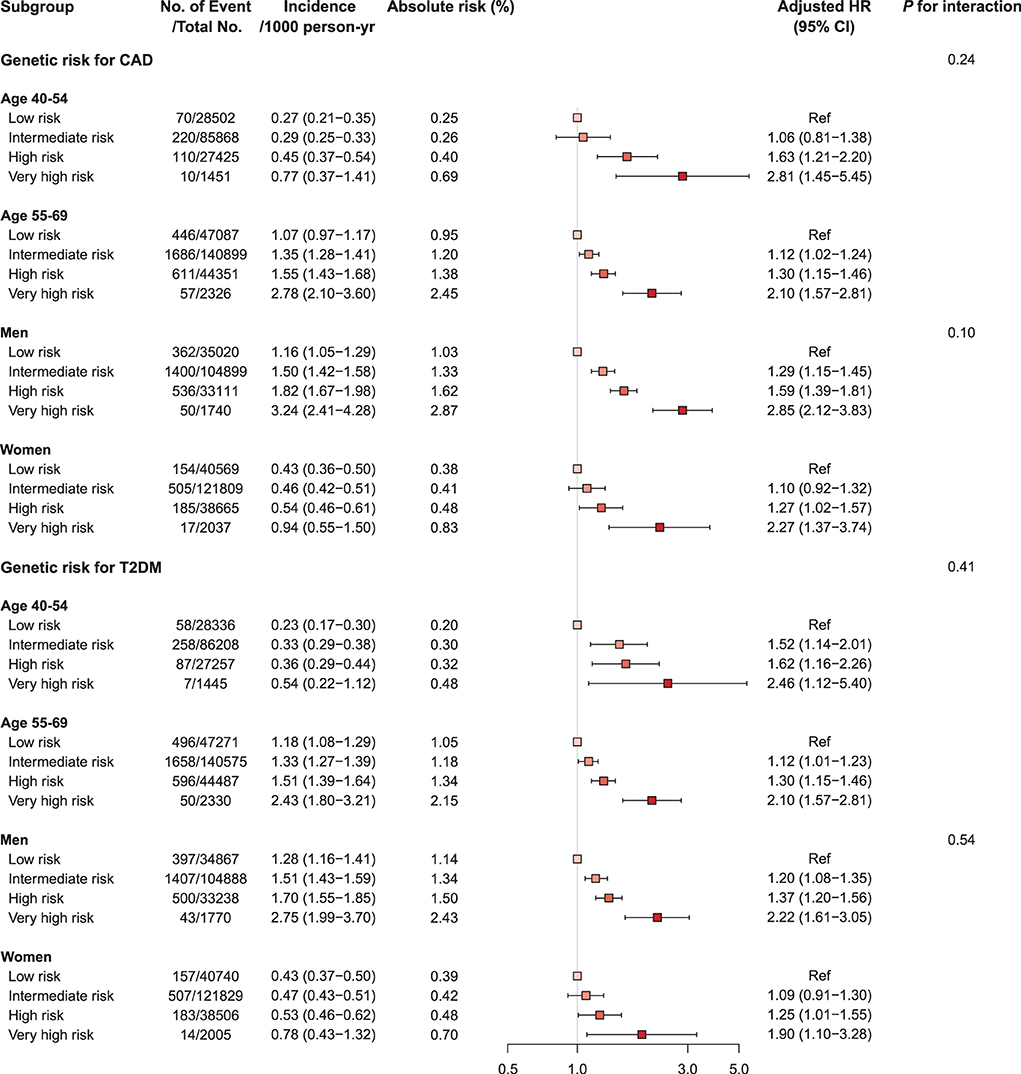
Figure 3. Forest plot of cardiovascular mortality according to genetic risk, age, and sex. Cox regression model was adjusted for age, sex, genotyping array, and first ten principal components of ancestry. p-values are for testing the interaction between each genetic risk category, age, and sex. CAD, coronary artery disease; CI, confidence interval; HR, hazard ratio; T2DM, type 2 diabetes mellitus.
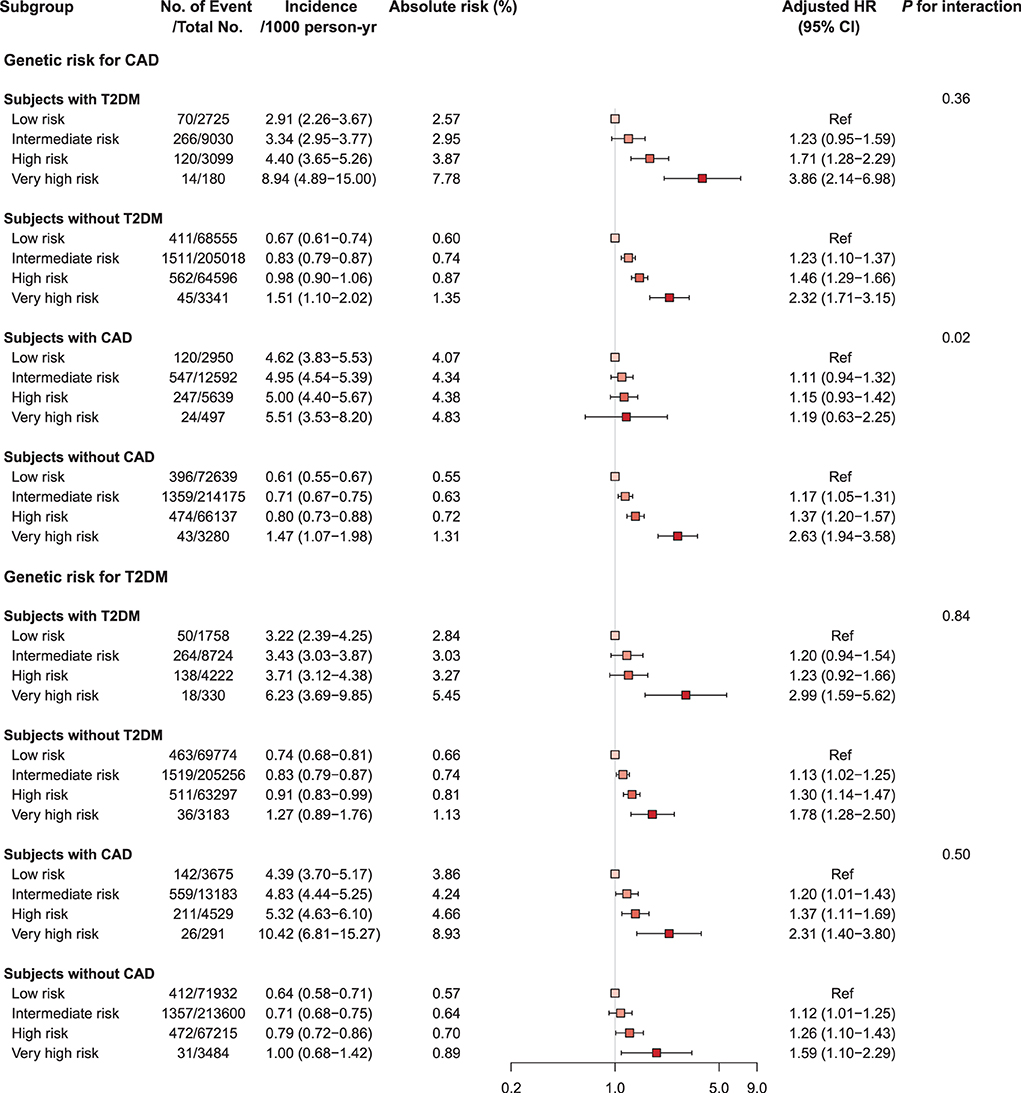
Figure 4. Forest plot of cardiovascular mortality according to genetic risk and PRS-related disease status. PRS, polygenic risk score. Cox regression model was adjusted for age, sex, genotyping array, and first ten principal components of ancestry. p-values are for testing the interaction between each genetic risk category and disease status. CAD, coronary artery disease; CI, confidence interval; HR, hazard ratio; T2DM, type 2 diabetes mellitus.
Discussion
In this prospective nationwide cohort study, we comprehensively explored the prognostic utility of PRS in predicting CV mortality. We found that the genetic risk of CAD and of T2DM was the independent predictive factor for CV mortality. Furthermore, participants at very high genetic risk for CAD or T2DM, equivalent to 1% of the total population, had a 2.6- and 2.1-fold increased risk of CV mortality, respectively, relative to those at low genetic risk. These associations were attenuated but still evident even after an adjustment for a wide range of mortality-related clinical and lifestyle behavior factors. In all genetic risk groups, adherence to a favorable lifestyle was associated with a reduced risk of CV mortality. In groups with very high genetic risk for CAD or T2DM, CV mortality risk remained substantially high even with a favorable lifestyle.
Previous studies have identified an association between mortality and genetic risk scores incorporating CVD-related variants. Two studies reported that CV mortality is significantly associated with a PRS calculated with a small number of variants (10, 11). Recently, Meisner et al. described a significant association between CAD PRS and CAD-specific mortality using the UK Biobank dataset (21), and Damask et al. likewise reported that those with high genetic risk for CAD had 50% higher risk of major adverse cardiac events (22). Fewer studies have investigated the association of genetic risk of diabetes with mortality. A previous longitudinal cohort study by Leong et al. reported a borderline significant association between genetic risk for T2DM and all-cause mortality (9). Others have suggested that genetic risk scores associated with hyperglycemia or diabetes could not predict mortality or age of death (21). In the context of CVD, diabetes and hyperglycemia are the well-known major risk factors for CVD and related mortality (23), but controversy exists regarding the effect of genetic variants associated with diabetes risk on CV mortality. Previous studies have suggested a shared genetic basis between T2DM and CAD with a complex bidirectional relationship (24), and several Mendelian randomization studies supported the hypothesis that genetic mechanisms linked with the risk of T2DM may have causal roles in the etiology of CVD (25, 26). Our results expand on previous research by demonstrating the significant and robust association of genetic risk of T2DM, as well as CAD with mortality, using a large dataset composed of UK Biobank participants. In the model fully adjusted for established clinical CV risk factors, the HRs of PRSs were attenuated, but both genetic scores remained to be significant independent predictors of future CV mortality. Our finding implies that although the traditional clinical risk factors included as covariates in this study influence CV mortality, there are multiple additional pathways related to PRS that affect CV risk and mortality, such as subclinical atherosclerosis, subclinical metabolic derangement, and clinical events, related to CV mortality during follow-up periods (27, 28).
In our study, we found that genetic risk and modifiable lifestyle habits were independently associated with the risk of CV mortality. To the best of our knowledge, this is the first study to investigate the associations of lifestyle habits and genetic risk with CV mortality. Previous studies have mainly targeted the interaction between genetic risk and lifestyle habits for specific diseases. For example, Khera et al. suggested that both genetic factors and adherence to an unfavorable lifestyle conjointly contributed to an increased risk of CAD (18). Other studies reported that combined unfavorable lifestyle behaviors and genetic risk had an additive deleterious effect on the risk of developing CAD, stroke, and T2DM (4, 7, 29). The analyses in this study showed that the risk of CV mortality was reduced by adherence to a favorable lifestyle, even in high genetic risk groups. These findings indicate the potential benefits of lifestyle modifications, such as diet, weight control, regular physical activity, and smoking cessation, in preventing CV mortality, regardless of genetic risk. Our analysis showed that over 30% of CV mortality might have been reduced if all participants would have adhered to favorable lifestyle behavior. In particular, patients could adjust their smoking habits, as current smoking was the most deleterious risk factor for CV mortality out of the behaviors we analyzed. The effect of lifestyle intervention in individuals at high genetic risk for CV mortality should be verified in future studies.
Several studies have reported that the effect of genetic risk on disease onset or mortality varies according to age and sex. For example, Tada et al. reported that PRS risk for CVD was higher in younger individuals than in older ones (11), and Mars et al. suggested that higher genetic risk scores were associated with early onset of diseases, including CAD and T2DM (6). An early onset of CVD in a high genetic risk group can be consistent with an increased risk of CV mortality in a young age group. Regarding risk and sex, while men have a higher rate of CVD incidence and mortality in the general population, the risks for CVD and its mortality have been shown to be greater in women with diabetes than in men with diabetes (29, 30). However, we observed no significant interactions between PRS, age, and sex with regard to CV mortality. Our results indicated that the effects of PRSs on CV mortality were not stronger in women than in men, even among participants with diabetes.
This study also found that, among participants with T2DM at baseline, PRSs for T2DM and CAD were significantly associated with increased risk of CV mortality. Consistent with our findings, Cox et al. showed in 2014 that a genetic risk score based on SNPs associated with CVD in patients with T2DM was associated with all-cause and CV mortalities (8). Meanwhile, among individuals in our study with CAD at baseline, PRS for CAD was not associated with CV mortality. This finding is in line with the previous results that PRSs determined using SNPs associated with CAD are not associated with recurrent CV events (31). Individuals with a medical history of CAD used more aggressive medications, such as aspirin and lipid-lowering agents for secondary prevention; such post-event care efforts might reduce the impact of factors mediated by genetic risk for CAD on CV mortality. A previous post hoc analysis of clinical trials has shown that high genetic risk of CAD can be mitigated by statin treatment (32).
Several limitations of our study should be considered. First, our study could be affected by competing risk from other cause-specific mortalities, such as cancer. To account for the potential effect of competing risk of death from other causes, we adjusted for the presence at baseline of major comorbidities such as cancer, chronic lung disease, and chronic liver disease. In addition, we performed sensitivity analysis after excluding those participants who had major comorbidities at baseline, such as chronic liver disease, lung disease, kidney disease, or cancer. Second, our definition of comorbidity included self-reported physician-made diagnosis, which may be incomplete and fail to include all true patients with comorbidities. However, self-reported disease status or history was obtained through a verbal interview with a trained nurse and was found to be the strongest predictor of all-cause mortality in men in a previous study (33). Third, lifestyle behaviors were based on a single measurement at baseline and were non-randomized. Fourth, the predictive power of PRS can be further refined using summary statistics from larger GWASs, which may change the estimated risk of CV mortality in individuals at high genetic risk. Fifth, information on lifestyle, disease history, and cause of death was mainly based on the self-report and/or diagnostic code, and thus, measurement error may exist. Finally, the association between genetic risk and CV mortality was not validated in external cohorts nor in non-European populations. Further studies with other cohorts are warranted to verify the generalizability of our findings.
Conclusion
In summary, our study used a large, well-phenotyped, prospective cohort drawn from the UK Biobank to assess the association between PRSs and CV mortality. We found that PRSs for CAD and T2DM are the independent predictive factors for future CV mortality for this white, middle-aged cohort. We were able to adjust for various demographic, clinical, and lifestyle factors to control for potential confounding factors for mortality. The associations remained robust after an adjustment for a wide range of clinical variables, including traditional CV risk factors. PRS thus has prognostic clinical utility in identifying people at risk for CVD and mortality. Furthermore, due to the considerable number of participants, we were also able to perform the analyses stratified by lifestyle risk. Our findings suggest that PRS-based risk assessment could be useful among individuals with high genetic risk for CAD or T2DM who need intensive behavioral or therapeutic interventions to prevent CV mortality. PRS can stratify high risk groups associated with an increased risk of CV death, as well as specific diseases, at an early age or early stage of disease. This stratification could be used for early clinical support and lifelong intervention and thereby enable precision medicine. Further studies are needed to verify the efficacy of intervention or cost-effectiveness of risk assessment based on PRS.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions. The UK Biobank dataset was obtained from the UK Biobank (Application Number 33002), and a full list of the variables are available online. Data cannot be shared publicly due to the violation of patient privacy and the absence of informed consent for data sharing. Requests to access these datasets should be directed to https://biobank.ndph.ox.ac.uk/.
Ethics statement
The studies involving human participants were reviewed and approved by the UK Biobank was approved by the National Research Ethics Committee (June 17, 2011 [RES reference 11/NW/0382]; extended on May 10, 2016 [RES reference 16/NW/0274]). The patients/participants provided their written informed consent to participate in this study.
Author contributions
J-SY developed the study design, wrote the manuscript, and analyzed and interpreted data. S-HJ analyzed data, interpreted data, and contributed to discussion. MS analyzed data and revised the manuscript. BX and W-YP revised the manuscript. AK, H-HW, and DK interpreted results, contributed to discussion, and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (no. 2022R1A2C2009998) and by the National Institute of General Medical Sciences (NIGMS) R01 GM138597.
Acknowledgments
The authors thank the participants who contributed their data in the UK Biobank study.
Conflict of interest
Author W-YP was employed by a commercial company, GENINUS.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.919374/full#supplementary-material
Abbreviations
CAD, coronary artery disease; CCI, Charlson Comorbidity Index; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; GWAS, genome-wide association study; PAF, population attributable fraction; PC, principal component; PRS, polygenic risk score; SNP, single-nucleotide polymorphism; T2DM, type 2 diabetes mellitus; ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; HR, hazard ratio; ICD, international statistical classification of disease; QC, quality control.
References
1. Members WG, Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, et al. Heart disease and stroke statistics−2012 update: a report from the American Heart Association. Circulation. (2012) 125:e2–220. doi: 10.1161/CIR.0b013e31823ac046
2. Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. (1998) 339:229–34. doi: 10.1056/NEJM199807233390404
3. Vistisen D, Witte DR, Brunner EJ, Kivimäki M, Tabák A, Jørgensen ME, et al. Risk of cardiovascular disease and death in individuals with prediabetes defined by different criteria: the whitehall II study. Diabetes Care. (2018) 41:899–906. doi: 10.2337/dc17-2530
4. Han X, Wei Y, Hu H, Wang J, Li Z, Wang F, et al. Genetic risk, a healthy lifestyle, and type 2 diabetes: the dongfeng-tongji cohort study. J Clin Endocrinol Metab. (2020) 105:1242–50. doi: 10.1210/clinem/dgz325
5. Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, et al. Genome-Wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. (2018) 50:1219–24. doi: 10.1038/s41588-018-0183-z
6. Mars N, Koskela JT, Ripatti P, Kiiskinen TT, Havulinna AS, Lindbohm JV, et al. Polygenic and clinical risk scores and their impact on age at onset and prediction of cardiometabolic diseases and common cancers. Nat Med. (2020) 26:549–57. doi: 10.1038/s41591-020-0800-0
7. Said MA, Verweij N, van der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK Biobank Study. JAMA cardiology. (2018) 3:693–702. doi: 10.1001/jamacardio.2018.1717
8. Cox AJ, Hsu F-C, Ng MC, Langefeld CD, Freedman BI, Carr JJ, et al. Genetic risk score associations with cardiovascular disease and mortality in the diabetes heart study. Diabetes Care. (2014) 37:1157–64. doi: 10.2337/dc13-1514
9. Leong A, Porneala B, Dupuis J, Florez JC, Meigs JB. Type 2 diabetes genetic predisposition, obesity, and all-cause mortality risk in the US: a multiethnic analysis. Diabetes Care. (2016) 39:539–46. doi: 10.2337/dc15-2080
10. Pereira A, Mendonca MI, Sousa AC, Borges S, Freitas S, Henriques E, et al. Genetic risk score and cardiovascular mortality in a Southern European population with coronary artery disease. Int J Clin Pract. (2017) 71. doi: 10.1111/ijcp.12956
11. Tada H, Melander O, Louie JZ, Catanese JJ, Rowland CM, Devlin JJ, et al. Risk prediction by genetic risk scores for coronary heart disease is independent of self-reported family history. Eur Heart J. (2016) 37:561–7. doi: 10.1093/eurheartj/ehv462
12. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK biobank resource with deep phenotyping and genomic data. Nature. (2018) 562:203–9. doi: 10.1038/s41586-018-0579-z
13. Elliott P, Peakman TC. The UK biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. (2008) 37:234–44. doi: 10.1093/ije/dym276
14. Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. (2009) 5:e1000529. doi: 10.1371/journal.pgen.1000529
15. Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive 1000 genomes–based genome-wide association meta-analysis of coronary Artery disease. Nature Genetics. (2015) 47:1121–30. doi: 10.1038/ng.3396
16. Scott RA, Scott LJ, Mägi R, Marullo L, Gaulton KJ, Kaakinen M, et al. An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes. (2017) 66:2888–902. doi: 10.2337/db16-1253
17. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. (2010) 121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703
18. Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. (2016) 375:2349–58. doi: 10.1056/NEJMoa1605086
19. Brusselaers N, Lagergren J. The Charlson comorbidity index in registry-based research. Methods Inf Med. (2017) 56:401–6. .
20. Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro III AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
21. Meisner A, Kundu P, Zhang YD, Lan LV, Kim S, Ghandwani D, et al. Combined utility of 25 disease and risk factor polygenic risk scores for stratifying risk of all-cause mortality. Am J Hum Genet. (2020) 107:418–31. doi: 10.1016/j.ajhg.2020.07.002
22. Damask A, Steg PG, Schwartz GG, Szarek M, Hagström E, Badimon L, et al. Patients with high genome-wide polygenic risk scores for coronary artery disease may receive greater clinical benefit from alirocumab treatment in the odyssey outcomes trial. Circulation. (2020) 141:624–36. doi: 10.1161/CIRCULATIONAHA.119.044434
23. Tancredi M, Rosengren A, Svensson A-M, Kosiborod M, Pivodic A, Gudbjörnsdottir S, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. (2015) 373:1720–32. doi: 10.1056/NEJMoa1504347
24. Zheng Q, Jiang J, Huo Y, Chen D. Genetic predisposition to type 2 diabetes is associated with severity of coronary artery disease in patients with acute coronary syndromes. Cardiovasc Diabetol. (2019) 18:131. doi: 10.1186/s12933-019-0930-1
25. Ross S, Gerstein HC, Eikelboom J, Anand SS, Yusuf S, Paré G. Mendelian randomization analysis supports the causal role of dysglycaemia and diabetes in the risk of coronary artery disease. Eur Heart J. (2015) 36:1454–62. doi: 10.1093/eurheartj/ehv083
26. Li Y, Pan A, Wang DD, Liu X, Dhana K, Franco OH, et al. Impact of healthy lifestyle factors on life expectancies in the us population. Circulation. (2018) 138:345–55. doi: 10.1161/CIRCULATIONAHA.117.032047
27. Gan W, Bragg F, Walters RG, Millwood IY, Lin K, Chen Y, et al. Genetic predisposition to type 2 diabetes and risk of subclinical atherosclerosis and cardiovascular diseases among 160,000 Chinese adults. Diabetes. (2019) 68:2155–64. doi: 10.2337/db19-0224
28. Meigs JB. The genetic epidemiology of type 2 diabetes: opportunities for health translation. Curr Diab Rep. (2019) 19:1–8. doi: 10.1007/s11892-019-1173-y
29. Rutten-Jacobs LC, Larsson SC, Malik R, Rannikmäe K, Sudlow CL, Dichgans M, et al. Genetic risk, incident stroke, and the benefits of adhering to a healthy lifestyle: cohort study of 306 473 UK Biobank Participants. BMJ. (2018) 363. doi: 10.1136/bmj.k4168
30. Collaboration ERF. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. (2011) 364:829–41. doi: 10.1056/NEJMoa1008862
31. Labos C, Martinez SC, Wang RHL, Lenzini PA, Pilote L, Bogaty P, et al. Utility of a genetic risk score to predict recurrent cardiovascular events 1 year after an acute coronary syndrome: a pooled analysis of the Risca, Praxy, and Triumph Cohorts. Atherosclerosis. (2015) 242:261–7. doi: 10.1016/j.atherosclerosis.2015.07.029
32. Mega JL, Stitziel NO, Smith JG, Chasman DI, Caulfield MJ, Devlin JJ, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. (2015) 385:2264–71.
Keywords: polygenic risk score, lifestyle, cardiovascular mortality, coronary artery disease, type 2 diabetes mellitus
Citation: Yun J-S, Jung S-H, Shivakumar M, Xiao B, Khera AV, Park W-Y, Won H-H and Kim D (2022) Associations between polygenic risk of coronary artery disease and type 2 diabetes, lifestyle, and cardiovascular mortality: A prospective UK Biobank study. Front. Cardiovasc. Med. 9:919374. doi: 10.3389/fcvm.2022.919374
Received: 13 April 2022; Accepted: 11 July 2022;
Published: 17 August 2022.
Edited by:
Georges Nemer, Hamad Bin Khalifa University, QatarReviewed by:
Venkataraghavan Ramamoorthy, Baptist Health South Florida, United StatesEmily Baker, Cardiff University, United Kingdom
Copyright © 2022 Yun, Jung, Shivakumar, Xiao, Khera, Park, Won and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-Hee Won, d29uaGhAc2trdS5lZHU=; Dokyoon Kim, ZG9reW9vbi5raW1AcGVubm1lZGljaW5lLnVwZW5uLmVkdQ==
†These authors have contributed equally to this work and share first authorship
 Jae-Seung Yun
Jae-Seung Yun Sang-Hyuk Jung
Sang-Hyuk Jung Manu Shivakumar1,4,5
Manu Shivakumar1,4,5 Woong-Yang Park
Woong-Yang Park Hong-Hee Won
Hong-Hee Won