
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 23 January 2023
Sec. Heart Valve Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.917064
Purpose: The present study aimed to investigate the association of renin–angiotensin system inhibitors (RASi) with short- and long-term mortality in patients with aortic stenosis (AS).
Methods: A systematic search was performed in PubMed, Embase, and Cochrane library databases for relevant studies published before March 2022. Studies meeting the inclusion criteria were included to assess the effect of RASi on short-term (≤30 days) and long-term (≥1 year) mortality in patients with AS.
Results: A total of 11 studies were included in the meta-analysis. Our results demonstrated that RASi reduced short-term mortality (OR = 0.76, 95% CI 0.63–0.93, p = 0.008) after aortic valve replacement (AVR). Subgroup analysis revealed that RASi was still associated with lower short-term mortality after transcatheter aortic valve replacement (TAVR); however, the association was relatively weak in patients who underwent surgical aortic valve replacement (SAVR). For long-term mortality, the pooled OR was 1.04 (95% CI 0.88–1.24, p = 0.63) after sensitivity analysis in patients who did not undergo AVR. In addition, our study confirmed that RASi significantly reduced long-term mortality (OR = 0.57, 95% CI 0.44–0.74, p < 0.0001) in patients who underwent AVR. Subgroup analysis showed that both TAVR and SAVR groups treated with RASi had lower long-term mortality.
Conclusion: Renin–angiotensin system inhibitors did not change long-term mortality in AS patients who did not undergo AVR. However, RASi reduced short- and long-term mortality in patients who underwent AVR.
Aortic stenosis (AS) is a common primary valve disease in the aging population, affecting 2–7% of subjects older than 65 years (1, 2). AS is a progressive disease without obvious symptoms in the early stages. It is associated with increased cardiovascular morbidity and mortality (3, 4). To date, the appropriate timing of aortic valve replacement (AVR), including surgical aortic valve replacement (SAVR) and transcatheter aortic valve replacement (TAVR), is the main treatment strategy of AS with documented survival benefits (5, 6).
Even in its early stage, AS leads to left ventricular hypertrophy and fibrosis through several mechanisms, one of which is renin–angiotensin system (RAS) activation (7, 8). Severe left ventricular hypertrophy and fibrosis were associated with poor prognosis in patients with AS (9, 10). Herein, treatment with renin–angiotensin system inhibitors (RASi), including angiotensin-converting enzyme inhibitors (ACEi) and angiotensin-receptor blockers (ARBs), attenuates cardiac remodeling and myocardial hypertrophy and fibrosis in patients with AS (11, 12). However, the effect of RASi on prognosis has not been well characterized, and the results of previous studies in this field have been inconsistent (2, 13, 14).
Therefore, this meta-analysis of available randomized and observational studies aimed to explore whether treatment with RASi is associated with short- and long-term mortality in patients with AS.
We performed this meta-analysis in accordance with the Cochrane Handbook for Systematic Review of Intervention. In addition, the meta-analysis has been reported according to the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) statement (15). The preferred reporting items for systematic reviews and meta-analyses statement (PRISMA) checklist is showed in Supplementary Table 2.
A systematic search was performed in PubMed, Embase, and Cochrane library databases among articles published before March 2022. The following Medical Subject Heading (MeSH) terms or free texts were used for searching: renin-angiotensin system inhibitors, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aortic valve stenoses, (stenoses, aortic valve), (stenosis, aortic valve), (valve stenoses, aortic), (valve stenosis, aortic), aortic stenosis, (stenoses, aortic), (stenosis, aortic). We also reviewed the reference lists of retrieved articles for additional studies.
Studies meeting the following inclusion criteria were selected: (1) randomized controlled trials, prospective cohort studies, and retrospective cohort studies reporting the effect of RASi on short-term (≤30 days) or long-term (≥1 year) all-cause mortality in patients with AS; (2) provided the number of all-cause death; and (3) performed in adults more than 18 years. Reviews, comments, conference abstracts, and editorials were all excluded.
Two investigators independently extracted the following information with standardized data abstraction form from eligible studies: first author’s name, publication year, study design, the number of patients, the number of all-cause mortality, baseline characteristics, and echocardiographic data. The quality of the studies included in this meta-analysis was assessed by two independent investigators using the 9-star Newcastle–Ottawa Scale (NOS) and Revised Jadad’s Scale. Any discrepancies regarding data extraction and quality assessment were resolved by discussing with a third researcher under the supervision of the senior researcher.
The overall odd ratios (ORs) with 95% confidence intervals (CIs) for all-cause mortality were calculated using the reported number of all-cause death in the RASi group and non-RASi group in eligible studies. We calculated the trial-specific ORs using a fixed- or random-effects model depending on the heterogeneity of data included in this meta-analysis. The heterogeneity of data was evaluated by Cochran’s Q test and I2 statistic. The fixed-effects model was used in the absence of significant heterogeneity (p > 0.1, I2 < 50%) among eligible studies. In the presence of significant heterogeneity (p < 0.1, I2 ≥ 50%) among studies, a sensitivity analysis or subgroup analysis was performed to explore the sources of heterogeneity. The funnel plot was not performed due to the limited number of studies included in the present meta-analysis. The pooled analyses in the present study were performed using Review Manager, version 5.3 (Cochrane Collaboration, Copenhagen, Denmark). All tests were 2-tailed, and p-values were considered statistically significant at <0.05.
In total, 581 articles were identified by a systematic search in PubMed, Embase, and Cochrane library databases, and 1 article was identified from reference lists of the eligible studies. After removing duplicates and excluding non-relevant articles, 27 articles remained for full-text assessment. According to the inclusion criteria, 11 studies were used in this meta-analysis (2, 11–14, 16–21). The detailed flow diagram of this study is presented in Figure 1.
The quality of eligible studies assessed by NOS and Revised Jadad’s Scale is shown in Supplementary Table 1. The mean NOS score was 8 stars, and Revised Jadad’s score was 4, indicating satisfactory study quality.
As can be seen in Table 1, 10 of 11 studies included in this meta-analysis were cohort studies (2, 12–14, 16–21) and 1 of 11 was a randomized controlled trial (11). These 11 eligible studies consisted of 33,858 patients with AS, including 16,299 RASi users and 17,559 non-RASi users. The range follow-up length varied from 1 to 8 years.
The demographic, clinical, and echocardiographic data of patients are presented in Tables 2, 3. The mean age of patients ranged from 67.2 to 84.8 years and more than half were male. In addition, more than half of the patients had hypertension, and the majority of patients had preserved ejection fraction.
Five studies comprising 20,666 patients with AS reported the effect of RASi on short-term mortality (11, 16–19). However, the studies were all conducted in patients with AS who underwent AVR. After pooling data from 10,800 RASi users and 10,586 non-RASi users by the fixed-effects model (p = 0.65, I2 = 0%), we found that the RASi group had a lower risk of short-term mortality (OR = 0.76, 95% CI 0.63–0.93, p = 0.008, Figure 2) after AVR.
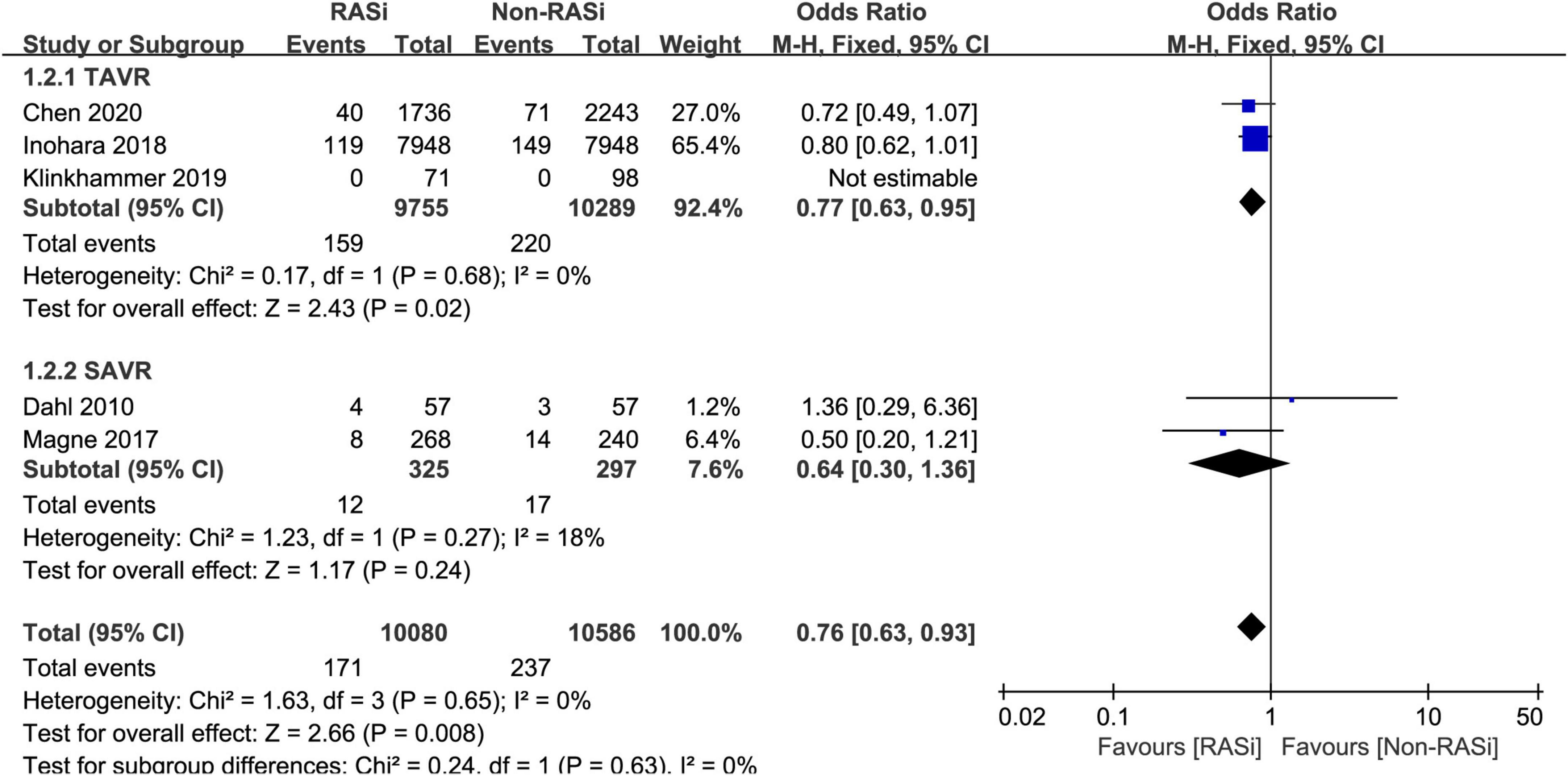
Figure 2. Forest plot comparing short-term mortality between patients with RASi-treated and non-RASi-treated AS who underwent AVR. RASi, renin–angiotensin system inhibitors; AS, aortic stenosis; AVR, aortic valve replacement. SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Subsequently, we performed a subgroup analysis to investigate the effect of RASi on short-term mortality in patients who underwent SAVR (11, 19) and TAVR (16–18). In patients with AS, receiving RASi was associated with a lower risk of mortality (OR = 0.77, 95% CI 0.63–0.95, p = 0.02, Figure 2) within 30 days after TAVR. Among patients who underwent SAVR, the RASi group also had lower 30-day mortality (OR = 0.64, 95% CI 0.30 to 1.36, p = 0.24, Figure 2); however, it was not statistically significant.
Three studies reported the effect of RASi on long-term mortality in patients with AS who did not undergo AVR (2, 13, 14). After pooling the 3 studies with a random-effects model, we found that there is no increased risk of long-term mortality in patients with AS treated with RASi (OR = 0.82, 95% CI 0.50–1.34, p = 0.43, Figure 3A). Due to the remarkable heterogeneity (p < 0.00001, I2 = 93%), a sensitivity analysis was performed. After excluding the study causing the heterogeneity (14), our pooled results still demonstrated that RASi did not increase the risk of long-term mortality (OR = 1.04, 95% CI 0.88–1.24, p = 0.63, Figure 3B).
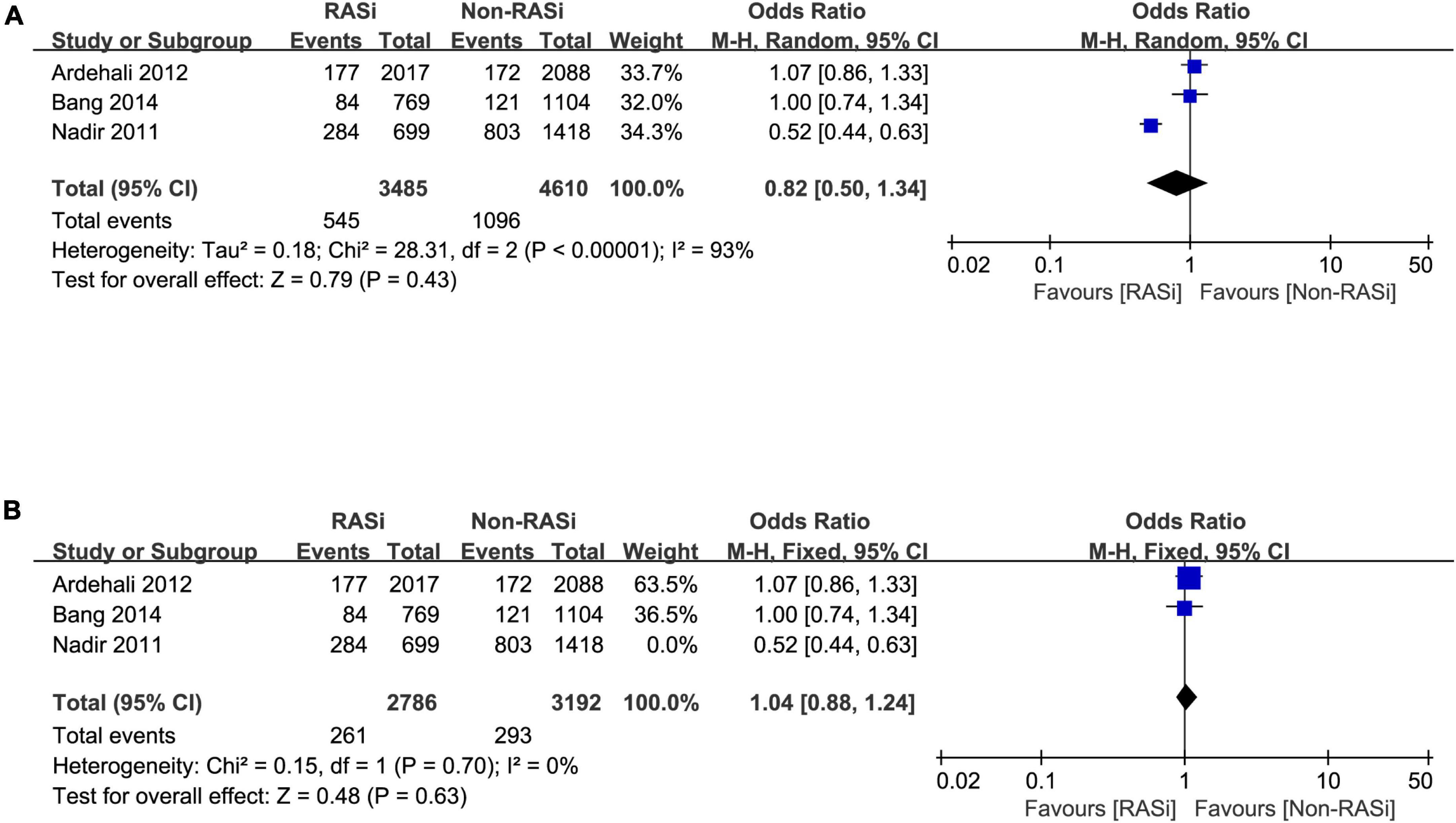
Figure 3. Forest plot comparing long-term mortality between patients with RASi-treated and non-RASi-treated AS who did not undergo AVR (A). Forest plot comparing long-term mortality between patients with RASi-treated and non-RASi-treated AS who did not undergo AVR after sensitivity analysis (B). RASi, renin–angiotensin system inhibitors; AS, aortic stenosis; AVR, aortic valve replacement.
As can be seen in Figure 4, our results also confirmed that treatment with RASi was associated with lower long-term mortality (OR = 0.57, 95% CI 0.44–0.74, p < 0.0001) in patients who underwent AVR, after pooling 8 studies with 25,763 patients by a random-effects model (11, 12, 16–21).
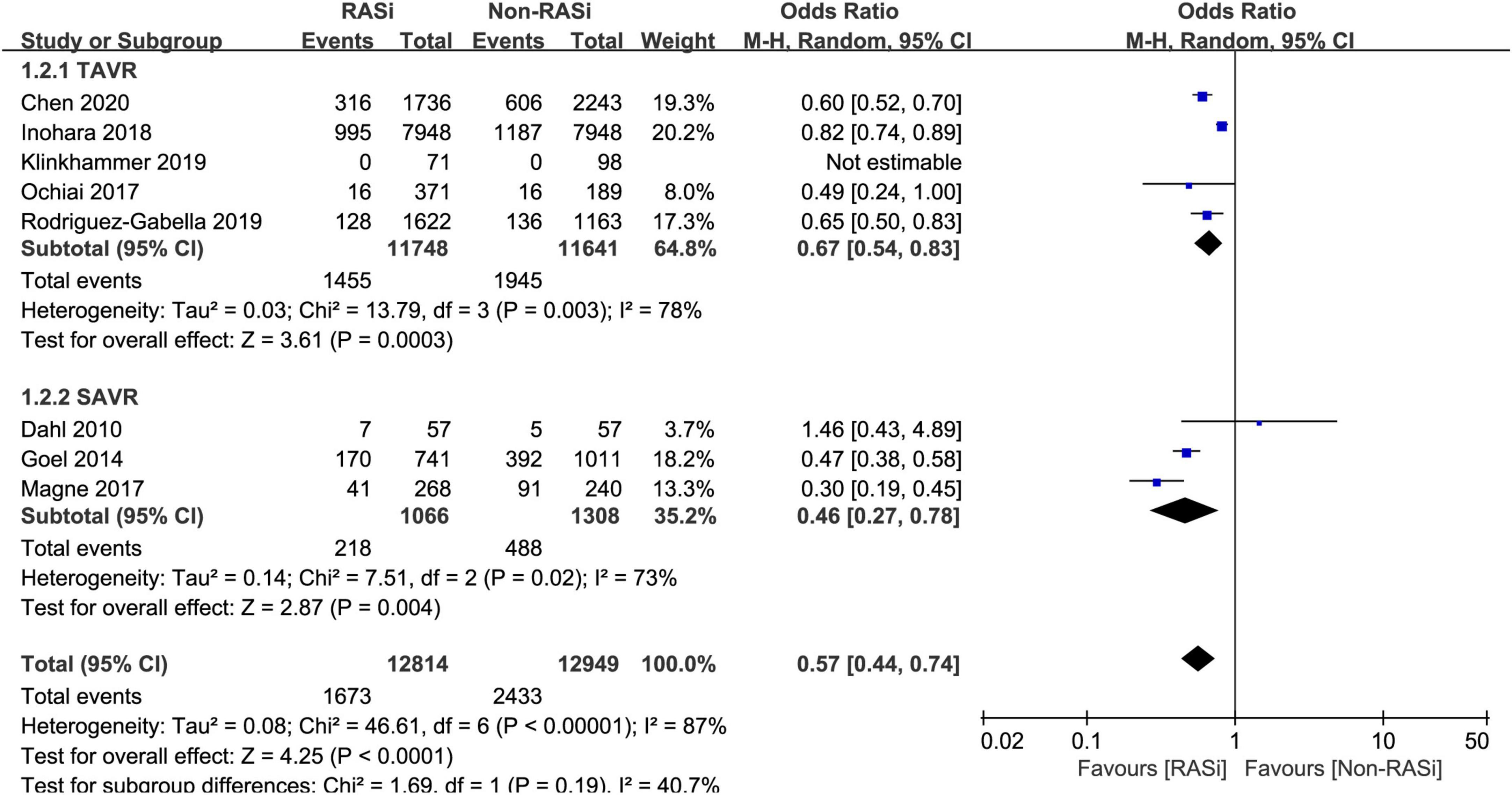
Figure 4. Forest plot comparing long-term mortality between patients with RASi-treated and non-RASi-treated AS who underwent AVR. RASi, renin–angiotensin system inhibitors; AS, aortic stenosis; AVR, aortic valve replacement. SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Meanwhile, a subgroup analysis was conducted to investigate the effect of RASi on long-term mortality in patients who underwent SAVR (11, 19, 20) and TAVR (12, 16–18, 21). Our pooled analysis indicated that treatment with RASi in patients who underwent TAVR was significantly associated with lower long-term mortality (OR = 0.67, 95% CI 0.54–0.83, p = 0.0003, Figure 4). Among patients who underwent SAVR, the negative association still persisted (OR = 0.46, 95% CI 0.27–0.78, p = 0.004, Figure 4).
Based on a visual inspection of funnel plots (Supplementary Figures 1–3), there was no obvious evidence of publication bias among the included studies. In addition, we performed a sensitivity analysis for long- or short-term all-cause mortality and found that the pooled results remained stable after the sensitivity analysis.
This meta-analysis of 11 clinical trials had three significant findings. First, treatment with RASi reduced short-term mortality in patients who underwent AVR. Subgroup analysis demonstrated that the association persisted in patients with AS who were treated with TAVR. Second, RASi was safe for patients with AS who did not undergo AVR and did not increase long-term mortality. Third, among patients who underwent AVR, using RASi was associated with a lower risk of long-term mortality. After subgroup analysis, the association still persisted in patients with AS who underwent TAVR or SAVR.
Previously, treatment with drugs like RASi was considered unsafe and even contraindicated in patients with significant AS (22, 23). These drugs were assumed to induce severe hypotension due to the vasodilator induced by them and the fixed obstruction at left ventricular outflow in patients with AS (22). Although this risk has never been confirmed by clinical evidence, many studies have suggested that treatment with RASi is well tolerated in patients with mild-to-severe AS (24, 25). In addition, some studies even reported the beneficial effects of RASi on hemodynamics, left ventricular hypertrophy, and AS progression and prognosis (24, 26–28).
To date, most studies measuring the effects of RASi on the prognosis of AS were non-randomized controlled trials and reported controversial results (2, 13, 14). The meta-analysis by Andersson and Abdulla (29) indicated that treatment with RASi does not increase long-term mortality in patients with AS. The study did not measure the effect of RASi on the prognosis of AS in patients who underwent AVR.
After pooling 3 studies (16–18), our results demonstrated that RASi reduced short-term mortality after TAVR in patients with AS. A similar association was not observed in patients who underwent SAVR. We assume that the discrepancies are largely due to 2 studies included in the SAVR group with a limited sample size (11, 19). Therefore, multicenter randomized controlled trials with large sample sizes are needed to further investigate the effects of RASi on short-term prognosis in patients who undergo SAVR. To date, the effect of RASi on short-term mortality was poorly understood in patients with AS who did not undergo AVR. Although Ardehail et al. (13) reported that RASi did not change short-term mortality (90 days) in patients who did not undergo AVR, the study was non-randomized. In addition, the high proportion of male participants (about 95%) and higher prevalence of hypertension and diabetes mellitus in the RASi group all could affect the results of that study (13). Therefore, future prospective, randomized controlled trials are needed to further explore whether RASi can improve short-term survival in AS patients without indication for AVR.
Consistent with the meta-analysis by Andersson and Abdulla (29), our present study also showed that RASi did not change long-term mortality in patients with AS who did not undergo AVR. Although RASi did not change long-term mortality, previous studies reported that RASi can postpone AS progression and reduce the need for AVR (26–29). Therefore, RASi may be considered in AS patients without indications for AVR. In addition, our meta-analysis of 8 studies (11, 12, 16–21) revealed that RASi reduced long-term mortality in patients who underwent AVR. Subgroup analysis further confirmed that the association persisted in both TAVR and SAVR groups. The reason behind the different effects of RASi on long-term mortality in patients who underwent AVR or not remains unknown. We hypothesize that the contradictory findings are largely due to the following reasons. First, RASi can be associated with the greater regression of left ventricular hypertrophy and fibrosis in patients who underwent AVR compared with those who did not undergo AVR. Second, increased sympathetic activity, which is associated with cardiovascular diseases (30), is more effectively inhibited by RASi in patients who underwent AVR. Third, aortic regurgitation or mitral regurgitation, which is associated with left ventricular remodeling (12, 31, 32), is significantly less severe in patients who underwent AVR. Furthermore, the association between RASi and better clinical outcomes might be mediated by decreasing afterload, valvulo-arterial impedance, systemic vascular resistance, vascular calcification, inflammatory cytokine levels, and endothelial dysfunction (33–36).
There are some limitations in our present study that need to be noted. First, most of the included studies were retrospective cohorts; therefore, our pooled results could be affected by unmeasured confounding variables. Second, due to the heterogeneity of the included trials, a random-effects model was used in the present study. Third, the limited number of studies included in this meta-analysis did not allow us to conduct more subgroup analyses and sensitivity or meta-regression analyses for the outcomes. Finally, the majority of included studies were conducted in highly experienced cardiovascular centers, which could limit the generalizability of our findings to other centers with less expertise.
The present study demonstrated that RASi was well tolerated in patients with AS. Furthermore, treatment with RASi significantly reduced short- and long-term mortality in AS patients who underwent AVR. To further confirm these findings, large-scale, randomized controlled trials are warranted.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
HL and QL were responsible for the study conception and design. YG and XK reviewed studies, extracted data, and drafted the manuscript. YG and LZ assessed the quality of the studies included. FG, HZ, and XK performed a systematic search and checked the data. All authors read and approved the final manuscript.
This study was supported by a grant from the Capital’s Funds for Health Improvement and Research (2022-1G-2063).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.917064/full#supplementary-material
AS, aortic stenosis; AVR, aortic valve replacement; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement; RAS, renin–angiotensin system; RASi, renin–angiotensin system inhibitors; ACEi, angiotensin-converting enzyme inhibitors; ARBs, angiotensin-receptor blockers; MeSH, Medical Subject Heading; NOS, Newcastle–Ottawa Scale.
1. Carabello B, Paulus W. Aortic stenosis. Lancet. (2009) 373:956. doi: 10.1016/S0140-6736(09)60211-7
2. Bang C, Greve A, Køber L, Rossebø A, Ray S, Boman K, et al. Renin-angiotensin system inhibition is not associated with increased sudden cardiac death, cardiovascular mortality or all-cause mortality in patients with aortic stenosis. Int J Cardiol. (2014) 175:492–8. doi: 10.1016/j.ijcard.2014.06.013
3. Otto C, Lind B, Kitzman D, Gersh B, Siscovick D. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. (1999) 341:142–7. doi: 10.1056/NEJM199907153410302
4. Otto C. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol. (2006) 47:2141–51. doi: 10.1016/j.jacc.2006.03.002
5. Baumgartner H, Falk V, Bax J, De Bonis M, Hamm C, Holm P, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2017) 38:2739–91.
6. Nishimura R, Otto C, Bonow R, Carabello B, Erwin J, Fleisher L, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the american college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. (2017) 70:252–89. doi: 10.1016/j.jacc.2017.03.011
7. Fielitz J, Hein S, Mitrovic V, Pregla R, Zurbrügg H, Warnecke C, et al. Activation of the cardiac renin-angiotensin system and increased myocardial collagen expression in human aortic valve disease. J Am Coll Cardiol. (2001) 37:1443–9. doi: 10.1016/s0735-1097(01)01170-6
8. Zaid R, Barker C, Little S, Nagueh S. Pre and post-operative diastolic dysfunction in patients with valvular heart disease: diagnosis and therapeutic implications. J Am Coll Cardiol. (2013) 62:1922–30. doi: 10.1016/j.jacc.2013.08.1619
9. Dweck M, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. (2011) 58:1271–9. doi: 10.1016/j.jacc.2011.03.064
10. Cioffi G, Faggiano P, Vizzardi E, Tarantini L, Cramariuc D, Gerdts E, et al. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart. (2011) 97:301–7. doi: 10.1136/hrt.2010.192997
11. Dahl J, Videbaek L, Poulsen M, Pellikka P, Veien K, Andersen L, et al. Effect of candesartan treatment on left ventricular remodeling after aortic valve replacement for aortic stenosis. Am J Cardiol. (2010) 106:713–9. doi: 10.1016/j.amjcard.2010.04.028
12. Ochiai T, Saito S, Yamanaka F, Shishido K, Tanaka Y, Yamabe T, et al. Renin-angiotensin system blockade therapy after transcatheter aortic valve implantation. Heart. (2018) 104:644–51. doi: 10.1136/heartjnl-2017-311738
13. Ardehali R, Leeper N, Wilson A, Heidenreich PA. The effect of angiotensin-converting enzyme inhibitors and statins on the progression of aortic sclerosis and mortality. J Heart Valve Dis. (2012) 21:337.
14. Nadir M, Wei L, Elder D, Libianto R, Lim T, Pauriah M, et al. Impact of renin-angiotensin system blockade therapy on outcome in aortic stenosis. J Am Coll Cardiol. (2011) 58:570–6. doi: 10.1016/j.jacc.2011.01.063
15. Stroup D, Berlin J, Morton S, Olkin I, Williamson G, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
16. Chen S, Redfors B, Nazif T, Kirtane A, Crowley A, Ben-Yehuda O, et al. Impact of renin-angiotensin system inhibitors on clinical outcomes in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement: an analysis of from the PARTNER 2 trial and registries. Eur Heart J. (2020) 41:943–54. doi: 10.1093/eurheartj/ehz769
17. Inohara T, Manandhar P, Kosinski A, Matsouaka R, Kohsaka S, Mentz R, et al. Association of renin angiotensin inhibitor treatment with mortality and heart failure readmission in patients with transcatheter aortic valve replacement. JAMA. (2018) 320:2231. doi: 10.1001/jama.2018.18077
18. Klinkhammer B. Renin-angiotensin system blockade after transcatheter aortic valve replacement (TAVR) improves intermediate survival. J Cardiovasc Thorac Res. (2019) 11:176–81. doi: 10.15171/jcvtr.2019.30
19. Magne J, Guinot B, Le Guyader A, Bégot E, Marsaud J, Mohty D, et al. Relation between renin angiotensin system blockers and survival following isolated aortic valve replacement for aortic stenosis. Am J Cardiol. (2018) 121:455–60. doi: 10.1016/j.amjcard.2017.11.013
20. Goel S, Aksoy O, Gupta S, Houghtaling P, Tuzcu E, Marwick T, et al. Renin-angiotensin system blockade therapy after surgical aortic valve replacement for severe aortic stenosis: a cohort study. Ann Intern Med. (2014) 161:699–710. doi: 10.7326/M13-1505
21. Rodriguez-Gabella T, Catalá P, Muñoz-García A, Nombela-Franco L, Del Valle R, Gutiérrez E, et al. Renin-angiotensin system inhibition following transcatheter aortic valve replacement. J Am Coll Cardiol. (2019) 74:631–41. doi: 10.1016/j.jacc.2019.05.055
22. Cox N, Abdul-Hamid A, Mulley G. Why deny ACE inhibitors to patients with aortic stenosis? Lancet. (1998) 352:111–2. doi: 10.1016/S0140-6736(98)85016-2
23. Cox N, Abdul-Hamid A, Mulley G. Aortic stenosis and ACE inhibitors. Lancet. (1998) 352:1392. doi: 10.1016/s0140-6736(05)60791-x
24. Dalsgaard M, Iversen K, Kjaergaard J, Grande P, Goetze J, Clemmensen P, et al. Short-term hemodynamic effect of angiotensin-converting enzyme inhibition in patients with severe aortic stenosis: a placebo-controlled, randomized study. Am Heart J. (2014) 167:226. doi: 10.1016/j.ahj.2013.11.002
25. Chockalingam A, Venkatesan S, Subramaniam T, Jagannathan V, Elangovan S, Alagesan R, et al. Safety and efficacy of angiotensin-converting enzyme inhibitors in symptomatic severe aortic stenosis: symptomatic cardiac obstruction–pilot study of enalapril in aortic stenosis (SCOPE-AS). Am Heart J. (2004) 147:740. doi: 10.1016/j.ahj.2003.10.017
26. Wakabayashi K, Wakabayashi K, Tsujino T, Tsujino T, Naito Y, Naito Y, et al. Administration of angiotensin-converting enzyme inhibitors is associated with slow progression of mild aortic stenosis in Japanese patients. Heart Vessels. (2011) 26:252–7. doi: 10.1007/s00380-010-0052-x
27. Bull S, Loudon M, Francis J, Joseph J, Gerry S, Karamitsos T, et al. A prospective, double-blind, randomized controlled trial of the angiotensin-converting enzyme inhibitor Ramipril In Aortic Stenosis (RIAS trial). Eur Heart J Cardiovasc Imaging. (2015) 16:834–41. doi: 10.1093/ehjci/jev043
28. Goh S, Sia C, Ngiam N, Tan B, Lee P, Tay E, et al. Effect of renin-angiotensin blockers on left ventricular remodeling in severe aortic stenosis. Am J Cardiol. (2017) 119:1839–45. doi: 10.1016/j.amjcard.2017.02.037
29. Andersson C, Abdulla J. Is the use of renin-angiotensin system inhibitors in patients with aortic valve stenosis safe and of prognostic benefit? A systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother. (2017) 3:21–7. doi: 10.1093/ehjcvp/pvw027
30. Marina N, Teschemacher A, Kasparov S, Gourine A. Glia, sympathetic activity and cardiovascular disease. Exp Physiol. (2016) 101:565–76.
31. Une D, Mesana L, Chan V, Maklin M, Chan R, Masters R, et al. Clinical impact of changes in left ventricular function after aortic valve replacement. Circulation. (2015) 132:741–7. doi: 10.1161/CIRCULATIONAHA.115.015371
32. Hiendlmayr B, Nakda J, Elsaid O, Wang X, Flynn A. Timing of surgical intervention for aortic regurgitation. Curr Treat Options Cardiovasc Med. (2016) 18:63. doi: 10.1007/s11936-016-0485-3
33. Marquis-Gravel G, Redfors B, Leon M, Généreux P. Medical treatment of aortic stenosis. Circulation. (2016) 134:1766–84. doi: 10.1161/CIRCULATIONAHA.116.023997
34. Inohara T, Marquis-Gravel G. Concentration-dependent renin-angiotensin system inhibition effects after transcatheter aortic valve replacement: important evidence, but more data are needed. Can J Cardiol. (2021) 37:370–1. doi: 10.1016/j.cjca.2020.09.005
35. Goel S, Kleiman N, Zoghbi W, Reardon M, Kapadia S. Renin-angiotensin system blockade in aortic stenosis: implications before and after aortic valve replacement. J Am Heart Assoc. (2020) 9:e016911. doi: 10.1161/JAHA.120.016911
Keywords: renin-angiotensin system inhibitors, aortic stenosis, transcatheter aortic valve replacement, surgical aortic valve replacement, short-term mortality, long-term mortality
Citation: Guan Y, Kong X, Zhu H, Li H, Zhao L, Guo F and Lv Q (2023) Association of renin–angiotensin system inhibitors use with short- and long-term mortality in patients with aortic stenosis: A systematic review and meta-analysis. Front. Cardiovasc. Med. 9:917064. doi: 10.3389/fcvm.2022.917064
Received: 10 April 2022; Accepted: 30 December 2022;
Published: 23 January 2023.
Edited by:
Vinod H. Thourani, Piedmont Heart Institute, United StatesReviewed by:
Antonino S. Rubino, University of Campania Luigi Vanvitelli, ItalyCopyright © 2023 Guan, Kong, Zhu, Li, Zhao, Guo and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Li,  ZHJfbGlob25nQHNpbmEuY29t
ZHJfbGlob25nQHNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.