- 1Department of Nursing, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Background: Venous thromboembolism (VTE) can be fatal if not treated promptly, and individual studies have reported wide variability in rates of VTE associated with peripherally inserted central catheters (PICC). We thus conducted this meta-analysis to investigate the overall incidence and risk of developing PICC-related VTE in hospitalized patients.
Methods: We searched PubMed, Embase, Scopus, and Web of Science databases from inception until January 26, 2022. In studies with a non-comparison arm, the pooled incidence of PICC-related VTE was calculated. The pooled odds ratio (OR) was calculated to assess the risk of VTE in the studies that compared PICC to the central venous catheter (CVC). The Newcastle-Ottawa Scale was used to assess methodological quality.
Results: A total of 75 articles (58 without a comparison arm and 17 with), including 109292 patients, were included in the meta-analysis. The overall pooled incidence of symptomatic VTE was 3.7% (95% CI: 3.1–4.4) in non-comparative studies. In the subgroup meta-analysis, the incidence of VTE was highest in patients who were in a critical care setting (10.6%; 95% CI: 5.0–17.7). Meta-analysis of comparative studies revealed that PICC was associated with a statistically significant increase in the odds of VTE events compared with CVC (OR, 2.48; 95% CI, 1.83–3.37; P < 0.01). However, in subgroup analysis stratified by the study design, there was no significant difference in VTE events between the PICC and CVC in randomized controlled trials (OR, 2.28; 95% CI, 0.77–6.74; P = 0.13).
Conclusion: Best practice standards such as PICC tip verification and VTE prophylaxis can help reduce the incidence and risk of PICC-related VTE. The risk-benefit of inserting PICC should be carefully weighed, especially in critically ill patients. Cautious interpretation of our results is important owing to substantial heterogeneity among the studies included in this study.
Introduction
The peripherally inserted central catheter (PICC) or PICC line is a 50–60 cm long, thin, flexible tube inserted in the arm vein with the tip of the catheter positioned at the lower third of the superior vena cava (1–3). PICC, which is safer to insert than traditional central venous catheters (CVCs), is frequently used in inpatient and outpatient settings (2). In recent years, PICCs have gained popularity in medical practice over other CVCs due to several advantages, including ease of insertion, safety, cost-effectiveness, multiple uses for extended periods, and a reduction in central line-associated blood stream infection (CLABSI) (3, 4). In addition, the growth of nurse-led PICC teams has made the use of PICCs more convenient and accessible in various settings (5).
Despite the aforementioned benefits, the widespread use of PICC exposes patients to a number of high-risk complications, such as the risk of VTE and CLABSI, which increase mortality, morbidity, length of hospital stay, and medical expenses (6, 7). VTE, which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), is a potentially fatal condition in hospitalized patients. Accumulating evidence suggests that PICC insertion has been associated with a high risk of VTE, specifically arm DVT and PE (8–10). The documented incidence of symptomatic PICC-associated VTE ranges from 6 to 25% (9, 11, 12), while asymptomatic VTE ranges from 35 to 71.9% (13, 14).
In 2013, Chopra et al. (15) conducted a systematic review and meta-analysis to assess the risk of VTE associated with PICC. They found that the incidence of PICC-related DVT was highest in critically ill patients (13.91%), followed by cancer patients (6.67%). However, the studies in their meta-analysis varied greatly because they included many studies in which the PICC tip location was not reported. PICC tip position is an important predictor of catheter-related thrombosis, and verifying tip position at the cavoatrial junction appears to be associated with a significant decrease in DVT (16, 17). Furthermore, one-third of the studies included in the meta-analysis by Chopra et al. were only in abstract form, which may have overestimated the overall result. The results presented in conference abstracts are questionable because systematic reviewers are unable to extract sufficient information on study design, methods, risk of bias, outcomes, and detailed results (18). A recently published meta-analysis that assessed the thrombotic rate associated with PICCs in patients who had catheter insertion performed with proper tip location verification found that the pooled DVT rate was 2.4%, with the thrombotic rate being higher in onco-hematologic patients (5.9%) (19). Nonetheless, this study completely neglected retrospective studies on the same topic. As a result, these findings do not represent a reliable estimate of PICC-related thrombotic events. Despite advances in modern vascular techniques over the years, the precise estimation of the incidence and risk of VTE associated with PICC in hospitalized patients remains unclear.
Therefore, we conducted a systematic review and meta-analysis of studies published up-to-date to estimate the incidence of PICC-related VTE and compare this risk between PICC and CVCs in hospitalized patients. The objective was only to include studies in which the catheter tip position was confirmed in order to assess the precise incidence and risk of PICC-related VTE among hospital patients.
Materials and methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was used to conduct this systemic review and meta-analysis (20). This is a systematic review and meta-analysis with only secondary data analysis, so no ethical approval or consent is required.
Literature search and search strategies
The two authors (AP and HD) independently performed a systematic literature search of PubMed, Embase, Scopus, and Web of Science databases up to January 26, 2022, without language restriction. To include all relevant articles, the following search terms were combined with Boolean operators: PICC, peripherally inserted central venous catheter, PICC line, PICC, venous thromboembolism, pulmonary embolism, deep vein thrombosis, deep vein thrombi, and upper-extremity deep vein thrombosis. The detailed search strategy is presented in Supplementary Appendix 1. A manual reference search was also conducted to identify additional potentially eligible studies.
Study selection and eligibility criteria
We imported all the searched articles into the EndNote X 8.0 software and excluded duplicate studies. The titles and abstracts of all articles retrieved from the search were screened independently by two authors. The same investigators reviewed the full text of potentially eligible studies. Disagreements were resolved through discussion among the authors. The inclusion criteria were as follows: (a) study population: studies with participants ≥ 18 years of age and PICC placed in veins of the upper arm, (b) The studies reporting VTE events (DVT, PE, or both) after the insertion of PICC, (c) studies in which the catheter tip position was confirmed using radiographic imaging of the chest or fluoroscopy or any other method following PICC placement. The exclusion criteria were as follows: (a) studies with pediatric cases or pregnant women, (b) studies reporting complications after PICC is inserted into leg veins, and studies reporting complications such as phlebitis or thrombophlebitis but not VTE. We also excluded review articles, letters, comments, case reports, editorials, and conference abstracts. All records were screened for eligible studies by two investigators (AP and HD). Disagreements between reviewers during full-text screening were resolved by consensus with a third reviewer (MG).
Data extraction and outcome measures
Two authors independently extracted data, and a Microsoft Excel spreadsheet was used to record the data. The following data were extracted for each included study: author, publication year, geographical location, study design, sample size, number of DVT or PE or both, PICC indication, method of VTE diagnosis, and use of DVT prophylaxis. We divided the eligible studies into non-comparison studies (studies reporting PICC-related VTE occurrence in PICC recipients) and comparison studies (studies in which PICCs were compared with other CVCs in terms of venous thromboembolism). The primary outcome was the development of VTE, which included DVT and PE. DVT was defined as thrombosis of the deep veins of the upper arm detected using Doppler ultrasound, compression ultrasonography, or venography. The occurrence of PE after PICC insertion was determined based on diagnoses reported in individual studies. We contacted the study authors to obtain missing or ambiguous information.
Quality assessment
The Newcastle–Ottawa scale was used independently by two authors (AP and MG) to assess the quality of the eligible studies. Each of the included studies was evaluated in the three domains listed below: (1) selection of exposed and non- exposed cohorts (four items: representativeness of the exposed cohort, selection of non-exposed cohort, ascertainment of the exposure, and the outcome present at the start of the study); (2) comparability (one item: comparability of cohorts based on the design of the analysis); and (3) outcome of interest (three items: assessment of outcome, length of follow-up, and adequacy of follow-up). Comparative studies with stars in all domains were deemed high quality. For non-comparison studies, the comparability of the cohort domain was excluded, and stars in all domains except comparability were deemed high quality. Studies with four to eight stars were considered moderate quality, while those with less than four stars were deemed low quality.
Statistical analysis
Meta-analysis was conducted separately for the non-comparison and comparison studies. A Freeman-Tukey double arcsine transformation with a random effect model was used to calculate the weighted proportion of VTE with corresponding 95% confidence intervals (CIs) in non-comparison studies. The total number of PICC-associated DVTs was divided by the total number of PICCs placed to calculate the incidence of PICC-related DVTs, as some studies reported data on DVT events per PICC rather than per patient. For comparison studies, the OR with corresponding 95% CIs was calculated, and the results were pooled using the DerSimonian and Laird inverse-variance-weighted random-effects models as there was variability between included studies. The I2 statistic and Cochran’s Q test were used to assess heterogeneity between included studies. According to the I2 statistic, values of < 25, 25–75%, and >75% indicate low, moderate, and high levels of heterogeneity, respectively. Publication bias was assessed visually using funnel plots and quantitatively using Begg’s and Egger’s regression tests. When there was publication bias, trim and fill analysis was performed to correct the funnel plot asymmetry. Finally, a sensitivity analysis was conducted to assess the impact of each study on the overall pooled estimates by leave-one-out meta-analysis (by removing one study at a time). All tests were two-sided, and a p-value of less than 0.05 was considered statistically significant. All analyses were performed in the ‘‘meta’’ package using R version 4.1.0 for Windows1.
Prespecified subgroup analyses
The subgroup analysis were performed according to VTE type (DVT vs. DVT/PE), setting (critical care or non-critical care), patient population (oncology or non-oncology), study design (prospective vs. retrospective), study location, DVT prophylaxis (yes or no or not reported), and publication year.
Results
Search results and study characteristics
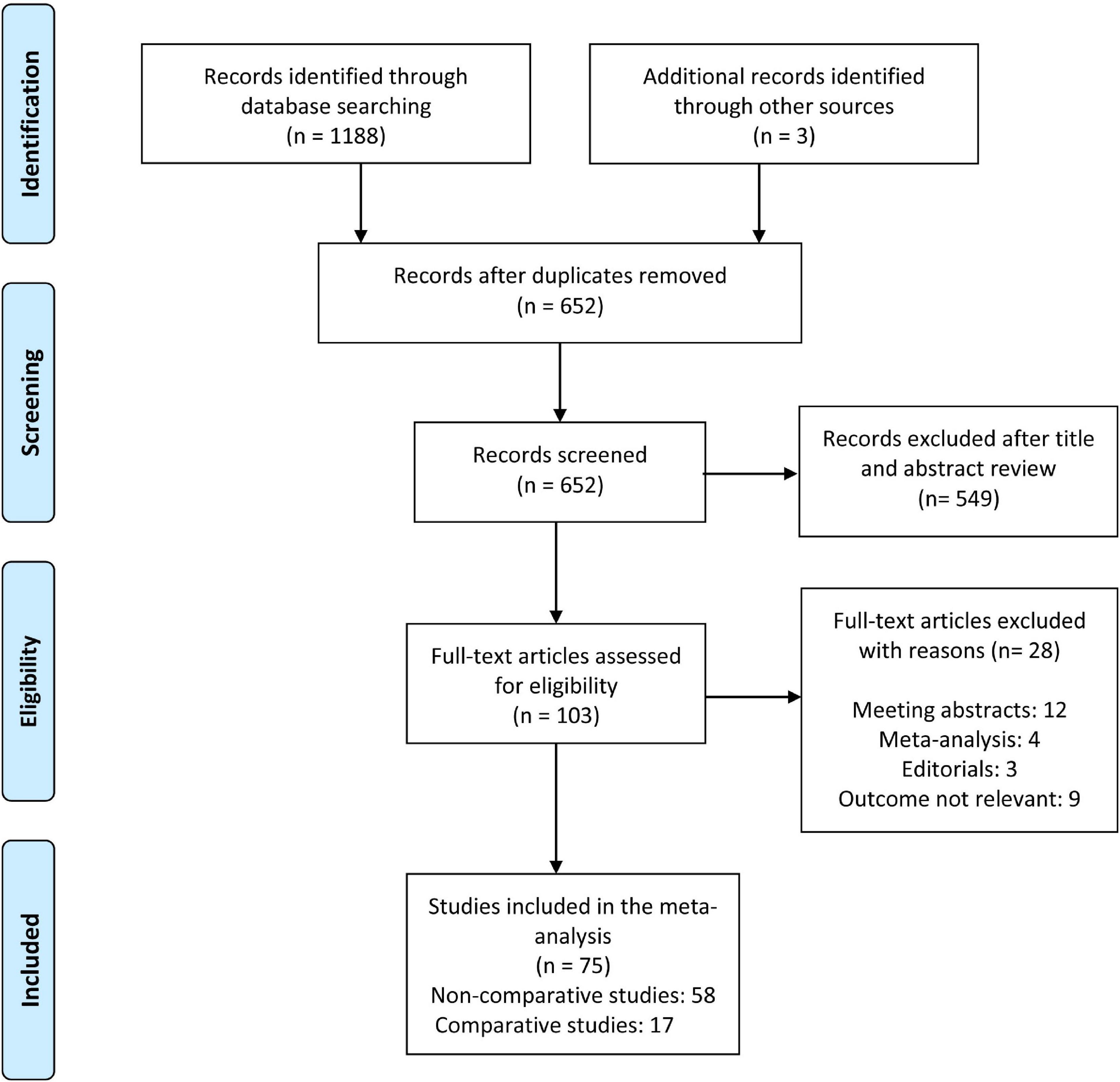
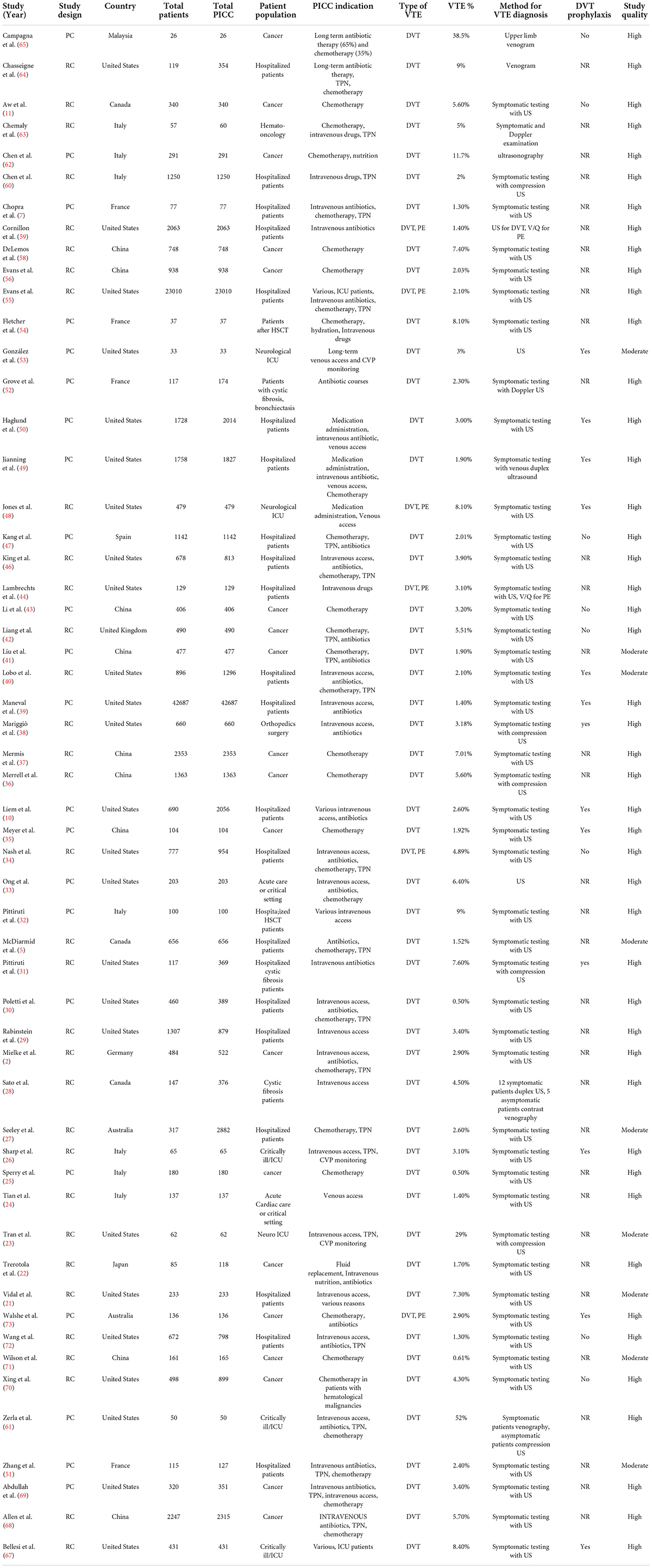
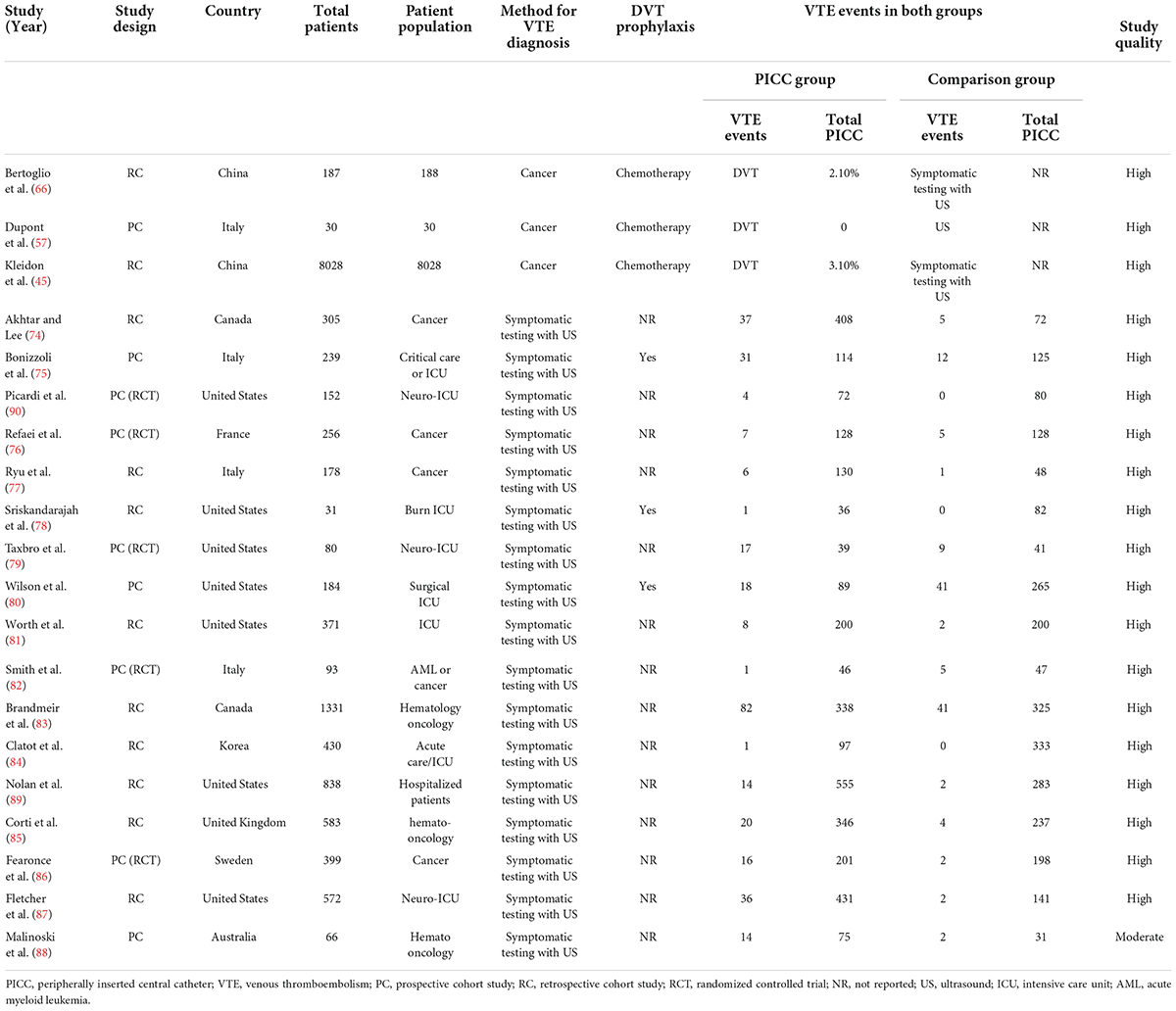
The search yielded 1188 citations, as well as three studies identified from an additional source. After removing duplicates and screening titles and abstracts, ultimately, 103 articles were assessed for full-text review. After excluding 28 ineligible studies, a total of 75 articles were included in the meta-analysis. Figure 1 depicts the flowchart of the study selection process. Fifty eight non-comparative studies (2, 5, 7, 10, 11, 21–73) included 103351 patients who underwent 109163 PICC insertions (Table 1), while seventeen comparative studies (74–90) with 5941 patients compared PICCs with CVCs (Table 2). For non-comparison studies, only thirteen provided data on anticoagulant prophylaxis for VTE prevention (Table 1). The sample sizes ranged widely across non-comparison studies (median: 373 patients; range: 26–42,661). Among 58 non-comparative studies, 25 were conducted in the United States, 11 in China, 8 in Italy, 4 in France, and ten elsewhere. Of the 58 studies included, 35 were retrospective, and 23 were prospective studies (Table 1). The median sample size for studies with a comparison arm was 256 (range: 31–1,331). Seven of the 17 comparative studies were conducted in the United States, 3 in Italy, 2 in Canada, and 5 in other countries. Among the 17 included comparative studies, there were nine retrospective studies, five prospective RCTs, and three prospective studies (Table 2).
Quality of the studies
The quality of the included comparison and non-comparison studies was evaluated using the Newcastle-Ottawa scale. Sixteen of the 17 comparison studies were of high quality, while one was of moderate quality. Of the 58 non-comparison studies, 49 were of high quality, and nine were of moderate quality (Supplementary Table 1).
Meta-analysis of the incidence of peripherally inserted central catheters-related venous thromboembolism
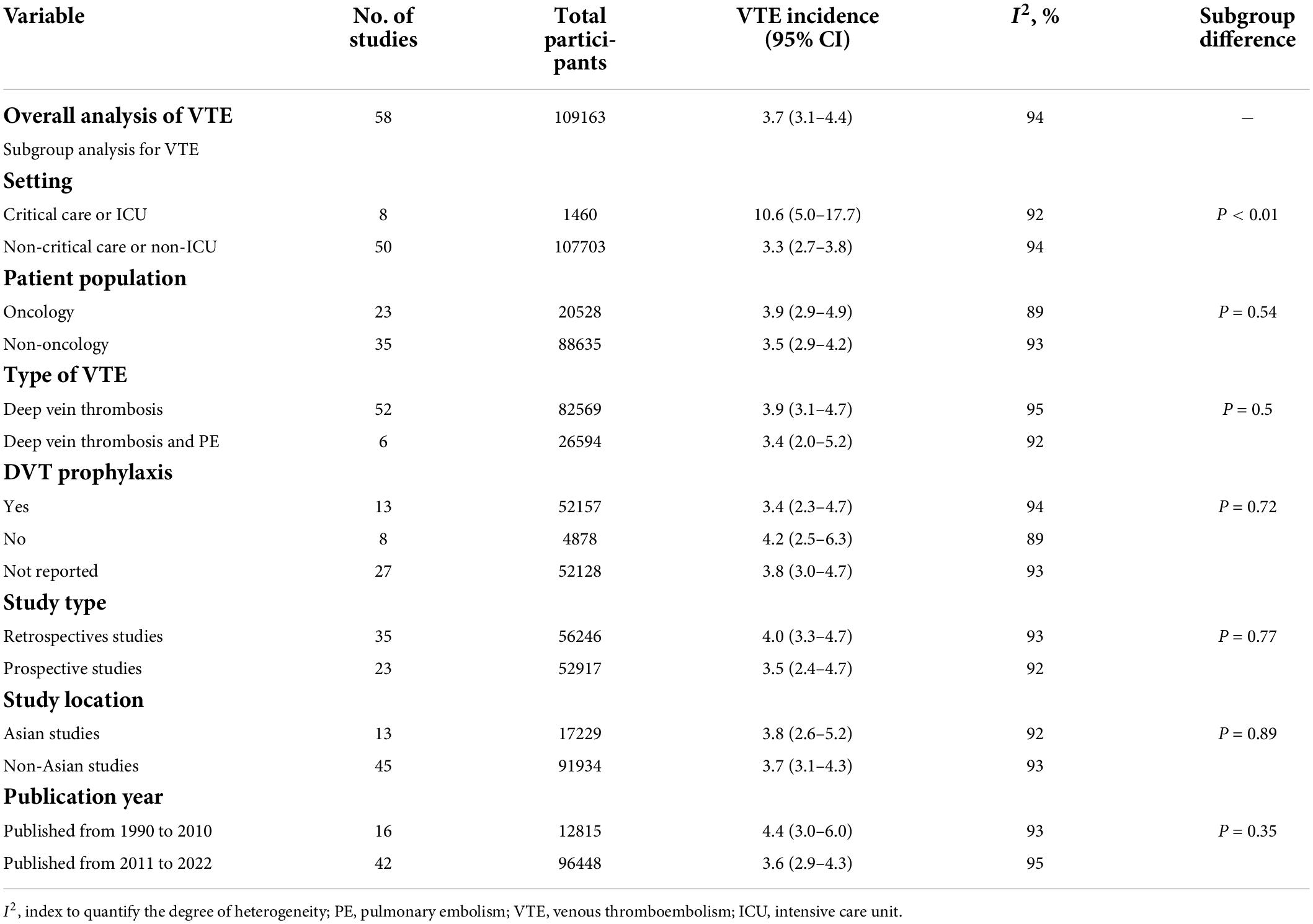
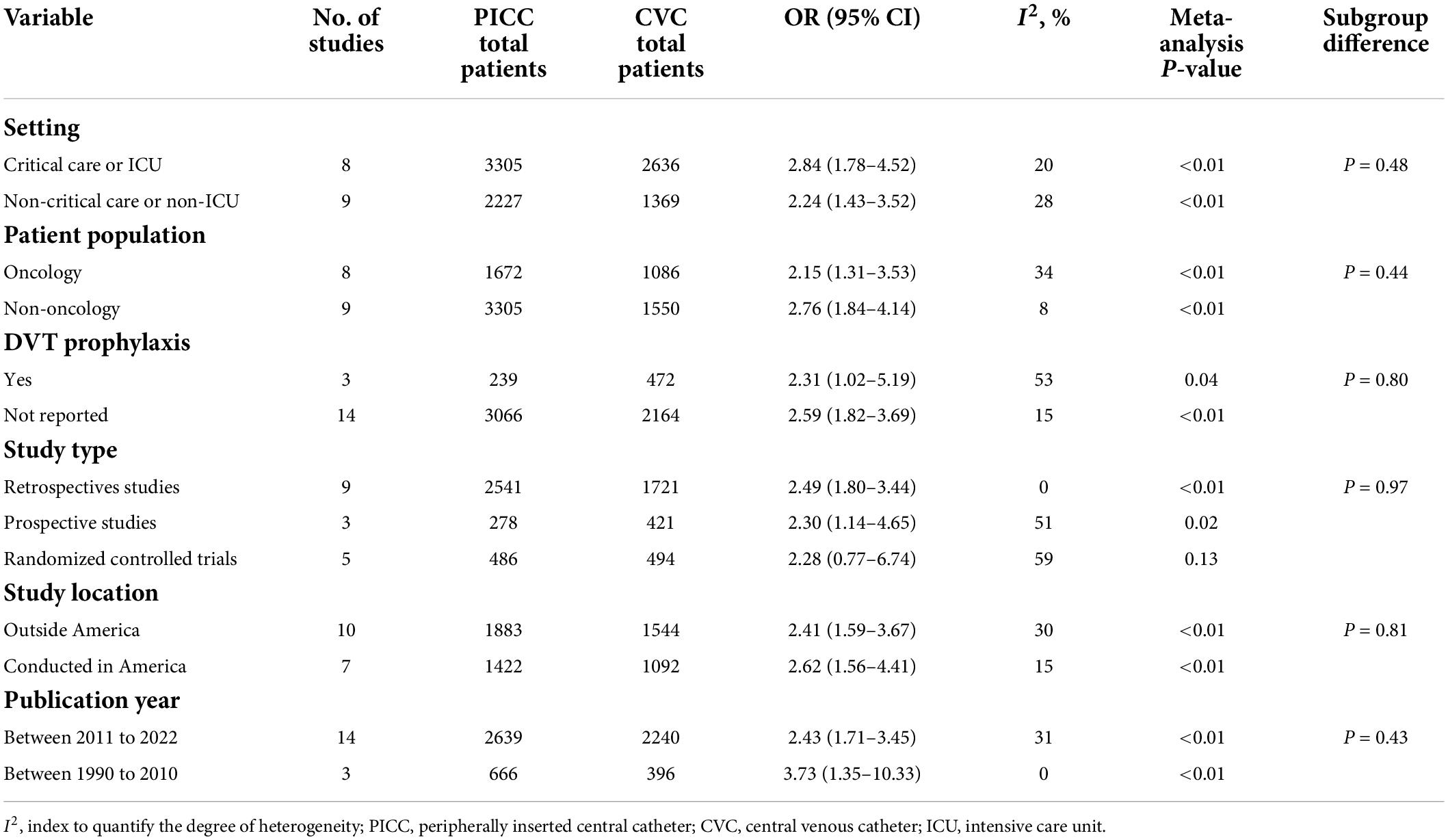
Fifty-eight studies reported VTE incidence among patients after PICC insertion, and the overall pooled incidence of VTE was 3.7% (95% CI: 3.1–4.4), with high heterogeneity (I2 = 94%, P < 0.01) (Figure 2). Visual inspection of asymmetry in the funnel plot (Supplementary Figure 1) and the Egger’s test (t = 5.29; P < 0.001) revealed evidence of publication bias. The trim-and-fill method was used to account for publication bias in these studies, and the funnel plot became symmetrical after imputing 24 unpublished studies (Supplementary Figure 2). Furthermore, sensitivity analysis revealed the robustness of our finding, with the recalculated pooled incidence of VTE after excluding each study ranging from 3.5% (95% CI: 3.0–4.1) to 3.9% (95% CI: 3.2–4.6) (Figure 3).
Figure 2. Forest plot showing the pooled incidence of PICC-related VTE among hospitalized patients in non-comparative studies. PICC, peripherally inserted central catheter; VTE, venous thromboembolism.
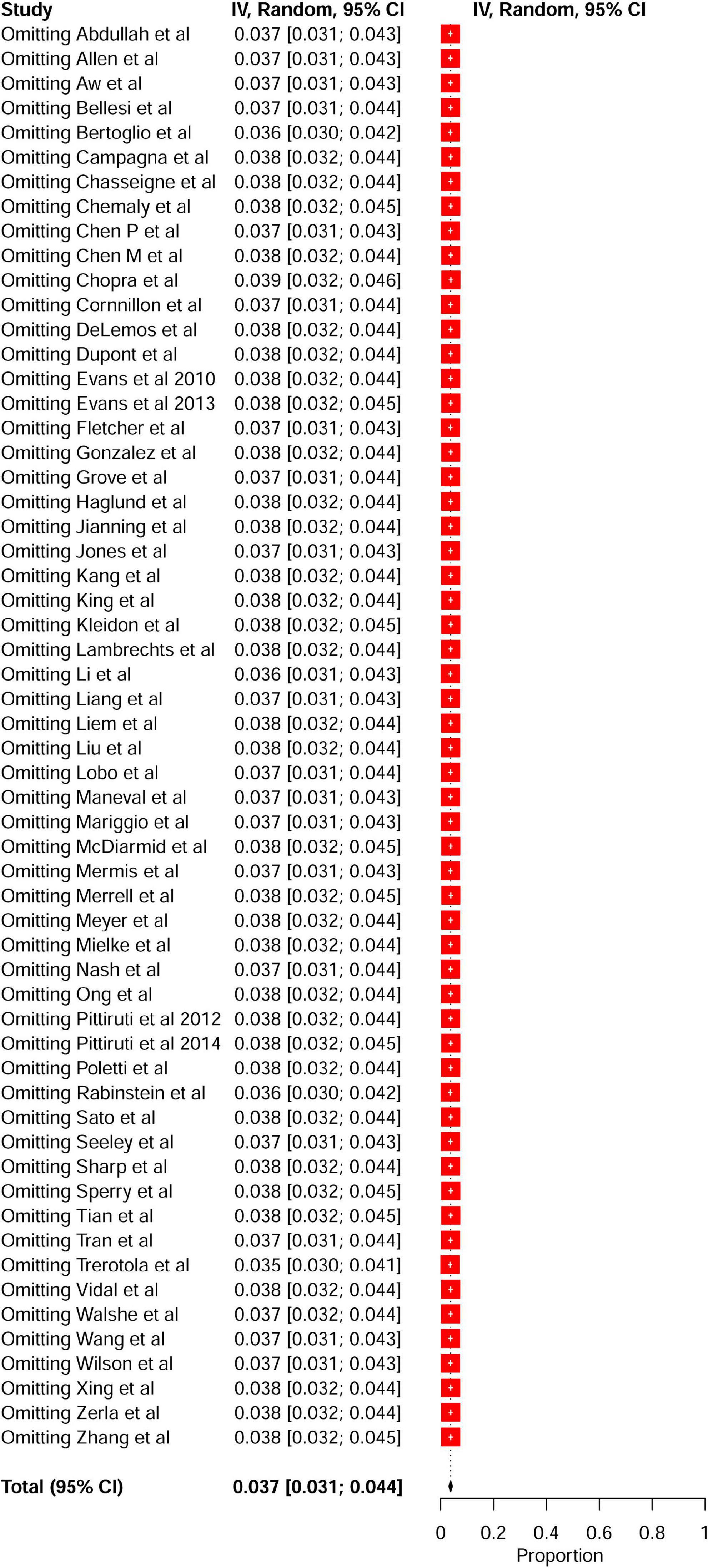
Figure 3. Leave-one-out sensitivity analysis of the pooled incidence of PICC-related VTE among hospitalized patients. PICC, peripherally inserted central catheter; VTE, venous thromboembolism.
A subgroup meta-analysis was also performed to analyze the incidence of PICC-related VTE based on the study setting, patient population, type of VTE, DVT prophylaxis, study design, study location, and publication year. Subgroup analyses based on the study setting showed that the incidence of VTE in critical care or intensive care unit (ICU) settings was 10.6% (95% CI: 5.0–17.7), whereas the incidence of VTE in non-critical care or non-ICU patients was 3.3% (95% CI: 2.7–3.8) (Table 3 and Supplementary Figure 3). On subgroup analysis stratified by patient population, the pooled incidence of VTE was 3.9% (95% CI: 2.9–4.9) in oncology patients and 3.5% for non-oncology patients (95% CI: 2.9–4.2) (Table 3 and Supplementary Figure 4). When data were analyzed based on the type of VTE, the pooled incidence of DVT was 3.9% (95% CI: 3.1–4.7), while the incidence of DVT/PE was 3.4% (95% CI: 2.0–5.2) (Table 3 and Supplementary Figure 5). The subgroup analysis of DVT prophylaxis revealed similar pooled proportions of VTE among three different subgroups, i.e., the pooled incidence of VTE was 3.4% (95% CI: 2.3–4.7) in studies that reported DVT prophylaxis, 3.8% (95% CI: 3.0–4.7) in studies that did not report DVT prophylaxis, and 4.2% (95% CI: 2.5–6.3) in studies that used no DVT prophylaxis (Table 3 and Supplementary Figure 6). Subgroup analyses stratified by study design showed that the incidence of PICC-related VTE was 4% (95% CI: 3.3–4.7) in retrospective studies and 3.5% (95% CI: 2.4–4.7) in prospective studies (Table 3 and Supplementary Figure 7). According to subgroup analyses stratified by study location, the incidence of PICC-related VTE was 3.7% (95% CI: 3.1–4.3) in non-Asian studies and 3.8% (95% CI: 2.6–5.2) in Asian studies (Table 3 and Supplementary Figure 8). Further subgroup analysis stratified by publication year indicated that the incidence of VTE was 4.4% (95% CI: 3.0–6.0) in studies published from 1990 to 2010 and 3.6% (95% CI: 2.9–4.3) in studies published from 2011 to 2022 (Table 3 and Supplementary Figure 9). The summary results of the overall and subgroup meta-analyses are presented in Table 3.
Meta-analysis of the risk of venous thromboembolism associated with peripherally inserted central catheters
A meta-analysis of 17 comparison studies showed that PICC was associated with a statistically significant increase in the odds of VTE events when compared with CVC (OR, 2.48; 95% CI, 1.83–3.37; P < 0.01) (Figure 4). No significant heterogeneity was detected across the included studies (I2 = 20%, P = 0.22) (Figure 4). Visual inspection of funnel plot symmetry (Supplementary Figure 10) followed by the Egger test (t = 1.3; P = 0.21) revealed no publication bias. The leave-one-out sensitivity analyses showed that the pooled estimated OR for VTE associated with PICC ranged between 2.33 (95% CI: 1.75–3.1) and 2.69 (95% CI: 1.99–3.63), indicating that individual studies had no significant effect on estimates (Figure 5).
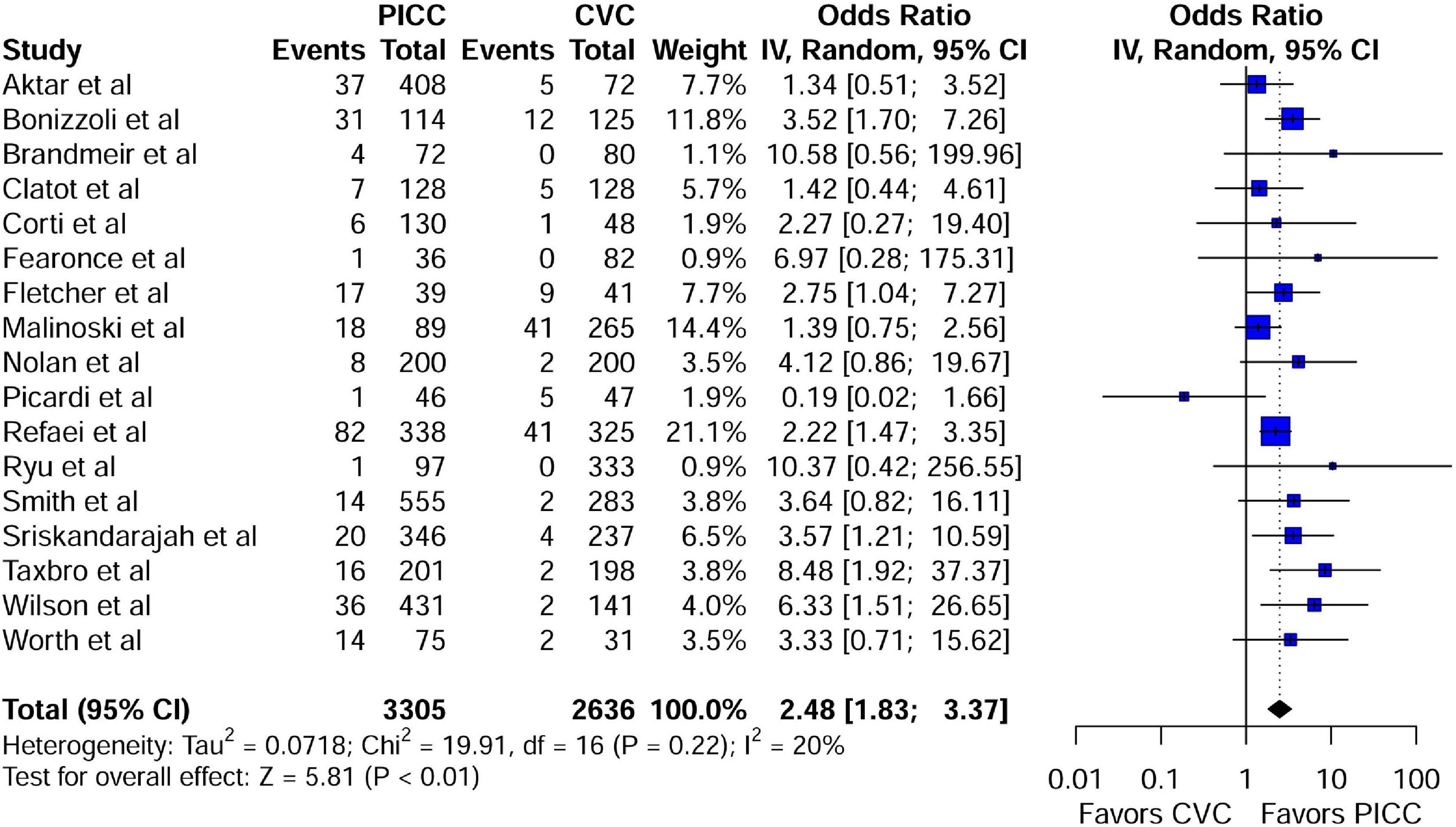
Figure 4. Forest plot depicting the risk of VTE between the peripherally inserted central catheter and central venous catheters in studies with a comparison arm. PICC, peripherally inserted central catheter; CVC, central venous catheter.
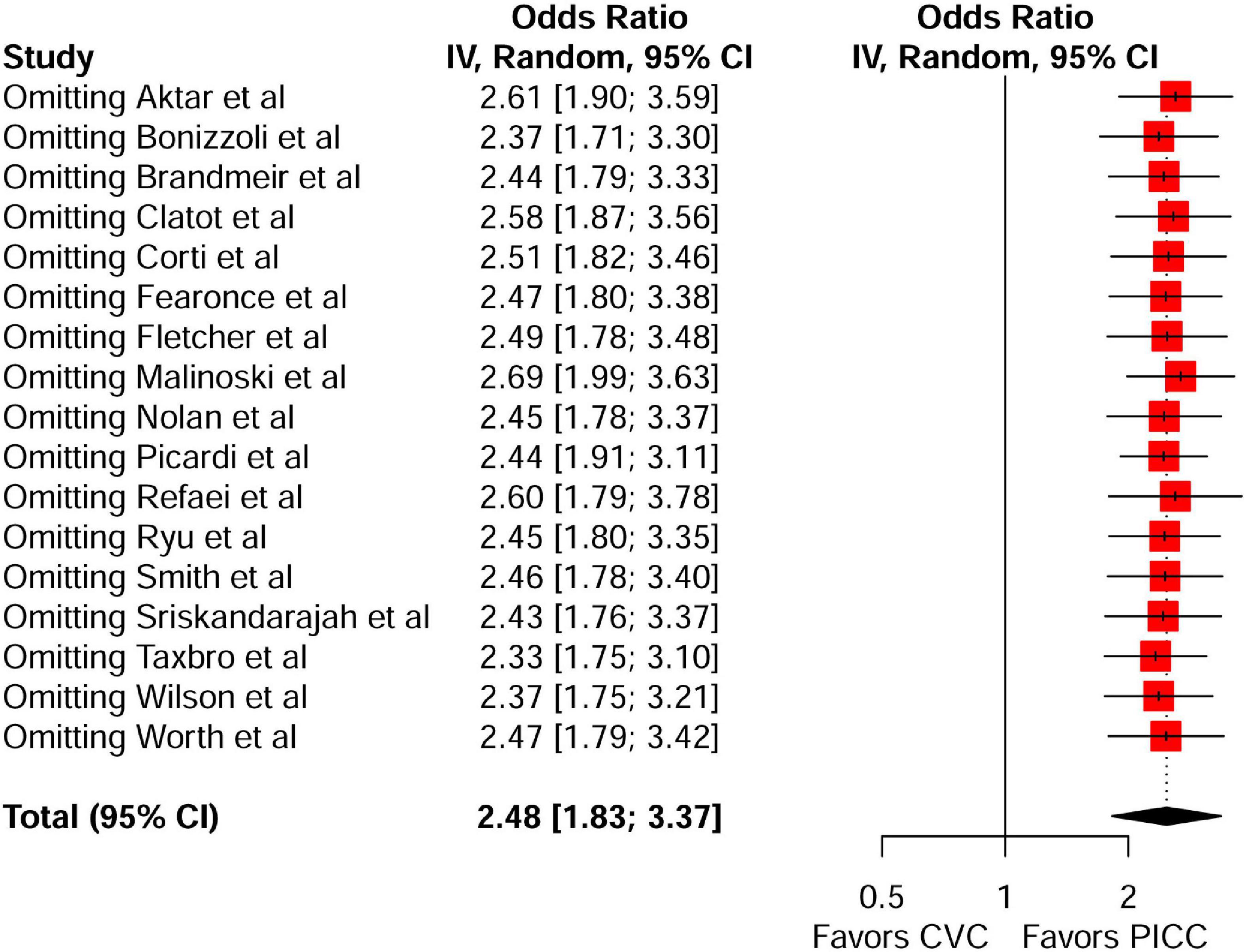
Figure 5. Leave-one-out sensitivity analysis of the risk of VTE between the peripherally inserted central catheter and central venous catheters in studies with a comparison arm. VTE, venous thromboembolism; PICC, peripherally inserted central catheter; CVC, central venous catheter.
Subgroup analyses were performed according to study setting, patient population, DVT prophylaxis, study design, study location, and publication year. According to subgroup analysis stratified by study setting, PICC was associated with a statistically significant increase in the odds of VTE events in both critical care/ICU (OR, 2.84; 95% CI: 1.78–4.52; P < 0.01) and non-critical care/non-ICU (OR, 2.24; 95% CI: 1.43–3.52; P < 0.01) subgroups (Table 4 and Supplementary Figure 11). Similarly, on subgroup analysis stratified by patient population, PICC was associated with a significant increase in the odds of VTE events in oncology (OR, 2.15; 95% CI: 1.31–3.53; P < 0.01) and non-oncology (OR, 2.76; 95% CI: 1.84–4.14; P < 0.01) subgroups (Table 4 and Supplementary Figure 12). Furthermore, a subgroup analysis by DVT prophylaxis revealed a significant difference in VTE events between the PICC and CVC in studies where DVT prophylaxis was not reported (OR, 2.59; 95% CI: 1.82–2.59; P < 0.01). However, in studies where VTE prophylaxis was reported, there was no significant difference in VTE events between the PICC and CVC (OR, 2.31; 95% CI: 1.02–5.19; P = 0.04) (Table 4 and Supplementary Figure 13). Subgroup analysis stratified by study design revealed that there were significant differences in the VTE events between PICC and CVC in retrospective (OR, 2.49; 95% CI: 1.80–3.33; P < 0.01) and prospective studies (OR, 2.30; 95% CI: 1.14–4.65; P = 0.02) but not in RTCs (OR, 2.28; 95% CI: 0.77–6.74; P = 0.13) (Table 4 and Supplementary Figure 14). Subgroup analysis by study location revealed that the American studies had a significantly higher risk of PICC-related VTE than the non-American studies (OR, 2.62; 95% CI: 1.56–4.41 vs. OR, 2.41; 95% CI: 1.59–3.67; P < 0.01) (Table 4 and Supplementary Figure 15). Furthermore, subgroup analysis by publication year (studies published from 2011 to 2022 and studies published from 1990 to 2010) revealed significant differences in the risk of VTE between PICC and CVC in both subgroups (P < 0.01) (Table 4 and Supplementary Figure 16).
Discussion
To the best of our knowledge, this systematic review and meta-analysis assessing the incidence and risk of VTE associated with PICC in the hospitalized patient is the largest to date, with more than three times the number of patients included in any previous meta-analysis (15, 19). Furthermore, we only included studies in which the catheter tip location was confirmed in order to determine the exact incidence and risk of PICC-related VTE in hospitalized patients. This meta-analysis demonstrated that in non-comparison studies, the overall incidence of PICC-related symptomatic VTE was 3.7% (95% CI: 3.1–4.4) in hospitalized patients and 10.6% in critically ill or ICU patients. Nevertheless, there is significant heterogeneity across studies, most likely due to differences in study design, high variability in sample size, study location, measurement instruments, and timing of outcome measurements. The meta-analysis by Chopra et al. (15) showed that the weighted incidence of PICC-related symptomatic VTE was 4.30%. Moreover, the incidence of PICC-associated VTE was higher in critically ill patients (13.91%). In this study, we found a lower incidence of PICC-related VTE than in the meta-analysis by Chopra et al. (15). The possible reasons for this finding are as follows: (i) Catheter-related complications are highly dependent on the insertion technique, and the majority of the studies included by Chopra et al. (15) differed substantially in terms of insertion technique. In this meta-analysis, studies only with proper PICC tip location verification were included, so the incidence of VTE was lower than in the meta-analysis by Chopra et al. (15). (ii) Furthermore, their meta-analysis included studies that differed significantly in terms of approach to VTE diagnosis (symptomatic vs. asymptomatic DVT), catheter insertion techniques and outcomes that could have affected pooled estimates (15). (iii) The study by Chopra et al. (91) was conducted in 2012, PICC-related complications have been reduced in recent years due to the use of evidence-based intervention techniques and vascular access specialist team approach. We included 42 studies published after 2011 (i.e., the modern vascular access era) in the pooled analysis, which may have resulted in a lower incidence of PICC-related VTE. A recent meta-analysis by Balsorano et al. (19) investigated the thrombotic rate associated with PICCs and found that the pooled DVT rate was 2.4%, with the thrombotic rate being higher in onco-hematologic patients (5.9%). Although this meta-analysis included patients for whom catheter insertion was performed with ultrasound guidance and proper tip location verification, there were some limitations, including a search strategy period limited to studies published between January 2010 and November 2018. Furthermore, they excluded retrospective studies and limited their research to only prospective studies.
A meta-analysis of comparative studies in this study showed that PICCs were associated with an increased risk of VTE compared with CVCs. A similar finding was also observed for PICC-related VTE risk in a study by Chopra et al. (15). This finding is consistent with prior studies, which showed that PICCs were associated with an increased risk of VTE compared with CVCs (78–80). A previous meta-analysis found that the pooled OR for PICC-related VTE was 2.55 (95% CI: 1.54–4.23). The present study also showed a comparable odds ratio (OR, 2.48; 95% CI: 1.83–3.37). Ample evidence suggests that risk factors such as a recent cancer diagnosis, PICC size, a prior history of PICC-related venous thrombosis or DVT, obesity, catheter tip location, and chemotherapeutic agents infused through the PICC line are linked to PICC-related VTE (9, 43, 62). Although the exact mechanisms underlying catheter-related thrombosis are unclear, increased cross-sectional diameter of the peripheral veins occupied by PICC may result in venous stasis (92). Additionally, catheter misplacement or migration of the catheter tip may also cause vascular endothelium damage (93).
In light of the increased risk of DVT, it is unclear whether PICC recipients should receive pharmacological DVT prophylaxis on a regular basis. A meta-analysis of 15 RCTs of anticoagulant prophylaxis in patients with CVCs revealed that anticoagulant prophylaxis effectively prevents catheter-associated DVT (94). However, its efficacy in preventing symptomatic VTE and PE remains unknown. Similarly, a recent meta-analysis concluded that pharmacological DVT prophylaxis reduces the risk of both asymptomatic and clinically detected VTE (95). Notably, in this study, there was no significant difference in VTE risk between the PICC and CVC in the subgroup analysis (OR, 2.31; 95% CI: 1.02–5.19; P = 0.04). This finding is consistent with a previous meta-analysis, which found no statistically significant difference in the risk of VTE associated with PICC (15). Similarly, the evidence-based guidelines for the management of VTE published by the American Society of Hematology recommend against routine VTE prophylaxis in patients in long-term care or outpatients with low VTE risk (96). However, pharmacological VTE prophylaxis is recommended in acutely or critically ill inpatients with acceptable bleeding risk.
Of note, subgroup analysis based on the study design showed that there was no significant difference in VTE events between PICC and CVC in prospective studies (OR, 2.30; 95% CI: 1.14–4.65; P = 0.02) and RCTs (OR, 2.28; 95% CI: 0.77–6.74; P = 0.13) included in this meta-analysis. Retrospective studies are prone to selection and recall biases, which can impact on overall results. Our findings support the need for future well-designed prospective studies and RCTs to elucidate the true picture of the risk of VTE associated with PICC.
Limitations and strengths
This meta-analysis has several limitations. First, due to the lack of a comparison group in the majority of included studies, we were unable to estimate pooled ORs of PICC-related VTE in those studies. Second, the inclusion of retrospective studies in this meta-analysis could have resulted in selection and recall biases. However, more prospective studies were included in this meta-analysis than in previous meta-analyses. Third, despite using subgroup analysis, there was substantial heterogeneity in studies with a non-comparison arm. Heterogeneity was due to differences in the study design, population, and other basic characteristics of the studies. Additionally, we could not conduct a meta-regression analysis to account for heterogeneity due to insufficient data. Fourth, many primary studies did not report data on VTE prophylaxis. Therefore, our finding regarding the use of VTE prophylaxis cannot be interpreted as a generalizable estimate.
Despite the aforementioned limitations, this meta-analysis has several strengths. First, this systematic review and meta-analysis is the most comprehensive to date because it includes the largest number (n = 75) of studies (both comparison and non-comparison studies) investigating the incidence and risk of VTE associated with PICCs in hospitalized patients. Second, in this meta-analysis, we included five comparative prospective RCTs, whereas previous meta-analyses did not include any RCTs. Third, this study only included studies published in peer-reviewed journals, whereas previous meta-analyses included one-third of the studies only in abstract form. Finally, sensitivity analysis of both comparison and non-comparison studies showed that our findings are robust.
Current clinical challenges and future research
Peripherally inserted central catheters -related VTE rates were extremely variable in individual studies included in this meta-analysis due to wide variation in the study population, study design, VTE diagnostic method, and definition of VTE events across studies (28, 29, 31, 45). The majority of primary studies ignored catheter size, and the use of ultrasound for PICC insertion was inconsistent in many studies. Because the majority of PICC-related thrombotic events occur within 2–3 weeks of PICC insertion (97), future RCTs should include preplanned follow-up for catheter-related complications to reduce the impact of attrition bias in true risk detection for thrombotic events. Future studies with adequate follow-up duration, uniform definitions of VTE, and standardized risk assessment for PICC-related VTE are required. Low-molecular-weight heparins (LMWHs) are used to prevent both primary and secondary catheter-related thrombosis. The clinical practice guidelines for the management of catheter-related thrombosis from the International Initiative on Thrombosis and Cancer (ITAC), the American Society of Clinical Oncology (ASCO), the American Society of Hematology (ASH), and the National Comprehensive Cancer Network (NCCN) all recommend anticoagulation for at least 3 months or as long as the catheter is kept in place (98). However, in patients who are at high risk of bleeding, catheter removal should be considered instead of using anticoagulation for the treatment of catheter-related thrombosis (98). There are several active ongoing clinical trials (ClinicalTrials.gov) on thromboprophylaxis for VTE (for e.g., NCT04924322, NCT05029063). A recently published two-center prospective TRIM-line pilot trial (99) enrolled 105 patients with active cancer and a newly inserted CVC demonstrated that symptomatic thrombotic complications occurred in 5.8% of the rivaroxaban group compared to 9.4% of the control group. The major symptomatic VTE rate in the rivaroxaban and control groups was 3.9 and 9.4%, respectively. The findings of this study revealed that thrombotic complications are common in patients with cancer and a newly inserted CVC. The ongoing phase III study (NCT05029063) is recruiting 1828 participants to determine the efficacy and safety of rivaroxaban for preventing VTE in cancer patients following the insertion of CVC. The primary outcome measure for this study is the number of major VTE in the patient population and major bleeding episodes. Secondary outcomes include fatal VTE, a composite of major VTE and major bleeding, and proximal upper and lower extremity CVC-related DVT. The results of this trial are expected in late 2026. This study is expected to provide important data on the prevention of VTE. Additionally, future research is required to improve strategies for addressing current clinical challenges in this patient population, such as diagnostic and therapeutic management and risk stratification.
Conclusion
In conclusion, this meta-analysis showed that the overall incidence of PICC-related symptomatic VTE was 3.7% in hospitalized patients. This incidence rate was higher in critically ill or ICU patients. PICCs are associated with an increased risk of VTE when compared to the CVC. Clinicians should perform a comprehensive risk-benefit analysis before PICC placement in critically ill patients. Further research is needed to address the heterogeneity and limitations of current evidence and to better understand the risk of VTE associated with PICC.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
AP, HD, and QZ: conceptualization. AP, HD, MG, and CW: data curation. AP, HD, HH, and MG: formal analysis and visualization. AP, HD, and CW: investigation. AP, HD, and QZ: project administration. QZ and AP: supervision. QZ, MG, AP, and HD: validation and writing. AP, HD, CW, HH, QZ, and MG: review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Chongqing Municipal Education Commission (Grant/Award Number: yjg211006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.917572/full#supplementary-material
Footnotes
References
1. Jonczyk M, Gebauer B, Schnapauff D, Rotzinger R, Hamm B, Collettini F. Peripherally inserted central catheters: dependency of radiation exposure from puncture site and level of training. Acta Radiol. (2018) 59:688–93. doi: 10.1177/0284185117730101
2. Mielke D, Wittig A, Teichgräber U. Peripherally inserted central venous catheter (PICC) in outpatient and inpatient oncological treatment. Support Care Cancer. (2020) 28:4753–60. doi: 10.1007/s00520-019-05276-0
3. Johansson E, Hammarskjöld F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Oncol. (2013) 52:886–92. doi: 10.3109/0284186X.2013.773072
4. Wang K, Zhong J, Huang N, Zhou Y. Economic evaluation of peripherally inserted central catheter and other venous access devices: a scoping review. J Vasc Access. (2020) 21:826–37. doi: 10.1177/1129729819895737
5. McDiarmid S, Scrivens N, Carrier M, Sabri E, Toye B, Huebsch L, et al. Outcomes in a nurse-led peripherally inserted central catheter program: a retrospective cohort study. CMAJ Open. (2017) 5:E535–9. doi: 10.9778/cmajo.20170010
6. Krein SL, Saint S, Trautner BW, Kuhn L, Colozzi J, Ratz D, et al. Patient-reported complications related to peripherally inserted central catheters: a multicentre prospective cohort study. BMJ Qual Saf. (2019) 28:574–81. doi: 10.1136/bmjqs-2018-008726
7. Chopra V, Kaatz S, Grant P, Swaminathan L, Boldenow T, Conlon A, et al. Risk of venous thromboembolism following peripherally inserted central catheter exchange: an analysis of 23,000 hospitalized patients. Am J Med. (2018) 131:651–60. doi: 10.1016/j.amjmed.2018.01.017
8. Winters JP, Callas PW, Cushman M, Repp AB, Zakai NA. Central venous catheters and upper extremity deep vein thrombosis in medical inpatients: the medical inpatients and thrombosis (MITH) Study. J Thromb Haemost. (2015) 13:2155–60. doi: 10.1111/jth.13131
9. Al-Asadi O, Almusarhed M, Eldeeb H. Predictive risk factors of venous thromboembolism (VTE) associated with peripherally inserted central catheters (PICC) in ambulant solid cancer patients: retrospective single centre cohort study. Thromb J. (2019) 17:2. doi: 10.1186/s12959-019-0191-y
10. Liem TK, Yanit KE, Moseley SE, Landry GJ, Deloughery TG, Rumwell CA, et al. Peripherally inserted central catheter usage patterns and associated symptomatic upper extremity venous thrombosis. J Vasc Surg. (2012) 55:761–7. doi: 10.1016/j.jvs.2011.10.005
11. Aw A, Carrier M, Koczerginski J, McDiarmid S, Tay J. Incidence and predictive factors of symptomatic thrombosis related to peripherally inserted central catheters in chemotherapy patients. Thromb Res. (2012) 130:323–6. doi: 10.1016/j.thromres.2012.02.048
12. O’Brien J, Paquet F, Lindsay R, Valenti D. Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. (2013) 10:864–8. doi: 10.1016/j.jacr.2013.06.003
13. Itkin M, Mondshein JI, Stavropoulos SW, Shlansky-Goldberg RD, Soulen MC, Trerotola SO. Peripherally inserted central catheter thrombosis - Reverse tapered versus nontapered catheters: a randomized controlled study. J Vasc Interv Radiol. (2014) 25:85–91.e1. doi: 10.1016/j.jvir.2013.10.009
14. Paauw JD, Borders H, Ingalls N, Boomstra S, Lambke S, Fedeson B, et al. The incidence of PICC line-associated thrombosis with and without the use of prophylactic anticoagulants. J Parenter Enteral Nutr. (2008) 32:443–7. doi: 10.1177/0148607108319801
15. Chopra V, Anand S, Hickner A. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis (vol 382, pg 311, 2013). Lancet. (2013) 382:1328. doi: 10.1016/S0140-6736(13)60592-9
16. Fallouh N, McGuirk HM, Flanders SA, Chopra V. Peripherally inserted central catheter-associated deep vein thrombosis: a narrative review. Am J Med. (2015) 128:722–38. doi: 10.1016/j.amjmed.2015.01.027
17. Menéndez JJ, Verdú C, Calderón B, Gómez-Zamora A, Schüffelmann C, de la Cruz JJ, et al. Incidence and risk factors of superficial and deep vein thrombosis associated with peripherally inserted central catheters in children. J Thromb Haemost. (2016) 14:2158–68. doi: 10.1111/jth.13478
18. Scherer RW, Saldanha IJ. How should systematic reviewers handle conference abstracts? A view from the trenches. Syst Rev. (2019) 8:264. doi: 10.1186/s13643-019-1188-0
19. Balsorano P, Virgili G, Villa G, Pittiruti M, Romagnoli S, De Gaudio AR, et al. Peripherally inserted central catheter–related thrombosis rate in modern vascular access era—when insertion technique matters: a systematic review and meta-analysis. J Vasc Access. (2020) 21:45–54. doi: 10.1177/1129729819852203
20. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
21. Vidal V, Muller C, Jacquier A, Giorgi R, Le Corroller T, Gaubert JY, et al. Évaluation prospective des complications des PICCs. J Radiol. (2008) 89:495–8. doi: 10.1016/S0221-0363(08)71453-7
22. Trerotola SO, Stavropoulos SW, Mondschein JI, Patel AA, Fishman N, Fuchs B, et al. Triple-lumen peripherally inserted central catheter in patients in the critical care unit: prospective evaluation. Radiology. (2010) 256:312–20. doi: 10.1148/radiol.10091860
23. Tran H, Arellano M, Chamsuddin A, Flowers C, Heffner LT, Langston A, et al. Deep venous thromboses in patients with hematological malignancies after peripherally inserted central venous catheters. Leuk Lymphoma. (2010) 51:1473–7. doi: 10.3109/10428194.2010.481065
24. Tian G, Zhu Y, Qi L, Guo F, Xu H. Efficacy of multifaceted interventions in reducing complications of peripherally inserted central catheter in adult oncology patients. Support Care Cancer. (2010) 18:1293–8. doi: 10.1007/s00520-009-0747-7
25. Sperry BW, Roskos M, Oskoui R. The effect of laterality on venous thromboembolism formation after peripherally inserted central catheter placement. J Vasc Access. (2013) 13:91–5. doi: 10.5301/jva.5000014
26. Sharp R, Cummings M, Fielder A, Mikocka-Walus A, Grech C, Esterman A. The catheter to vein ratio and rates of symptomatic venous thromboembolism in patients with a peripherally inserted central catheter (PICC): a prospective cohort study. Int J Nurs Stud. (2015) 52:677–85. doi: 10.1016/j.ijnurstu.2014.12.002
27. Seeley MA, Santiago M, Shott S. Prediction tool for thrombi associated with peripherally inserted central catheters. J Infus Nurs. (2007) 30:280–6. doi: 10.1097/01.NAN.0000292570.62763.3f
28. Sato R, Kumai T, Hayashi R, Komatsuda H, Kishibe K, Takahara M, et al. Peripherally inserted central venous catheters provide safe and easy central venous access in patients with head and neck cancer. Pract Otorhinolaryngol. (2021) 114:801–5. doi: 10.5631/jibirin.114.801
29. Rabinstein AA, Hellickson JD, Macedo TA, Lewis BD, Mandrekar J, McBane RD. Sequential pneumatic compression in the arm in neurocritical patients with a peripherally inserted central venous catheter: a randomized trial. Neurocrit Care. (2020) 32:187–92. doi: 10.1007/s12028-019-00765-w
30. Poletti F, Coccino C, Monolo D, Crespi P, Ciccioli G, Cordio G, et al. Efficacy and safety of peripherally inserted central venous catheters in acute cardiac care management. J Vasc Access. (2018) 19:455–60. doi: 10.1177/1129729818758984
31. Pittiruti M, Emoli A, Porta P, Marche B, DeAngelis R, Scoppettuolo G. A prospective, randomized comparison of three different types of valved and non-valved peripherally inserted central catheters. J Vasc Access. (2014) 15:519–23. doi: 10.5301/jva.5000280
32. Pittiruti M, Brutti A, Celentano D, Pomponi M, Biasucci DG, Annetta MG, et al. Clinical experience with power-injectable PICCs in intensive care patients. Crit Care. (2012) 16:R21. doi: 10.1186/cc11181
33. Ong B, Gibbs H, Catchpole I, Hetherington R, Harper J. Peripherally inserted central catheters and upper extremity deep vein thrombosis. Australas Radiol. (2006) 50:451–4. doi: 10.1111/j.1440-1673.2006.01623.x
34. Nash EF, Helm EJ, Stephenson A, Tullis E. Incidence of deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. J Vasc Interv Radiol. (2009) 20:347–51. doi: 10.1016/j.jvir.2008.11.018
35. Meyer BM. Managing peripherally inserted central catheter thrombosis risk: a guide for clinical best practice. J Assoc Vasc Access. (2011) 16:144–7. doi: 10.2309/java.16-3-3
36. Merrell SW, Peatross BG, Grossman MD, Sullivan JJ, Harker WG. Peripherally inserted central venous catheters - Low-risk alternatives for ongoing venous access. West J Med. (1994) 160:25–30.
37. Mermis JD, Strom JC, Greenwood JP, Low DM, He J, Stites SW, et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. (2014) 11:1404–10. doi: 10.1513/AnnalsATS.201404-175OC
38. Mariggiò E, Iori AP, Micozzi A, Chistolini A, Latagliata R, Berneschi P, et al. Peripherally inserted central catheters in allogeneic hematopoietic stem cell transplant recipients. Support Care Cancer. (2020) 28:4193–9. doi: 10.1007/s00520-019-05269-z
39. Maneval RE, Clemence BJ. Risk factors associated with catheter-related upper extremity deep vein thrombosis in patients with peripherally inserted central venous catheters: a prospective observational cohort study: part 2. J Infus Nurs. (2014) 37:260–8. doi: 10.1097/NAN.0000000000000042
40. Lobo BL, Vaidean G, Broyles J, Reaves AB, Shorr RI. Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. (2009) 4:417–22. doi: 10.1002/jhm.442
41. Liu K, Zhou Y, Xie W, Gu Z, Jin Y, Ye X, et al. Handgrip exercise reduces peripherally-inserted central catheter-related venous thrombosis in patients with solid cancers: a randomized controlled trial. Int J Nurs Stud. (2018) 86:99–106. doi: 10.1016/j.ijnurstu.2018.06.004
42. Liang Y-J, He Y, Li J-M, Chen L-M, Chen L-P, Wang C, et al. The incidence and predictors of symptomatic venous thromboembolism associated with peripherally inserted central catheters in patients with nasopharyngeal carcinoma. Onco Targets Ther. (2018) 11:3119–27. doi: 10.2147/OTT.S164723
43. Li X, Wang G, Yan K, Yin S, Wang H, Wang Y, et al. The incidence, risk factors, and patterns of peripherally inserted central catheter-related venous thrombosis in cancer patients followed up by ultrasound. Cancer Manag Res. (2021) 13:4329–40. doi: 10.2147/CMAR.S301458
44. Lambrechts MJ, Spence BS, Harris SM, Gilmore A, Marjara J, Si Z, et al. What are the risk factors for an upper extremity deep venous thrombosis after orthopaedic irrigation and debridement and peripherally inserted central catheter placement? Mo Med. (2021) 118:374–80.
45. Kleidon TM, Horowitz J, Rickard CM, Ullman AJ, Marsh N, Schults J, et al. Peripherally inserted central catheter thrombosis after placement via electrocardiography vs traditional methods. Am J Med. (2021) 134:e79–88. doi: 10.1016/j.amjmed.2020.06.010
46. King MM, Rasnake MS, Rodriguez RG, Riley NJ, Stamm JA. Peripherally inserted central venous catheter-associated thrombosis: retrospective analysis of clinical risk factors in adult patients. South Med J. (2006) 99:1073–7. doi: 10.1097/01.smj.0000240707.22171.12
47. Kang J, Chen W, Sun W, Ge R, Li H, Ma E, et al. Peripherally inserted central catheter-related complications in cancer patients: a prospective study of over 50,000 catheter days. J Vasc Access. (2017) 18:153–7. doi: 10.5301/jva.5000670
48. Jones D, Wismayer K, Bozas G, Palmer J, Elliott M, Maraveyas A. The risk of venous thromboembolism associated with peripherally inserted central catheters in ambulant cancer patients. Thromb J. (2017) 15:25. doi: 10.1186/s12959-017-0148-y
49. Jianning W, Zhimin W, Peiyi H, Ping D, Huang X, Xu Y. The ultrasound monitoring to the venous thromboembolism associated with peripherally inserted central catheters in chemotherapy patients: a prospective cohort study. J Assoc Vasc Access. (2018) 23:221–8. doi: 10.1016/j.java.2018.09.001
50. Haglund NA, Cox ZL, Lee JT, Song Y, Keebler ME, Disalvo TG, et al. Are Peripherally inserted central catheters associated with increased risk of adverse events in status 1B patients awaiting transplantation on continuous intravenous milrinone? J Card Fail. (2014) 20:630–7. doi: 10.1016/j.cardfail.2014.06.004
51. Zhang X, Huang JJ, Xia Y, Li CF, Wang Y, Liu PP, et al. High risk of deep vein thrombosis associated with peripherally inserted central catheters in lymphoma. Oncotarget. (2016) 7:35404–11. doi: 10.18632/oncotarget.8639
52. Grove JR, Pevec WC. Venous thrombosis related to peripherally inserted central catheters. J Vasc Interv Radiol. (2000) 11:837–40. doi: 10.1016/S1051-0443(07)61797-7
53. González S, Jiménez P, Saavedra P, MacÍas D, Loza A, León C, et al. Five-year outcome of peripherally inserted central catheters in adults: a separated infectious and thrombotic complications analysis. Infect Control Hosp Epidemiol. (2021) 42:833–41. doi: 10.1017/ice.2020.1300
54. Fletcher JJ, Stetler W, Wilson TJ. The clinical significance of peripherally inserted central venous catheter-related deep vein thrombosis. Neurocrit Care. (2011) 15:454–60. doi: 10.1007/s12028-011-9554-3
55. Evans RS, Sharp JH, Linford LH, Lloyd JF, Woller SC, Stevens SM, et al. Reduction of peripherally inserted central catheter-associated DVT. Chest. (2013) 143:627–33. doi: 10.1378/chest.12-0923
56. Evans RS, Sharp JH, Linford LH, Lloyd JF, Tripp JS, Jones JP, et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. (2010) 138:803–10. doi: 10.1378/chest.10-0154
57. Dupont C, Gouya H, Panzo R, Hubert D, Correas JM, Agrario L, et al. Complications of peripherally inserted central catheters in adults with cystic fibrosis or bronchiectasis. J Vasc Access. (2015) 16:245–9. doi: 10.5301/jva.5000347
58. DeLemos C, Abi-Nader J, Akins PT. Use of peripherally inserted central catheters as an alternative to central catheters in neurocritical care units. Crit Care Nurse. (2011) 31:70–5. doi: 10.4037/ccn2011911
59. Cornillon J, Martignoles JA, Tavernier-Tardy E, Gire M, Martinez P, Tranchan C, et al. Prospective evaluation of systematic use of peripherally inserted central catheters (PICC lines) for the home care after allogeneic hematopoietic stem cells transplantation. Support Care Cancer. (2017) 25:2843–7. doi: 10.1007/s00520-017-3699-3
60. Chen Y, Chen H, Yang J, Jin W, Fu D, Liu M, et al. Patterns and risk factors of peripherally inserted central venous catheter-related symptomatic thrombosis events in patients with malignant tumors receiving chemotherapy. J Vasc Surg Venous Lymphat Disord. (2020) 8:919–29. doi: 10.1016/j.jvsv.2020.01.010
61. Zerla PA, Canelli A, Cerne L, Caravella G, Gilardini A, De Luca G, et al. Evaluating safety, efficacy, and cost-effectiveness of picc securement by subcutaneously anchored stabilization device. J Vasc Access. (2017) 18:238–42. doi: 10.5301/jva.5000655
62. Chen P, Wan G, Zhu B. Incidence and risk factors of symptomatic thrombosis related to peripherally inserted central catheter in patients with lung cancer. J Adv Nurs. (2021) 77:1284–92. doi: 10.1111/jan.14666
63. Chemaly RF, De Parres JB, Rehm SJ, Adal KA, Lisgaris MV, Katz-Scott DS, et al. Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland clinic experience. Clin Infect Dis. (2002) 34:1179–83. doi: 10.1086/339808
64. Chasseigne V, Larbi A, Goupil J, Bouassida I, Buisson M, Beregi JP, et al. PICC management led by technicians: establishment of a cooperation program with radiologists and evaluation of complications. Diagn Interv Imaging. (2020) 101:7–14. doi: 10.1016/j.diii.2019.06.010
65. Campagna S, Gonella S, Berchialla P, Rigo C, Morano G, Zerla PA, et al. A retrospective study of the safety of over 100,000 peripherally-inserted central catheters days for parenteral supportive treatments. Res Nurs Health. (2019) 42:198–204. doi: 10.1002/nur.21939
66. Bertoglio S, Faccini B, Lalli L, Cafiero F, Bruzzi P. Peripherally inserted central catheters (PICCs) in cancer patients under chemotherapy: a prospective study on the incidence of complications and overall failures. J Surg Oncol. (2016) 113:708–14. doi: 10.1002/jso.24220
67. Bellesi S, Chiusolo P, De Pascale G, Pittiruti M, Scoppettuolo G, Metafuni E, et al. Peripherally inserted central catheters (PICCs) in the management of oncohematological patients submitted to autologous stem cell transplantation. Support Care Cancer. (2013) 21:531–5. doi: 10.1007/s00520-012-1554-0
68. Allen AW, Megargell JL, Brown DB, Lynch FC, Singh H, Singh Y, et al. Venous thrombosis associated with the placement of peripherally inserted central catheters. J Vasc Interv Radiol. (2000) 11:1309–14. doi: 10.1016/S1051-0443(07)61307-4
69. Abdullah BJJ, Mohammad N, Sangkar JV, Abd Aziz YF, Gan GG, Goh KY, et al. Incidence of upper limb venous thrombosis associated with peripherally inserted central catheters (PICC). Br J Radiol. (2005) 78:596–600. doi: 10.1259/bjr/32639616
70. Xing L, Adhikari VP, Liu H, Kong LQ, Liu SC, Li HY, et al. Diagnosis prevention and treatment for PICC-related upper extremity deep vein thrombosis in breast cancer patients. Asia Pac J Clin Oncol. (2012) 8:e12–6. doi: 10.1111/j.1743-7563.2011.01508.x
71. Wilson TJ, Brown DL, Meurer WJ, Stetler WR, Wilkinson DA, Fletcher JJ. Risk factors associated with peripherally inserted central venous catheter-related large vein thrombosis in neurological intensive care patients. Intensive Care Med. (2012) 38:272–8. doi: 10.1007/s00134-011-2418-7
72. Wang G, Wang H, Shen Y, Dong J, Wang X, Wang X, et al. Association between ABO blood group and venous thrombosis related to the peripherally inserted central catheters in cancer patients. J Vasc Access. (2021) 22:590–6. doi: 10.1177/1129729820954721
73. Walshe LJ, Malak SF, Eagan J, Sepkowitz KA. Complication rates among cancer patients with peripherally inserted central catheters. J Clin Oncol. (2002) 20:3276–81. doi: 10.1200/JCO.2002.11.135
74. Akhtar N, Lee L. Utilization and complications of central venous access devices in oncology patients. Curr Oncol. (2021) 28:367–77. doi: 10.3390/curroncol28010039
75. Bonizzoli M, Batacchi S, Cianchi G, Zagli G, Lapi F, Tucci V, et al. Peripherally inserted central venous catheters and central venous catheters related thrombosis in post-critical patients. Intensive Care Med. (2011) 37:284–9. doi: 10.1007/s00134-010-2043-x
76. Refaei M, Fernandes B, Brandwein J, Goodyear MD, Pokhrel A, Wu C. Incidence of catheter-related thrombosis in acute leukemia patients: a comparative, retrospective study of the safety of peripherally inserted vs. centrally inserted central venous catheters. Ann Hematol. (2016) 95:2057–64. doi: 10.1007/s00277-016-2798-4
77. Ryu DY, Lee SB, Kim GW, Kim JHA. Peripherally Inserted Central Catheter is a Safe and Reliable Alternative to Short-Term Central Venous Catheter for the Treatment of Trauma Patients. J Trauma Inj. (2019) 32:150–6. doi: 10.20408/jti.2019.015
78. Sriskandarajah P, Webb K, Chisholm D, Raobaikady R, Davis K, Pepper N, et al. Retrospective cohort analysis comparing the incidence of deep vein thromboses between peripherally-inserted and long-term skin tunneled venous catheters in hemato-oncology patients. Thromb J. (2015) 13:21. doi: 10.1186/s12959-015-0052-2
79. Taxbro K, Hammarskjöld F, Thelin B, Lewin F, Hagman H, Hanberger H, et al. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: an open-label, randomised, two-centre trial. Br J Anaesth. (2019) 122:734–41. doi: 10.1016/j.bja.2019.01.038
80. Wilson TJ, Stetler WR Jr., Fletcher JJ. Comparison of catheter-related large vein thrombosis in centrally inserted versus peripherally inserted central venous lines in the neurological intensive care unit. Clin Neurol Neurosurg. (2013) 115:879–82. doi: 10.1016/j.clineuro.2012.08.025
81. Worth LJ, Seymour JF, Slavin MA. Infective and thrombotic complications of central venous catheters in patients with hematological malignancy: prospective evaluation of nontunneled devices. Support Care Cancer. (2009) 17:811–8. doi: 10.1007/s00520-008-0561-7
82. Smith JR, Friedell ML, Cheatham ML, Martin SP, Cohen MJ, Horowitz JD. Peripherally inserted central catheters revisited. Am J Surg. (1998) 176:208–11. doi: 10.1016/s0002-9610(98)00121-4
83. Brandmeir NJ, Davanzo JR, Payne R, Sieg EP, Hamirani A, Tsay A, et al. Randomized trial of complications of peripherally and centrally inserted central lines in the neuro-intensive care unit: results of the NSPVC trial. Neurocrit Care. (2020) 32:400–6. doi: 10.1007/s12028-019-00843-z
84. Clatot F, Fontanilles M, Lefebvre L, Lequesne J, Veyret C, Alexandru C, et al. Randomised phase II trial evaluating the safety of peripherally inserted catheters versus implanted port catheters during adjuvant chemotherapy in patients with early breast cancer. Eur J Cancer. (2020) 126:116–24. doi: 10.1016/j.ejca.2019.11.022
85. Corti F, Brambilla M, Manglaviti S, Di Vico L, Pisanu MN, Facchinetti C, et al. Comparison of outcomes of central venous catheters in patients with solid and hematologic neoplasms: an Italian real-world analysis. Tumori. (2021) 107:17–25. doi: 10.1177/0300891620931172
86. Fearonce G, Faraklas I, Saffle JR, Cochran A. Peripherally inserted central venous catheters and central venous catheters in burn patients: a comparative review. J Burn Care Res. (2010) 31:31–5. doi: 10.1097/BCR.0b013e3181cb8eaa
87. Fletcher JJ, Wilson TJ, Rajajee V, Stetler WR, Jacobs TL, Sheehan KM, et al. Randomized trial of central venous catheter type and thrombosis in critically ill Neurologic Patients. Neurocrit Care. (2016) 25:20–8. doi: 10.1007/s12028-016-0247-9
88. Malinoski D, Ewing T, Bhakta A, Schutz R, Imayanagita B, Casas T, et al. Which central venous catheters have the highest rate of catheter-associated deep venous thrombosis: a prospective analysis of 2,128 catheter days in the surgical intensive care unit. J Trauma Acute Care Surg. (2013) 74:454–62. doi: 10.1097/TA.0b013e31827a0b2f
89. Nolan ME, Yadav H, Cawcutt KA, Cartin-Ceba R. Complication rates among peripherally inserted central venous catheters and centrally inserted central catheters in the medical intensive care unit. J Crit Care. (2016) 31:238–42. doi: 10.1016/j.jcrc.2015.09.024
90. Picardi M, Della Pepa R, Cerchione C, Pugliese N, Mortaruolo C, Trastulli F, et al. A frontline approach with peripherally inserted versus centrally inserted central venous catheters for remission induction chemotherapy phase of acute myeloid leukemia: a randomized comparison. Clin Lymphoma Myeloma Leuk. (2019) 19:e184–94. doi: 10.1016/j.clml.2018.12.008
91. Yi X, Chen J, Li J, Feng L, Wang Y, Zhu J-A, et al. Risk factors associated with PICC-related upper extremity venous thrombosis in cancer patients. J Clin Nurs. (2014) 23:837–43. doi: 10.1111/jocn.12227
92. Nifong TP, McDevitt TJ. The effect of catheter to vein ratio on blood flow rates in a simulated model of peripherally inserted central venous catheters. Chest. (2011) 140:48–53. doi: 10.1378/chest.10-2637
93. Marnejon T, Angelo D, Abdou AA, Gemmel D. Risk factors for upper extremity venous thrombosis associated with peripherally inserted central venous catheters. J Vasc Access. (2012) 13:231–8. doi: 10.5301/jva.5000039
94. Kirkpatrick A, Rathbun S, Whitsett T, Raskob G. Prevention of central venous catheter-associated thrombosis: a meta-analysis. Am J Med. (2007) 120:.901.e1–13. doi: 10.1016/j.amjmed.2007.05.010
95. Horner D, Stevens JW, Pandor A, Nokes T, Keenan J, de Wit K, et al. Pharmacological thromboprophylaxis to prevent venous thromboembolism in patients with temporary lower limb immobilization after injury: systematic review and network meta-analysis. J Thromb Haemost. (2020) 18:422–38. doi: 10.1111/jth.14666
96. Schünemann HJ, Cushman M, Burnett AE, Kahn SR, Beyer-Westendorf J, Spencer FA, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. (2018) 2:3198–225. doi: 10.1182/bloodadvances.2018022954
97. Liu Y, Gao Y, Wei L, Chen W, Ma X, Song L. Peripherally inserted central catheter thrombosis incidence and risk factors in cancer patients: a double-center prospective investigation. Ther Clin Risk Manag. (2015) 11:153–60. doi: 10.2147/TCRM.S73379
98. Streiff MB, Abutalib SA, Farge D, Murphy M, Connors JM, Piazza G. Update on guidelines for the management of cancer-associated thrombosis. Oncologist. (2021) 26:e24–40. doi: 10.1002/onco.13596
Keywords: peripherally inserted central catheters, deep vein thrombosis, pulmonary embolism, meta-analysis, venous thromboembolism, central venous catheters
Citation: Puri A, Dai H, Giri M, Wu C, Huang H and Zhao Q (2022) The incidence and risk of venous thromboembolism associated with peripherally inserted central venous catheters in hospitalized patients: A systematic review and meta-analysis. Front. Cardiovasc. Med. 9:917572. doi: 10.3389/fcvm.2022.917572
Received: 12 April 2022; Accepted: 07 July 2022;
Published: 26 July 2022.
Edited by:
Avi Leader, Rabin Medical Center, IsraelReviewed by:
Marco Matteo Ciccone, University of Bari Aldo Moro, ItalyNicola Potere, University of Studies G. d’Annunzio Chieti and Pescara, Italy
Copyright © 2022 Puri, Dai, Giri, Wu, Huang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinghua Zhao, cWgyMDA2M0AxNjMuY29t
†These authors have contributed equally to this work
 Anju Puri1†
Anju Puri1† Mohan Giri
Mohan Giri Huanhuan Huang
Huanhuan Huang