- 1Department of Infectious Disease, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 2Department of General and Acute Care Medicine, Royal North Shore Hospital, Sydney, NSW, Australia
- 3Concord Clinical School, The University of Sydney, Sydney, NSW, Australia
- 4Division of the Renal and Metabolic, George Institute for Global Health, TheUniversity of New South Wales, Sydney, NSW, Australia
- 5Department of Renal Medicine, Concord Repatriation General Hospital, Concord, NSW, Australia
Aims: To perform a systematic review assessing the clinical manifestations and outcomes of cardiorenal syndrome or the presence of both cardiac and renal complications in the 2019 coronavirus disease (COVID-19) patients.
Methods: All relevant studies about cardiorenal syndrome or both cardiac and renal complications in COVID-19 patients were retrieved on PUBMED, MEDLINE, and EMBASE from December 1, 2019 to February 20, 2022.
Results: Our search identified 15 studies including 637 patients with a diagnosis of cardiorenal syndrome or evidence of both cardiac and renal complications followingSARS-CoV-2 infection. They were male predominant (66.2%, 422/637), with a mean age of 58 years old. Cardiac complications included myocardial injury (13 studies), heart failure (7 studies), arrhythmias (5 studies), or myocarditis and cardiomyopathy (2 studies). Renal complications manifested as acute kidney injury with or without oliguria. Patients with cardiorenal injury were often associated with significantly elevated levels of inflammatory markers (CRP, PCT, IL-6). Patients with a diagnosis of cardiorenal syndrome or evidence of both cardiac and renal complications had more severe disease and poorer prognosis (9 studies).
Conclusion: The presence of either cardiorenal syndrome or concurrent cardiac and renal complications had a significant impact on the severity of the disease and the mortality rate among patients with COVID-19 infection. Therefore, careful assessment and management of potential cardiac and renal complications in patients with COVID-19 infection are important to improve their outcomes.
Introduction
The 2019 coronavirus disease (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Current literature indicates that sepsis secondary to COVID-19 infection has typical pathophysiological characteristics, namely early cytokine storms and subsequent immunosuppressive stages (1). Sepsis is frequently associated with cardiovascular complications and acute kidney injury either in isolation or in combination (2).
Angiotensin-converting enzyme 2 (ACE-2) is thought to be the major cell entry receptor for SARS-CoV-2 (3). ACE-2 is also expressed in the heart and kidney, providing a link between coronavirus infection and potential cardiovascular and renal complications (4). A recent epidemiological study (5) demonstrated that acute myocardial injury, cardiac arrhythmias, and shock can occur in 7.2, 18.7, and 8.7% of COVID-19 patients, respectively. Renal involvement is also not uncommon in the course of COVID-19. More than 40% of patients admitted to hospitals with COVID-19 infection had proteinuria (6). Among critically ill patients, acute kidney injury (AKI) is common, affecting ~20–40% of patients infected with COVID-19 admitted to intensive care units (7).
Although COVID-19 is most commonly associated with COVID pneumonitis, it can also result in several extrapulmonary manifestations, such as thrombotic complications, acute cardiac injury (ACI), acute kidney injury (AKI), gastrointestinal symptoms, and hepatocellular injury (8).
Cardiorenal syndrome can occur in COVID-19 patients, precipitated by arrhythmias, ACI, and AKI (2). Cardiorenal syndrome comprises a spectrum of disorders involving both the heart and kidneys, in which acute or chronic dysfunction in one organ may induce acute or chronic dysfunction in the other (9).
Limited data is available when evaluating the outcomes of COVID-19 patients with cardiorenal syndrome. Thus, the objective of this systematic review is to analyze and summarize the available literature on COVID-19 patients with both cardiac and renal complications, or cardiorenal syndrome, to gain an improved understanding of these issues in COVID-19 patients.
Methods
Search Strategy
The literature search was conducted in PUBMED/MEDLINE and EMBASE databases from December 1, 2019 to February 20, 2022 using the following terms: (COVID-19 OR SARS-CoV-2 OR severe acute respiratory syndrome coronavirus 2) AND (acute kidney injury OR acute renal impairment OR acute renal failure OR renal replacement therapy) AND (cardiomyopathy OR CMP OR cardiomyopathies OR myocardiopathy OR cardiac injury OR myocarditis OR heart injury) in the title/abstract. We limited our search to articles written in English. The literature search was conducted independently by three authors (LL, YQC, and DWH). Additionally, all references of selected papers were searched manually. This systematic review followed instructions from the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) statement (10).
Criteria for Inclusion
We included human studies meeting the following criteria: (1) Patients with COVID-19 were confirmed through positive results for SARS-CoV-2 nucleic acid testing of nasopharyngeal or throat swab specimens; (2) Patients 18 years or older; (3) Patients diagnosed with cardiorenal syndrome or evidence of both cardiac and renal complications. The exclusion criteria applied to the studies were: (1) Pregnant or lactating women; (2) Study type: review, conference abstract, letter to the editor.
Data Extraction
The following variables were extracted from all included studies: first author, the country where the research was conducted, type of study, number of patients, mean age, gender, underlying comorbidities, cardiac and kidney clinical events (such as cardiac arrhythmia, cardiac injury defined as elevated troponin levels, heart failure defined as EF ≤ 40%, elevated BNP, or echocardiographic evidence of heart failure, myocarditis, oliguria, anuric, proteinuria, acute kidney injury defined as elevated serum creatinine level, tubular injury), laboratory findings, use of Angiotensin-Converting Enzyme Inhibitors (ACEI) or Angiotensin Receptor Blockers (ARB), and clinical outcomes. Three authors (LL, YQC, and DWH) independently performed data extraction. Any disagreements were discussed and resolved with the senior authors (AYW and WJQ).
Results
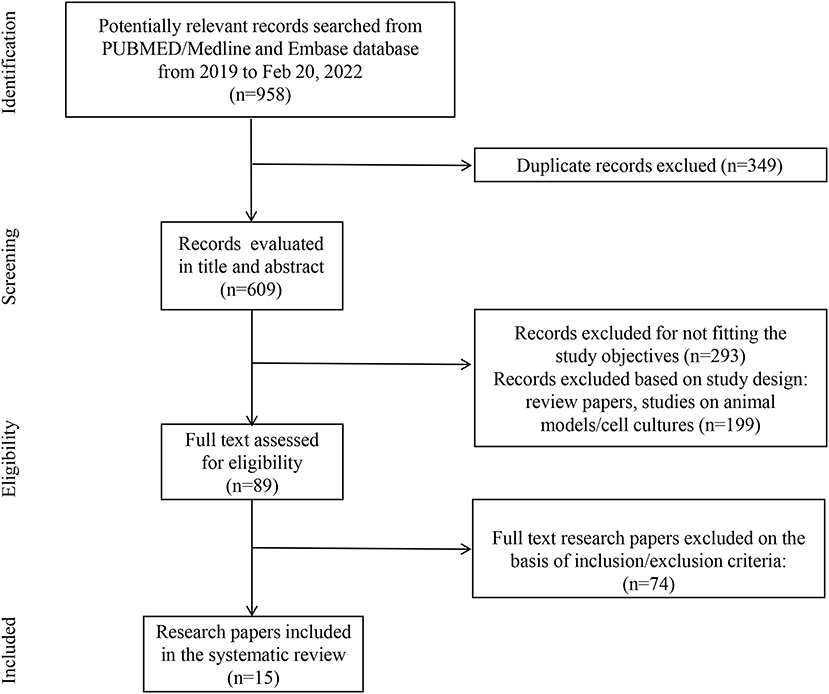
The search identified 15 studies and 637 patients with a diagnosis of cardiorenal syndrome or evidence of both cardiac and renal complications after SARS-CoV-2 infection. They were male predominant (66.2%, 422/637), with a mean age of 58 years old (Figure 1; Table 1).
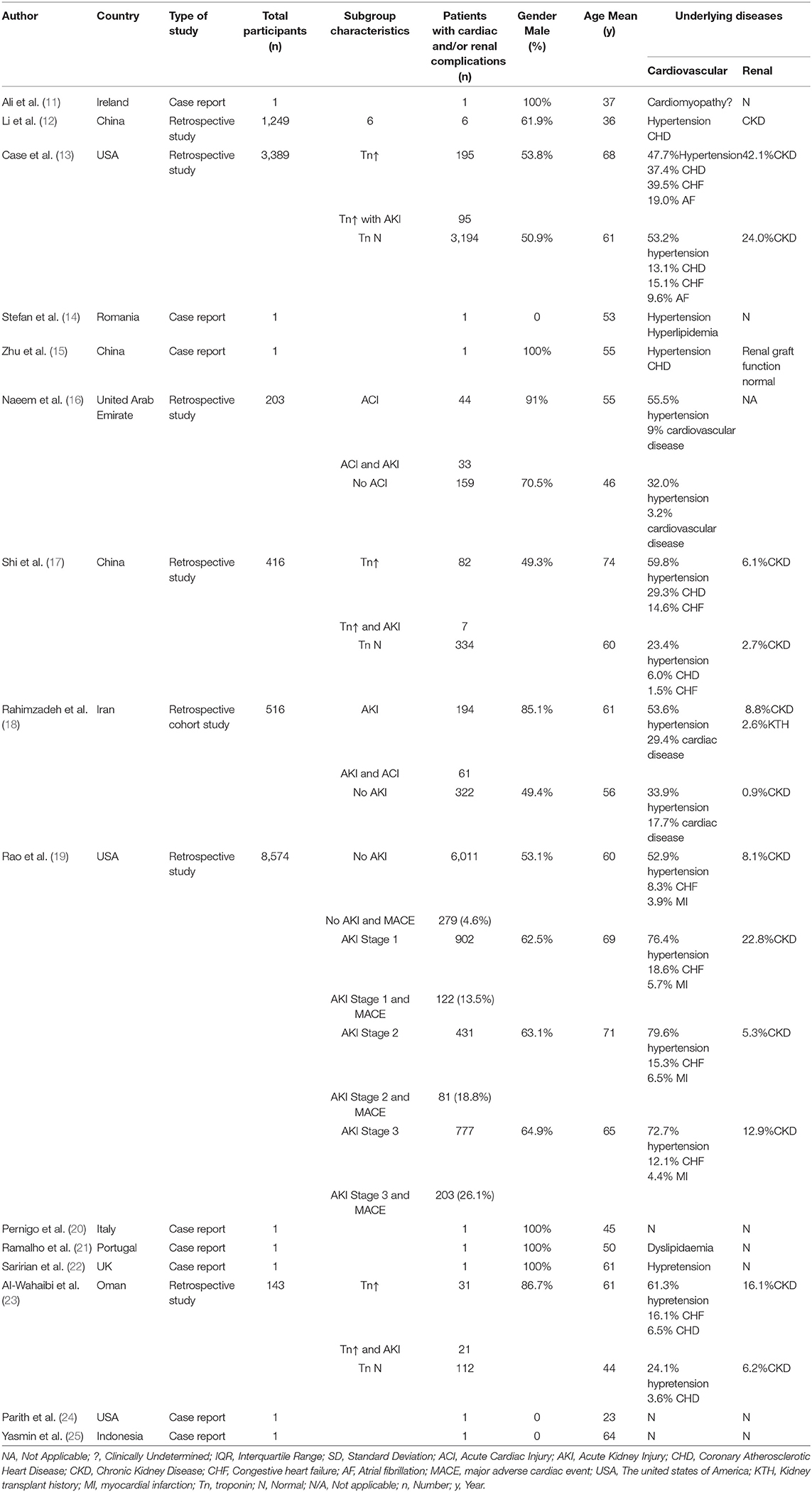
The studies were either retrospective (7 studies) or case reports (8 studies). Most patients had multiple comorbidities including hypertension, chronic heart failure, and chronic kidney disease before SARS-CoV-2 infection, but specific data were not provided (Table 2).
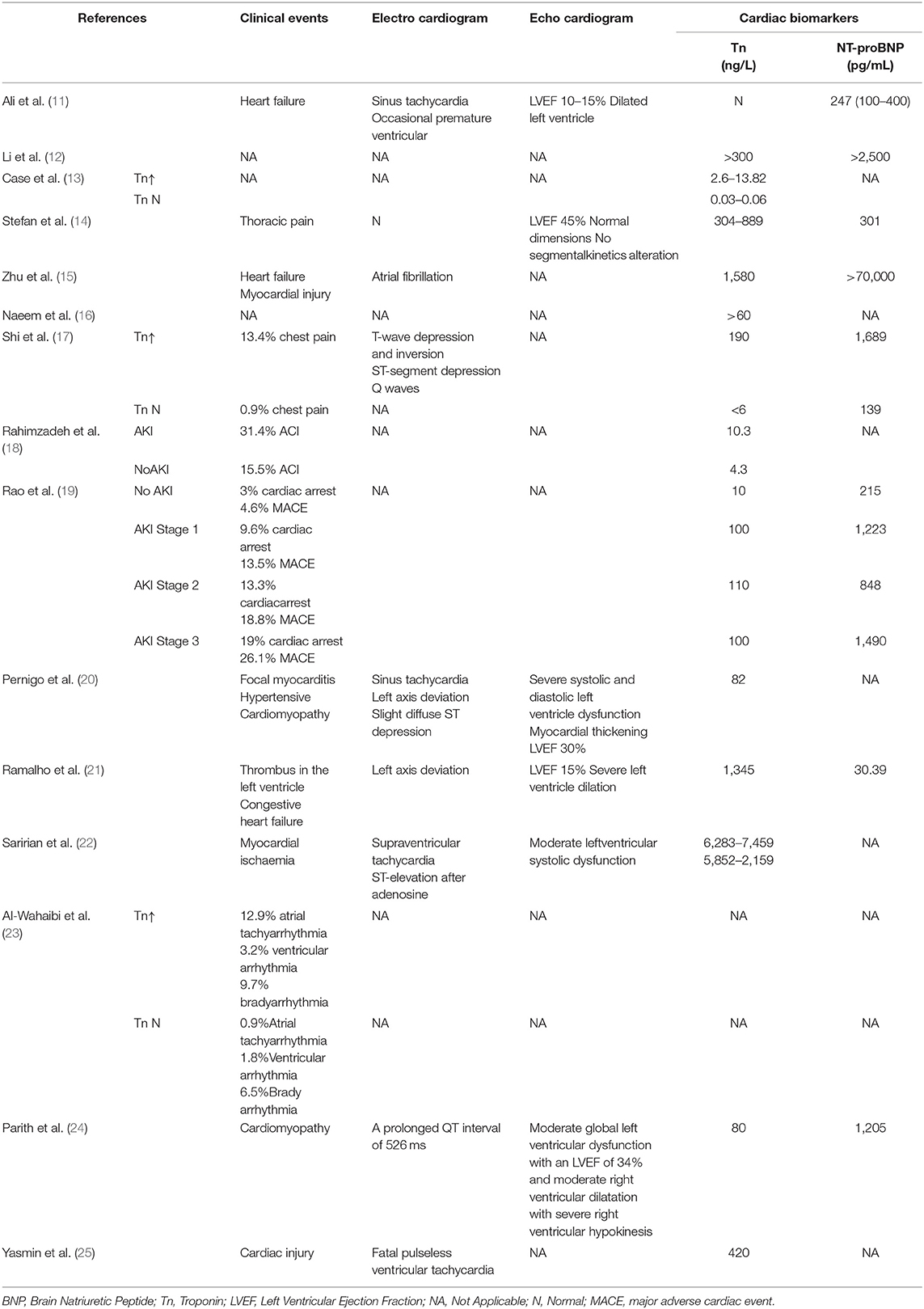
Table 2. Clinical and laboratory findings of the heart in COVID-19 patients with cardiac and renal complications.
Cardiac complications manifested as myocardial injury (13 studies), heart failure (7 studies), arrhythmia (5 studies), or myocarditis and cardiomyopathy (2 studies) (Table 2). Five studies demonstrated a reduction in left ventricular ejection fraction. Elevated troponin and brain natriuretic peptides were seen in 9 studies. Renal complications manifested as AKI with or without oliguria. However, severe AKI requiring dialysis therapy was not common (5 studies) (Table 3). Patients with cardiorenal injury were often associated with significantly elevated levels of inflammatory markers (CRP, PCT, IL-6) (Table 4). Use of ACEI/ARB occurred in 2 studies. Patients with a diagnosis of cardiorenal syndrome or evidence of both cardiac and renal complications had more severe disease and poorer prognosis (9 studies).
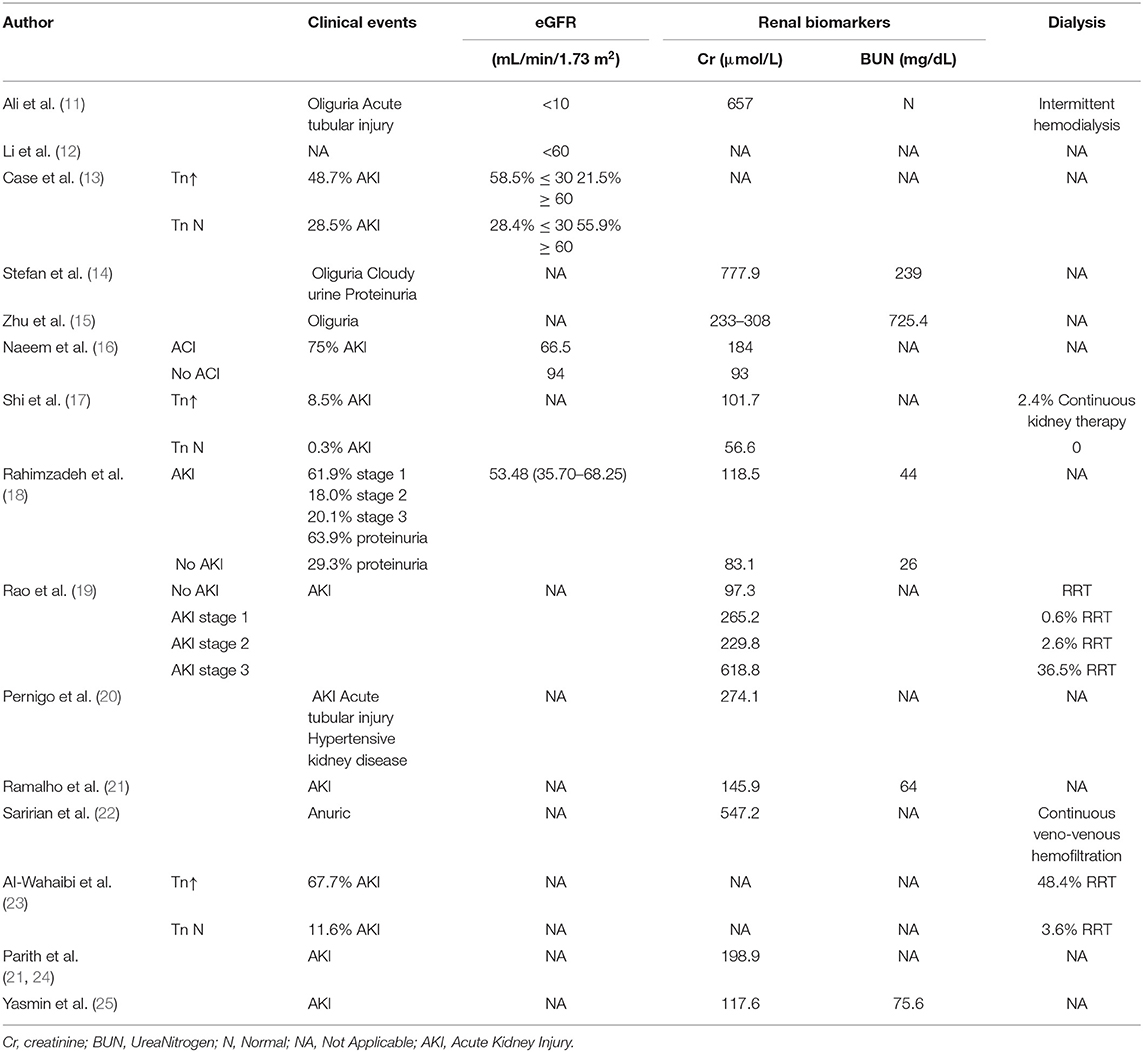
Table 3. Clinical and laboratory findings of the kidney in COVID-19 patients with cardiac and renal complications.
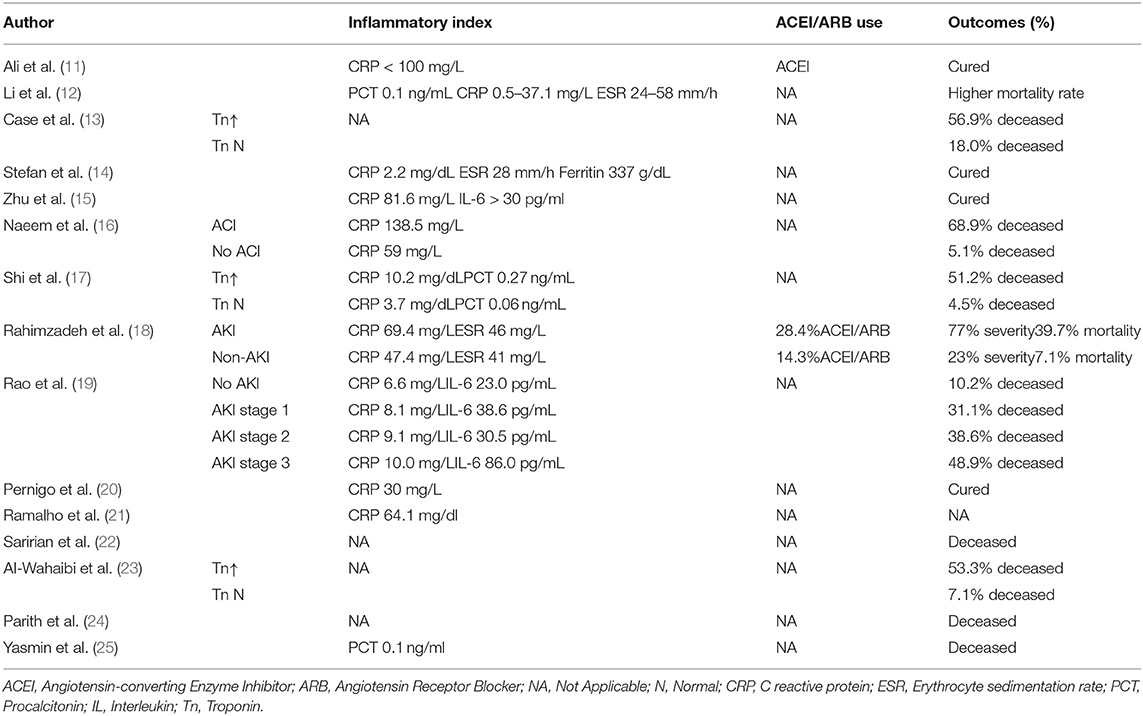
Table 4. Inflammatory index, ACEI/ARB use and the outcomes in COVID-19 patients with cardiac and renal complications.
Discussion
Patients who developed AKI were more likely to have a cardiac event suggesting a probable role of cardiorenal interaction in the renal dysfunction that occurs in COVID-19. AKI may result in volume overload and cardiac dysfunction, and vice versa since cardiomyopathy may lead to hypotension, renal hypoperfusion, and renal congestion resulting in renal dysfunction (26), and culminating in acute respiratory distress syndrome (ARDS). The cardiorenal syndrome is associated with increased morbidity and mortality in COVID-19 patients, as well as healthcare costs.
COVID-19 may affect the heart and kidney through several mechanisms (Figure 2). Firstly, new evidence suggests that SARS-CoV-2 may have direct cytopathic effects on the heart and kidney. ACE-2 is the receptor for SARS-CoV-2 to enter human cells, which is highly expressed in extrapulmonary tissues including the heart and kidney (27). Secondly, excessive release of cytokines due to viral infection, known as cytokine release syndrome or cytokine storm, is the mechanism leading to multiorgan damage in COVID-19. The presence of cytokine storms and pneumonia-related hypoxia can contribute to myocardial and renal ischemia due to changes between oxygen supply and demand. Furthermore, Li et al. (28) has reported that the kinetic changes of cytokines correlate with the prognosis of patients with severe COVID-19. Thirdly, thrombotic microangiopathy seen in COVID-19 may also lead to ACI and AKI. Systemic coagulation dysfunction appears to promote thrombosis with the observation of arterial events in patients with COVID-19, such as renal artery thrombosis or acute coronary syndrome.
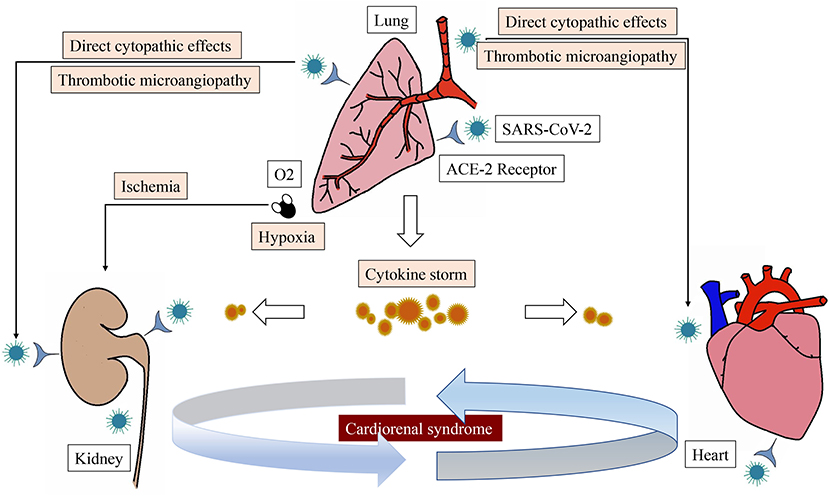
Figure 2. The main pathophysiological pathways of cardiorenal syndrome associated with SARS-CoV-2 infection.
Up to a fifth of COVID-19 patients have an acute myocardial injury (12–17% of cases) (29, 30). In patients with SARS-CoV-2 infection, the most common features of myocardial injury were ECG changes and elevated troponin. Echocardiography showed subclinical left ventricular diastolic dysfunction and even decreased ejection fraction (EF) in severe cases (5). As previously seen during coronavirus outbreaks, patients with a low EF are more likely to require mechanical ventilation (31). This is clinically important for hospitalized patients, as expert consensus recommends an early assessment and continuous cardiac monitoring to identify patients with cardiac injury and help predict further COVID-19 complications (32). High-sensitivity troponin is a useful cardiac monitoring tool in COVID-19. Zhou et al. (30) observed a gradual increase in high-sensitivity cardiac troponin I (hs-cTnI) levels in non-survivors (reaching the reference limit on day 11), while hs-cTnI levels in survivors remained low. Piccioni et al. (33) also identified that in patients with COVID-19, high-sensitivity troponin was a negative prognostic indicator. Increased cTnI levels may be associated with endotoxin production, which may be secondary to sepsis, an overall pro-inflammatory state, or direct myocardial infarction through ACE2 receptors in cardiac tissue (34). The increase of IL-6 was parallel to that of hs-cTnI, which increased the possibility of reflecting viral myocarditis. Existing data from China show that one-quarter to one-third of COVID-19 patients have severe heart failure. Zhou et al. (30) reported 23% of heart failure in their series of 191 patients with SARS-CoV-2, while Chen et al. (35) reported 27.5% (33/120) of increased N-terminal pro-B type natriuretic peptide (NT-proBNP).
Although early reports showed a low incidence of AKI (3–9%) among COVID-19 patients in a Chinese population (5), recent data has shown a higher incidence of renal abnormalities. The most prominent findings are proteinuria or hematuria. The most significant findings were albuminuria or hematuria, which was found by test paper evaluation in nearly one-third of patients on the first day of admission, and elevated serum creatinine and blood urea nitrogen in 15.5 and 14.1% of patients (6). Importantly, an elevation of any marker of kidney damage in COVID-19 patients is associated with significantly higher hospital mortality. Several mechanisms may contribute to the kidney injury seen with COVID-19. Other mechanisms that have been reported include sepsis, acute tubular necrosis caused by renal hypoperfusion, cytokine storm, alveolar injury caused by renal medulla hypoxia, cardiorenal syndrome, and rhabdomyolysis (26, 36–38). Magoon et al. has reported less common conditions such as immune-mediated glomerulonephritis and primary glomerular lesions that caused focal segmental glomerulosclerosis collapse (39). Moreover, the hypercoagulable state in COVID-19 may lead to thrombotic microangiopathy and peritubular and glomerular capillary obstruction (38, 40). AKI may also be the result or complication of COVID-19 treatment. Antiviral drugs can lead to tubulointerstitial diseases (41, 42), and biopsy confirmed oxalate nephropathy associated with vitamin C has been reported (43). Certain antibiotics/antibacterial agents have also been implicated in AKI in COVID-19 patients (44).
ACE-2 is the main entry point of most coronaviruses, and its binding domain has a high affinity with SARS-CoV-2. The coronavirus binds to the extracellular domain of ACE-2 on the host cell surface through its spike protein (S protein), and then invades the cells, resulting in the down-regulation of ACE-2 expression on the cell surface (3). After entering cells, viruses replicate and induce cytotoxicity, which may lead to organ failure. ACE-2 is widely expressed throughout the body, with the highest expression in the gastrointestinal tract and oral epithelium, and is highly expressed in the lung, kidney, and heart (45–47). As mentioned, ACE-2 is highly expressed in the proximal tubule of the kidney (3), which may allow for direct viral cell damage resulting in tissue injury and renal failure (2). On a cellular level, ACE-2 is widely expressed in cardiac fibroblasts, myocardial cells, and coronary artery endothelial cells (48). The use of an ACEI or ARB for antihypertensive treatment in a rat model has been shown to increase ACE-2 gene expression, protein levels, and activity in hearts (49–51), which may increase the chance of SARS-CoV-2 infection or the severity of COVID-19. Whether these drugs can increase the expression and activity of ACE-2 protein in humans remains controversial. In the absence of convincing clinical data, most professional organizations suggest that ACEI or ARB treatment should be continued for patients with heart failure who have or have the risk of SARS-CoV-2 infection.
Conclusions
Patients with cardiorenal syndrome or both cardiac and renal complications had a significant impact on the severity of the disease and mortality rate among patients with COVID-19. Therefore, emphasis should be placed on the risk factors for the development of cardiorenal syndrome, its pathophysiologic mechanisms, racial predilection, optimal therapy, and prevention in the COVID-19 patient population. However, there are limited data evaluating outcomes of COVID-19 patients with cardiorenal syndrome. Thus, further research in this area is needed.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
LL, AY, AW, and WQ designed the study. LL, YC, and DH performed the search, study selection, and data synthesis. LL wrote the first draft of the manuscript. AY, AW, and WQ revised the article. All authors contributed to the paper and approved the submitted version.
Funding
WQ was supported by Capital's Funds for Health Improvement and Research (2020-2-2027), and Funding support for key clinical projects in Beijing, China. AW was supported by National Heart Foundation Vanguard Grant, Australia.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.915533/full#supplementary-material
References
1. Remy KE, Brakenridge SC, Francois B, Daix T, Deutschman CS, Monneret G, et al. Immunotherapies for COVID-19: lessons learned from sepsis. Lancet Respir Med. (2020) 8:946–9. doi: 10.1016/S2213-2600(20)30217-4
2. Apetrii M, Enache S, Siriopol D, Burlacu A, Kanbay A, Kanbay M, et al. A brand-new cardiorenal syndrome in the COVID-19 setting. Clin Kidney J. (2020) 13:291–6. doi: 10.1093/ckj/sfaa082
3. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80.e8. doi: 10.1016/j.cell.2020.02.052
4. Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. (2020) 383:590–2. doi: 10.1056/NEJMc2011400
5. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
6. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. (2020) 97:829–38. doi: 10.1016/j.kint.2020.03.005
7. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. (2020) 323:2052–9. doi: 10.1001/jama.2020.6775
8. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. (2020) 26:1017–32. doi: 10.1038/s41591-020-0968-3
9. Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. (2019) 139:e840–78. doi: 10.1161/CIR.0000000000000664
10. Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. (2011) 39:91–2. doi: 10.1016/j.jcms.2010.11.001
11. Ali UA, Sadiq MS, Yunus MJ. Cardiorenal syndrome in COVID-19. BMJ Case Rep. (2021) 14:e241914. doi: 10.1136/bcr-2021-241914
12. Li WX, Xu W, Huang CL, Fei L, Xie XD, Li Q, et al. Acute cardiac injury and acute kidney injury associated with severity and mortality in patients with COVID-19. Eur Rev Med Pharmacol Sci. (2021) 25:2114–22. doi: 10.26355/eurrev_202102_25117
13. Case BC, Yerasi C, Forrestal BJ, Chezar-Azerrad C, Shea C, Rappaport H, et al. The impact of COVID-19 patients with troponin elevation on renal impairment and clinical outcome. Cardiovasc Revascular Med. (2021) 33:45–8. doi: 10.1016/j.carrev.2021.05.004
14. Stefan MF, Magda S L, Vinereanu D. COVID-19 presented as acute kidney injury with secondary myocardial damage. J Infect Public Health. (2021) 14:371–3. doi: 10.1016/j.jiph.2020.12.031
15. Zhu L, Shu H, Li H, Qiu T, Zhou J, Chen G. Slow recovery from critical coronavirus disease 2019 pneumonia in an immunosuppressed renal transplant recipient with early acute cardiorenal syndrome. Cardiorenal Med. (2020) 10:470–5. doi: 10.1159/000510916
16. Naeem KB, Hachim MY, Hachim IY, Chkhis A, Quadros R, Hannawi H, et al. Acute cardiac injury is associated with adverse outcomes, including mortality in COVID-19 patients. A single-center experience. Saudi Med J. (2020) 41:1204–10. doi: 10.15537/smj.2020.11.25466
17. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. (2020) 5:802–10. doi: 10.1001/jamacardio.2020.0950
18. Rahimzadeh H, Kazemian S, Rahbar M, Farrokhpour H, Montazeri M, Kafan S, et al. The risk factors and clinical outcomes associated with acute kidney injury in patients with COVID-19: data from a large cohort in Iran. Kidney Blood Press Res. (2021) 46:620–8. doi: 10.1159/000517581
19. Rao A, Ranka S, Ayers C, Hendren N, Rosenblatt A, Alger HM, et al. Association of kidney disease with outcomes in COVID-19: results from the american heart association COVID-19 cardiovascular disease registry. J Am Heart Assoc. (2021) 10:e020910. doi: 10.1161/JAHA.121.020910
20. Pernigo M, Triggiani M, Gavazzi E, Papa I, Vaccari A, Fisogni S, et al. Acute heart failure due to COVID-19 related myocardial injury and de novo hypertensive cardiomyopathy: a challenging diagnosis. Monaldi Arch Chest Dis. (2021) 92:10.4081. doi: 10.4081/monaldi.2021.1778
21. Ramalho C, Almeida M, Gomes F, Silva M, Rodrigues S. Cardiac abnormalities in COVID-19 patients: should a cardiac echocardiogram be routine? Eur J Case Rep Intern Med. (2021) 8:002559. doi: 10.12890/2021_002559
22. Saririan M, Armstrong R, George JC, Olechowski B, O'Connor S, Byrd JB, et al. ST-segment elevation in patients presenting with COVID-19: case series. Eur Heart J Case Rep. (2021) 5:ytaa553. doi: 10.1093/ehjcr/ytaa553
23. Al-Wahaibi K, Al-Wahshi Y, Mohamed Elfadil O. Myocardial injury is associated with higher morbidity and mortality in patients with 2019 novel coronavirus disease (COVID-19). SN Compreh Clin Med. (2020) 9:1–7. doi: 10.1007/s42399-020-00569-6
24. Wongkittichote P, Watson JR, Leonard JM, Toolan ER, Dickson PI, Grange DK. Fatal COVID-19 infection in a patient with long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: a case report. JIMD Rep. (2020) 56:40–5. doi: 10.1002/jmd2.12165
25. Yasmin Kusumawardhani N, Huang I, Martanto E, Sihite TA, Nugraha ES, Prodjosoewojo S, et al. Lethal arrhythmia (torsade de pointes) in COVID-19: an event synergistically induced by viral associated cardiac injury, hyperinflammatory response, and treatment drug? Clin Med Insights Case Rep. (2020) 13:1179547620972397. doi: 10.1177/1179547620972397
26. Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. (2020) 16:308–10. doi: 10.1038/s41581-020-0284-7
27. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. (2020) 9:45. doi: 10.1186/s40249-020-00662-x
28. Li Q, Xu W, Li WX, Huang CL, Chen L. Dynamics of cytokines and lymphocyte subsets associated with the poor prognosis of severe COVID-19. Eur Rev Med Pharmacol Sci. (2020) 24:12536–44. doi: 10.26355/eurrev_202012_24051
29. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
30. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
31. Li SS, Cheng CW, Fu CL, Chan YH, Lee MP, Chan JW, et al. Left ventricular performance in patients with severe acute respiratory syndrome: a 30-day echocardiographic follow-up study. Circulation. (2003) 108:1798–803. doi: 10.1161/01.CIR.0000094737.21775.32
32. Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. (2020) 116:1666–87. doi: 10.1093/cvr/cvaa106
33. Piccioni A, Brigida M, Loria V, Zanza C, Longhitano Y, Zaccaria R, et al. Role of troponin in COVID-19 pandemic: a review of literature. Eur Rev Med Pharmacol Sci. (2020) 24:10293–300. doi: 10.26355/eurrev_202010_23254
34. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. (2018) 72:2231–64. doi: 10.1016/j.jacc.2018.08.1038
35. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. (2020) 45:230–2. doi: 10.1007/s00059-020-04909-z
36. Sharma RK, Li J, Krishnan S, Richards EM, Raizada MK, Mohandas R. Angiotensin-converting enzyme 2 and COVID-19 in cardiorenal diseases. Clin Sci. (2021) 135:1–17. doi: 10.1042/CS20200482
37. Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. (2020) 97:824–8. doi: 10.1016/j.kint.2020.03.001
38. Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. (2020) 98:219–27. doi: 10.1016/j.kint.2020.04.003
39. Magoon S, Bichu P, Malhotra V, Alhashimi F, Hu Y, Khanna S, et al. COVID-19-related glomerulopathy: a report of 2 cases of collapsing focal segmental glomerulosclerosis. Kidney Med. (2020) 2:488–92. doi: 10.1016/j.xkme.2020.05.004
40. Xia P, Wen Y, Duan Y, Su H, Cao W, Xiao M, et al. Clinicopathological features and outcomes of acute kidney injury in critically ill COVID-19 with prolonged disease course: a retrospective cohort. J Am Soc Nephrol. (2020) 31:2205–21. doi: 10.1681/ASN.2020040426
41. Binois Y, Hachad H, Salem JE, Charpentier J, Lebrun-Vignes B, Pène F, et al. Acute kidney injury associated with lopinavir/ritonavir combined therapy in patients with COVID-19. Kidney Int Rep. (2020) 5:1787–90. doi: 10.1016/j.ekir.2020.07.035
42. Arrestier R, Stehle T, Letavernier E, Mekontso-Dessap A. Lopinavir-ritonavir associated acute kidney injury is not related to crystalluria in critically ill COVID-19 patients. Kidney Int Rep. (2020) 5:2119. doi: 10.1016/j.ekir.2020.08.021
43. Fontana F, Cazzato S, Giovanella S, Ballestri M, Leonelli M, Mori G, et al. Oxalate nephropathy caused by excessive vitamin C administration in 2 patients with COVID-19. Kidney Int Rep. (2020) 5:1815–22. doi: 10.1016/j.ekir.2020.07.008
44. Izzedine H, Jhaveri KD, Perazella MA. COVID-19 therapeutic options for patients with kidney disease. Kidney Int. (2020) 97:1297–8. doi: 10.1016/j.kint.2020.03.015
45. Sharma RK, Stevens BR, Obukhov AG, Grant MB, Oudit GY, Li Q, et al. ACE2 (angiotensin-converting enzyme 2) in cardiopulmonary diseases: ramifications for the control of SARS-CoV-2. Hypertension. (2020) 76:651–61. doi: 10.1161/HYPERTENSIONAHA.120.15595
46. Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. (2020) 126:1456–74. doi: 10.1161/CIRCRESAHA.120.317015
47. Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. (2000) 87:E1–9. doi: 10.1161/01.RES.87.5.e1
48. Guo J, Huang Z, Lin L, Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. (2020) 9:e016219. doi: 10.1161/JAHA.120.016219
49. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. (2005) 111:2605–10. doi: 10.1161/CIRCULATIONAHA.104.510461
50. Ocaranza MP, Godoy I, Jalil JE, Varas M, Collantes P, Pinto M, et al. Enalapril attenuates downregulation of angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension. (2006) 48:572–8. doi: 10.1161/01.HYP.0000237862.94083.45
Keywords: cardiorenal syndrome (CRS), COVID-19, SARS-CoV-2, cardiac complications, renal complications
Citation: Lin L, Chen Y, Han D, Yang A, Wang AY and Qi W (2022) Cardiorenal Syndrome in COVID-19 Patients: A Systematic Review. Front. Cardiovasc. Med. 9:915533. doi: 10.3389/fcvm.2022.915533
Received: 08 April 2022; Accepted: 03 June 2022;
Published: 28 June 2022.
Edited by:
Dragos Cretoiu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Natalia G. Vallianou, Evaggelismos General Hospital, GreeceConrado Roberto Hoffmann Filho, Hospital Regional Hans Dieter Schmidt, Brazil
Copyright © 2022 Lin, Chen, Han, Yang, Wang and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amanda Y. Wang, YXdhbmdAZ2VvcmdlaW5zdGl0dXRlLm9yZy5hdQ==; Wenjie Qi, cWlfd2VuamllQGNjbXUuZWR1LmNu
 Ling Lin
Ling Lin Yangqin Chen
Yangqin Chen Dongwan Han
Dongwan Han Andrew Yang
Andrew Yang Amanda Y. Wang
Amanda Y. Wang Wenjie Qi
Wenjie Qi