- 1Department of Cardiology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 2Montefiore Medical Center, Bronx, NY, United States
Aims: To date, the prognostic effects of permanent pacemaker implantation (PPI) after transcatheter aortic valve replacement (TAVR) remain controversial. The purpose of this meta-analysis was to investigate the mid- (1 year) to long-term (> 1 year) clinical and echocardiographic effects of post-procedural PPI in patients after TAVR.
Methods: PubMed, Embase, Web of Science, and Cochrane Library databases were systematically searched from the establishment of databases up to 1 December 2021. Studies comparing clinical and echocardiographic outcomes between patients with and without post-TAVR PPI of ≥ 1-year follow-up were collected for further meta-analysis.
Results: A total of 39 studies comprising of 83,082 patients were included in this meta-analysis. At mid-term follow-up (1 year), the pooled results demonstrated a higher risk of all-cause mortality in patients with post-procedural PPI than those without following TAVR (relative risk (RR), 1.17; 95% CI, 1.10–1.24; P < 0.00001). No significant differences were observed in cardiovascular mortality (RR, 0.86; 95% CI, 0.71–1.03; P = 0.10) or heart failure rehospitalization (RR, 0.91; 95% CI, 0.58–1.44; P = 0.69) at 1-year follow-up. At long-term follow-up (> 1 year), post-TAVR PPI had negative effects on all-cause mortality (RR, 1.18; 95% CI, 1.09–1.28; P < 0.0001) and heart failure rehospitalization (RR, 1.42; 95% CI, 1.18–1.71; P = 0.0002). There was no difference in long-term cardiovascular mortality between the two groups (RR, 1.15; 95% CI, 0.97–1.36; P = 0.11). Left ventricular ejection fraction (LVEF) was not significantly different at baseline (mean difference, 1.40; 95% CI, –0.13–2.93; P = 0.07), but was significantly lower in the PPI group at 1-year follow-up (mean difference, –3.57; 95% CI, –4.88 to –2.26; P < 0.00001).
Conclusion: Our meta-analysis provides evidence that post-TAVR PPI has negative clinical and echocardiographic effects on patients at mid- to long-term follow-up. Further studies are urgently needed to explore the cause of these complications and optimize the treatment and management of patients requiring permanent pacing after TAVR.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021289935], identifier [CRD42021289935].
Introduction
Transcatheter aortic valve replacement (TAVR) has become a well-established therapy for patients with severe aortic stenosis and high risk for surgical aortic valve replacement (1, 2). Recent randomized controlled trials provided evidence to extend the application of TAVR to low-risk patients (3, 4). Despite technological advances and clinical experience accumulation, atrioventricular node, and infranodal tissues remain easily impaired during the implantation of the valve prosthesis. Conduction abnormalities (e.g., high-degree atrioventricular block and new-onset persistent left bundle branch block) are frequently observed after TAVR, and patients often require permanent pacemaker implantation (PPI) (5). The application of post-TAVR PPI was reported in approximately 2.3–37.7% of patients, and the rates largely vary according to the types and generations of the transcatheter valves (6).
Cardiac pacing is a recommended therapy to reduce the risk of death related to severe bradycardia arrhythmias. However, traditional right ventricular pacing (RVP) can cause electrical and mechanical dyssynchrony (7, 8), thus increasing the risk of mortality and heart failure hospitalization (9–11). Currently, it remains controversial whether the application of PPI could influence the clinical symptoms and survival outcomes after TAVR (12). Previous meta-analyses were limited by a small number of studies or lack of long-term follow-up (13, 14). This meta-analysis aims to investigate the mid- to long-term clinical and echocardiographic outcomes of post-procedural PPI in patients after TAVR.
Methods
Search Strategy
We performed a systematic literature search in PubMed, Embase, Web of Science, and Cochrane Library from the establishment of databases up to 1 December 2021 by two investigators (Shun Xu and Enrui Zhang) independently. The following strategy was used in PubMed: ((((((“Transcatheter Aortic Valve Replacement” [Mesh]) OR (Transcatheter Aortic Valve Replacement [Title/Abstract])) OR (Transcatheter Aortic Valve Implantation [Title/Abstract])) OR (TAVR [Title/Abstract])) OR (TAVR [Title/Abstract]))) AND (((((“Cardiac Pacing, Artificial” [Mesh]) OR (pacing [Title/Abstract])) OR (pace [Title/Abstract])) OR ((“Pacemaker, Artificial” [Mesh]) OR (pacemaker [Title/Abstract])))). The searching strategies for Embase, Web of Science, and Cochrane Library were provided in Supplementary Table 1. We also manually screened reference lists of retrieved reviews, reports, and other relevant publications to identify additional pertinent studies.
Study Design
The protocol of this meta-analysis has been registered in PROSPERO (Registration ID: CRD42021289935). Clinical studies were eligible if they met the following inclusion criteria: (1) studies comparing clinical and echocardiographic outcomes between patients with and without post-procedural PPI after TAVR, including all-cause mortality, cardiovascular mortality, heart failure rehospitalization, and left ventricular ejection fraction (LVEF); (2) studies with a follow-up of ≥ 1 year; (3) studies with full texts published in English in peer-reviewed journals. We only included the study containing the most data for multiple publications of the same trial. We excluded review articles, case reports, letters, editorials, articles lacking outcomes of interest, studies without detailed data, and studies with a follow-up of < 1 year. Importantly, we also excluded studies that failed to distinguish patients with PPI before TAVR. Two independent investigators (Shun Xu and Enrui Zhang) assess eligibility by screening and reviewing article titles, abstracts, and full texts. Any disagreement about eligibility was clarified via consulting a third investigator (Jinyu Sun).
Data Extraction and Quality Assessment
Two investigators (Shun Xu and Enrui Zhang) independently extracted data for each eligible study. Any disagreement was resolved through discussion with a third investigator (Jinyu Sun) to reach a consensus. The following characteristics were included: first author, year of publication, inclusion period, number and region of centers, sample size, PPI criteria, patient demographic characteristics, and the following mid-term (1 year) to long-term (> 1 year) outcomes, including all-cause mortality, cardiovascular mortality, heart failure rehospitalization, and LVEF.
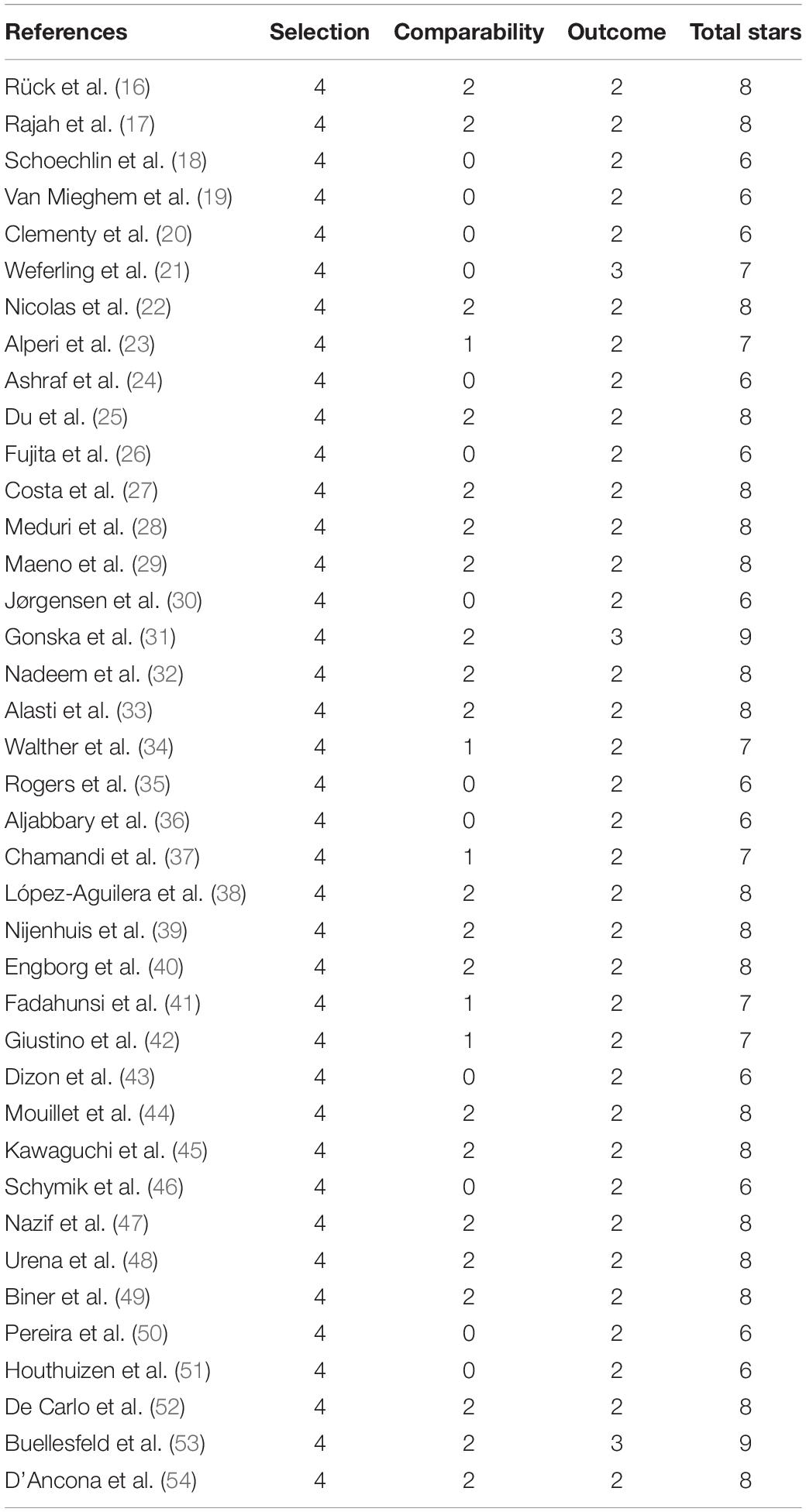
The quality of studies involved was assessed by two investigators (Shun Xu and Enrui Zhang) independently using the Newcastle-Ottawa Scale (NOS). The NOS tool involved three aspects, and a maximum of 9 stars can be allotted to each study: the selection of cohorts (0–4 stars), the comparability of cohorts (0–2 stars), and the assessment of the outcome (0–3 stars). A NOS score ≥ 6 stars indicated moderate-to-high quality, while a NOS score < 6 stars indicated low quality. Discrepancies were resolved by consulting a third investigator (Jinyu Sun) to reach a consensus.
Statistical Analysis
Continuous variables were presented as mean ± standard deviation, and categorical variables were presented as frequencies or percentages. Relative risk (RR) with corresponding 95% confidence intervals (CIs) for each endpoint was calculated and analyzed for categorical variable outcomes. Continuous data were summarized as a mean difference with 95% CI. P-value < 0.05 was considered statistically significant. The heterogeneity between studies was quantified by I-squared (I2) statistic, with a fixed-effects model adopted when the I2-value was < 50% and a random-effects model applied otherwise. Review Manager version 5.3 was used for all the statistical analyses. The meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (15).
Results
Study Selection and Quality Assessment
Figure 1 shows the flow chart of the study selection. A total of 9,852 records were initially identified from the databases according to the searching strategies, including 1,842 from PubMed, 4,782 from Embase, 3,053 from Web of Science, and 175 from Cochrane Library. After title and abstract screening, a total of 4,321 duplicates and 5,461 irrelevant records were excluded, the remaining 70 full-text articles to be reviewed for eligibility. Of those, 22 studies were excluded for having no outcomes of interest or without provided data. Two studies were excluded due to failing to distinguish patients with PPI before TAVR. One study was excluded because the follow-up duration was less than 1 year. Six case reports were also excluded. Finally, 39 studies containing 83,082 patients were included for further analysis (16–54) (Table 1).
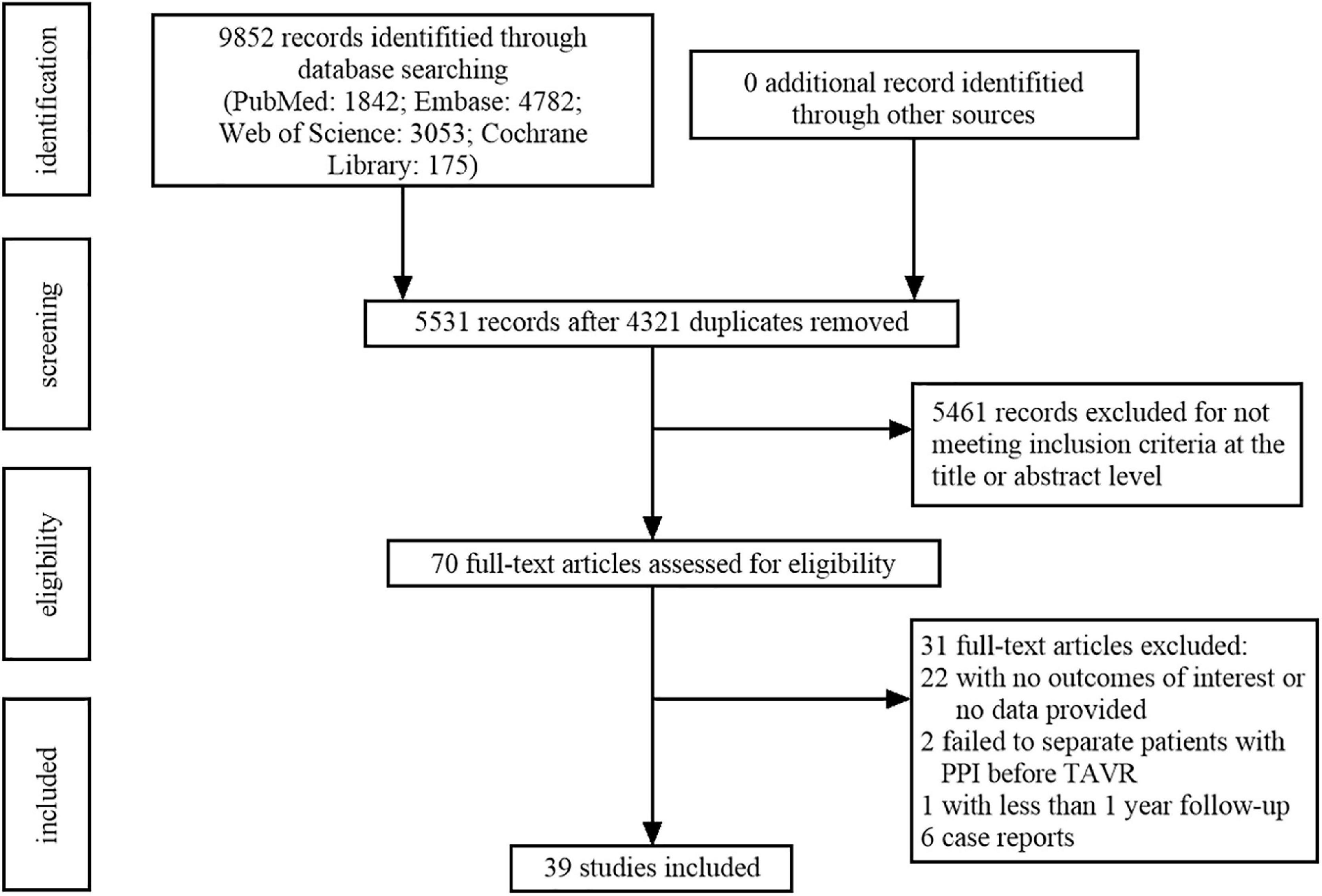
Figure 1. Flowchart of study selection based on the Preferred Reported Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.
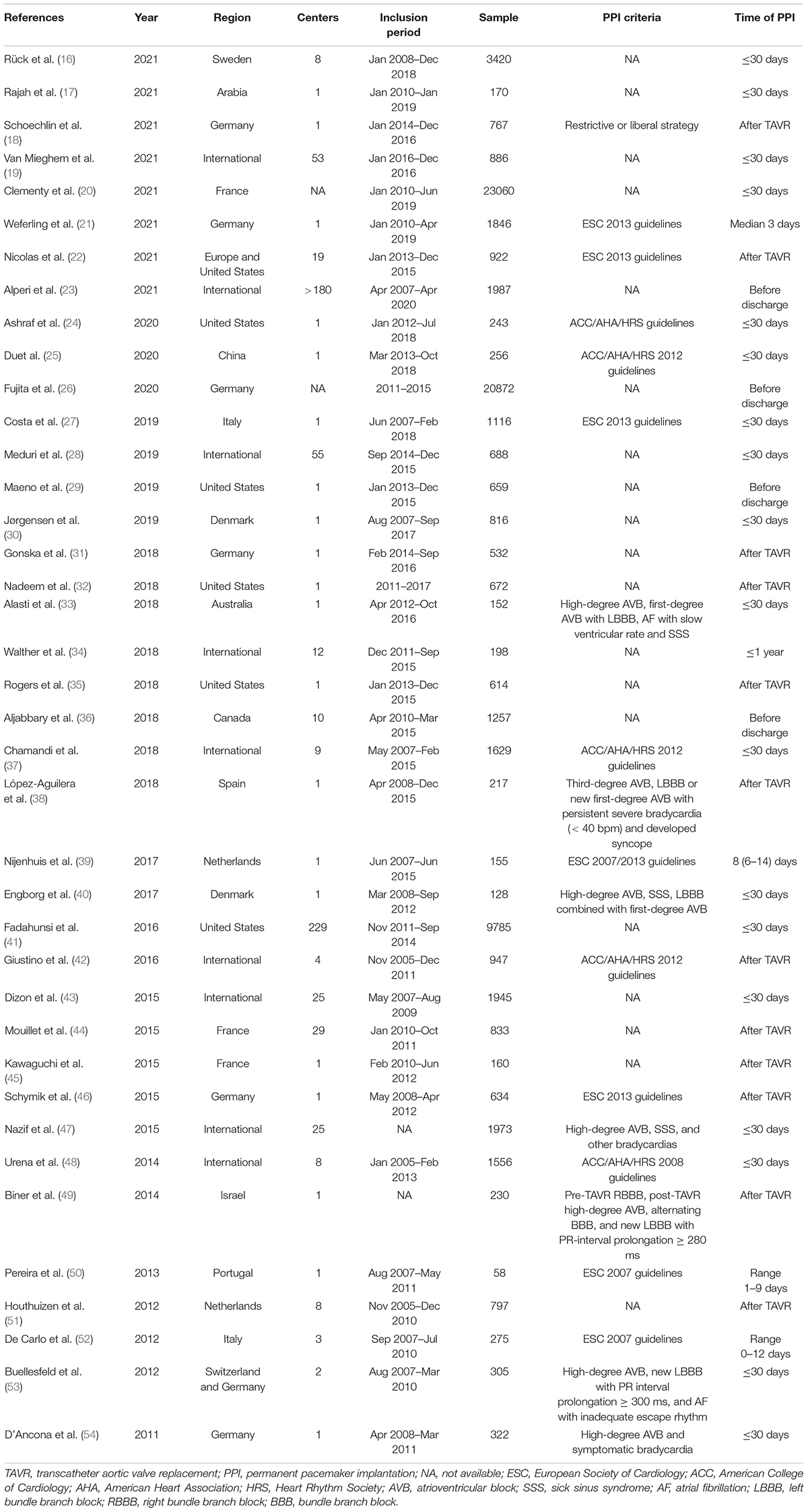
Table 1. Summary of studies evaluating mid- to long-term clinical and echocardiographic effects of post-TAVR PPI.
All included studies had moderate-to-high quality while none had less than 6 points according to NOS: two with 9 points, nineteen with 8 points, six with 7 points, and 12 with 6 points. The details of the quality assessment are shown in Table 2.
Mid-Term (1 Year) Clinical Effects of Post-procedural Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement
The risk of mid-term all-cause mortality was pooled from 27 studies that included 49,579 patients, and 7,235 patients were implanted with permanent pacemakers after TAVR. There were 1,197 of 7,235 (16.54%) cases of all-cause mortality in the PPI group while 6,285 of 42,344 (14.84%) cases in the no PPI group. The pooled results demonstrated that patients with PPI had a higher risk of death than those without PPI following TAVR (RR, 1.17; 95% CI, 1.10–1.24; P < 0.00001; I2 = 22%; Figure 2A). After pooling the results from nine studies, no significant difference in mid-term cardiovascular death was observed (RR, 0.86; 95% CI, 0.71–1.03; P = 0.10; I2 = 0%; Figure 2B). The risk of 1-year heart failure rehospitalization was assessed in five studies using a random-effects model. As shown in Figure 2C, no significant difference was observed in heart failure rehospitalization (RR, 0.91; 95% CI, 0.58–1.44; P = 0.69; I2 = 83%).
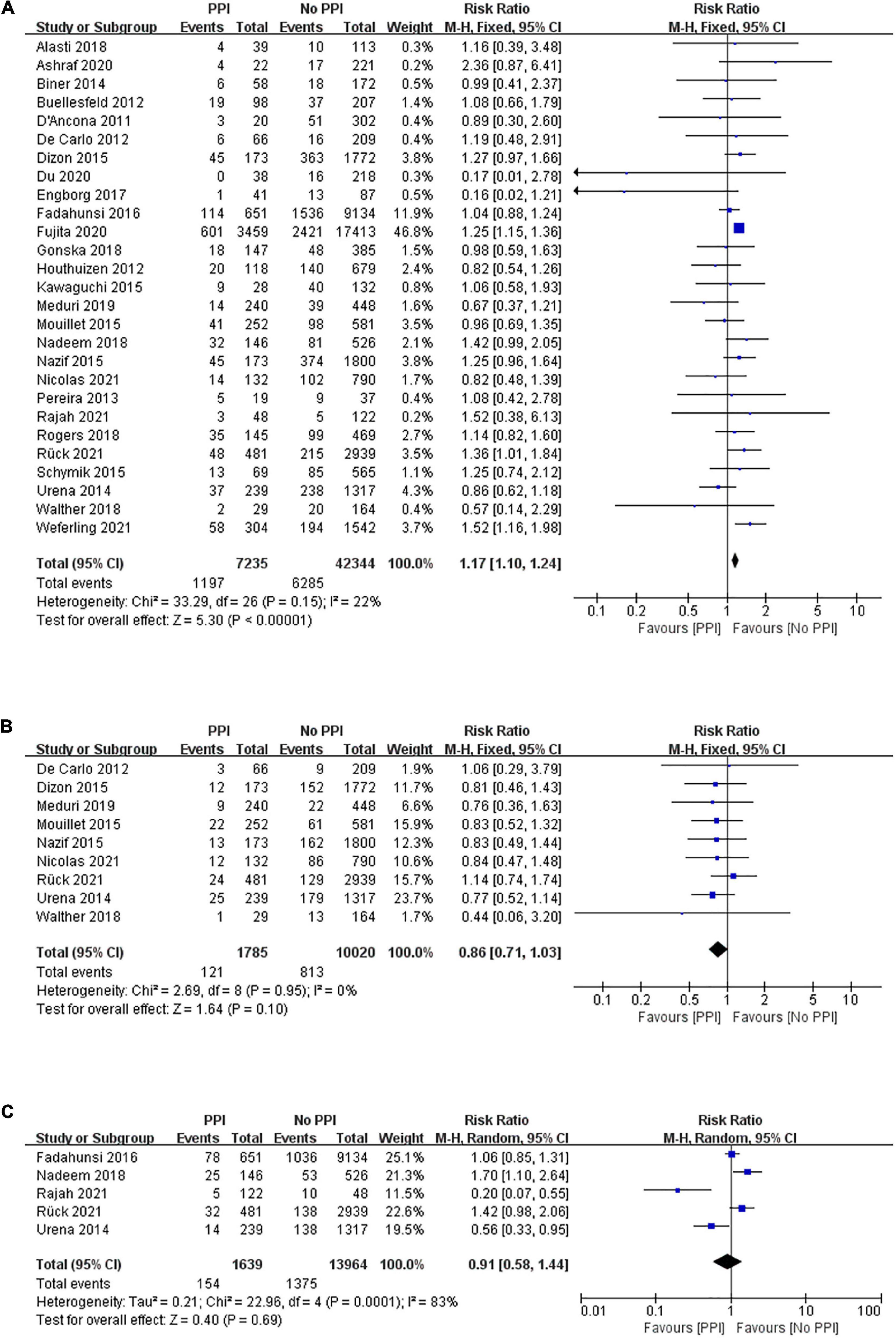
Figure 2. Forest plot of the risk of mid-term (1 year) (A) all-cause mortality, (B) cardiovascular mortality, and (C) heart failure rehospitalization in patients with post-procedural permanent pacemaker implantation (PPI) after transcatheter aortic valve replacement (TAVR).
Long-Term (> 1 Year) Clinical Effects of Post-procedural Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement
Long-term mortality between patients with and without PPI after TAVR was reported in 18 studies enrolling 39,172 patients with a mean follow-up period of 2.59 years. A random-effects model was applied, and patients with PPI after TAVR had a higher risk of all-cause mortality than those without PPI after TAVR (RR, 1.18; 95% CI, 1.09–1.28; P < 0.0001; I2 = 57%; Figure 3A). However, there was no statistical difference in long-term risk of cardiovascular mortality between the two groups (RR, 1.15; 95% CI, 0.97–1.36; P = 0.11; I2 = 59%; Figure 3B) after a mean follow-up of 2.12 years. Seven studies demonstrated a deleterious effect of PPI on heart failure rehospitalization after a mean follow-up of 2.16 years (RR, 1.42; 95% CI, 1.18–1.71; P = 0.0002; I2 = 76%; Figure 3C).
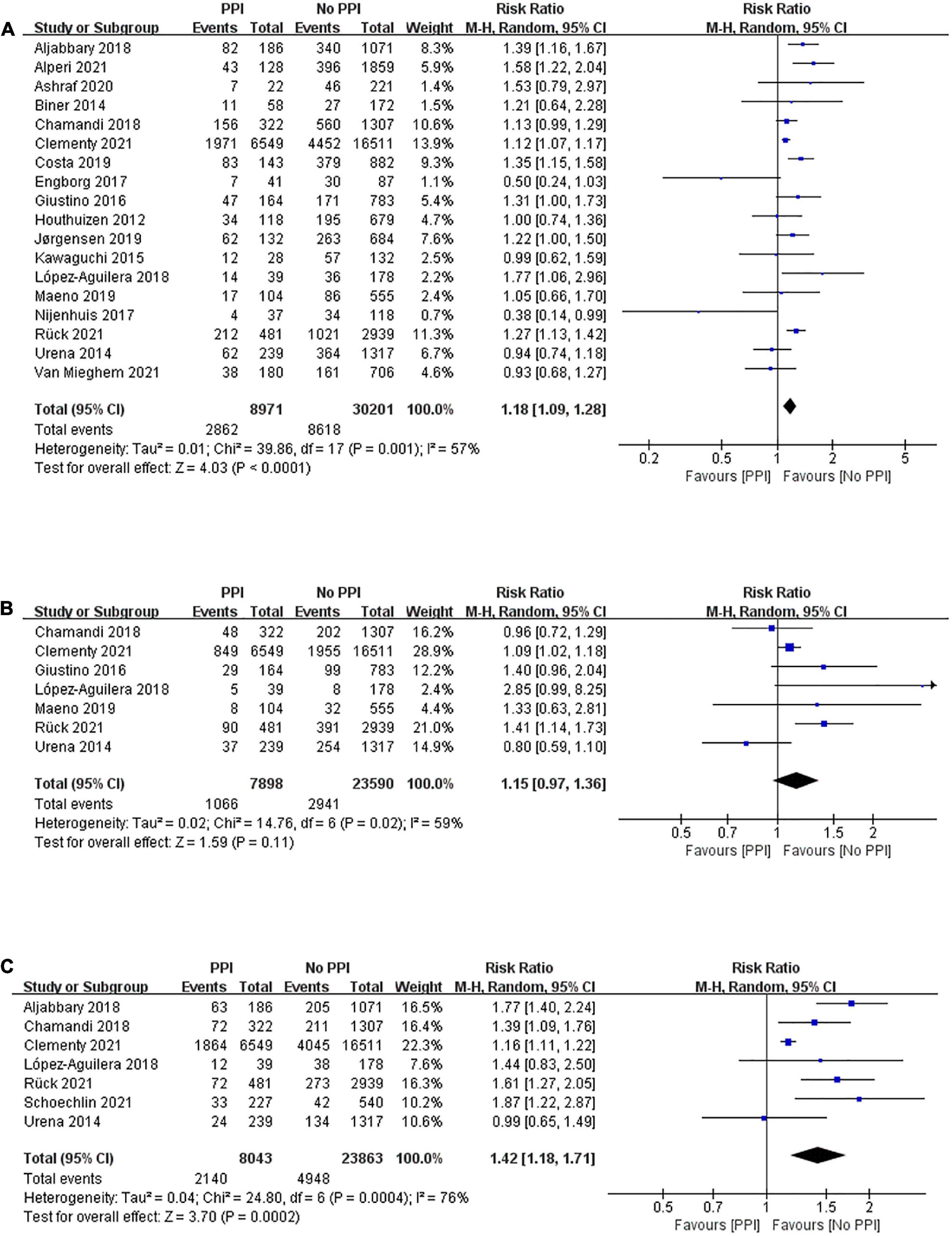
Figure 3. Forest plot of the risk of long-term (> 1 year) (A) all-cause mortality, (B) cardiovascular mortality, and (C) heart failure rehospitalization in patients with post-procedural PPI after TAVR.
Echocardiographic Effects of Post-procedural Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement
Two studies reported LVEF both at baseline and 1-year follow-up. Figure 4A shows no significant difference in LVEF between the two groups at baseline (mean difference, 1.40; 95% CI, –0.13 to 2.93; P = 0.07; I2 = 0%). LVEF at 1-year follow-up after TAVR was assessed using a fixed-effect model, and the overall value of LVEF was significantly greater in the no PPI group than in the PPI group (mean difference, –3.57; 95% CI, –4.88 to –2.26; P < 0.00001; I2 = 0%; Figure 4B).
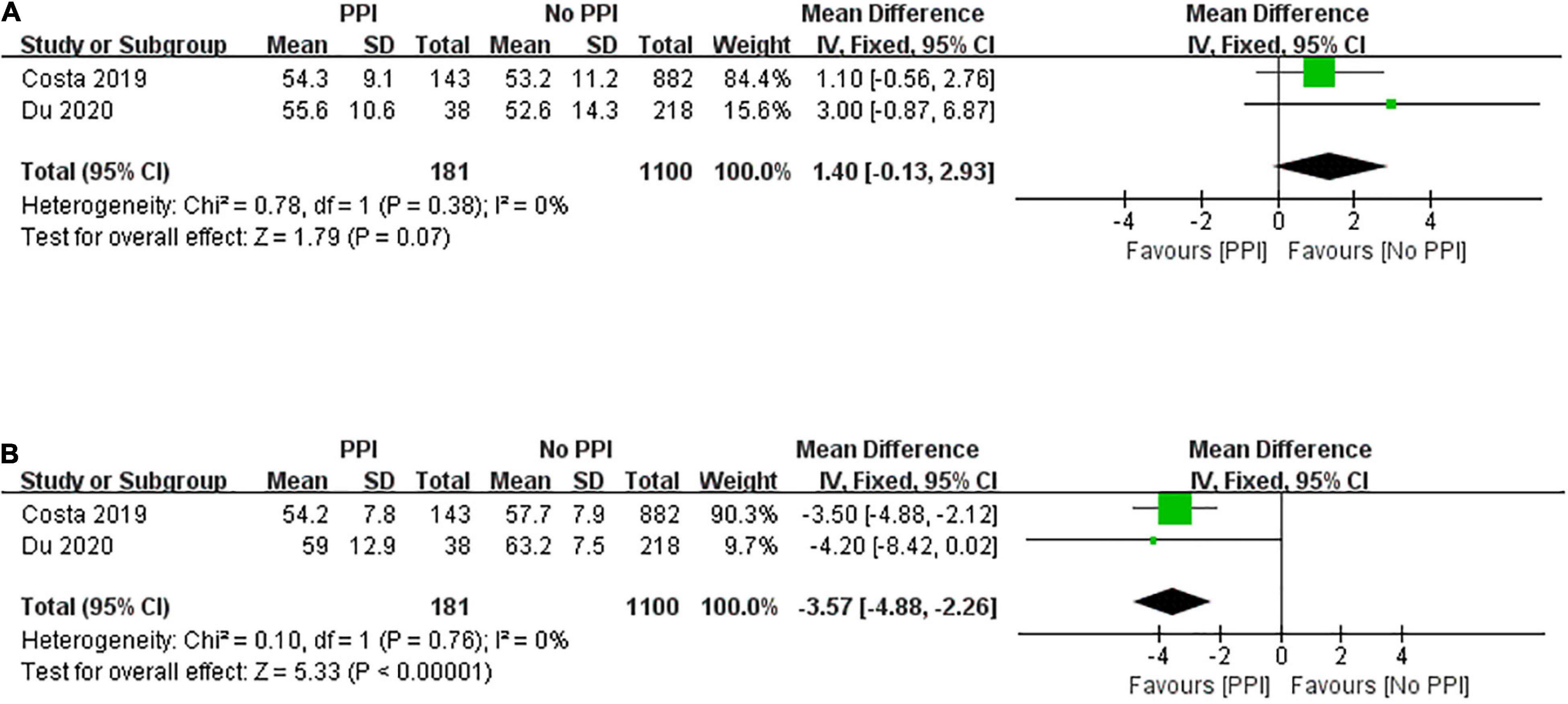
Figure 4. Forest plot of left ventricular ejection fraction at baseline (A) and 1-year follow-up (B) between patients with and without post-procedural PPI after TAVR.
Discussion
The results of this meta-analysis can be summarized as follows: (1) patients with post-procedural PPI show a higher risk of all-cause mortality at mid-term follow-up after TAVR; (2) post-TAVR PPI is associated with an increased risk of all-cause mortality and heart failure rehospitalization at long-term follow-up; and (3) post-procedural PPI adversely affect LVEF recovery on patients undergoing TAVR.
Twenty years after the first procedure in 2002 (55), TAVR has become a first-line treatment for patients with symptomatic severe aortic stenosis regardless of the estimated surgical risk (1–4). Although TAVR technology has matured significantly over the years, conduction abnormalities remain one of the major complications to be resolved. Currently, there is insufficient evidence to support that the newer-generation devices could reduce the rate of post-procedural PPI (56, 57). The underlying pathophysiological mechanisms compose of direct trauma, hemorrhage, inflammation, and ischemic injury of the conduction system during the expansion of the valve prosthesis (5). With accumulating TAVR cases, it is important to investigate the mid- to long-term clinical and echocardiographic outcomes of post-procedural PPI after TAVR.
Numerous studies have confirmed that RVP can negatively impact left ventricular function and increase the risk of the occurrence of atrial fibrillation (10, 58–60). The detrimental effects of RVP may elevate the risk of mortality and heart failure rehospitalization. As shown in our study, the pooled results revealed that patients undergoing PPI after TAVR had a higher risk of death at both mid- and long-term follow-up. They were also more likely to be hospitalized for heart failure during long-term follow-up. Similarly, a recent study containing the largest sample size reported that PPI after TAVR was independently associated with higher mortality and heart failure rehospitalization rate during follow-up, which was based on the entire France nationwide-level population (20).
We observed no significant difference in cardiovascular mortality and 1-year risk of heart failure rehospitalization between the two groups in our meta-analysis. The potential protective effects of PPI with respect to lethal bradyarrhythmias may counterbalance the negative effects of ventricular pacing. After the improvement of aortic stenosis, hemodynamic improvement of left ventricular function may compensate for the potential deleterious effects of ventricular pacing in such patients. In addition, implanting biventricular pacemakers in patients after TAVR may partially offset adverse effects linked to RVP.
Inconsistent with our results, few previous meta-analyses showed significant impacts of PPI after TAVR on clinical outcomes (13, 61, 62), except for a study by Faroux et al. (14), which was the first meta-analysis to reveal a significantly higher risk of all-cause death and heart failure rehospitalization in patients with PPI post-TAVR at 1-year follow-up. There are several explanations underlying the conflicting results in different studies. The small number of samples and short follow-up time may account for the distinct results. The occurrence and severity of pacing-induced cardiomyopathy are associated with ventricular pacing burden and duration, especially in patients with long-term pacing percentage ≥ 40% (11, 63, 64). Studies on TAVR have shown that new-onset conduction disturbances after TAVR may recover during follow-up, and about half of the patients requiring post-TAVR PPI are not pacing-dependent eventually (65–67). This may also partly explain why there was no significant difference in 1-year heart failure rehospitalization rates between the two groups.
Conduction disturbances occur commonly after TAVR, and an expert consensus algorithm was provided for managing post-TAVR conduction disturbances, but the optimal management of this complication is still unknown (68, 69). Schoechlin et al. (18) compared patients’ outcomes between different PPI implantation indications and revealed that the restrictive PPI strategy they adopted reduces the PPI rate significantly and is safe after a follow-up of 3 years. In consideration of the mid- to long-term negative effects demonstrated in our meta-analysis, we recommended adopting a relatively restrictive PPI strategy after TAVR, but the detailed indications and management need to be further explored. Furthermore, His-Purkinje system pacing (HPSP) allows for electrical stimulation signaling through the physiological conduction system, which has the potential to prevent pacing-induced dyssynchrony, heart failure hospitalization, and mortality (70–73). Previous studies have confirmed the feasibility and safety of HPSP in patients after TAVR. De Pooter et al. (74) found that the valve prosthesis can serve as an anatomical landmark for the implantation of the His-bundle lead. A multicenter study by Vijayaraman et al. (75) revealed that left bundle branch pacing had a higher success rate than His-bundle pacing after TAVR, with more ideal pacing parameters. Eleven patients with reduced left ventricular function who underwent HPSP successfully in this study showed significant LVEF improvement from 35 to 42% during follow-up. However, there is no systematic large-scale study evaluating the clinical and echocardiographic effects of HPSP in patients undergoing TAVR. Therefore, further studies are needed to focus on this area.
Limitations
Several limitations of our meta-analysis should be acknowledged. First, most studies included in our meta-analysis were retrospective observational studies. Thus, prospective, multi-center, randomized comparative studies are urgently needed. Second, TAVR technology has developed over time, and the types of valve prostheses are different. Patients included in the prior studies might have different PPI inclusion criteria compared with later ones so the heterogeneity among studies was relatively high in our study. Third, we had inadequate numbers of studies reporting ventricular pacing percentage to assess any significance of pacing-induced cardiomyopathy. We also do not have enough information to study other complications of PPI, such as infection, pneumothorax, and pocket hematoma, which may result in significant clinical consequences outside of mortality. Last but not least, our study is a meta-analysis, and we lack access to individual patient data which may provide more information.
Conclusion
The present meta-analysis provides evidence that post-TAVR PPI has negative clinical and echocardiographic effects at mid- to long-term follow-up. This study highlights the importance of identifying patients at high risk of developing conduction disturbances and requiring PPI after TAVR. Cardiologists should optimize treatment strategies and management of these patients. TAVR technology should also improve to reduce the incidence of such complications.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
SX, EZ, JS, and JZ contributed to the conception and designed the study. SX, EZ, and JS extracted the data and evaluated the quality. SX and EZ analyzed the data and wrote the manuscript. ZQ, FZ, YW, XH, and JZ critically reviewed and revised the manuscript. All authors have read and approved the final version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (grant no. 82070521, PI: JZ) and the Clinical Competence Improvement Project of Jiangsu Province Hospital (grant no. JSPH-MA-2020-3, PI: JZ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.911234/full#supplementary-material
Abbreviations
TAVR, transcatheter aortic valve replacement; PPI, permanent pacemaker implantation; RVP, right ventricular pacing; LVEF, left ventricular ejection fraction; NOS, Newcastle-Ottawa Scale; RR, relative risk; CI, confidence intervals; HPSP, His-Purkinje system pacing.
References
1. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43:561–632. doi: 10.1093/eurheartj/ehab395
2. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2021) 77:450–500. doi: 10.1016/j.jacc.2020.11.035
3. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. (2019) 380:1706–15. doi: 10.1056/NEJMoa1816885
4. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. (2019) 380:1695–705. doi: 10.1056/NEJMoa1814052
5. van der Boon RM, Nuis RJ, Van Mieghem NM, Jordaens L, Rodés-Cabau J, van Domburg RT, et al. New conduction abnormalities after TAVI–frequency and causes. Nat Rev Cardiol. (2012) 9:454–63. doi: 10.1038/nrcardio.2012.58
6. van Rosendael PJ, Delgado V, Bax JJ. Pacemaker implantation rate after transcatheter aortic valve implantation with early and new-generation devices: a systematic review. Eur Heart J. (2018) 39:2003–13. doi: 10.1093/eurheartj/ehx785
7. Tops LF, Schalij MJ, Bax JJ. The effects of right ventricular apical pacing on ventricular function and dyssynchrony implications for therapy. J Am Coll Cardiol. (2009) 54:764–76. doi: 10.1016/j.jacc.2009.06.006
8. Tse HF, Lau CP. Long-term effect of right ventricular pacing on myocardial perfusion and function. J Am Coll Cardiol. (1997) 29:744–9. doi: 10.1016/s0735-1097(96)00586-4
9. Riahi S, Nielsen JC, Hjortshøj S, Thomsen PE, Højberg S, Møller M, et al. Heart failure in patients with sick sinus syndrome treated with single lead atrial or dual-chamber pacing: no association with pacing mode or right ventricular pacing site. Europace. (2012) 14:1475–82. doi: 10.1093/europace/eus069
10. Kaye GC, Linker NJ, Marwick TH, Pollock L, Graham L, Pouliot E, et al. Effect of right ventricular pacing lead site on left ventricular function in patients with high-grade atrioventricular block: results of the protect-pace study. Eur Heart J. (2015) 36:856–62. doi: 10.1093/eurheartj/ehu304
11. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. (2003) 107:2932–7. doi: 10.1161/01.Cir.0000072769.17295.B1
12. Sammour Y, Krishnaswamy A, Kumar A, Puri R, Tarakji KG, Bazarbashi N, et al. Incidence, predictors, and implications of permanent pacemaker requirement after transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2021) 14:115–34. doi: 10.1016/j.jcin.2020.09.063
13. Regueiro A, Abdul-Jawad Altisent O, Del Trigo M, Campelo-Parada F, Puri R, Urena M, et al. Impact of new-onset left bundle branch block and periprocedural permanent pacemaker implantation on clinical outcomes in patients undergoing transcatheter aortic valve replacement: a systematic review and meta-analysis. Circ Cardiovasc Interv. (2016) 9:e003635. doi: 10.1161/circinterventions.115.003635
14. Faroux L, Chen S, Muntané-Carol G, Regueiro A, Philippon F, Sondergaard L, et al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: a systematic review and meta-analysis. Eur Heart J. (2020) 41:2771–81. doi: 10.1093/eurheartj/ehz924
15. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
16. Rück A, Saleh N, Glaser N. Outcomes following permanent pacemaker implantation after transcatheter aortic valve replacement: SWEDEHEART observational study. JACC Cardiovasc Interv. (2021) 14:2173–81. doi: 10.1016/j.jcin.2021.07.043
17. Rajah FT, Alaamiri AA, Mahmoodurrahman M, Alhowaish TS, Aldosari SF, Hussain AO, et al. Incidence, predictors, and clinical outcomes of permanent pacemaker insertion following transcatheter aortic valve implantation in an Arab population. J Interv Card Electrophysiol. (2021) 63:545–54. doi: 10.1007/s10840-021-01039-2
18. Schoechlin S, Minners J, Schulz U, Eichenlaub M, Ruile P, Neumann FJ, et al. Three-year outcome after transcatheter aortic valve implantation: comparison of a restrictive versus a liberal strategy for pacemaker implantation. Heart Rhythm. (2021) 18:2040–7. doi: 10.1016/j.hrthm.2021.08.011
19. Van Mieghem NM, Windecker S, Manoharan G, Bosmans J, Bleiziffer S, Modine T, et al. Final 3-year clinical outcomes following transcatheter aortic valve implantation with a supra-annular self-expanding repositionable valve in a real-world setting: results from the multicenter FORWARD study. Catheter Cardiovasc Interv. (2022) 99:171–8. doi: 10.1002/ccd.29889
20. Clementy N, Bisson A, Bodin A, Herbert J, Lacour T, Etienne CS, et al. Outcomes associated with pacemaker implantation following transcatheter aortic valve replacement: a nationwide cohort study. Heart Rhythm. (2021) 18:2027–32. doi: 10.1016/j.hrthm.2021.06.1175
21. Weferling M, Liebetrau C, Renker M, Fischer-Rasokat U, Choi YH, Hamm CW, et al. Right bundle branch block is not associated with worse short- and mid-term outcome after transcatheter aortic valve implantation. PLoS One. (2021) 16:e0253332. doi: 10.1371/journal.pone.0253332
22. Nicolas J, Guedeney P, Claessen BE, Mehilli J, Petronio AS, Sartori S, et al. Incidence, predictors and clinical impact of permanent pacemaker insertion in women following transcatheter aortic valve implantation: insights from a prospective multinational registry. Catheter Cardiovasc Interv. (2021) 98:E908–17. doi: 10.1002/ccd.29807
23. Alperi A, Rodés-Cabau J, Simonato M, Tchetche D, Charbonnier G, Ribeiro HB, et al. Permanent pacemaker implantation following valve-in-valve transcatheter aortic valve replacement: VIVID registry. J Am Coll Cardiol. (2021) 77:2263–73. doi: 10.1016/j.jacc.2021.03.228
24. Ashraf H, Fortuin FD, Sweeney J, DeValeria PA, Lanza LA, Ramsay G, et al. Development of advanced conduction disturbances following balloon-expandable transcatheter aortic valve replacement leads to poorer clinical outcomes. J Arrhythm. (2020) 36:755–61. doi: 10.1002/joa3.12383
25. Du F, Zhu Q, Jiang J, Chen H, Liu X, Wang J. Incidence and predictors of permanent pacemaker implantation in patients who underwent transcatheter aortic valve replacement: observation of a Chinese population. Cardiology. (2020) 145:27–34. doi: 10.1159/000502792
26. Fujita B, Schmidt T, Bleiziffer S, Bauer T, Beckmann A, Bekeredjian R, et al. Impact of new pacemaker implantation following surgical and transcatheter aortic valve replacement on 1-year outcome. Eur J Cardio Thorac Surg. (2020) 57:151–9. doi: 10.1093/ejcts/ezz168
27. Costa G, Zappulla P, Barbanti M, Cirasa A, Todaro D, Rapisarda G, et al. Pacemaker dependency after transcatheter aortic valve implantation: incidence, predictors and long-term outcomes. EuroIntervention. (2019) 15:875–83. doi: 10.4244/eij-d-18-01060
28. Meduri CU, Kereiakes DJ, Rajagopal V, Makkar RR, O’Hair D, Linke A, et al. Pacemaker implantation and dependency after transcatheter aortic valve replacement in the REPRISE III trial. J Am Heart Assoc. (2019) 8:e012594. doi: 10.1161/jaha.119.012594
29. Maeno Y, Abramowitz Y, Israr S, Yoon SH, Kubo S, Nomura T, et al. Prognostic impact of permanent pacemaker implantation in patients with low left ventricular ejection fraction following transcatheter aortic valve replacement. J Invasive Cardiol. (2019) 31:E15–22.
30. Jørgensen TH, De Backer O, Gerds TA, Bieliauskas G, Svendsen JH, Søndergaard L. Mortality and heart failure hospitalization in patients with conduction abnormalities after transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2019) 12:52–61. doi: 10.1016/j.jcin.2018.10.053
31. Gonska B, Keßler M, Wöhrle J, Rottbauer W, Seeger J. Influence of permanent pacemaker implantation after transcatheter aortic valve implantation with new-generation devices. Neth Heart J. (2018) 26:620–7. doi: 10.1007/s12471-018-1194-1
32. Nadeem F, Tsushima T, Ladas TP, Thomas RB, Patel SM, Saric P, et al. Impact of right ventricular pacing in patients who underwent implantation of permanent pacemaker after transcatheter aortic valve implantation. Am J Cardiol. (2018) 122:1712–7. doi: 10.1016/j.amjcard.2018.07.046
33. Alasti M, Rashid H, Rangasamy K, Kotschet E, Adam D, Alison J, et al. Long-term pacemaker dependency and impact of pacing on mortality following transcatheter aortic valve replacement with the LOTUS valve. Catheter Cardiovasc Interv. (2018) 92:777–82. doi: 10.1002/ccd.27463
34. Walther T, Manoharan G, Linke A, Möllmann H, Holzhey D, Worthley SG, et al. Incidence of new-onset left bundle branch block and predictors of new permanent pacemaker following transcatheter aortic valve replacement with the Portico™ valve. Eur J Cardio Thorac Surg. (2018) 54:467–74. doi: 10.1093/ejcts/ezy078
35. Rogers T, Devraj M, Thomaides A, Steinvil A, Lipinski MJ, Buchanan KD, et al. Utility of invasive electrophysiology studies in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. Am J Cardiol. (2018) 121:1351–7. doi: 10.1016/j.amjcard.2018.02.015
36. Aljabbary T, Qiu F, Masih S, Fang J, Elbaz-Greener G, Austin PC, et al. Association of clinical and economic outcomes with permanent pacemaker implantation after transcatheter aortic valve replacement. JAMA Netw Open. (2018) 1:e180088. doi: 10.1001/jamanetworkopen.2018.0088
37. Chamandi C, Barbanti M, Munoz-Garcia A, Latib A, Nombela-Franco L, Gutiérrez-Ibanez E, et al. Long-term outcomes in patients with new permanent pacemaker implantation following transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2018) 11:301–10. doi: 10.1016/j.jcin.2017.10.032
38. López-Aguilera J, Segura Saint-Gerons JM, Sánchez Fernández J, Mazuelos Bellido F, Pan Álvarez-Ossorio M, Suárez de Lezo J, et al. Long-term clinical impact of permanent cardiac pacing after transcatheter aortic valve implantation with the CoreValve prosthesis: a single center experience. Europace. (2018) 20:993–1000. doi: 10.1093/europace/eux046
39. Nijenhuis VJ, Van Dijk VF, Chaldoupi SM, Balt JC, Ten Berg JM. Severe conduction defects requiring permanent pacemaker implantation in patients with a new-onset left bundle branch block after transcatheter aortic valve implantation. Europace. (2017) 19:1015–21. doi: 10.1093/europace/euw174
40. Engborg J, Riechel-Sarup C, Gerke O, Mickley H, Sandgaard NC, Nissen H, et al. Effect of permanent pacemaker on mortality after transcatheter aortic valve replacement. Scand Cardiovasc J. (2017) 51:40–6. doi: 10.1080/14017431.2016.1236982
41. Fadahunsi OO, Olowoyeye A, Ukaigwe A, Li Z, Vora AN, Vemulapalli S, et al. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement: analysis from the U.S. society of thoracic surgeons/American college of cardiology TVT registry. JACC Cardiovasc Interv. (2016) 9:2189–99. doi: 10.1016/j.jcin.2016.07.026
42. Giustino G, Van der Boon RM, Molina-Martin de Nicolas J, Dumonteil N, Chieffo A, de Jaegere PP, et al. Impact of permanent pacemaker on mortality after transcatheter aortic valve implantation: the PRAGMATIC (Pooled Rotterdam-Milan-Toulouse in collaboration) pacemaker substudy. EuroIntervention. (2016) 12:1185–93. doi: 10.4244/eijv12i9a192
43. Dizon JM, Nazif TM, Hess PL, Biviano A, Garan H, Douglas PS, et al. Chronic pacing and adverse outcomes after transcatheter aortic valve implantation. Heart. (2015) 101:1665–71. doi: 10.1136/heartjnl-2015-307666
44. Mouillet G, Lellouche N, Yamamoto M, Oguri A, Dubois-Rande JL, Van Belle E, et al. Outcomes following pacemaker implantation after transcatheter aortic valve implantation with CoreValve(®) devices: results from the FRANCE 2 registry. Catheter Cardiovasc Interv. (2015) 86:E158–66. doi: 10.1002/ccd.25818
45. Kawaguchi AT, D’Allessandro C, Collet JP, Cluzel P, Makri R, Leprince P. Ventricular conduction defects after transcatheter aortic valve implantation: a single-institute analysis. Artif Organs. (2015) 39:409–15. doi: 10.1111/aor.12393
46. Schymik G, Tzamalis P, Bramlage P, Heimeshoff M, Würth A, Wondraschek R, et al. Clinical impact of a new left bundle branch block following TAVI implantation: 1-year results of the TAVIK cohort. Clin Res Cardiol. (2015) 104:351–62. doi: 10.1007/s00392-014-0791-2
47. Nazif TM, Dizon JM, Hahn RT, Xu K, Babaliaros V, Douglas PS, et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: the PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc Interv. (2015) 8:60–9. doi: 10.1016/j.jcin.2014.07.022
48. Urena M, Webb JG, Tamburino C, Muñoz-García AJ, Cheema A, Dager AE, et al. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation. (2014) 129:1233–43. doi: 10.1161/circulationaha.113.005479
49. Biner S, Michowitz Y, Leshem-Rubinow E, Topilsky Y, Ben-Assa E, Shimiaie J, et al. Hemodynamic impact and outcome of permanent pacemaker implantation following transcatheter aortic valve implantation. Am J Cardiol. (2014) 113:132–7. doi: 10.1016/j.amjcard.2013.09.030
50. Pereira E, Ferreira N, Caeiro D, Primo J, Adão L, Oliveira M, et al. Transcatheter aortic valve implantation and requirements of pacing over time. Pacing Clin Electrophysiol. (2013) 36:559–69. doi: 10.1111/pace.12104
51. Houthuizen P, Van Garsse LA, Poels TT, de Jaegere P, van der Boon RM, Swinkels BM, et al. Left bundle-branch block induced by transcatheter aortic valve implantation increases risk of death. Circulation. (2012) 126:720–8. doi: 10.1161/circulationaha.112.101055
52. De Carlo M, Giannini C, Bedogni F, Klugmann S, Brambilla N, De Marco F, et al. Safety of a conservative strategy of permanent pacemaker implantation after transcatheter aortic CoreValve implantation. Am Heart J. (2012) 163:492–9. doi: 10.1016/j.ahj.2011.12.009
53. Buellesfeld L, Stortecky S, Heg D, Hausen S, Mueller R, Wenaweser P, et al. Impact of permanent pacemaker implantation on clinical outcome among patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol. (2012) 60:493–501. doi: 10.1016/j.jacc.2012.03.054
54. D’Ancona G, Pasic M, Unbehaun A, Hetzer R. Permanent pacemaker implantation after transapical transcatheter aortic valve implantation. Interact Cardiovasc Thorac Surg. (2011) 13:373–6. doi: 10.1510/icvts.2011.274456
55. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. (2002) 106:3006–8. doi: 10.1161/01.cir.0000047200.36165.b8
56. Auffret V, Puri R, Urena M, Chamandi C, Rodriguez-Gabella T, Philippon F, et al. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation. (2017) 136:1049–69. doi: 10.1161/circulationaha.117.028352
57. Minha S, Yarkoni Y, Segev A, Finkelstein A, Danenberg H, Fefer P, et al. Comparison of permanent pacemaker implantation rate after first and second generation of transcatheter aortic valve implantation-A retrospective cohort study. Catheter Cardiovasc Interv. (2021) 98:E990–9. doi: 10.1002/ccd.29891
58. Lamas GA, Orav EJ, Stambler BS, Ellenbogen KA, Sgarbossa EB, Huang SK, et al. Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. Pacemaker selection in the elderly investigators. N Engl J Med. (1998) 338:1097–104. doi: 10.1056/nejm199804163381602
59. Lamas GA, Lee KL, Sweeney MO, Silverman R, Leon A, Yee R, et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med. (2002) 346:1854–62. doi: 10.1056/NEJMoa013040
60. Toff WD, Camm AJ, Skehan JD. Single-chamber versus dual-chamber pacing for high-grade atrioventricular block. N Engl J Med. (2005) 353:145–55. doi: 10.1056/NEJMoa042283
61. Mohananey D, Jobanputra Y, Kumar A, Krishnaswamy A, Mick S, White JM, et al. Clinical and echocardiographic outcomes following permanent pacemaker implantation after transcatheter aortic valve replacement: meta-analysis and meta-regression. Circ Cardiovasc Interv. (2017) 10:e005046. doi: 10.1161/circinterventions.117.005046
62. Ueshima D, Nai Fovino L, Mojoli M, Napodano M, Fraccaro C, Tarantini G. The interplay between permanent pacemaker implantation and mortality in patients treated by transcatheter aortic valve implantation: a systematic review and meta-analysis. Catheter Cardiovasc Interv. (2018) 92:E159–67. doi: 10.1002/ccd.27681
63. De Sisti A, Márquez MF, Tonet J, Bonny A, Frank R, Hidden-Lucet F. Adverse effects of long-term right ventricular apical pacing and identification of patients at risk of atrial fibrillation and heart failure. Pacing Clin Electrophysiol. (2012) 35:1035–43. doi: 10.1111/j.1540-8159.2012.03371.x
64. Sweeney MO, Hellkamp AS. Heart failure during cardiac pacing. Circulation. (2006) 113:2082–8. doi: 10.1161/circulationaha.105.608356
65. Urena M, Mok M, Serra V, Dumont E, Nombela-Franco L, DeLarochellière R, et al. Predictive factors and long-term clinical consequences of persistent left bundle branch block following transcatheter aortic valve implantation with a balloon-expandable valve. J Am Coll Cardiol. (2012) 60:1743–52. doi: 10.1016/j.jacc.2012.07.035
66. van der Boon RM, Van Mieghem NM, Theuns DA, Nuis RJ, Nauta ST, Serruys PW, et al. Pacemaker dependency after transcatheter aortic valve implantation with the self-expanding Medtronic CoreValve system. Int J Cardiol. (2013) 168:1269–73. doi: 10.1016/j.ijcard.2012.11.115
67. Testa L, Latib A, De Marco F, De Carlo M, Agnifili M, Latini RA, et al. Clinical impact of persistent left bundle-branch block after transcatheter aortic valve implantation with CoreValve revalving system. Circulation. (2013) 127:1300–7. doi: 10.1161/circulationaha.112.001099
68. Rodés-Cabau J, Ellenbogen KA, Krahn AD, Latib A, Mack M, Mittal S, et al. Management of conduction disturbances associated with transcatheter aortic valve replacement: JACC scientific expert panel. J Am Coll Cardiol. (2019) 74:1086–106. doi: 10.1016/j.jacc.2019.07.014
69. Malebranche D, Bartkowiak J, Ryffel C, Bernhard B, Elsmaan M, Nozica N, et al. Validation of the 2019 expert consensus algorithm for the management of conduction disturbances after TAVR. JACC Cardiovasc Interv. (2021) 14:981–91. doi: 10.1016/j.jcin.2021.03.010
70. Abdelrahman M, Subzposh FA, Beer D, Durr B, Naperkowski A, Sun H, et al. Clinical outcomes of his bundle pacing compared to right ventricular pacing. J Am Coll Cardiol. (2018) 71:2319–30. doi: 10.1016/j.jacc.2018.02.048
71. Upadhyay GA, Vijayaraman P, Nayak HM, Verma N, Dandamudi G, Sharma PS, et al. His corrective pacing or biventricular pacing for cardiac resynchronization in heart failure. J Am Coll Cardiol. (2019) 74:157–9. doi: 10.1016/j.jacc.2019.04.026
72. Huang W, Wu S, Vijayaraman P, Su L, Chen X, Cai B, et al. Cardiac resynchronization therapy in patients with non-ischemic cardiomyopathy using left bundle branch pacing. JACC Clin Electrophysiol. (2020) 6:849–58. doi: 10.1016/j.jacep.2020.04.011
73. Sun JY, Sha YQ, Sun QY, Qiu Y, Shao B, Ni YH, et al. The long-term therapeutic effects of His-Purkinje system pacing on bradycardia and cardiac conduction dysfunction compared with right ventricular pacing: a systematic review and meta-analysis. J Cardiovasc Electrophysiol. (2020) 31:1202–10. doi: 10.1111/jce.14445
74. De Pooter J, Gauthey A, Calle S, Noel A, Kefer J, Marchandise S, et al. Feasibility of his-bundle pacing in patients with conduction disorders following transcatheter aortic valve replacement. J Cardiovasc Electrophysiol. (2020) 31:813–21. doi: 10.1111/jce.14371
Keywords: transcatheter aortic valve replacement, permanent pacemaker implantation, mortality, heart failure rehospitalization, left ventricular ejection fraction, meta-analysis
Citation: Xu S, Zhang E, Qian Z, Sun J, Zou F, Wang Y, Hou X and Zou J (2022) Mid- to Long-Term Clinical and Echocardiographic Effects of Post-procedural Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 9:911234. doi: 10.3389/fcvm.2022.911234
Received: 02 April 2022; Accepted: 30 May 2022;
Published: 28 June 2022.
Edited by:
Giuseppe Tarantini, University of Padua, ItalyReviewed by:
Chiara Fraccaro, University Hospital of Padua, ItalyAleksander Dokollari, Lankenau Medical Center, United States
Copyright © 2022 Xu, Zhang, Qian, Sun, Zou, Wang, Hou and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangang Zou, amd6b3VAbmptdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Shun Xu
Shun Xu Enrui Zhang
Enrui Zhang Zhiyong Qian
Zhiyong Qian Jinyu Sun
Jinyu Sun Fengwei Zou2
Fengwei Zou2 Jiangang Zou
Jiangang Zou