
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 18 July 2022
Sec. Heart Failure and Transplantation
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.907664
This article is part of the Research Topic Palliative Care for People Living with Heart and Lung Disease View all 15 articles
Chronic obstructive pulmonary disease (COPD) is a disabling condition associated with progressive airflow limitation and lung tissue damage; its main symptoms are breathlessness, fatigue, cough, and sputum production. In the advanced stage of the disease, these symptoms may severely impact on a person's physical and psychological functioning, with some also developing chronic respiratory failure, associated with blood gas abnormalities. Non-pharmacological interventions can improve quality of life and functioning in the management of people living with advanced COPD. This article will provide an overview of common non-pharmacological methods used in the symptomatic management of severe COPD, including: breathlessness and fatigue management strategies, anxiety management, pulmonary rehabilitation (PR) and physical activity (PA), neuromuscular electrical stimulation (NMES), airway clearance techniques (ACTs), nutrition and non-invasive ventilation (NIV). The importance of a holistic and multi-disciplinary approach to people living with COPD will be discussed.
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of chronic morbidity and mortality worldwide (1). COPD leads to mucous hypersecretion (chronic bronchitis), tissue destruction (emphysema) and small airway chronic inflammation and fibrosis (bronchiolitis) as well as systemic inflammation (2, 3). The progressive nature of the disease leads to severe poorly reversible airflow obstruction despite optimal bronchodilation therapy. This results in increased airway resistance and compliance and in consequence to air trapping, hyperinflation and flattening of the diaphragm (2, 3). The changes in mechanics of breathing in COPD lead to increased effort of breathing and energy expenditure at rest (4, 5). Further consequence of advanced COPD may be gas exchange abnormalities causing chronic hypoxaemia or nocturnal hypercapnia. The impact of systemic inflammation on other systems in the body are becoming more evident in advance stages of COPD. These consequences may include cachexia, skeletal muscle atrophy, osteoporosis, increased risk of cardiovascular disease or neuropsychiatric disorders (3). With the progression of the disease, the more frequent and more severe acute exacerbations lead to an increased risk of hospitalisations and deterioration of the function (6).
Consequently, COPD causes persistent and progressive respiratory and non-respiratory disabling symptoms, such as breathlessness, fatigue, cough, and/or sputum production (7, 8). It is also common for people with COPD to experience anxiety and depression (9). These symptoms negatively affect individuals with COPD including their health-related quality of life, activities of daily living, physical activity, and sleep (10). It is crucial to evaluate not only the intensity of these symptoms but also their impact on daily functioning and participation in family and social life. Importantly, patients with COPD had a 2-fold increased risk of frailty (11–13), which can affect prognosis and management in advanced COPD. Therefore, people suffering from severe COPD require a holistic and multi-disciplinary approach that involves a variety of healthcare professionals, including physicians, nurses, physiotherapists, occupational therapists, psychologists, and social workers (14). Palliative care is a multidisciplinary approach which focuses on patient's symptom management and improvement in quality of life, therefore it has to be incorporated earlier into the management of COPD (15).
This article provides an overview of methods used in palliative care for the non-pharmacological management of symptomatic patients with severe COPD, including the management of breathlessness, fatigue and anxiety, pulmonary rehabilitation (PR), neuromuscular electrical stimulation (NMES), management of sputum clearance, nutrition and chronic respiratory failure (e.g., non-invasive ventilation).
Breathlessness is the most common symptom in severe COPD (16), its prevalence is greater in the end stage of COPD (17–19). There may be several factors that contribute to the sensation of breathlessness (20). Management of this symptom should be multifactorial, based on an assessment of the patient to identify any elements contributing to the subjective sensation of breathlessness. The ‘Breathing, Thinking, Functioning’ model used by the Cambridge Breathlessness Intervention Service (CBIS), has been developed by Spathis and colleagues (21, 22). The model presents three features of the vicious cycle of breathlessness: (1) inefficient breathing, (2) thinking (including anxiety and distress), and (3) reduced function leading to muscle deconditioning (22, 23). With this model, it is possible to create categories of interventions to reduce breathlessness (see Figure 1) (21). Additionally, this model emphasized the need to implement multifactorial strategies to manage breathlessness.
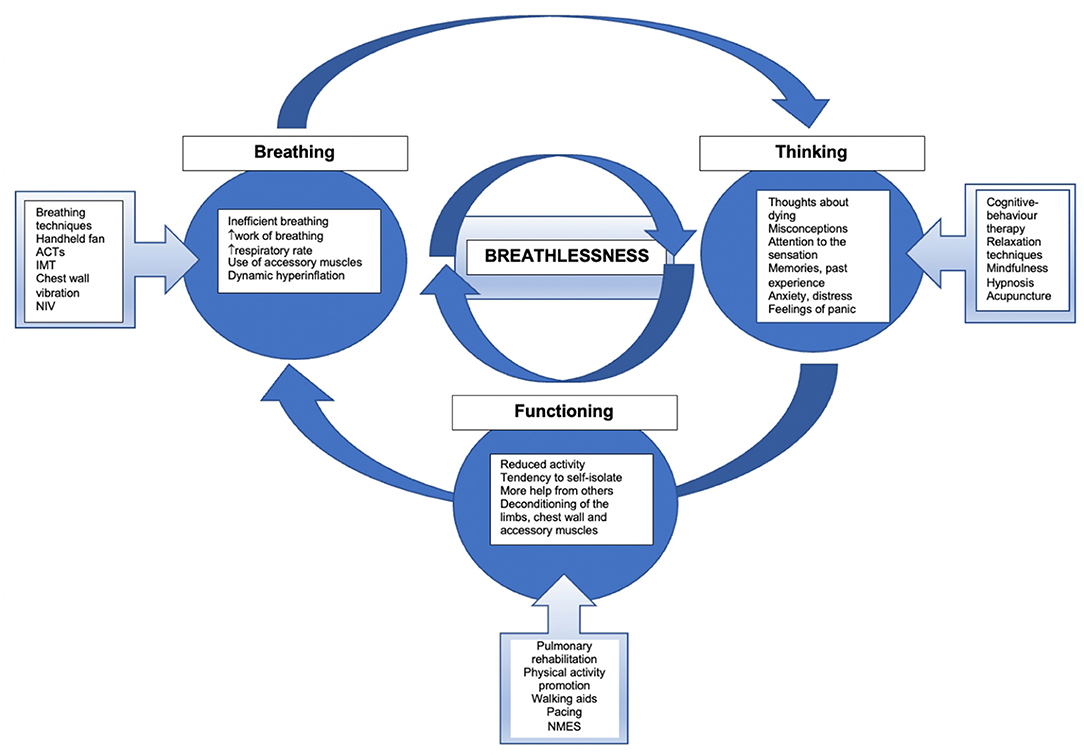
Figure 1. The model of breathlessness and management approaches. Adopted from Spathis et al. (21). ACTs, airway clearance techniques; IMT, inspiratory muscle training; NIV, non-invasive ventilation; NMES, neuromuscular electrical stimulation.
Breathlessness progresses with time, may intensify with advancement of the disease, and often negatively impacts on function (24). Individuals with advanced COPD will experience breathlessness on a regular basis. Their breathlessness may be triggered by exertion, for example during activities of daily living or a change in emotional state. Strategies to manage an acute onset of breathlessness may include positioning, breathing techniques, panic management, and desensitization (20).
The “leaning forward” position is frequently used in clinical practice for the management of breathlessness triggered by activities of daily living or during rehabilitation. The theory behind this technique proposes that fixing the shoulder girdle, reduces activity of both the scalenes and sternomastoid muscles whilst increasing both transdiaphragmatic pressure (via diaphragmatic recruitment) and thoraco-abdominal movements (25–29). Using the forward lean to improve efficiency of respiratory muscles and decrease work of breathing is thought to lead to quicker recovery from breathlessness.
Although evidence supporting the effectiveness of breathing techniques varies, depending on the specific technique in question (30), their use is recommended to help breathless people gain better control of their breathing (31). Purse-lip breathing (PLB) technique has one of the strongest evidence-bases to support its use. The technique requires to inhale slowly through the nose and exhale through the mouth with the puckered lips, which alters respiratory mechanics (32). The increased resistance from half-opened lips on expiration physiologically generates an extrinsic positive end expiratory pressure (extrinsic PEEP), which decreases airway collapse by reducing the Bernoulli effect (33). This leads to decreased “air trapping” in patients with emphysema resulting in a reduction of hyperinflation.
Applying PLB lowers oxygen consumption, respiratory rate (RR) and reduces breathlessness in people with COPD (34). It shows to improve inspiratory capacity (IC) at rest (35) and reduced level dynamic hyperinflation during activity (36). PLB used on exercise shows to improve exercise tolerance (33, 37), reduce respiratory rate (RR) and improves recovery time in people with COPD (38).
Other breathing techniques, such as Breathing Control (BC), Blow as you go (BAYG) or Paced breathing, have less evidence to support their effectiveness, but some patients find them helpful in managing their breathlessness either on exercise or during recovery (39).
However, not all breathing techniques are beneficial for the management of breathlessness in severe COPD; diaphragmatic breathing may increase dyspnoea (40) by increasing chest wall asynchronicity (41, 42), leading to increase work of breathing. Another technique, slow deep breathing may predispose the diaphragm to fatigue (43). Therefore, clinical guidelines do not recommend use of either of these techniques for patients with advanced COPD (31).
Walking aids may be used to help with management of breathlessness during ambulation and enable patients with severe COPD to stay active and independent. The rollator frames on exertion may reduce work of breathing by maintaining the lean forward position during activity (44). Evidence from a research study by Probst at al. (45) shows rollator frames can significantly increase walking distance, whilst reducing exertional dyspnoea in patients with COPD. The effect on respiratory function was demonstrated with improvements in oxygen uptake, tidal volume and minute ventilation. Similarly, the use of gutter frames with elderly COPD patients have been shown to increase walking distance and reduce oxygen desaturation during ambulation (46).
The benefits of utilizing cool, flowing air on the facial skin for patients with COPD is has long been known, with many patients reporting having benefited (47). The mechanism of action is explained in part through stimulation of facial temperature receptors (48) and modulation of central perception of breathlessness (49). Although a systematic literature review from 2008 showed insufficient data to support the evidence for fans' effectiveness (50), the authors emphasized that more research is necessary on selecting and identifying those who might benefit from using handheld fans (51, 52). Subsequently a number of studies showed that a cool draft of air from a hand-held fan directed to the face can be helpful in reducing the sensation of breathlessness in patients with advanced COPD (53–55). Moreover, there is data that suggests that using a handheld fan increases physical activity (56). Some authors indicate that future research should explore the relationship between handheld fan characteristics and relief of breathlessness (57). The authors researching handheld fans for breathlessness emphasize their acceptability to patients, relative inexpense, portability and ability to give patients more control; and recommended their use as part of palliative management to support patients' self-management and independence (53, 54, 58, 59).
There are also some strategies to improve chronic breathlessness in the longer term. This includes exercise training or more comprehensive programmes such as pulmonary rehabilitation (PR) (60). Furthermore, in patients with COPD with dysfunctional breathing patterns, breathing retraining programmes may be considered. However, a systematic review by Holland et al. (30) demonstrated inconsistent evidence about improvement in breathlessness.
There is also evidence that Inspiratory Muscle Training (IMT) in moderate-to-severe COPD improves dyspnoea and quality of life (61, 62). A recent systematic review (63) presented results from 23 studies, which all indicated that IMT training decreased dyspnoea. However, there were some indications that improvement was limited to patients with pre-existing respiratory muscle weakness.
The sensation of fatigue may be defined in various ways, including as tiredness (64), a lack of energy (65), exhaustion or weakness (2). The mechanism of subjective fatigue in COPD is complex and multidimensional (66, 67).
Exercise training alone or as a part of PR has been found beneficial in managing fatigue. A recent literature review demonstrated that any type of exercise could reduce fatigue (68). It has been also established that pulmonary rehabilitation reduces fatigue (60, 65), in particularly general, physical and reduce motivation (69).
One study investigated the effect of an 8-week progressive muscle relaxation programme on fatigue (70). It showed reduced fatigue and an improvement in sleep quality following the programme. There are some indications that sleep quality influences fatigue (71). Other fatigue management strategies reported by a qualitative study included pacing, protection, energy conservation, keeping active, resting or planning daily living and prioritizing (71, 72).
Energy conservation involves modifying an activity or the environment to decrease the level of energy required to complete a task. Pacing and energy conservation are also used for management of fatigue (73). A recent randomized controlled trial of a 2-week training programme involving energy conservation techniques (ECT) for COPD patients (74), demonstrated that after the programme there was lower level of desaturation and decrease in the metabolic equivalent of task (MET) while performing activities of daily living. Another observational study showed that ECT decreased heart rate, oxygen uptake, minute ventilation and dyspnoea. Although, ECT are recommended for management of fatigue in clinical practice, there is no evidence to demonstrate decrease of fatigue with this intervention.
Feelings of anxiety are common in patients with advanced COPD (9). More intense breathlessness is associated with greater levels of anxiety (20). These symptoms negatively affect patients' physical functioning and increase their social isolation (75–78). Currently, we observe a growing number of studies addressing the issue of the use of non-pharmacological methods in the treatment of COPD patients affected by anxiety. The evaluated interventions included: relaxation (79, 80), hypnosis (81), cognitive behavioral therapy (82), and mindfulness (83). One study investigated also breathing techniques and found out them beneficial in managing anxiety (84). A recent systematic review, demonstrated significant, clinically relevant improvement in anxiety and depression following PR programme (85). Further research is needed to determine which interventions are the most effective and could be an efficacious add-on to standard PR programs or stand-alone treatment.
Pulmonary Rehabilitation (PR) is an important part of integrated care for patients with COPD. PR is a comprehensive programme which includes a variety of non-pharmacological interventions, exercise training and education (86). It has proved to be highly effective in management symptoms, improving QoL, physical and psychological functioning of patients with COPD (60, 85). In advance disease, people with COPD may experience frequent exacerbations and are at greater risk of frailty. Patients who complete PR after acute exacerbation would also have a lower risk of hospital readmission and mortality (87). Furthermore, COPD patients with frailty and risk of frailty showed benefit from PR, but they are more likely not to complete the programme (88).
Despite undisputable benefits from PR, in advanced stage of the disease, there may be number of barriers which could make attending programme difficult. The recent clinical report indicated that patients may struggle to complete post-exacerbation PR due to transport issues, advance disease, co-morbidities such as anxiety, poor motivation, and high fatigue (89). These patients are often fragile and may not always have the sufficient reserve to initiate the programme. It may be difficult for these patients to spend time outside home in late stage of disease, which may require effort and may create additional stress.
Nevertheless, the interventions aiming to reducing sedentary lifestyle and increase physical activity (PA) should still be considered. The evidence for different exercise-based PA-enhancing interventions is inconsistent. Behavioral change using tele-coaching or coaching, pedometers, applications, walking and home exercise programmes has been suggested to boost PA in patients with COPD (90, 91).
Impaired muscle function and decreased cross-sectional muscle mass are common features in people with severe COPD, which affect the respiratory and the skeletal muscles, especially of the lower limbs (92, 93). Patients with advanced COPD who are severely affected by muscle weakness, including those who are housebound, may benefit from Neuromuscular Electrical Stimulation (NMES) (94). NMES usually is applied to the quadriceps muscle and improves impaired muscle function and structure by increasing cross-sectional muscle mass, muscle force, endurance, and exercise tolerance as well as reducing dyspnoea (94–96). Moreover, some studies report that NMES promotes a reduction of the perceived sensation of dyspnea during exercise in patients with COPD (97). For people admitted to an intensive care or high dependency unit with an acute exacerbation of COPD, research suggests NMES combined with conventional exercise may reduce the time taken for patients to first sit out of bed (98).
The effectiveness of NMES in adults with advanced COPD and other diseases was analyzed in two Cochrane Systematic Reviews (50, 98). The authors conclude that there is a high strength of evidence that NMES may be an effective treatment for muscle weakness in adults with advanced progressive disease.
For some COPD patients, cough and sputum may be a burden, especially during exacerbations. When the patient experiences difficulties with sputum expectoration, advice and support may be required. There are several airway clearance techniques (ACTs) recognized as effective methods (99, 100). Application of ACTs decreases breathlessness, lower need for ventilatory assistance and Positive Expiratory Pressure (PEP) devices improve sputum volume expectoration and decrease hospital length of stay for COPD patients admitted due to acute exacerbation (101, 102). In a recent review, significant improvements in the rate of exacerbation frequency at 6 months of ACTs use was demonstrated (100). Therefore, it would be important to review if sputum is cleared effectively and identify potential need for management with appropriate ACTs in COPD patients.
Many people in advanced stages of COPD are underweight and may demonstrate sign of cachexia. Evidence suggests that 25–40% of all COPD patients have low body weight, 25% of patients have moderate to severe weight loss, and 35% of patients with extremely low fat-free mass (FFM) index (103). This has a negative effect on muscle mass and function and impacts exercise tolerance. Therefore, the European Respiratory Society (ERS) recommends that nutritional interventions should be considered as a single treatment or integrated with exercise training (104). Especially, patients with negative energy balance, may benefit from energy- and protein-enriched diet and the evidence suggests that nutritional supplementation promotes weight gain among patients with COPD (105). Furthermore, because exercise increases energy expenditure, it is suggested to assess the nutritional status of COPD patients before starting Pulmonary Rehabilitation (86). In patients with weight abnormalities, dietary counseling and food fortification or nutritional supplementation should be considered. Some authors suggest that smaller volumes of food may be more appropriate to optimize energy intake (106). Education and advice on nutrition are indicated as methods that bring a short-term effect on improving intake (106).
Due to the small airway disease and lung hyperinflation, the diaphragm muscle is flattened, which may lead to its atrophy and greater fatiguability in severe COPD (107). Sleep hypoventilation is also observed in some people in advanced COPD (108). These factors may lead to a development of the type 2 respiratory failure. Therefore, these patients may benefit from nocturnal non-invasive ventilation (NIV) to support their respiratory muscle. The American Thoracic Society (ATS) Clinical Practice Guidelines recommend the use of nocturnal NIV in addition to usual care for patients with chronic stable hypercapnic COPD (109). A systematic review on the use of NIV in severe stable COPD concluded that bilevel non-invasive positive pressure ventilation may have an adjunctive role in the management of chronic respiratory failure through attenuation of compromised respiratory function and improvement in health-related outcomes (110). There is also evidence that long-term NIV added to home oxygen therapy reduces risk of readmission and death (111). Figure 2 highlights the key benefits from NIV. Furthermore, it is important to consider the application of appropriate therapeutic pressures, which is the key factor guaranteeing clinical effectiveness for carbon dioxide level reduction (112). However, McEvoy et al. (113) emphasizes that whilst nocturnal NIV in stable oxygen-dependent patients with hypercapnic COPD may improve survival, this appears to be at the cost of worsening quality of life. Hence, it is important to take into consideration individual patient preferences and agree on the treatment plan collaboratively.
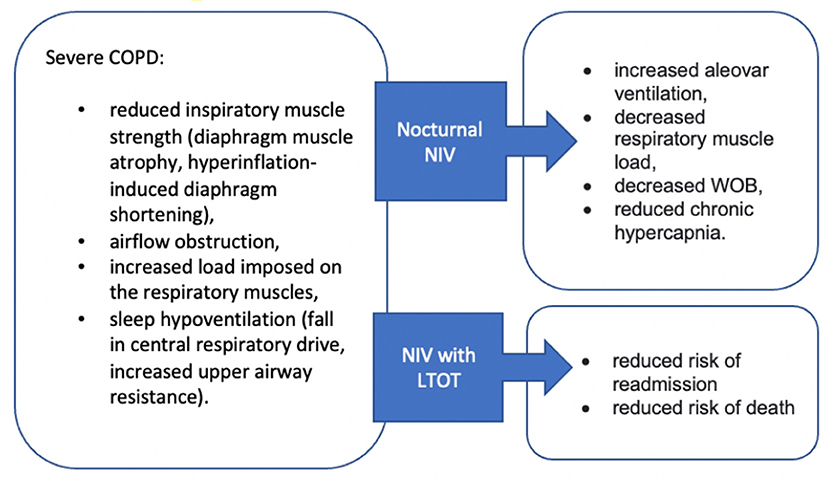
Figure 2. Effectiveness of Non-invasive ventilation in patients with advanced COPD. NIV, non-invasive ventilation; WOB, work of breathing.
This article discussed several non-pharmacological interventions used to manage symptoms and clinical problems arising in the palliative care for patients with advanced COPD. The summary of these various interventions, their evidence and clinical practice recommendations are presented in Table 1.
For patients in the advance stage of COPD, whilst a ceiling effect in pharmacological treatment is often reached, there is a range of management strategies which could be used to improve their quality of life, as it was presented it this article. There are several interventions suggested for relief of symptoms in clinical practice, but not all the methods have a strong evidence-base to support their effectiveness. However, palliative care does not always fit the Evidence-Based Medicine framework (122). Whereas breathlessness received the greatest attention and there is a wide body of evidence to support management of this symptom. Hence, there are specially designed services to address this problem. Other symptoms, such as fatigue, may be acknowledged, but there are not always specifically treated or may lack the complex management approach. This is potentially the reason why, the palliative care for COPD patients is often fragmented and interdisciplinary approach not always well-coordinated. The palliative care for patients with COPD should be a key part of the long-term management plan and a gold standard of care in advanced COPD. Therefore, there is a need for more research into management of symptoms other than breathlessness and development of more complex management programmes for palliative management in COPD.
Both authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Quaderi SA, Hurst JR. The unmet global burden of COPD. Glob Health Epidemiol Genom. (2018) 6:3. doi: 10.1017/gheg.2018.1
2. MacNee W. Pathology, pathogenesis, and pathophysiology. BMJ. (2006) 332:1202. doi: 10.1136/bmj.332.7551.1202
3. Bourdin A, Burgel PR, Chanez P, Garcia G, Perez T, Roche N. Recent advances in COPD: pathophysiology, respiratory physiology and clinical aspects, including comorbidities. Eur Respir Rev. (2009) 18:198–212. doi: 10.1183/09059180.00005509
4. Schols AM, Fredrix EW, Soeters PB, Westerterp KR, Wouters EF. Resting energy expenditure in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. (1991) 54:983–7. doi: 10.1093/ajcn/54.6.983
5. Loring SH, Garcia-Jacques M, Malhotra A. Pulmonary characteristics in COPD and mechanisms of increased work of breathing. J Appl Physiol. (2009) 107:309–14. doi: 10.1152/japplphysiol.00008.2009
6. Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. (2010) 19:113–8. doi: 10.1183/09059180.00002610
7. Kinsman RA, Yaroush RA, Fernandez E, Dirks JF, Schocket M, Fukuhara J. Symptoms and experiences in chronic bronchitis and emphysema. Chest. (1983) 83:755–61. doi: 10.1378/chest.83.5.755
8. Walke LM, Byers AL, Tinetti ME, Dubin JA, McCorkle M, Fried TR. Range and severity of symptoms over time among older adults with chronic obstructive pulmonary disease and heart failure. Arch Intern Med. (2007) 167:2503–8. doi: 10.1001/archinte.167.22.2503
9. Hill K, Geist R, Goldstein RS, Lacasse Y. Anxiety and depression in end-stage COPD. Eur Respir J. (2008) 3:667–77. doi: 10.1183/09031936.00125707
10. Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Respir Res. (2017) 1:67. doi: 10.1186/s12931-017-0548-3
11. Marengoni A, Vetrano DL, Manes-Gravina E, Bernabei R, Onder G, Palmer K. The relationship between COPD and frailty: a systematic review and meta-analysis of observational studies. Chest. (2018) 154:21–40. doi: 10.1016/j.chest.2018.02.014
12. Gephine S, Mucci P, Grosbois JM, Maltais F, Saey D. Physical frailty in COPD patients with chronic respiratory failure. Int J Chron Obstruct Pulmon Dis. (2021) 16:1381–92. doi: 10.2147/COPD.S295885
13. Antoniu SA, Boiculese LV, Prunoiu V. Frailty, a dimension of impaired functional status in advanced COPD: utility and clinical applicability. Medicina. (2021) 57:474. doi: 10.3390/medicina57050474
14. Kuzma AM, Meli Y, Meldrum C, Jellen P, Butler-Lebair M, Koczen-Doyle D et al. Multidisciplinary care of the patient with chronic obstructive pulmonary disease. Proc Am Thorac Soc. (2008) 5:567–71. doi: 10.1513/pats.200708-125ET
15. Vermylen JH, Szmuilowicz E, Kalhan R. Palliative care in COPD: an unmet area for quality improvement. Int J Chron Obstruct Pulmon Dis. (2015) 10:1543–51. doi: 10.2147/COPD.S74641
16. Kessler R, Partridge MR, Miravitlles M, Cazzola M, Vogelmeier C, Leynaud D et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. (2011) 37:264–72. doi: 10.1183/09031936.00051110
17. Elkington H, White P, Addington-Hall J, Higgs R, Edmonds P. The healthcare needs of chronic obstructive pulmonary disease patients in the last year of life. Palliat Med. (2005) 19:485–91. doi: 10.1191/0269216305pm1056oa
18. Jones I, Kirby A, Ormiston P, Loomba Y, Chan KK, Rout J et al. The needs of patients dying of chronic obstructive pulmonary disease in the community. Fam Pract. (2004) 21:310–3. doi: 10.1093/fampra/cmh317
19. Rocker GM, Sinuff T, Horton R, Hernandez P. Advanced chronic obstructive pulmonary disease: innovative approaches to palliation. J Palliat Med. (2007) 10:783–97. doi: 10.1089/jpm.2007.9951
20. Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J et al. American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. (2012) 185:435–52. doi: 10.1164/rccm.201111-2042ST
21. Spathis A, Booth S, Moffat C, Hurst R, Ryan R, Chin C, et al. The Breathing, Thinking, Functioning clinical model: a proposal to facilitate evidence-based breathlessness management in chronic respiratory disease. NPJ Prim Care Respir Med. (2017) 27:27. doi: 10.1038/s41533-017-0024-z
22. Booth S. Cambridge Breathlessness Intervention Service (CBIS). Prog Palliat Care. (2013) 21:224–8. doi: 10.1179/1743291X13Y.0000000058
23. Chin C, Booth S. Managing breathlessness: a palliative care approach. Postgrad Med J. (2016) 92:393–400. doi: 10.1136/postgradmedj-2015-133578
24. Currow DC, Abernethy AP, Ko DN. The active identification and management of chronic refractory breathlessness is a human right. Thorax. (2014) 69:393–4. doi: 10.1136/thoraxjnl-2013-204701
25. O'Neill S, McCarthy DS. Postural relief of dyspnoea in severe chronic airflow limitation: relationship to respiratory muscle strength. Thorax. (1983) 38:595–600. doi: 10.1136/thx.38.8.595
26. Sharp JT, Druz WS, Moisan T, Foster J, Machnach W. Postural relief of dyspnea in severe chronic obstructive pulmonary disease. Am Rev Respir Dis. (1980) 122:201–11.
27. Delgado HR, Braun SR, Skatrud JB, Reddan WG, Pegelow DF. Chest wall and abdominal motion during exercise in patients with COPD. Am Rev Respir Dis. (1982) 126:200–05.
28. Barach AL. Chronic obstructive lung disease: postural relief of dyspnea. Arch Phys Med Rehabil. (1974) 55:494–504.
29. Kim KS, Byun MK, Lee WH, Cynn HS, Kwon OY Yi CH. Effects of breathing maneuver and sitting posture on muscle activity in inspiratory accessory muscles in patients with chronic obstructive pulmonary disease. Multidiscip Respir Med. (2012) 7:9. doi: 10.1186/2049-6958-7-9
30. Holland AE, Hill CJ, Jones AY, McDonald CF. Breathing exercises for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2012) 10:CD008250. doi: 10.1002/14651858.CD008250.pub2
31. Bott J, Blumenthal S, Buxton, Ellum S, Falconer C, Garrod R M, et al. Guidelines for the physiotherapy management of the adult, medical, spontaneously breathing patient. Thorax. (2009) 64:1–51. doi: 10.1136/thx.2008.110726
32. Spahija J, de Marchie M, Grassino A. Effects of imposed pursed-lips breathing on respiratory mechanics and dyspnea at rest and during exercise in COPD. Chest. (2005) 128:640–50. doi: 10.1378/chest.128.2.640
33. Bhatt SP, Luqman-Arafath TK, Gupta AK, Mohan A, Stoltzfus JC, Dey T et al. Volitional pursed lips breathing in patients with stable chronic obstructive pulmonary disease improves exercise capacity. Chron Respir Dis. (2013) 10:5–10. doi: 10.1177/1479972312464244
34. Jones AY, Dean E, Chow CC. Comparison of the oxygen cost of breathing exercises and spontaneous breathing in patients with stable chronic obstructive pulmonary disease. Phys Ther. (2003) 83:424–31. doi: 10.1093/ptj/83.5.424
35. Visser FJ, Ramlal S, Dekhuijzen PN, Heijdra YF. Pursed-lips breathing improves inspiratory capacity in chronic obstructive pulmonary disease. Respiration. (2011) 5:372–8. doi: 10.1159/000319036
36. de Araujo CL, Karloh M, Dos Reis CM, Palu M, Mayer AF. Pursed-lips breathing reduces dynamic hyperinflation induced by activities of daily living test in patients with chronic obstructive pulmonary disease: a randomized cross-over study. J Rehabil Med. (2015) 10:957–62. doi: 10.2340/16501977-2008
37. Cabral LF, D'Elia Tda C, Marins Dde S, Zin WA, Guimaraes FS. Pursed lip breathing improves exercise tolerance in COPD: a randomized crossover study. Eur J Phys Rehabil Med. (2015) 51:79–88.
38. Garrod R, Dallimore K, Cook J, Davies V, Quade K. An evaluation of the acute impact of pursed lips breathing on walking distance in nonspontaneous pursed lips breathing chronic obstructive pulmonary disease patients. Chron Respir Dis. (2005) 2:67–72. doi: 10.1191/1479972305cd068oa
39. Brien SB, Lewith GT, Thomas M. Patient coping strategies in COPD across disease severity and quality of life: a qualitative study. NPJ Prim Care Respir Med. (2016) 26:16051. doi: 10.1038/npjpcrm.2016.51
40. Vitacca M, Clini E, Bianchi L, Ambrosino N. Acute effects of deep diaphragmatic breathing in COPD patients with chronic respiratory insufficiency. Eur Respir J. (1998) 11:408–15. doi: 10.1183/09031936.98.11020408
41. Mendes LP, Moraes KS, Hoffman M, Vieira DS, Ribeiro-Samora GA, Lage SM. Effects of Diaphragmatic Breathing With and Without Pursed-Lips Breathing in Subjects With COPD. Respir Care. (2019) 64:136–44. doi: 10.4187/respcare.06319
42. Gosselink RA, Wagenaar RC, Rijswijk H, Sargeant AJ, Decramer ML. Diaphragmatic breathing reduces efficiency of breathing in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (1995) 151:1136–42. doi: 10.1164/ajrccm.151.4.7697243
43. Bellemare F, Grassino A. Force reserve of the diaphragm in patients with chronic obstructive pulmonary disease. J Appl Physiol Respir Environ Exerc Physiol. (1983) 55:8–15. doi: 10.1152/jappl.1983.55.1.8
44. Booth S, Moffat C, Burkin J, Galbraith S, Bausewein C. Nonpharmacological interventions for breathlessness. Curr Opin Support Palliat Care. (2011) 5:77–86. doi: 10.1097/SPC.0b013e3283460c93
45. Probst VS, Troosters T, Coosemans I, Spruit MA, Pitta Fde O, Decramer R et al. Mechanisms of improvement in exercise capacity using a rollator in patients with COPD. Chest. (2004) 126:1102–7. doi: 10.1378/chest.126.4.1102
46. Roomi J, Yohannes AM, Connolly MJ. The effect of walking aids on exercise capacity and oxygenation in elderly patients with chronic obstructive pulmonary disease. Age Ageing. (1998) 27:703–6. doi: 10.1093/ageing/27.6.703
47. Bourke SJ, Peel ET. Integrated Palliative Care of Respiratory Disease. London: Springer. (2013). doi: 10.1007/978-1-4471-2230-2
48. Swan F, Booth S. The role of airflow for the relief of chronic refractory breathlessness. Curr Opin Support Palliat Care. (2015) 9:206–11. doi: 10.1097/SPC.0000000000000160
49. Johnson MJ, Simpson MI, Currow DC, Millman RE, Hart SP, Green G. Magnetoencephalography to investigate central perception of exercise-induced breathlessness in people with chronic lung disease: a feasibility pilot. BMJ Open. (2015) 5:e007535. doi: 10.1136/bmjopen-2014-007535
50. Bausewein C, Booth S, Gysels M, Higginson I. Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database Syst Rev. (2008) CD005623. doi: 10.1002/14651858.CD005623.pub2
51. Bausewein C, Booth S, Gysels M, Kuhnbach R, Higginson I. Effectiveness of a hand-held fan for breathlessness: a randomized phase II trial. BMC Palliat Care. (2010) 19:22. doi: 10.1186/1472-684X-9-22
52. Luckett T, Phillips J, Johnson MJ, Farquhar M, Swan F, Assen T. Contributions of a hand-held fan to self-management of chronic breathlessness. Eur Respir J. (2017) 50:1700262. doi: 10.1183/13993003.00262-2017
53. Galbraith S, Fagan P, Perkins P, Lynch A, Booth S. Does the use of a handheld fan improve chronic dyspnea? A randomized, controlled, crossover trial. J Pain Symptom Manage. (2010) 39:831–8. doi: 10.1016/j.jpainsymman.2009.09.024
54. Kamal AH, Maguire JM, Wheeler JL, Currow DC, Abernethy AP. Dyspnea review for the palliative care professional: treatment goals and therapeutic options. J Palliat Med. (2012) 15:106–14. doi: 10.1089/jpm.2011.0110
55. Swan F, Newey A, Bland M, Allgar V, Booth S, Bausewein C et al. Airflow relieves chronic breathlessness in people with advanced disease: An exploratory systematic review and meta-analyses. Palliat Med. (2019) 33:618–33. doi: 10.1177/0269216319835393
56. Barnes-Harris M, Allgar V, Booth S, Currow D, Hart S, Phillips J et al. Battery operated fan and chronic breathlessness: does it help? BMJ Support Palliat Care. (2019) 9:478–81. doi: 10.1136/spcare-2019-mariecuriepalliativecare.8
57. Smith TA, Cho JG, Roberts MM, Swami V, Wheatley JR. Hand-held fans: physical properties and perceptions of patients with COPD. J Pain Symptom Manage. (2022) 63:e9–e16. doi: 10.1016/j.jpainsymman.2021.07.006
58. Qian MYY, Politis J, Thompson M, Wong D, Le B, Irving L et al. Individualized breathlessness interventions may improve outcomes in patients with advanced COPD. Respirology. (2018) 23:1146–51. doi: 10.1111/resp.13324
59. Booth S, Burkin J, Moffat C, Spathis A. Managing breathlessness in clinical practice. London: Springer. (2014). doi: 10.1007/978-1-4471-4754-1
60. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2015) 23:CD003793. doi: 10.1002/14651858.CD003793.pub3
61. Lötters F, van Tol B, Kwakkel G. Gosselink. Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. Eur Respir J. (2002) 20:570–6. doi: 10.1183/09031936.02.00237402
62. O'Brien K, Geddes EL, Reid WD, Brooks D, Crowe J. Inspiratory muscle training compared with other rehabilitation interventions in chronic obstructive pulmonary disease: a systematic review update. J Cardiopulm Rehabil Prev. (2008) 28:128–41. doi: 10.1097/01.HCR.0000314208.40170.00
63. Beaumont M, Forget P, Couturaud F, Reychler G. Effects of inspiratory muscle training in COPD patients: A systematic review and meta-analysis. Clin Respir J. (2018) 12:2178–88. doi: 10.1111/crj.12905
64. Vandevoorde J, Verbanck S, Gijssels L, Schuermans D, Devroey D, De Backer J et al. Early detection of COPD: a case finding study in general practice. Respir Med. (2007) 101:525–30. doi: 10.1016/j.rmed.2006.06.027
65. Gift AG, Shepard CE. Fatigue and other symptoms in patients with chronic obstructive pulmonary disease: do women and men differ? J Obstet Gynecol Neonatal Nurs. (1999) 28:201–8. doi: 10.1111/j.1552-6909.1999.tb01985.x
66. Lewko A, Bidgood P, Jewell A, Garrod R. A comprehensive literature review of COPD Related Fatigue. Curr Respir Med Rev. (2012) 8:370–82. doi: 10.2174/157339812803832476
67. Lewko A, Bidgood PL, Garrod R. Evaluation of psychological and physiological predictors of fatigue in patients with COPD. BMC Pulm Med. (2009) 21:47. doi: 10.1186/1471-2466-9-47
68. Li LSK, Butler S, Goldstein R, Brooks D. Comparing the impact of different exercise interventions on fatigue in individuals with COPD: A systematic review and meta-analysis. Chron Respir Dis. (2019) 16:1479973119894855. doi: 10.1177/1479973119894855
69. Lewko A, Bidgood PL, Jewell A, Garrod R. Evaluation of multidimensional COPD-related subjective fatigue following a pulmonary rehabilitation programme. Respir Med. (2014) 108:95–102. doi: 10.1016/j.rmed.2013.09.003
70. Seyedi Chegeni P, Gholami M, Azargoon A, Hossein Pour AH, Birjandi R, Norollahi H. The effect of progressive muscle relaxation on the management of fatigue and quality of sleep in patients with chronic obstructive pulmonary disease: A randomized controlled clinical trial. Complement Ther Clin Pract. (2018) 31:64–70. doi: 10.1016/j.ctcp.2018.01.010
71. Stridsman C, Lindberg A, Skär L. Fatigue in chronic obstructive pulmonary disease: a qualitative study of people's experiences. Scand J Caring Sci. (2014) 28:130–8. doi: 10.1111/scs.12033
72. Small S, Lamb M. Fatigue in chronic illness: the experience of individuals with chronic obstructive pulmonary disease and with asthma. J Adv Nurs. (1999) 30:469–78. doi: 10.1046/j.1365-2648.1999.01102.x
73. Navarro T. Quality of Life. In: Blackler L, Jones C, Mooney C (Eds.), Managing Chronic Obstructive Pulmonary Disease. Chichester, England: John Wiley & Sons. (2007) p. 113–120. doi: 10.1002/9780470697603.ch6
74. Wingårdh ASL, Göransson C, Larsson S, Slinde F, Vanfleteren LEGW. Effectiveness of Energy Conservation Techniques in Patients with COPD. Respiration. (2020) 99:409–16. doi: 10.1159/000506816
75. Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. (2014) 23:345–9. doi: 10.1183/09059180.00007813
76. Hynninen KM, Breitve MH, Wiborg AB, Pallesen S, Nordhaus IH. Psychological characteristics of patients with chronic obstructive pulmonary disease: a review. J Psychosom Res. (2005) 59:429–43. doi: 10.1016/j.jpsychores.2005.04.007
77. Maurer J, Rebbapragada V, Borson S, Goldstein R, Kunik ME, Yohannes AM, et al. ACCP Workshop Panel on Anxiety and Depression in COPD. Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest. (2008) 134:43S−56S. doi: 10.1378/chest.08-0342
78. Eisner MD, Blanc PD, Yelin EH, Katz PP, Sanchez G, Iribarren C, et al. Influence of anxiety on health outcomes in COPD. Thorax. (2010) 65:229–34. doi: 10.1136/thx.2009.126201
79. Hyland ME, Halpin DM, Blake S, Seamark C, Pinnuck M, Ward D et al. Preference for different relaxation techniques by COPD patients: comparison between six techniques. Int J Chron Obstruct Pulmon Dis. (2016) 11:2315–9. doi: 10.2147/COPD.S113108
80. Reaves C, Angosta AD. The relaxation response: Influence on psychological and physiological responses in patients with COPD. Appl Nurs Res. (2021) 57:151351. doi: 10.1016/j.apnr.2020.151351
81. Anlló H, Herer B, Delignières A, Ghergan A, Bocahu Y, Segundo I, et al. Hypnosis for the management of COPD-related anxiety and dyspnoea in pulmonary rehabilitation: rationale and design for a cluster-randomised, active-control trial (HYPNOBPCO_2). ERJ Open Res. (2021) 8:00565–2021. doi: 10.1183/23120541.00565-2021
82. Heslop-Marshall K, Baker C, Carrick-Sen D, Newton J, Echevarria C, Stenton C, et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res. (2018) 4:00094–2018. doi: 10.1183/23120541.00094-2018
83. Farver-Vestergaard I, O'Toole MS, O'Connor M, Løkke A., Bendstrup, Basdeo SA, et al. Mindfulness-based cognitive therapy in COPD: a cluster randomised controlled trial. Eur Respir J. (2018) 51:1702082. doi: 10.1183/13993003.02082-2017
84. Valenza MC, Valenza-Peña G, Torres-Sánchez I, González-Jiménez E, Conde-Valero A, Valenza-Demet G, et al. Effectiveness of controlled breathing techniques on anxiety and depression in hospitalized patients with COPD: a randomized clinical Trial. Respir Care. (2014) 59:209–15. doi: 10.4187/respcare.02565
85. Gordon CS, Waller JW., Cook RM, Cavalera SL, Lim WT, Osadnik CR, et al. Effect of pulmonary rehabilitation on symptoms of anxiety and depression in COPD: a systematic review and meta-analysis. Chest. (2019) 156:80–91. doi: 10.1016/j.chest.2019.04.009
86. Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. ATS/ERS task force on pulmonary rehabilitation. An official american thoracic society/European respiratory society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. (2013) 188:e13–64. doi: 10.1164/rccm.201309-1634ST
87. Puhan MA, Gimeno-Santos E, Cates CJ, Troosters T. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2016). doi: 10.1002/14651858.CD005305.pub4
88. Maddocks M, Kon SSC, Canavan JL, Jones SE, Nolan CM, Labey A, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. (2016) 71:988–95. doi: 10.1136/thoraxjnl-2016-208460
89. Lewko A, Mansell SK, Irvin-Sellers M. Effectiveness and feasibility of post-exacerbation pulmonary rehabilitation (PEPR) in a real-world clinical setting: a quality improvement project. Physiotherapy Review. (2021) 3:12–23. doi: 10.5114/phr.2021.109027
90. Spruit MA, Rochester CL, Pitta F, Kenn K, Schols AMWJ, Hart N et al. Pulmonary rehabilitation, physical activity, respiratory failure and palliative respiratory care. Thorax. (2019) 74:693–9. doi: 10.1136/thoraxjnl-2018-212044
91. Mantoani LC, Rubio N, McKinstry B, MacNee W, Rabinovich RA. Interventions to modify physical activity in patients with COPD: a systematic review. Eur Respir J. (2016) 48:69–81. doi: 10.1183/13993003.01744-2015
92. Barreiro E, Gea J. Respiratory and Limb Muscle Dysfunction in COPD. COPD. (2015) 12:413–26. doi: 10.3109/15412555.2014.974737
93. Engelen MPKJ, Jonker R, Thaden JJ, Ten Have GAM, Jeon MS, Desarathy S et al. Comprehensive metabolic flux analysis to explain skeletal muscle weakness in COPD. Clin Nutr. (2020) 39:3056–65. doi: 10.1016/j.clnu.2020.01.010
94. Neder JA, Sword D, Ward SA, Mackay E, Cochrane LM, Clark CJ, et al. Home based neuromuscular electrical stimulation as a new rehabilitative strategy for severely disabled patients with chronic obstructive pulmonary disease (COPD). Thorax. (2002) 4:333–7. doi: 10.1136/thorax.57.4.333
95. Vivodtzev I, Debigaré R, Gagnon P, Mainguy V, Saey D, Dubé A, et al. Functional and muscular effects of neuromuscular electrical stimulation in patients with severe COPD: a randomized clinical trial. Chest. (2012) 141:716–25. doi: 10.1378/chest.11-0839
96. Hill K, Cavalheri V, Mathur S, Roig M, Janaudis-Ferreira T, Robles P, et al. Neuromuscular electrostimulation for adults with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2018) 5:CD010821. doi: 10.1002/14651858.CD010821.pub2
97. Vieira PJ, Chiappa AM, Cipriano G Jr, Umpierre D, Arena R, Chiappa GR, et al. Neuromuscular electrical stimulation improves clinical and physiological function in COPD patients. Respir Med. (2014) 108:609–20. doi: 10.1016/j.rmed.2013.12.013
98. Jones S, Man WD, Gao W, Higginson IJ, Wilcock A, Maddocks M. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease Cochrane Database Syst Rev. (2016) 10:CD009419. doi: 10.1002/14651858.CD009419.pub3
99. Belli S, Prince I, Savio G, Paracchini E, Cattaneo D, Bianchi M, et al. Airway Clearance Techniques: The Right Choice for the Right Patient. Front Med. (2021) 8:544826. doi: 10.3389/fmed.2021.544826
100. Ides K, Vissers D, De Backer L, Leemans G, De Backer W. Airway clearance in COPD: need for a breath of fresh air? A systematic review. COPD. (2011) 8:196–205. doi: 10.3109/15412555.2011.560582
101. Osadnik CR, McDonald CF, Jones AP, Holland AEl. Airway clearance techniques for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2012) 14:CD008328. doi: 10.1002/14651858.CD008328.pub2
102. Daynes E, Jones AW, Greening NJ, Singh SJ. The use of airway clearance devices in the management of chronic obstructive pulmonary disease. A systematic review and meta-analysis of randomized controlled trials. Ann Am Thorac Soc. (2021) 18:308–20. doi: 10.1513/AnnalsATS.202005-482OC
103. Rawal G, Yadav S. Nutrition in chronic obstructive pulmonary disease: a review. J Transl Int Med. (2015) 3:151–4. doi: 10.1515/jtim-2015-0021
104. Schols AM, Ferreira IM, Franssen FM, Gosker HR, Janssens W, Muscartioli M, et al. Nutritional assessment and therapy in COPD: a European Respiratory Society statement. Eur Respir J. (2014) 44:1504–20. doi: 10.1183/09031936.00070914
105. Ferreira IM, Brooks D, White J, Goldstein R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2012) 12:CD000998. doi: 10.1002/14651858.CD000998.pub3
106. Keogh E, Mark Williams E. Managing malnutrition in COPD: a review. Respir Med. (2021) 176:106248. doi: 10.1016/j.rmed.2020.106248
107. Gea J, Pascual S, Casadevall C, Orozco-Levi M, Barreiro E. Muscle dysfunction in chronic obstructive pulmonary disease: update on causes and biological findings. J Thorac Dis. (2015) 7:E418–38. doi: 10.3978/j.issn.2072-1439.2015.08.04
108. D'Cruz RF, Murphy PB, Kaltsakas G. Sleep disordered breathing and chronic obstructive pulmonary disease: a narrative review on classification, pathophysiology and clinical outcomes. J Thorac Dis. (2020) 12:S202–16. doi: 10.21037/jtd-cus-2020-006
109. Macrea M, Oczkowski S, Rochwerg B, Branson RD, Celli B, Coleman JM et al. Long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2020) 202:e74–87. doi: 10.1164/rccm.202006-2382ST
110. Kolodziej MA, Jensen L, Rowe B, Sin D. Systematic review of noninvasive positive pressure ventilation in severe stable COPD. Eur Respir J. (2007) 30:293–306. doi: 10.1183/09031936.00145106
111. Murphy PB, Rehal S, Arbane G, Bourke S, Calverley PMA, Crook AM et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA. (2017) 317:2177–86. doi: 10.1001/jama.2017.4451
112. Windish W, Storre JH. Chronic NIV in COPD. In: Handbook Noninvasive Ventilation. Simonds AK (eds). European Respiratory Society. (2015) p. 190–196.
113. McEvoy RD, Pierce RJ, Hillman D, Esterman A, Ellis EE, Catcheside PG, et al. Australian trial of non-invasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax. (2009) 64:561–6. doi: 10.1136/thx.2008.108274
114. Holland AE, Mahal A, Hill CJ, Lee AL, Burge AT, Cox NS et al. Home-based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial. Thorax. (2017) 72:57–65. doi: 10.1136/thoraxjnl-2016-208514
115. Grosbois JM, Gicquello A, Langlois C, Le Rouzic O, Bart F, Wallaert B et al. Long-term evaluation of home-based pulmonary rehabilitation in patients with COPD. Int J Chron Obstruct Pulmon Dis. (2015) 25:2037–44. doi: 10.2147/COPD.S90534
116. COPD Working Group. Pulmonary rehabilitation for patients with chronic pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser. (2012) 12:1–75.
117. Roberts SE, Stern M, Schreuder FM, Watson T. The use of pursed lips breathing in stable chronic obstructive pulmonary disease: a systematic review of the evidence. Phys Ther Rev. (2009) 14:240–6. doi: 10.1179/174328809X452908
118. Mayer AF, Karloh M, Dos Santos K, de Araujo CLP, Gulart AA. Effects of acute use of pursed-lips breathing during exercise in patients with COPD: a systematic review and meta-analysis. Physiotherapy. (2018) 104:9–17. doi: 10.1016/j.physio.2017.08.007
119. Borge CR, Hagen KB, Mengshoel AM, Omenaas E, Moum T, Wahl AK. Effects of controlled breathing exercises and respiratory muscle training in people with chronic obstructive pulmonary disease: results from evaluating the quality of evidence in systematic reviews. BMC Pulm Med. (2014) 14:184. doi: 10.1186/1471-2466-14-184
120. Langer D, Hendriks E, Burtin C, Probst V, van der Schans CP, Paterson WJ et al. A clinical practice guideline for physiotherapists treating patients with chronic obstructive pulmonary disease based on a systematic review of available evidence. Clin Rehabil. (2009) 23:445–62. doi: 10.1177/0269215509103507
121. Sillen MJH, Speksnijder CM, Eterman RA, Janssen PP, Wagers SS, Wouters EFM et al. Effects of neuromuscular electrical stimulation of muscles of ambulation in patients with chronic heart failure or COPD: a systematic review of the English-language literature. Chest. (2009) 136:44–61. doi: 10.1378/chest.08-2481
Keywords: palliative care, non-pharmacological management, COPD, narrative review, physiotherapy
Citation: Pyszora A and Lewko A (2022) Non-pharmacological Management in Palliative Care for Patients With Advanced COPD. Front. Cardiovasc. Med. 9:907664. doi: 10.3389/fcvm.2022.907664
Received: 30 March 2022; Accepted: 31 May 2022;
Published: 18 July 2022.
Edited by:
Piotr Z. Sobanski, Schwyz Hospital, SwitzerlandReviewed by:
Ting Yang, China-Japan Friendship Hospital, ChinaCopyright © 2022 Pyszora and Lewko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Pyszora, YW5uYS5weXN6b3JhQGNtLnVtay5wbA==
†ORCID: Anna Pyszora orcid.org/0000-0001-8431-4653
Agnieszka Lewko orcid.org/0000-0001-5688-0762
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.