
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 12 July 2022
Sec. Atherosclerosis and Vascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.908040
Background: Uremic cardiomyopathy is commonly presented in chronic kidney disease (CKD), and it severely affects the prognosis of patients with CKD. In the past few decades, the investigation of uremic cardiomyopathy has developed rapidly. However, no report has summarized the situation of uremic cardiomyopathy research to date. This study aimed to evaluate the state of uremic cardiomyopathy research in the last 30 years and identify important topics and achievements, as well as emerging trends through bibliometric analysis.
Materials and Methods: Publications related to uremic cardiomyopathy were collected from Science Citation Index Expanded. HistCite, VOSviewer, CiteSpace, and the Bibliometrix Package were used for bibliometric analysis and visualization, including the analysis of the overall distribution of the annual publication, leading countries, and active institutions and authors, core journals, co-cited references, and keywords.
Results: A total of 2,403 studies related to uremic cardiomyopathy were obtained, and progress related to uremic cardiomyopathy was slower in past 3 years. A total of 10,077 authors from 2,697 institutions in 89 countries or regions reported investigations on uremic cardiomyopathy. The United States of America was the most productive and the most cited country. Myles Wolf, Joseph I Shapiro, and Carmine Zoccali published most articles in uremic cardiomyopathy, and journals in nephrology possessed core status in the field. Phosphate metabolism was the hotspot in uremic cardiomyopathy research in recent years, and future progress may concentrate on phosphate metabolism, endogenous natriuretic factors, and novel biomarkers.
Conclusion: The United States of America and European countries played central roles in uremic cardiomyopathy research, while Chinese scholars should be more involved in this field. Global publications on uremic cardiomyopathy have entered platform stage, and the fibroblast growth factor-23-klotho axis remained a hotspot in this field. Endogenous natriuretic factors and novel biomarkers may be potential directions in future investigations.
Cardiovascular disease is a leading cause of death in patients with chronic kidney disease (CKD) (1). The most common feature of cardiovascular changes in CKD is uremic cardiomyopathy (2), which presents even in the early stages of CKD and in up to 75% of patients with predialysis (3–5). Uremic cardiomyopathy contributes to more severe cardiovascular anomalies, including diastolic dysfunction, arrhythmia, and even sudden death (6). Hence, uremic cardiomyopathy has been widely concerned and is considered as a main therapeutic target in patients with CKD with cardiovascular complications.
Several factors are highly involved in the emergence of uremic cardiomyopathy, including hypertension, anemia, renin-angiotensin system overactivation, microinflammation, CKD mineral-bone disorder (CKD-MBD), and uremic toxins (7). Importantly, these factors constitute the basis of uremic cardiomyopathy treatment. Since Parfrey et al. (8) observed the regression of LVH after renal transplantation, preventing uremic cardiomyopathy has become a possibility. Subsequently, recombinant human erythropoietin (9), hemodialysis (10), and spironolactone (11) were all found to cause uremic cardiomyopathy regression in patients with CKD. In 2011, Faul et al. (12) identified that fibroblast growth factor 23 (FGF-23), which is elevated in CKD, can directly induce LVH. This further links CKD-MBD to uremic cardiomyopathy and implies another therapeutic strategy for uremic cardiomyopathy. However, the lack of full understanding of the pathophysiological process of uremic cardiomyopathy makes the related progress slower. For example, sustained erythropoietin treatment may bring side effects (e.g., hypertension and thrombosis) and increase mortality (13), and FGF-23 neutralization failed to mitigate LVH but increased aortic calcification and the risk of mortality in CKD rats (14). Hence, comprehension of the developmental and potential directions of uremic cardiomyopathy to deepen our understanding and propose novel therapeutic strategies in this field are required.
Bibliometric analysis is “the application of mathematical and statistical methods to books and other media of communication” (15), and it can be used as an analytic model for quantitatively analyzing scientific publications (16), which are medium of scientific information. As an analytic model, bibliometric analysis can quickly reveal the knowledge network, research topic evolvement, and potential research directions in a certain research field (17). Liu et al. (18) analyzed podocyte injury research through bibliometric analysis and identified the most prolific country, top contributing authors, and most popular journals in this field. Most importantly, they also provided research topic evolvement, namely, “diabetic nephropathy” to “autophagy, microRNA, and inflammation,” and identified that traditional Chinese medicine may be a potential direction in future podocyte injury research. Hence, bibliometrics can act as a critical approach to provide an in-depth evaluation of the development of the uremic cardiomyopathy field, which may guide us in future work. To our knowledge, no bibliometric analysis of uremic cardiomyopathy has been performed. We aim to analyze publications on uremic cardiomyopathy and evaluate the status and emerging trends in this field.
Although PubMed contains more biomedicine-related publications compared to web of science (WoS), the latter has a more complete citation network than PubMed, and is critical for co-citation analysis. WoS almost includes the most influential publications in uremic cardiomyopathy, which avoids the omission of important studies as far as possible. In addition, HistCite, VOSviewer, CiteSpace, and Bibliometrix packages are most suitable for analyzing datasets derived from WoS. Based on these, we chose a dataset derived from WoS as our analytic target. WoS’ topic search is equivalent to a search model of words in the title, abstracts, author keywords, and keywords plus, thus, we chose topic search to precisely obtain the topic. As LVH is the main feature of uremic cardiomyopathy (7), we also searched “[(chronic kidney disease) OR (chronic renal failure)] AND [(cardiac hypertrophy) OR (left ventricular hypertrophy)]” in addition to “(uremic cardiomyopathy) OR (uremic cardiac hypertrophy).” Importantly, CKD and LVH can be jointly observed in Fabry disease, which is a lysosomal storage disease that affects both the kidney and the heart (19). Hence, we excluded Fabry disease in our dataset.
We performed a literature search on the Science Citation Index Expanded (SCIE), and the search formula was as follows: “((((chronic NEAR/0 kidney NEAR/0 disease) OR (chronic NEAR/0 renal NEAR/0 failure)) AND ((cardiac NEAR/0 hypertrophy) OR (left NEAR/0 ventricular NEAR/0 hypertrophy)) OR ((uremic NEAR/0 cardiac NEAR/0 hypertrophy) OR (uremic NEAR/0 cardiomyopathy))) NOT (Fabry))”. The publication year was restricted to 1990–2021, and the document types were “articles” or “reviews”. In addition, the article language was restricted to English. All the information, including publication number and related titles, authors, affiliations, countries, keywords, journals, publication year, references, and citations, were collected for analysis. We finally got 2,403 publications. To avoid deviations from updates, all the above operations were completed within 1 day, and on May 22, 2022.
HistCite (version 12.03.17) (20) was used to analyze the publication number, total global citation score (TGCS), and total local citation score (TLCS) for each publication year, active countries, active institutions, active authors, and core journals. TGCS is the number of citations in the SCIE, while TLCS is the number of citations in the current publication set.
Co-citation and co-occurrence networks were completed by VOSviewer (version 1.6.18) (21). Collaboration between countries, institutions, and authors and mutual citation between journals were analyzed by co-citation networks. Keywords occurrence in various publications was analyzed by co-occurrence networks. The size of the nodes in networks represents publication number, and the thickness of the connecting line in networks means the strength of the node-link. The threshold (the minimum number of documents and the maximum number of total link strength) of co-citation and co-occurrence networks were presented in corresponding parts. The size of the circle of an item was proportional to its number of publications, and the width of the line between the two items was proportional to the magnitude of linkages. In addition, the same color indicates close cooperation, and the total link strength of an item reflects the degree of cooperation with others.
CiteSpace (version 5.8.R3) (22) was used to analyze the knowledge domain in uremic cardiomyopathy, including cluster, timeline view, and reference burst analysis. Time slicing was set as from 1990 January to 2021 December, and 2 years per slice. Node type was set as References, and selection criteria were set as top 50 levels of most cited or occurred items from each slice. Modularity Q and mean silhouette were used to evaluate the reliability of clustering; Q > 0.3 and mean silhouette of >0.5 indicate ample clustering structure and convincing clustering results, respectively.
Author’s production over time and the thematic evolution analysis were performed by R language-based the Bibliometrix Package (4.1.0 Package) (23), which can show the publications of most 10 productive authors for the last 20 years and identify research topic evolvement, respectively.
A total of 2,403 studies related to uremic cardiomyopathy were obtained from SCIE, including 1,810 articles and 593 reviews (Supplementary Table 1). From only one publication in 1990 to 155 publications in 2021, global publications in the field demonstrated an overt growth trend (Figure 1A), and this can be divided into three stages according to the annual production status: Initial stage (1990–1996), and growing stage (1997–2014), and platform stage (2015–2021). The number of publications was less than 30 per year in the initial stage and a significant increase in publication number with an average growth rate of 12.90% per year in the growing stage. However, the average growth rate of publications on uremic cardiomyopathy dropped to 1.47% during the period from 2015 to 2021 (Figure 1A).
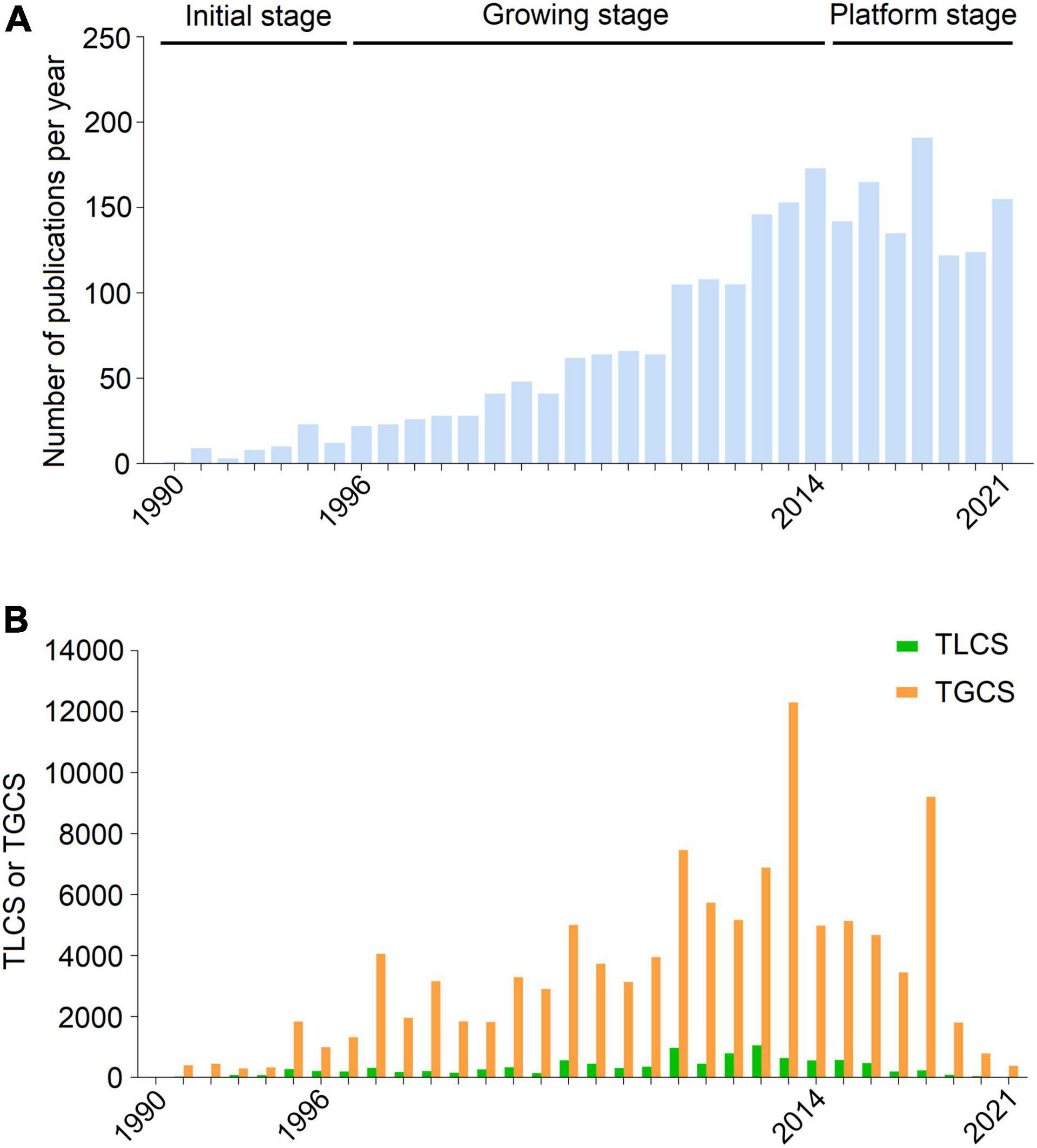
Figure 1. Publication outputs and citations on uremic cardiomyopathy. (A) Global annual production trends. (B) Annual total global citation score (TGCS) and total local citation score (TLCS) of publications on uremic cardiomyopathy.
Publications on uremic cardiomyopathy have been cited 90,649 times on SCIE, with an average of 45.16 times per article. Corresponding to publication number, the TGCS of publications was very low in the initial stage, while TGCS exhibited a gradually increasing trend from 1997 to 2014 and peaked in 2013. Since 2015, the TGCS was relatively stable (Figure 1B), further indicating the platform stage in this period.
A total of 89 countries and regions contributed to publications in this field, and the United States of America (USA) contributed the largest number of publications (839, 34.9% of all articles), followed by Italy (228, 9.5%), United Kingdom (UK) (213, 8.9%), Germany (203, 8.4%), and Japan (192, 8%) (Figure 2A and Table 1). Correspondingly, studies from the United States had the highest number of citations (50,802 citations), followed by those from Germany (18,429 citations), Italy (18,420 citations), the United Kingdom (18,057 citations), and Canada (14,399 citations), and the rest are all less than 10,000 citations (Figure 2B). Notably, although Japan, China, and Turkey had higher publication numbers, their total citations and average citations were significantly lower than others in the top 10 productive countries (Figures 2B,C), indicating a minor influence on their publications.
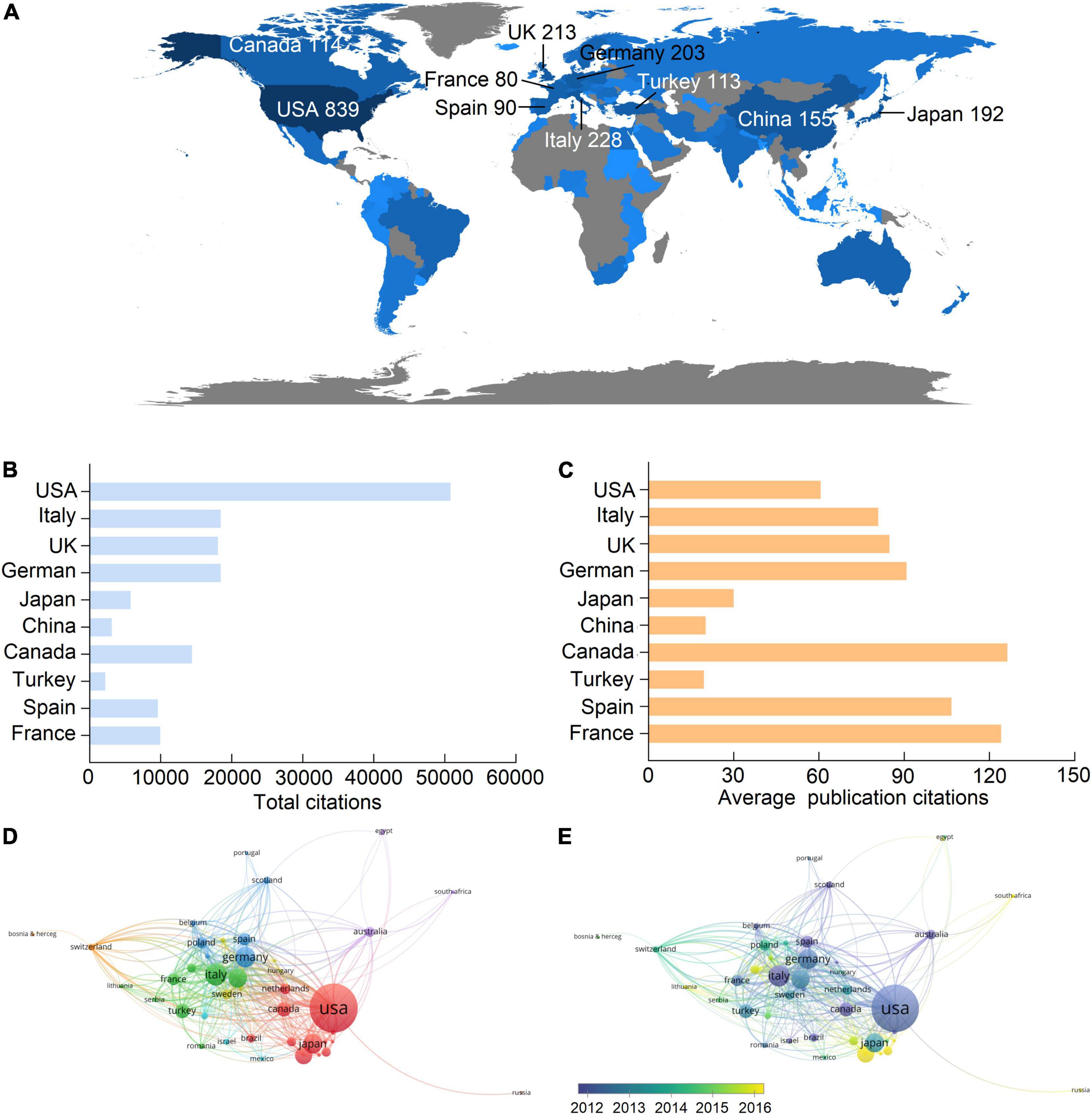
Figure 2. Leading countries in the uremic cardiomyopathy field. (A) Worldwide distribution of the publication. (B,C) Total citations and average publication citations of the top 10 productive countries. (D) Clustering of collaboration among countries. (E) Timeline visualization of collaboration among countries.
There were 44 countries with more than five publications that have been included in the co-authorship analysis, and one of them was not connected to others. Hence, 43 countries were used to analyze collaboration among countries. The highest total link strength was the United States (total link strength = 390 times), indicating the largest cooperative network led by it (Figure 2D). In this cooperative network, Germany (49), Canada (41), Italy (37), and China (33) had the closest cooperation with the United States (Figure 2D). Among the top 10 productive countries (Figure 2A and Table 1), China is the late starter in international cooperation in this field (Figure 2E).
There were 10,077 authors from 2,697 institutions who have published articles on uremic cardiomyopathy. The top 10 productive institutions were almost American universities, except Karolinska Institute. The University of Miami was the leading institution in publication, followed by the University of Pennsylvania, the University of Washington, Harvard University, and Northwestern University (Table 2). Both the TGCS and TLCS of the University of Miami (cited 6,022 times) were the highest in the top 10 active institutes (Table 2), indicating their leading role in uremic cardiomyopathy.
We analyzed the co-authorship of 121 institutions with more than 10 publications, while the items that were not connected were removed, leaving a total of 119 institutions. The University of Pennsylvania showed the most frequent collaboration with other institutions (total link strength = 186 times) (Figure 3A), which was followed by the University of Washington (162), the University of California, San Francisco (138), the University of Illinois (112), and Tulane University (101). Notably, the collaboration was mostly taking place within the institutions in the United States or the institutions in European (Figure 3B).
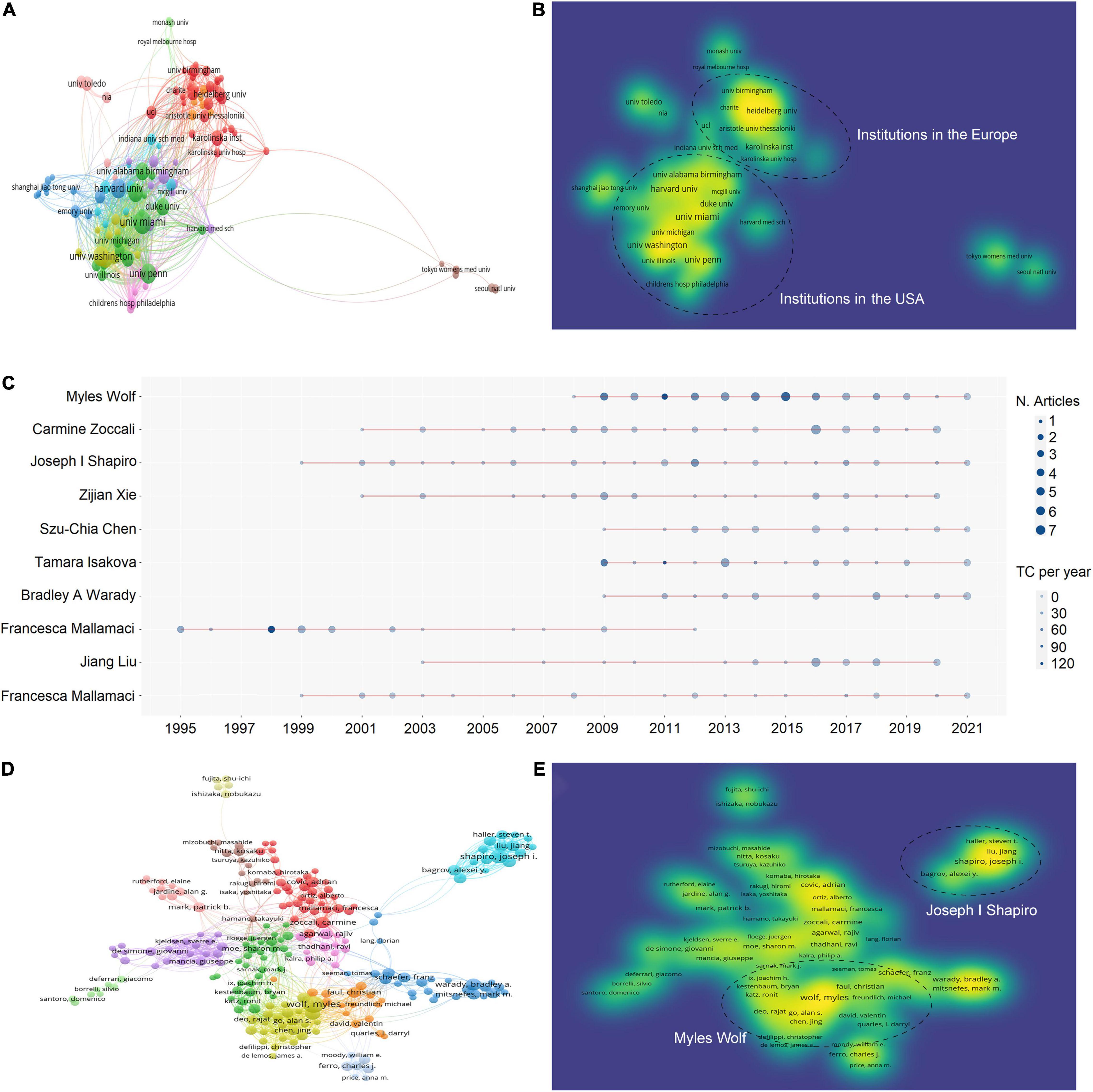
Figure 3. Active institutes and authors analysis. (A) Clustering of collaboration among institutes. (B) Density visualization of institutes based on the mean frequency of appearance and two groups with higher collaboration can be observed. (C) Timeline distribution of publications from the top 10 active authors. (D) Clustering of collaboration among authors. (E) Density visualization of authors based on the mean frequency of appearance and three groups with higher collaboration can be observed.
The top 10 active authors were Myles Wolf of the University of Miami, Joseph I Shapiro of Marshall University, Carmine Zoccali of National Research Council, Zijian Xie of Marshall University, Szu-Chia Chen of Kaohsiung Medical University, Tamara Isakova of Northwestern University, Bradley A. Warady of Children’s Mercy Hospital, Robert N. Foley of USRDS Coordinating Center, Jiang Liu of Marshall University, and Francesca Mallamaci of National Research Council of Italy (Table 3). Of the top 10 active authors, Carmine Zoccali and Francesca Mallamaci had the longest period in publication on uremic cardiomyopathy (Figure 3C), and they both focused mainly on clinical features of uremic cardiomyopathy.
Moreover, a total of 304 authors were co-authored in more than five publications, excluding 72 authors that were not connected. The two authors with the highest total link strength constructed the biggest two collaboration networks, namely, Myles Wolf (total link strength = 186 times) and Joseph I. Shapiro (138) (Figure 3D). The collaboration network of Myles Wolf is mainly concentrated on the relationship between phosphate metabolism and uremic cardiomyopathy, while the collaboration network of Joseph I. Shapiro is mainly concentrated on the relationship between Na+/K+-ATPase and uremic cardiomyopathy. These collaboration networks represent two main topics in uremic cardiomyopathy. Interestingly, the collaboration network of Joseph I. Shapiro demonstrated lower cooperation with other networks (Figure 3E).
Publications on uremic cardiomyopathy were presented in 500 journals. The top 10 journals with the highest publications were shown in Table 4, and about 28.3% of all publications were published in these journals. In addition, about 42% of all citations occurred in the top 10 journals with the highest publications, indicating their higher influence in the uremic cardiomyopathy field. It is noteworthy that most of these 10 journals are nephrology-related, except the Journal of Hypertension and PLoS ONE. American Journal of Kidney Diseases had the largest number of citations (8,559 citations), followed by the Journal of the American Society of Nephrology (8,447), Kidney International (7,854), Journal of Hypertension (6,576), and Nephrology Dialysis Transplantation (6,209).
A total of 553 journals were co-cited in more than 20 publications. Kidney International (total link strength = 1,080,090 times), Hypertension (893,963), Journal of the American Society of Nephrology (819,058), Circulation (788,881), and New England Journal of Medicine (742,818) showed the most co-citations with other journals (Figure 4A). Interestingly, journals in the nephrology field demonstrated a leading role in the co-citation network as indicated by the most complex citation network (Figure 4A) and the highest density in the co-citation network (Kidney International, Journal of the American Society of Nephrology, and American Journal of Kidney Diseases) (Figure 4B).
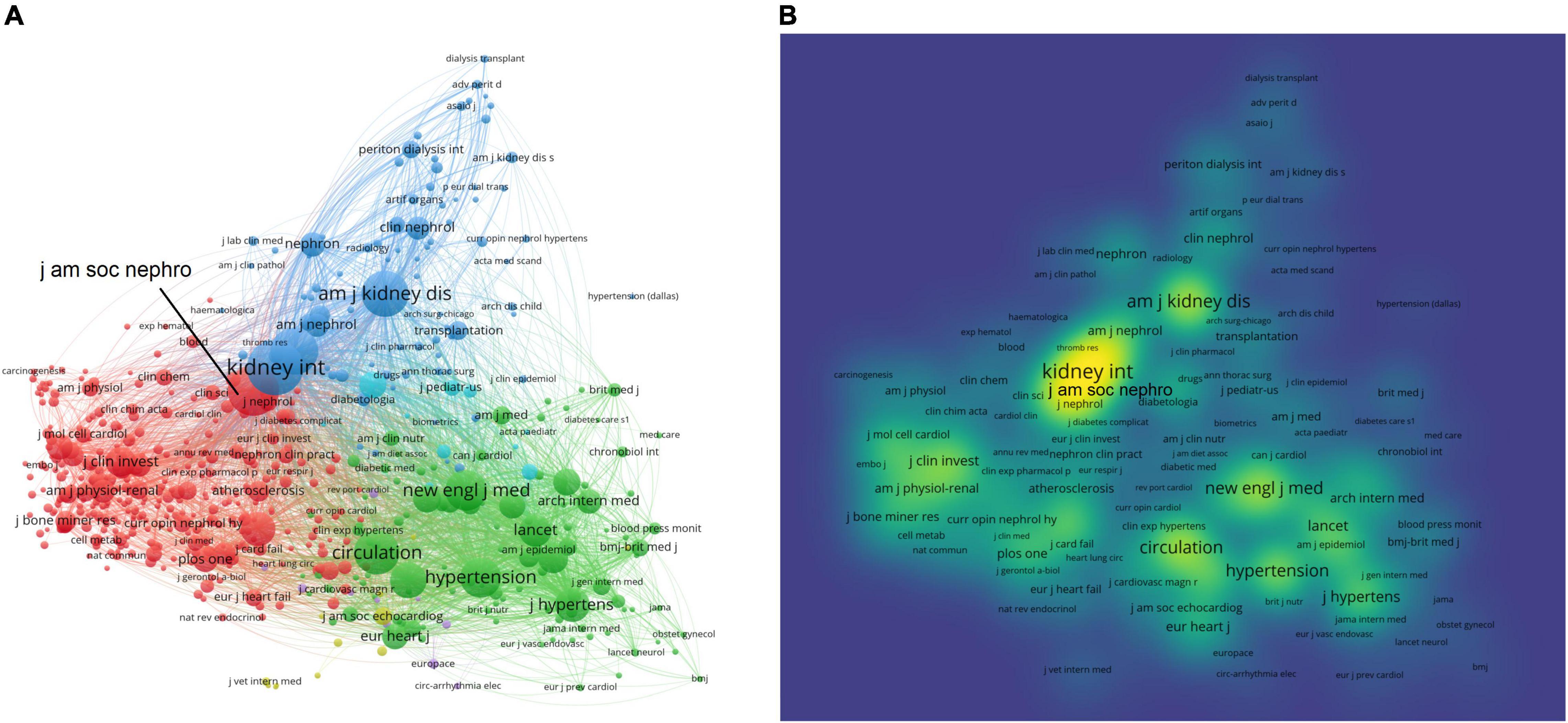
Figure 4. Core journals in the uremic cardiomyopathy field. (A) Clustering of co-citation among journals. (B) Density visualization of journals based on the mean frequency of appearance, and journals in nephrology possessed a core position in clustering.
The top 10 most cited publications were listed in Supplementary Table 2. Among these publications, there were two research articles, five guidelines, and three reviews, and seven publications were less cited by uremic cardiomyopathy-related publications (TLCS < 50), implying an indirect relationship with uremic cardiomyopathy. Thus, we further analyzed the top 10 most TLCS publications (Table 5). There were eight research articles and two review, and most of them were epidemiological reports, while three publications were pathophysiological investigations. Interestingly, these pathophysiological investigations all concentrated on the relationship between FGF-23 and uremic cardiomyopathy (12, 14, 24).
The visualization network of cited references demonstrated 13 clusters (Table 6), the modularity Q was 0.8555, and the mean silhouette value was 0.9436. We performed a visualized timeline for these clusters to identify the involvement of the research topic in the uremic cardiomyopathy field. “Anemia” and “rhuepo (recombinant human erythropoietin)” were early fields in uremic cardiomyopathy (Cluster ID #1 and ID #4, Figure 5A), which are attributed to the discovery of anemia-induced cardiac injury in CKD (25) and erythropoietin can mitigate LVH in patients with CKD (9). Later, CKD-MBD was considered a cardiovascular risk factor, and vitamin D and FGF-23 have gained wide concern (26) (Cluster ID #5, ID #7, and ID #19, Figure 5A), especially FGF-23 was found to correlate to LVH and can directly induce LVH (12, 27). In recent years, the hotspot of uremic cardiomyopathy was on “klotho” (Cluster ID #3, Figure 5A), which declines CKD and has FGF-23-dependent and FGF-23-independent protective effects on the myocardium (28).
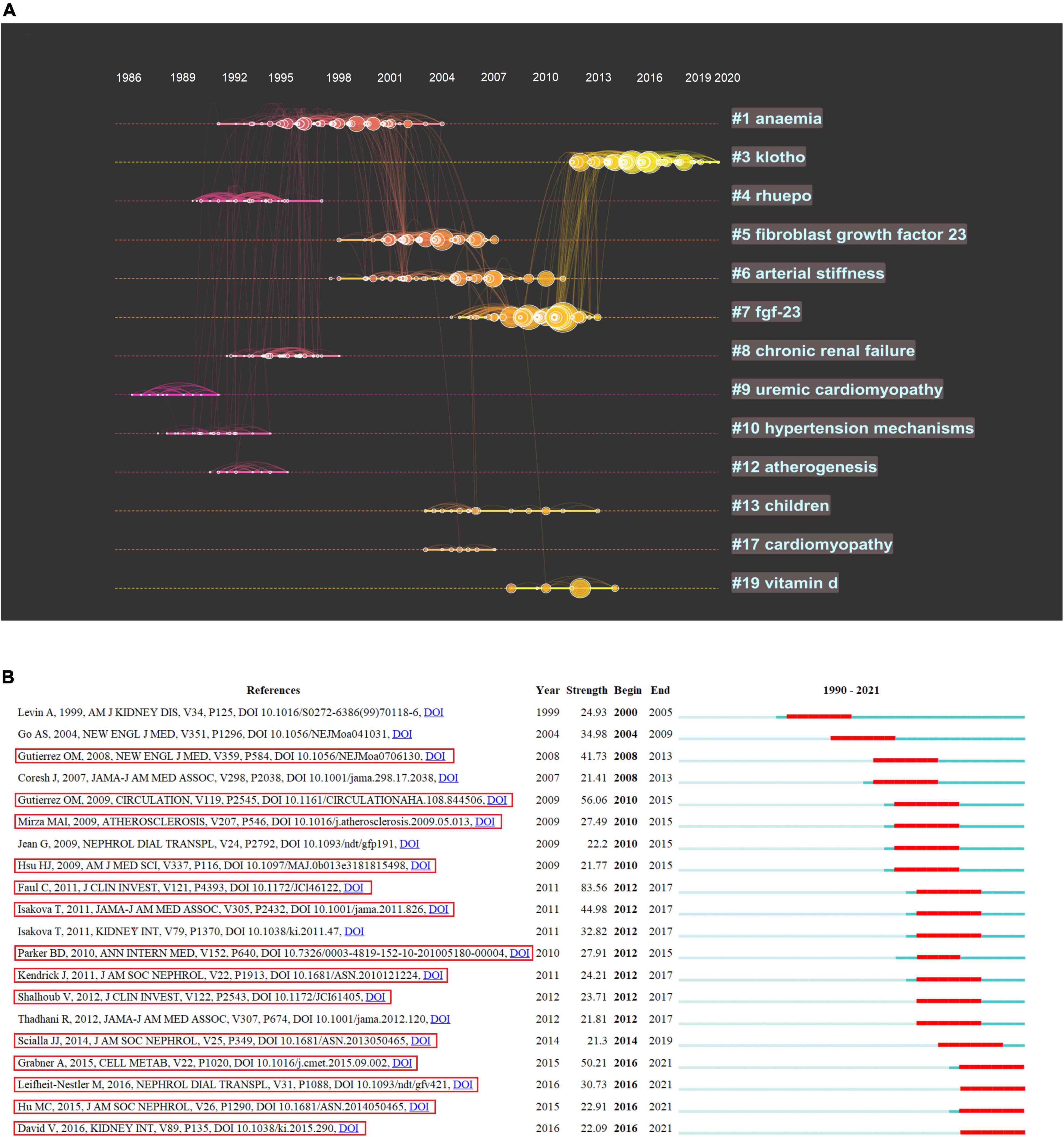
Figure 5. Co-cited reference analysis. (A) Timeline distribution of 13 clusters with the higher K values. (B) The top 20 references with the higher citation bursts, references with the red box is directly associated with FGF-23.
The term “burst” refers to references that frequently appear over some time and can reflect the hot topics in a certain period based on the topic of references. Reference burst detection of the top 20 references, with the strongest citation bursts, demonstrated that the publication of Faul et al. (12), Gutiérrez et al. (27), and Grabner et al. (24) are the top three burst strength (Figure 5B). Interestingly, these publications were all concentrated on the relationship between FGF-23 and uremic cardiomyopathy, and 14 references were directly correlated with FGF-23 or klotho, suggesting the specific role of calcium-phosphorus metabolism on uremic cardiomyopathy. The recent publications with higher citation bursts were coming from Hu et al. (29), Leifheit-Nestler et al. (30), and David et al. (31); Hu et al. and Leifheit-Nestler et al. demonstrated that LVH in CKD may be attributed to a deficiency of soluble klotho, thus, implying a potential therapeutic strategy in uremic cardiomyopathy. David et al. reported that inflammation and iron deficiency can upregulate FGF-23, which further indicates the association between inflammation, anemia, FGF-23, and cardiac injuries in patients with CKD.
Thematic evolution analysis of the author’s keywords showed that the initial stage of investigation on uremic cardiomyopathy was mainly focused on “renin-angiotensin system,” “hypertension,” “parathyroidectomy,” and “erythropoietin,” whereas, the main topics remained “hypertension” and “anemia” in the growing stage, indicating that the understanding on uremic cardiomyopathy was not deepened in this stage. In the platform stage, the main topic of uremic cardiomyopathy has gradually evolved toward “fibroblast growth factor-23,” which is a breakthrough in the uremic cardiomyopathy field (12, 27). In the past 3 years, “klotho” attracted the attention of scholars in uremic cardiomyopathy (Figure 6A). These results were consistent with the previous analysis (Figure 5A).
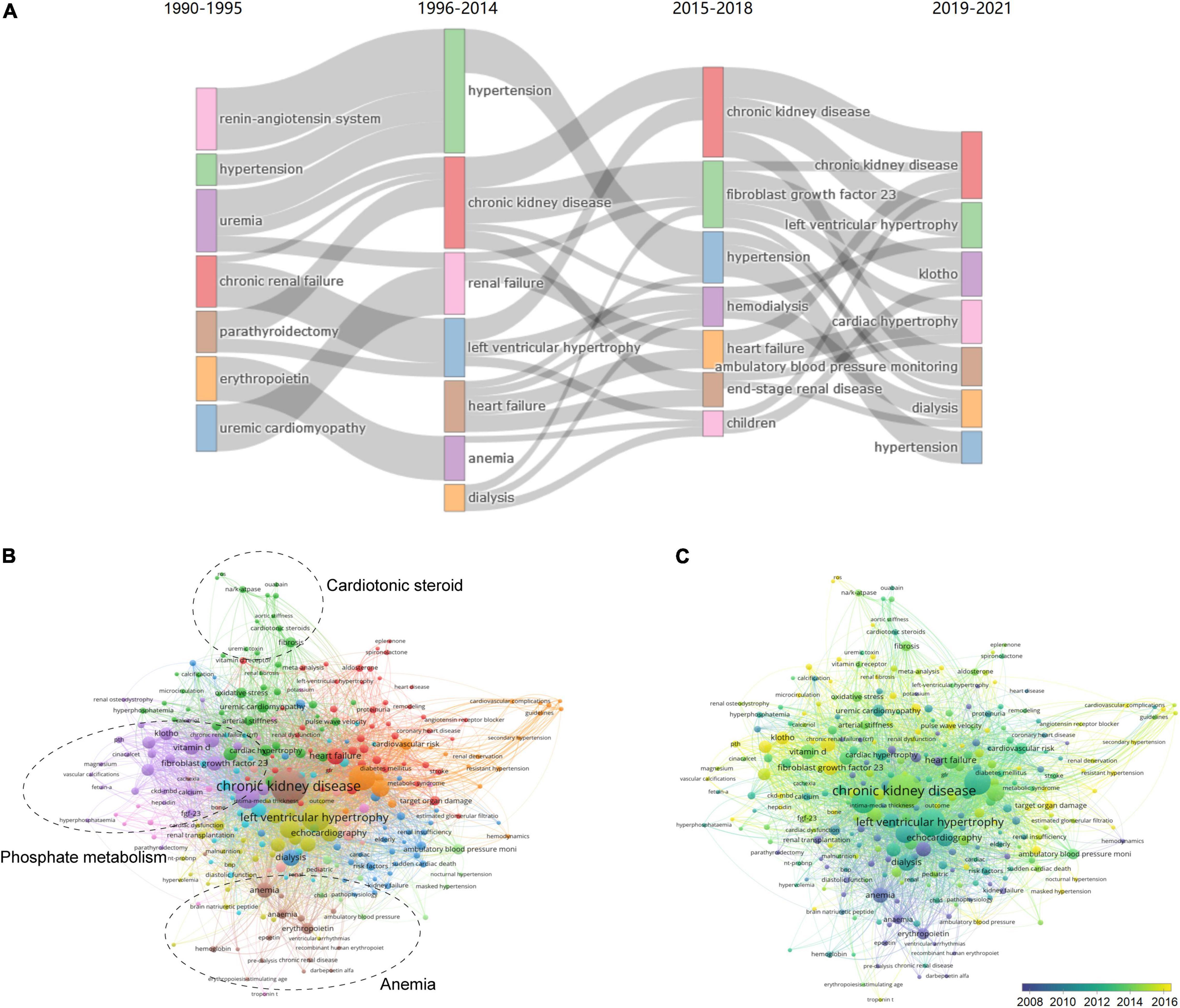
Figure 6. Analysis of keywords in publications and milestones of the uremic cardiomyopathy field. (A) Major keywords evolution of uremic cardiomyopathy research. (B) Clustering of co-occurrence among keywords. (C) Timeline visualization of co-occurrence among keywords.
A total of 283 keywords (set as author’s keywords) were identified as having occurred more than five times. It can be found that FGF-23, klotho, and vitamin D, which are all related to phosphate metabolism, constitute a critical part of uremic cardiomyopathy research (Figure 6B), and these were a relatively novel topic in this field as well (Figure 6C). In addition, cardiotonic steroids and Na+-K+-ATPase constitute another important part of uremic cardiomyopathy research (Figure 6B). Anemia, as a classical topic in uremic cardiomyopathy (Figure 6C), also occupied its domain (Figure 6B). Thus, these three parts were the leading topic in uremic cardiomyopathy.
Notably, we identified several potential novel aspects in uremic cardiomyopathy (Figure 6C), including Na+-K+-ATPase (occurrences: 16), reactive oxygen species (ROS) (5), troponin T (5), ambulatory blood pressure (10), and nocturnal hypertension (11), which were all less exposed in uremic cardiomyopathy but may have potential significance.
In this study, we analyzed the main knowledge domain and emerging trends of uremic cardiomyopathy through bibliometric analysis. The results showed that annual publications on uremic cardiomyopathy have entered a platform stage since its rise in 1996. CKD-related phosphate metabolism disorder remains the hotspot of uremic cardiomyopathy research, and the klotho is an emerging trend in recent years.
The United States was the most productive country (Figure 2A and Table 1); nine of the top 10 productive institutions and seven of the top 10 productive authors were from the United States (Tables 2, 3). The main research topic in uremic cardiomyopathy, the phosphate metabolism, was almost established by scholars from the United States (Figure 5B). Thus, the United States demonstrates a great impact on uremic cardiomyopathy research. In addition to the United States, scholars in Europe, as represented by Carmine Zoccali, possessed another critical part in uremic cardiomyopathy (Figures 3A,B). Compared to American scholars, European scholars mainly concentered on the clinical features and diagnosis of uremic cardiomyopathy (32, 33). Recently, Lv et al. (34) analyzed publications on cardiorenal syndrome and found that the United States and Europe still play central roles in this field. Although uremic cardiomyopathy only constitutes a small part of a cardiorenal syndrome, our result still partially confirmed their analysis.
Although China has the 6th rank in publications worldwide (Figure 2A and Table 1), the total and average publication citations are relatively lower in the top 10 productive countries (Figures 2B,C). Moreover, the lack of Chinese institutes and scholars in the top 10 productive institutes and authors can be observed (Figure 3), and China is a late starter in international cooperation (Figure 2E). These results indicate less influence of Chinese scholars on the uremic cardiomyopathy field, and it is manifestly unreasonable due to the existence of the largest population with CKD in China (35). In China, most nephrologists concentrate on glomerulonephritis, acute kidney injury, CKD, and dialysis (18, 36), while the investigations on uremic cardiomyopathy started fairly late, as indicated by the most influential study on uremic cardiomyopathy published by Jinghong Zhao’s group at 2015 (cited 105) (37). Therefore, Chinese scholars exhibit less influence in this field, and they should be more involved in uremic cardiomyopathy research with the aid of ample clinical resources.
Myles Wolf of the University of Miami plays a leading role in the investigations of the relationship between phosphate metabolism and uremic cardiomyopathy, and he is a pioneer of the most influential breakthrough in uremic cardiomyopathy (FGF-23 and uremic cardiomyopathy), as indicated by two studies from his group (27, 38). Based on this achievement, Myles Wolf established the largest co-authorship network in uremic cardiomyopathy (Figure 3D). Joseph I. Shapiro of Marshall University mainly concentrates on cardiotonic steroids and uremic cardiomyopathy; his group found that CKD-induced cardiotonic steroids elevation is sufficient to induce LVH and cardiac fibrosis through activating redox-sensitive Na+-K+-ATPase (39). However, although Joseph I. Shapiro published lots of studies on uremic cardiomyopathy, his group has failed to possess a central position in uremic cardiomyopathy (Figure 3D). This also influences the cooperation between Joseph I. Shapiro’s group and the outside group (Figure 3D). Notably, these two scholars both came from institutions in the United States, indicating the leading role of the United States in uremic cardiomyopathy research on the secondary side.
Based on the number, TLCS, TGCS, and H index, the Nephrology Dialysis Transplantation, American Journal of Kidney Diseases, Kidney International, and the Journal of the American Society of Nephrology are the most influential journals for scholars in the uremic cardiomyopathy field (Table 4). These results also imply that the journals in the nephrology domain, rather than journals in the cardiology domain, possess a central role in publishing uremic cardiomyopathy studies. Interestingly, we can also find highly influential publications in non-nephrology journals via citation burst analysis, including New England Journal of Medicine, Journal of the American Medical Association, Annals of Internal Medicine, Journal of Clinical Investigation, and Cell Metabolism (Figure 5B), indicating that interdisciplinary journals also publish breakthrough articles on uremic cardiomyopathy. This may be attributed to the interdisciplinary property of uremic cardiomyopathy, which attracts the attention of both cardiologists and nephrologists. Hence, it suggests that interdisciplinary collaboration is required for further investigations as well.
Uremic cardiomyopathy is a concept with a long history, and it can be dated back to the age of Richard Bright, who firstly reported CKD and observed pericarditis and cardiac hypertrophy in patients with CKD (40). Although the feature of uremic cardiomyopathy is observed earlier, the impact of this change on patients with CKD is unclear until the 1990s. In 1995, Foley et al. reported that LVH can affect mortality in patients with end-stage renal disease (2, 3), and this leads to a rapidly increasing trend of publications in the uremic cardiomyopathy field. Subsequently, anemia is found to be related to uremic cardiomyopathy (25), and the protective effect of erythropoietin makes it the earliest therapeutic strategy for uremic cardiomyopathy (9) (Figure 5A). However, erythropoietin treatment is recognized to cause hypertension in CKD (41), thus, it is not an ideal drug for uremic cardiomyopathy. Consistent with this, the analysis from Lv et al. (34) demonstrated that anemia is an early topic in cardiorenal syndrome research further implies anemia is an early recognized factor affecting both the kidney and the heart. Vitamin D supplementation moves into center stage of uremic cardiomyopathy due to its deficiency is linked to cardiovascular events (Figure 5A) (42). Unfortunately, Thadhani et al. demonstrated that 48-week vitamin D therapy is unable to mitigate LVH and improve diastolic dysfunction in patients with CKD (43); thus, the attraction of vitamin D drops rapidly around 2013 (Figure 5A).
Importantly, Gutiérrez et al. (27) reported that FGF-23 is independently associated with LVH in patients with CKD, and Faul et al. (12) further found that FGF-23 activates calcineurin signaling to induce LVH (Figure 5A). These results bring another therapeutic strategy for uremic cardiomyopathy; however, neutralizing FGF-23 improves hyperparathyroidism yet increases mortality, and the latter is caused by more severe hyperphosphatemia and vascular calcification (14). This temporarily delays the progress in FGF-23 and uremic cardiomyopathy. Fortunately, Grabner et al. (24) found that FGF-23 induces LVH via FGFR-4 instead of FGFR-1, which is critical for the phosphate metabolic effects of FGF-23, hence indicating that FGFR-4 inhibitor can be used to treat uremic cardiomyopathy. Strangely, there are no clinical trials based on FGFR-4 inhibitors have been conducted now. Most recent progress on uremic cardiomyopathy is mainly based on studies performed by Hu et al. (29) and Leifheit-Nestler et al. (30), who found that soluble klotho can alleviate LVH in CKD. Further investigations found the protective effects of soluble klotho are FGF-23-dependent and FGF-23-independent (28), thus, implying another therapeutic strategy for uremic cardiomyopathy (Figures 5A, 7).
Notably, the timeline view of references also showed that investigations related to uremic cardiomyopathy concentrate on both basic and clinical studies (Figure 5A), and this is confirmed by burst detection, which showed that most influential publications are presented in both basic and clinical journals (Figure 5B). Indeed, the investigations on uremic cardiomyopathy exhibit a good connection between basic and clinical studies, as indicated by a relatively rapid translation of basic to the clinic, and this brings a favorable developmental pattern to the uremic cardiomyopathy field.
Phosphate metabolism disorder exerts a central role in uremic cardiomyopathy, and it is now considered as a core to treat uremic cardiomyopathy (44). Relevant to this, studies on FGF-23 and its coreceptor klotho, which are closely associated with phosphate metabolism disorder, accounted for 40% (4/10) of publications with top 10 TLCS (Table 5). Lowering phosphate levels through dietary phosphate restriction, dialysis, and phosphate binders may mitigate uremic cardiomyopathy. Although phosphate binders are the only pharmacological treatment for hyperphosphatemia until now, Chue et al. (45) demonstrated that 40-week treatment wih phosphate binder, sevelamer, is unable to alleviate arterial stiffness, left ventricular mass, or any parameters of left ventricular systolic and diastolic function in patients with stage 3 CKD. In addition, hyperphosphatemia-induced FGF-23 elevation and klotho deficiency can also be therapeutic targets for uremic cardiomyopathy. Nevertheless, the complicated relationship between FGF-23/klotho and uremic cardiomyopathy hampers related progress (28). Soluble klotho supplementation and FGFR-4 blockade may act as therapeutic strategies as well (24, 29, 30). Hence, FGF-23 and klotho still receive wide attention in recent years (Figures 5A, 6A,C), and phosphate metabolism disorder will remain the hotspot of uremic cardiomyopathy in the coming time. Consistently, FGF-23 and klotho are the key nodes in Lv et al. (34) keyword occurrence analysis, and they also thought these should be important in future cardiorenal syndrome research.
Although phosphate metabolism disorder possesses the center stage of uremic cardiomyopathy, some potential aspects may progress this field as well. CKD is often accompanied by sodium retention, which causes a class of endogenous natriuretic factors, named endogenous cardiotonic steroids (eCTS), to promote renal sodium excretion (46). The eCTS can bound to Na+-K+-ATPase but does not affect its ion transport capacity (47), whereas, eCTS activates Src kinase, which is interacted with Na+-K+-ATPase, to activate prohypertrophic signaling activation and ROS generation, thus, promoting cardiac hypertrophy and fibrosis (48, 49). Based on these, targeting Na+-K+-ATPase or even eCTS to alleviate uremic cardiomyopathy may be effective.
Identifying novel biomarkers or risk factors of uremic cardiomyopathy is urgent in clinical practice. Serum troponin T and nocturnal hypertension may be novel indicators associated with heart failure in patients. Recently, Janus et al. (50) reported that a panel of brain natriuretic peptides, FGF-23, fibrinogen, and high-sensitive troponin T can be used to predict heart failure in patients with CKD. In addition, Fu et al. (51) found that nocturnal hypertension is associated with LVH in patients with CKD, indicating that ambulatory blood pressure monitoring may be used as an important indicator to evaluate the risk of uremic cardiomyopathy. Further studies are required to confirm these.
Our research has several limitations. Uremic cardiomyopathy is a clinical diagnosis with ambiguous diagnostic criteria. Although we enriched the search strategy as much as possible, such as adding “cardiac hypertrophy” and “left ventricular hypertrophy” as search terms. Still, fewer studies about uremic cardiomyopathy were not included in the analysis, such as case reports, which may give different results but are less included in SCIE. Moreover, most results were based on machine algorithms, which may have slight deviations. Finally, future analysis was based on the co-occurrence of keywords, whereas some potential aspects may temporarily not co-occur with uremic cardiomyopathy.
The original contributions presented in the study are included in this article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for this study in accordance with the local legislation and institutional requirements.
J-FB and AL designed the investigation. J-FB, Q-YS, and DZ collected the data. J-FB, P-PH, and J-JM analyzed the data. J-FB drafted the manuscript. AL revised the manuscript. All authors contributed to the article and approved the final version of the manuscript.
This work was supported by grants from the National Natural Science Foundation of China (81770727), Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme Grant (2017), the Natural Science Foundation of Guangdong Grant (2021A1515011376), and Key Project of Guangzhou Science Technology and Innovation Commission (20180402005).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.908040/full#supplementary-material
1. Go AS, Chertow GM, Fan D, Mcculloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. (2004) 351:1296–305. doi: 10.1056/NEJMoa041031
2. Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barré PE. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol. (1995) 5:2024–31. doi: 10.1681/ASN.V5122024
3. Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. (1995) 47:186–92. doi: 10.1038/ki.1995.22
4. Stewart GA, Gansevoort RT, Mark PB, Rooney E, Mcdonagh TA, Dargie HJ, et al. Electrocardiographic abnormalities and uremic cardiomyopathy. Kidney Int. (2005) 67:217–26. doi: 10.1111/j.1523-1755.2005.00072.x
5. Park M, Hsu CY, Li Y, Mishra RK, Martin KM, Rosas SE, et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol. (2012) 23:1725–34. doi: 10.1681/ASN.2012020145
6. Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. (2018) 15:387–407. doi: 10.1038/s41569-018-0007-y
7. Wang X, Shapiro J. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat Rev Nephrol. (2019) 15:159–75. doi: 10.1038/s41581-018-0101-8
8. Parfrey PS, Harnett JD, Foley RN, Kent GM, Murray DC, Barre PE, et al. Impact of renal transplantation on uremic cardiomyopathy. Transplantation. (1995) 60:908–14. doi: 10.1097/00007890-199511150-00005
9. Portolés J, Torralbo A, Martin P, Rodrigo J, Herrero JA, Barrientos A. Cardiovascular effects of recombinant human erythropoietin in predialysis patients. Am J Kidney Dis. (1997) 29:541–8. doi: 10.1016/S0272-6386(97)90335-8
10. Fagugli RM, Reboldi G, Quintaliani G, Pasini P, Ciao G, Cicconi B, et al. Short daily hemodialysis: blood pressure control and left ventricular mass reduction in hypertensive hemodialysis patients. Am J Kidney Dis. (2001) 38:371–6. doi: 10.1053/ajkd.2001.26103
11. Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. (2009) 54:505–12. doi: 10.1016/j.jacc.2009.03.066
12. Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. (2011) 121:4393–408. doi: 10.1172/JCI46122
13. Sanchis-Gomar F, Garcia-Gimenez JL, Pareja-Galeano H, Romagnoli M, Perez-Quilis C, Lippi G. Erythropoietin and the heart: physiological effects and the therapeutic perspective. Int J Cardiol. (2014) 171:116–25. doi: 10.1016/j.ijcard.2013.12.011
14. Shalhoub V, Shatzen EM, Ward SC, Davis J, Stevens J, Bi V, et al. FGF23 neutralization improves chronic kidney disease–associated hyperparathyroidism yet increases mortality. J Clin Invest. (2012) 122:2543–53. doi: 10.1172/JCI61405
17. Chen C, Song M. Visualizing a field of research: a methodology of systematic scientometric reviews. PLoS One. (2019) 14:e0223994. doi: 10.1371/journal.pone.0223994
18. Liu T, Yang L, Mao H, Ma F, Wang Y, Zhan Y. Knowledge domain and emerging trends in podocyte injury research from 1994 to 2021: a bibliometric and visualized analysis. Front Pharmacol. (2021) 12:772386. doi: 10.3389/fphar.2021.772386
19. Zarate YA, Hopkin RJ. Fabry’s disease. Lancet. (2008) 372:1427–35. doi: 10.1016/S0140-6736(08)61589-5
20. Garfield E, Paris SW, Stock WG. HistCite: a software tool for informetric analysis of citation linkage. NFD Inform Wiss Prax. (2006) 57:391–400.
21. Van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
22. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. (2004) 101(Suppl. 1):5303–10. doi: 10.1073/pnas.0307513100
23. Aria M, Cuccurullo C. Bibliometrix: an R-Tool for comprehensive science mapping analysis. J Informetr. (2017) 11:959–75. doi: 10.1016/j.joi.2017.08.007
24. Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, et al. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. (2015) 22:1020–32. doi: 10.1016/j.cmet.2015.09.002
25. Silverberg DS, Wexler D, Blum M, Tchebiner J, Sheps D, Keren G, et al. The correction of anemia in severe resistant heart failure with erythropoietin and intravenous iron prevents the progression of both the heart and the renal failure and markedly reduces hospitalization. Clin Nephrol. (2002) 58(Suppl. 1):S37–45.
26. De Albuquerque Suassuna PG, Sanders-Pinheiro H, De Paula RB. Uremic cardiomyopathy: a new piece in the chronic kidney disease-mineral and bone disorder puzzle. Front Med (Lausanne). (2018) 5:206. doi: 10.3389/fmed.2018.00206
27. Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. (2009) 119:2545–52. doi: 10.1161/CIRCULATIONAHA.108.844506
28. Bao JF, Hu PP, She QY, Li A. A land of controversy: fibroblast growth factor-23 and uremic cardiac hypertrophy. J Am Soc Nephrol. (2020) 31:1423–34. doi: 10.1681/ASN.2020010081
29. Hu MC, Shi M, Cho HJ, Adams-Huet B, Paek J, Hill K, et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol. (2015) 26:1290–302. doi: 10.1681/ASN.2014050465
30. Leifheit-Nestler M, Große Siemer R, Flasbart K, Richter B, Kirchhoff F, Ziegler WH, et al. Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol Dial Transplant. (2016) 31:1088–99. doi: 10.1093/ndt/gfv421
31. David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. (2016) 89:135–46. doi: 10.1038/ki.2015.290
32. Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G. Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis. (2005) 46:320–7. doi: 10.1053/j.ajkd.2005.04.031
33. Vickery S, Price CP, John RI, Abbas NA, Webb MC, Kempson ME, et al. B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis. (2005) 46:610–20. doi: 10.1053/j.ajkd.2005.06.017
34. Lv J, Li Y, Shi S, Liu S, Xu X, Wu H, et al. Frontier and hotspot evolution in cardiorenal syndrome: a bibliometric analysis from 2003 to 2022. Curr Probl Cardiol. (2022):101238. [Online ahead of print], doi: 10.1016/j.cpcardiol.2022.101238
35. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2020) 395:709–33. doi: 10.1016/S0140-6736(20)30045-3
37. Yang K, Wang C, Nie L, Zhao X, Gu J, Guan X, et al. Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J Am Soc Nephrol. (2015) 26:2434–46. doi: 10.1681/ASN.2014060543
38. Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. (2008) 359:584–92. doi: 10.1056/NEJMoa0706130
39. Liu J, Nie Y, Chaudhry M, Bai F, Chuang J, Sodhi K, et al. The redox-sensitive Na/K-ATPase signaling in uremic cardiomyopathy. Int J Mol Sci. (2020) 21:1256. doi: 10.3390/ijms21041256
40. Bright R. Cases and observations, illustrative of renal disease accompanied with the secretion of albuminous urine. Memoir the second. Guys Hosp Rep. (1840) 5:101–61, pp. 110-3.
41. Poux JM, Lartigue M, Chaisemartin RA, Galen FX, Leroux-Robert C. Uraemia is necessary for erythropoietin-induced hypertension in rats. Clin Exp Pharmacol Physiol. (1995) 22:769–71. doi: 10.1111/j.1440-1681.1995.tb01933.x
42. Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. (2008) 117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127
43. Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. J Am Med Assoc. (2012) 307:674–84. doi: 10.1001/jama.2012.120
44. Lunyera J, Scialla JJ. Update on chronic kidney disease mineral and bone disorder in cardiovascular disease. Semin Nephrol. (2018) 38:542–58. doi: 10.1016/j.semnephrol.2018.08.001
45. Chue CD, Townend JN, Moody WE, Zehnder D, Wall NA, Lorraine H, et al. Cardiovascular effects of sevelamer in stage 3 CKD. J Am Soc Nephrol. (2013) 24:842–52. doi: 10.1681/ASN.2012070719
46. Hamlyn JM. Natriuretic hormones, endogenous ouabain, and related sodium transport inhibitors. Front Endocrinol (Lausanne). (2014) 5:199. doi: 10.3389/fendo.2014.00199
47. Arnon A, Hamlyn JM, Blaustein MP. Ouabain augments Ca(2+) transients in arterial smooth muscle without raising cytosolic Na(+). Am J Physiol Heart Circ Physiol. (2000) 279:H679–91. doi: 10.1152/ajpheart.2000.279.2.H679
48. Elkareh J, Kennedy DJ, Yashaswi B, Vetteth S, Shidyak A, Kim EG, et al. Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension. (2007) 49:215–24. doi: 10.1161/01.HYP.0000252409.36927.05
49. Haller ST, Kennedy DJ, Shidyak A, Budny GV, Malhotra D, Fedorova OV, et al. Monoclonal antibody against marinobufagenin reverses cardiac fibrosis in rats with chronic renal failure. Am J Hypertens. (2012) 25:690–6. doi: 10.1038/ajh.2012.17
50. Janus SE, Hajjari J, Chami T, Mously H, Badhwar AK, Karnib M, et al. Multi-variable biomarker approach in identifying incident heart failure in chronic kidney disease: results from the Chronic Renal Insufficiency Cohort study. Eur J Heart Fail. (2022): [Online ahead of print], doi: 10.1002/ejhf.2543
51. Fu X, Ren H, Xie J, Wang W, Li Y, Gao P, et al. Association of nighttime masked uncontrolled hypertension with left ventricular hypertrophy and kidney function among patients with chronic kidney disease not receiving dialysis. JAMA Netw Open. (2022) 5:e2214460. doi: 10.1001/jamanetworkopen.2022.14460
52. Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. (1998) 32:S112–9. doi: 10.1053/ajkd.1998.v32.pm9820470
53. Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE. Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant. (1996) 11:1277–85. doi: 10.1093/oxfordjournals.ndt.a027540
Keywords: bibliometric analysis, uremic cardiomyopathy, CiteSpace, VOSviewer, HistCite, bibliometrix
Citation: Bao J-F, Hu P-P, She Q-Y, Zhang D, Mo J-J and Li A (2022) A Bibliometric and Visualized Analysis of Uremic Cardiomyopathy From 1990 to 2021. Front. Cardiovasc. Med. 9:908040. doi: 10.3389/fcvm.2022.908040
Received: 31 March 2022; Accepted: 16 June 2022;
Published: 12 July 2022.
Edited by:
Malcolm Koo, Tzu Chi University of Science and Technology, TaiwanCopyright © 2022 Bao, Hu, She, Zhang, Mo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aiqing Li, bGlhaXFpbmdAc211LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.