- 1Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Shanghai National Clinical Research Center for Metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai Key Laboratory for Endocrine Tumor-State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: Apolipoprotein B (apoB) and non-high-density lipoprotein cholesterol (non-HDL-C) have been shown to predict cardiovascular disease (CVD) even in the case of low levels of low-density lipoprotein cholesterol (LDL-C). We aimed to investigate whether the discordance between LDL-C and apoB or non-HDL-C was associated with arterial stiffness and elevated carotid intima-media thickness (CIMT) in middle-aged and elderly adults.
Methods: A total of 5,279 Chinese adults free of CVD at baseline were included and followed with a mean follow-up of 4.3 years. Arterial stiffness was measured by brachial-ankle pulse wave velocity (baPWV) and pulse pressure (PP). The associations of apoB, non-HDL-C, and LDL-C with arterial stiffness or elevated CIMT were examined with logistic regression models using either continuous scales by restricted cubic splines or categories of concordant and discordant values defined by medians.
Results: High apoB but not LDL-C was associated with elevated baPWV or PP. High apoB, non-HDL-C, and LDL-C were all associated with elevated CIMT (p < 0.05). Individuals with low levels of LDL-C and discordantly high apoB or non-HDL-C compared to those with concordantly low apoB or non-HDL-C demonstrated higher risks of elevated baPWV [ORs (95% CI) of 1.40 (1.03–1.91) and 1.56 (1.12–2.18), respectively] and elevated PP [ORs (95% CI) of 1.61 (1.19–2.18) and 1.55 (1.12–2.15), respectively]. While, discordant high LDL-C with low apoB was associated with an increased risk of elevated CIMT (OR, 1.74; 95% CI, 1.13–2.69).
Conclusion: Discordance analysis revealed that elevated apoB or non-HDL-C was a better predictor of risk of arterial stiffness, whereas LDL-C for elevated CIMT.
Introduction
Cardiovascular diseases (CVDs) remain the leading cause of health loss throughout the world (1). Multiple risk factors have been identified for CVDs, among which abnormal lipids account for a considerable proportion (2). Low-density lipoprotein cholesterol (LDL-C), an extensively studied lipid trait, has been recognized not only as a risk predictor but a causal factor for CVD (3). Definite reductions in the incidence of major vascular events could be obtained by lowering cholesterol, especially relying on the reduced number of LDL particles (4). However, a prospective meta-analysis shows that one in seven patients with cholesterol-lowering treatment had CVD events over 5 years (5). The existed “residual risk” among individuals with low LDL-C is partly attributed to an undeniable role of other lipid particles.
Samples from either the general population or large clinical studies show that a certain percentage of individuals have non-high-density lipoprotein cholesterol (non-HDL-C) or apolipoprotein B (apoB) above the recommended targets in those with low LDL-C (6). Non-HDL-C encompasses the cholesterol information of atherogenic lipid particles [LDL, very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), and lipoprotein (a)]. There is exactly one molecule apoB carried on the surface of each atherogenic lipid particle (7). ApoB provides a direct way to estimate the total number of these lipoproteins. Both apoB and non-HDL-C are proven to have a relation to CVD (8, 9). Meanwhile, Johannesen et al. have demonstrated that populations with LDL-C below the median but apoB or non-HDL-C above present increased risks of myocardial infarction [ORs (95% CI) of 1.49 (1.15–1.92) and 1.78 (1.35–2.34), respectively] and all-cause mortality [ORs (95% CI) of 1.21 (1.07–1.36) and 1.18 (1.02–1.36), respectively] compared with concordant low values (10), suggesting that such two lipid traits may be a better driver of cardiovascular risk rather than LDL-C alone.
Previous studies revealed that elevated carotid intima-media thickness (CIMT) and arterial stiffness measured by elevated brachial-ankle pulse wave velocity (baPWV) or elevated pulse pressure (PP) were all accompanied by a higher risk of CVD (11–13). These measurements are all reckoned as a prelude and a great threat to CVD health. Early detection and preventive intervention on subclinical cardiovascular events are crucial to reducing the CVD risk. However, few prospective studies evaluate the predictive values of discordant lipids in association with arterial stiffness and elevated CIMT among middle-aged and elderly adults.
To fulfill this knowledge gap, we conducted a discordance analysis on the associations of apoB, non-HDL-C, and LDL-C with the risk of elevated baPWV, elevated PP, or elevated CIMT in a community-based cohort study.
Materials and Methods
Study Population
The middle-aged and elderly study participants were from a prospective population-based cohort in Jiading District, Shanghai, China. The design and eligibility criteria of the overall study had been previously described in detail elsewhere (14). Briefly, the study was launched between March and August 2010 among 10,375 registered permanent residents aged 40 years or older, who all underwent a comprehensive survey comprising of a standard questionnaire and relevant biochemical measurements. For the current study, we excluded individuals who had a previous history of CVD (n = 850), defined as a composite endpoint of fatal or non-fatal myocardial infarction, stroke, hospitalization, or treatment for heart failure. From August 2014 to July 2015, all eligible participants were invited to attend a follow-up visit and 6,302 individuals complied. Given the investigated outcomes was an early stage of CVD, we further excluded 249 participants who developed CVD during the 5.4 years of follow-up (mean 4.3 years) to avoid influencing or even overestimating the target risk. Participants without baseline or follow-up information on baPWV, PP, or CIMT were not included, leaving 5,279 for this analysis. In addition, we excluded participants with baseline elevated baPWV (n = 1,317), elevated PP (n = 1,324), and elevated CIMT (n = 977) from analysis for each outcome. A detailed flowchart of the study is presented in Figure 1.
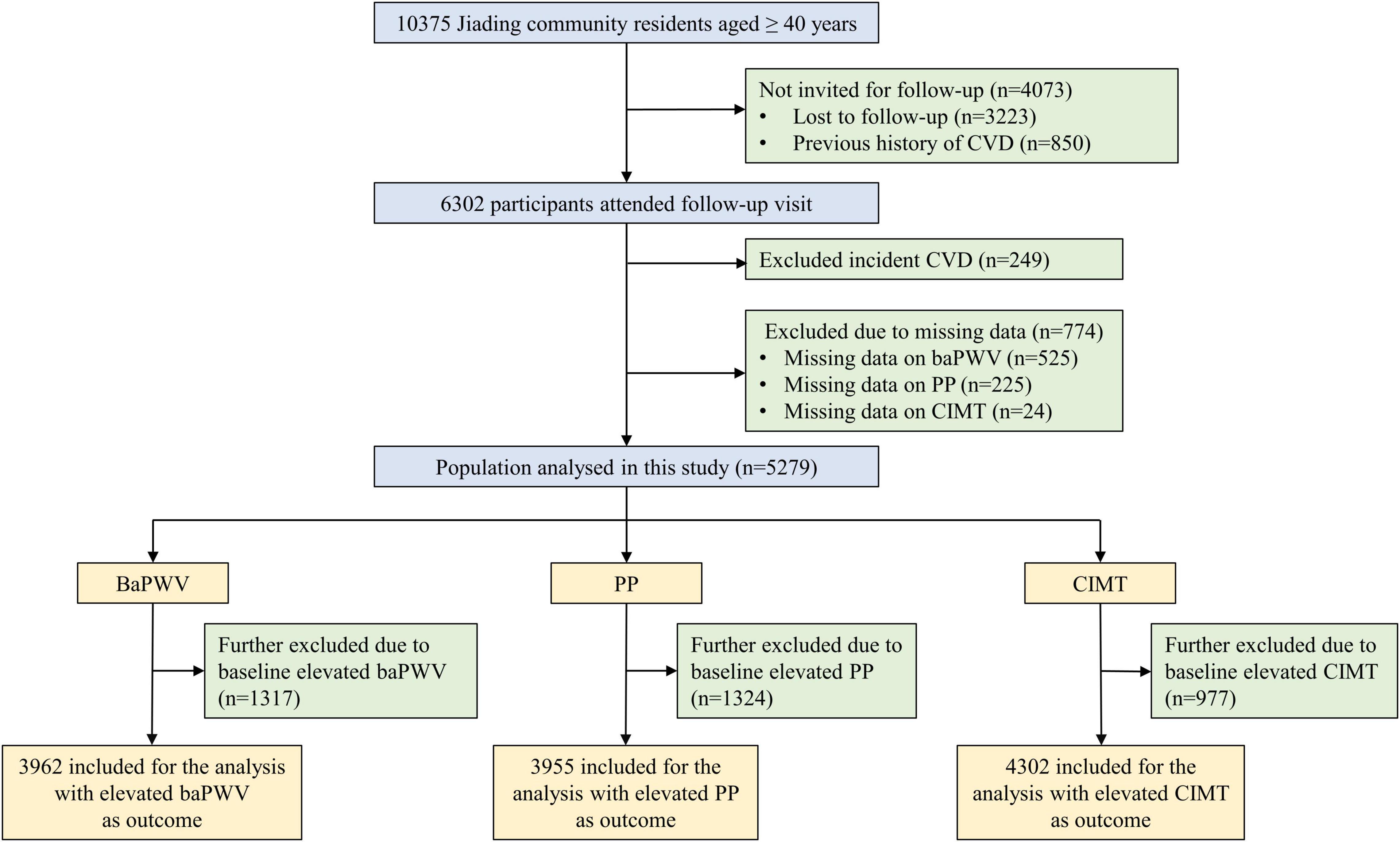
Figure 1. Flowchart of study procedure. CVD, cardiovascular disease; BaPWV, brachial-ankle pulse wave velocity; PP, pulse pressure; CIMT, carotid intima-media thickness.
The study protocol conformed to the Declaration of Helsinki and had been approved by the Institutional Review Board of the Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. All participants provided written informed consent.
Data Collection and Clinical Evaluation
A standardized, interviewer-administered questionnaire was used to collect information on demographic characteristics, current use of medications and lifestyle factors, etc. Current drinkers or smokers were defined as those who consumed alcohol once per week or smoked cigarettes once per day regularly during the past 6 months, respectively. We defined ideal physical activity as moderate-intensity ≥ 150 min/week or vigorous-intensity ≥ 75 min/week or moderate and vigorous-intensity ≥ 150 min/week using the International Physical Activity Questionnaire (15).
Each study participant was measured for body weight, height, and systolic and diastolic blood pressure (BP). Body mass index (BMI) was calculated as body weight (kg) divided by the square of body height (m). BP was measured three times using a calibrated automated electronic device (OMRON Model HEM-725 FUZZY, Omron Company, Dalian, China) on the non-dominant arm of seated participants who had rested after 5 min. The mean value of three BP readings was calculated for analysis. Pulse pressure (PP) was equal to systolic BP (SBP) minus diastolic BP (DBP).
BaPWV was determined at both baseline and follow-up by trained technicians using Colin VP-1000 [Model BP203RPE II, form PWV/ABI (ankle-brachial index); OMRON Colin Medical Instruments, Tokyo, Japan]. Pulse waves from both sides of the upper arms and ankles were obtained simultaneously through cuffs. The right and left baPWV were calculated by the time interval and the distance from ipsilateral arms to ankles. We applied the greater value of the right and left baPWV for analysis according to the definition in other large studies (16, 17).
CIMT measurements were performed once on the far wall of the right and left common carotid arteries close to the bifurcation by using a high-resolution B-mode tomographic ultrasound system (Esaote Biomedica SpA, Italy) with a linear 7.5-MHz transducer. The distance between the leading edge of the first and the second echogenic line at the end of diastole was regarded as CIMT. We adopted the greater value of the right and left CIMT for baseline and follow-up analysis (18, 19).
Under the condition that all participants were asked to fast after dinner, blood specimens were drawn the next morning and sent for biochemical tests including fasting plasma glucose, triglycerides, apoB, LDL-C, high-density lipoprotein cholesterol (HDL-C), and total cholesterol (TC). The lipid measurements were conducted using an autoanalyzer (Modular E170; Roche, Basel, Switzerland) in the College of American Pathologists (CAP)-certified central laboratory of the study strictly following the laboratory quality control procedures. Non-HDL-C was calculated by subtracting HDL-C from TC.
Outcome Assessment
A follow-up survey was conducted among all eligible study subjects to collect information on investigated outcomes according to the same standard protocols that were used during the baseline visit. Surrogate endpoints for cardiovascular disease, manifested by elevated baPWV, elevated PP, or elevated CIMT, were defined as the upper quartile of corresponding values at baseline or follow-up.
Statistical Analysis
Baseline sociodemographic and biochemical characteristics were described according to different lipid statuses. Continuous data were displayed as means ± standard deviations (SD) and categorical variables expressed as numbers (percentage). Test of difference across groups was conducted by the analysis of variance (continuous variables) or chi-square test (categorical variables).
Logistic regression models were used to analyze the associations between apoB, LDL-C, and non-HDL-C and the risk of elevated baPWV, PP, or CIMT. We adjusted multivariable models with baseline age, sex, BMI, smoking status, drinking status, physical activity, glucose-lowering therapy, and lipid-lowering therapy. When apoB, LDL-C, and non-HDL-C were on a continuous scale, we chose the lowest values as a reference to visualize and detect whether there were non-linear relationships between each protein/lipid variable and outcomes by using restricted cubic splines with three knots at the 5th, 50th, and 95th percentiles.
In the discordance analysis, individuals were stratified into categories based on less than median values or greater than or equal to median values of each lipid trait. Four mutually exclusive discordance/concordance categories according to lipid variables were presented: low/low, low/high, high/low, and high/high. Discordance was defined as low LDL-C with high apoB or non-HDL-C, or vice versa. Similarly, eight categories of discordant vs. concordant values of apoB vs. non-HDL-C vs. LDL-C were obtained using the above method. The multivariable-adjusted logistic regression model was further performed to assess whether the discordant/concordant categories were in relation to the risk of elevated baPWV, PP, or CIMT.
A two-tailed p < 0.05 was referred to be statistically significant. We used SAS version 9.4 (SAS Institute, Cary, NC) to conduct statistical analyses and R version 4.0.51 to plot restricted cubic splines and forest maps.
Results
Baseline Characteristics of Study Participants
Baseline characteristics of study participants are shown in Table 1. The median protein/lipid values, used as cutoffs to define discordant/concordant groups, were 95 mg/dl for apoB, 122 mg/dl for LDL-C, and 153 mg/dl for non-HDL-C in the baseline population (n = 5,279). There were significant differences in age, BMI, and systolic and diastolic BP across discordance/concordance groups. Among those with LDL-C below the median, 7.9 and 6.2% had a discordantly high apoB and non-HDL-C, respectively. Participants in such discordant groups more often were smokers and drinkers.
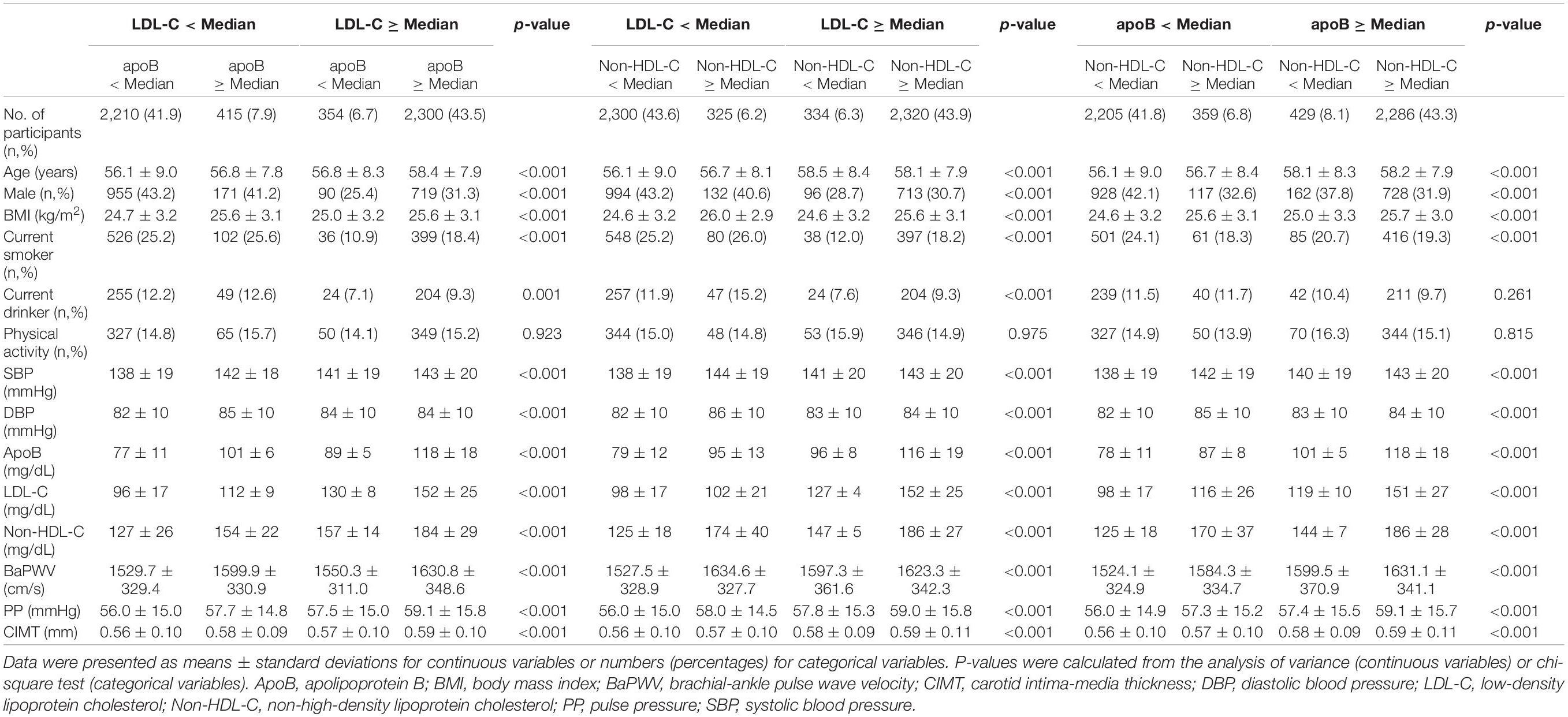
Table 1. Baseline characteristics of participants with concordant and discordant values of apoB vs. LDL-C, non-HDL-C vs. LDL-C, and apoB vs. non-HDL-C (n = 5,279).
Arterial Stiffness and Elevated Carotid Intima-Media Thickness for Lipid Traits Separately
There was no non-linear relationship between each protein/lipid trait and the risk of arterial stiffness or elevated CIMT (p for non-linearity > 0.05). The monotonically increasing trends existed between apoB with elevated baPWV (Figure 2A), elevated PP (Figure 2B), and elevated CIMT (Figure 2C) (p = 0.019, 0.021, and 0.001, respectively). But LDL-C had no significant relationship with arterial stiffness (p = 0.555 for elevated baPWV and p = 0.872 for elevated PP). As for non-HDL-C, there was a significant association with elevated baPWV (p = 0.028), but not with elevated PP (p = 0.066). Both LDL-C and non-HDL-C had a strong relationship with elevated CIMT (p = 0.002 and 0.003, respectively).
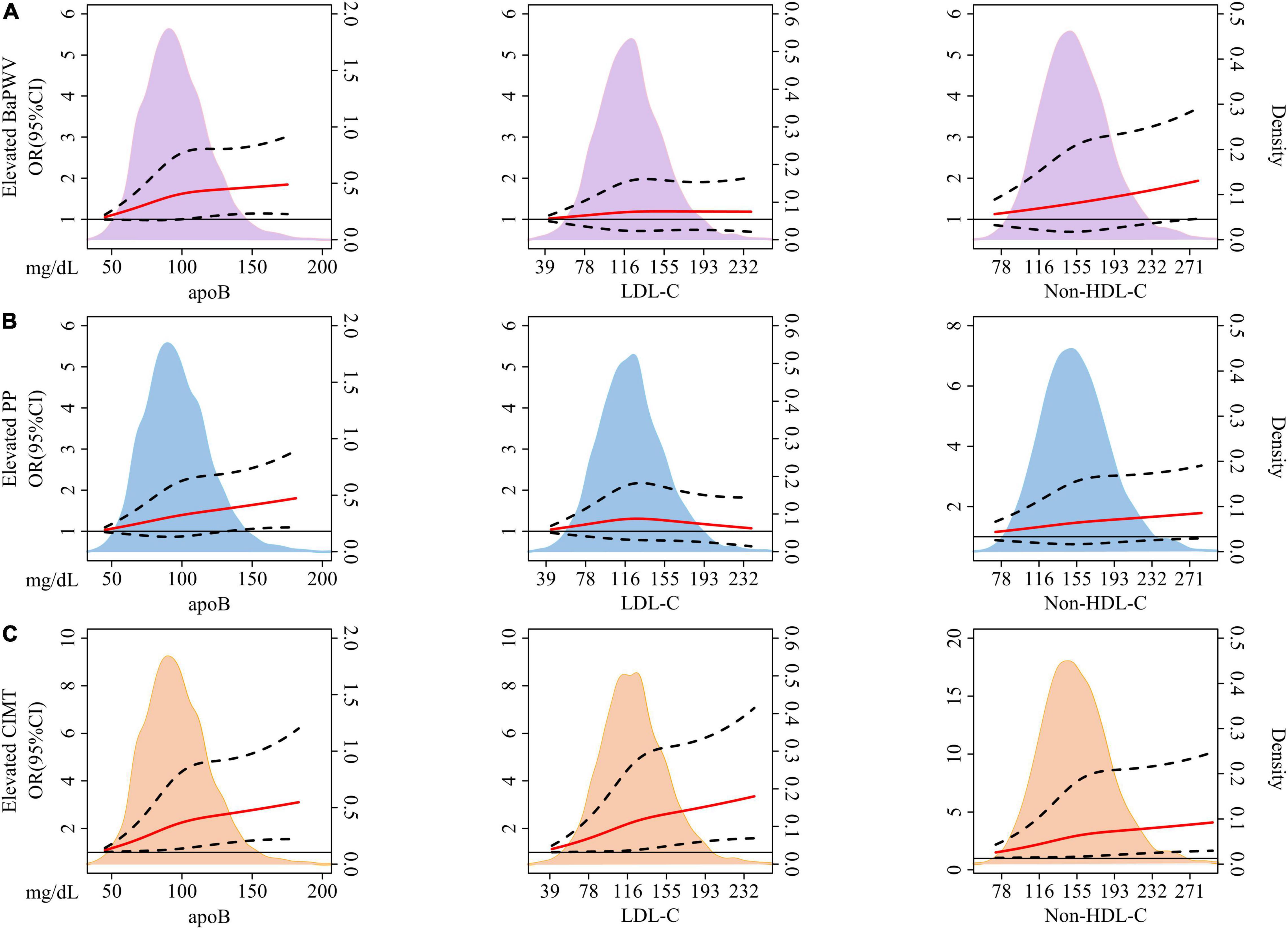
Figure 2. The association between baseline concentration of each lipid variable and incident elevated baPWV (A), PP (B), and CIMT (C) based on restricted cubic splines. The solid red lines represent a fitted relationship and dashed lines show 95% confidence intervals. Reference line for no association (Odds ratio: 1.0) is indicated by solid black line while areas of purple, blue, and orange represent the density distribution of lipid traits with incident elevated baPWV (A), PP (B), and CIMT (C) as ending points, respectively. Model was adjusted for baseline age, sex, BMI, smoking status, drinking status, physical activity, glucose-lowering therapy, and lipid-lowering therapy. ApoB, apolipoprotein B; LDL-C, low-density lipoprotein cholesterol; Non-HDL-C, non-high-density lipoprotein cholesterol; BaPWV, brachial-ankle pulse wave velocity; PP, pulse pressure; CIMT, carotid intima-media thickness; OR, odds ratio; CI, confidence interval; BMI, body mass index.
Multivariable adjusted odds ratios (ORs) and confidence intervals (CIs) of arterial stiffness and elevated CIMT according to per SD increase in the concentration of each lipid trait were shown in Table 2. ApoB presented significant associations with all investigated outcomes. The ORs (95% CI) were 1.10 (1.02–1.20) for elevated baPWV, 1.10 (1.01–1.19) for elevated PP, and 1.19 (1.07–1.32) for elevated CIMT. Non-HDL-C was associated with elevated baPWV (OR, 1.10; 95% CI, 1.01–1.19) and CIMT (OR, 1.17; 95% CI 1.05–1.29). Notably, LDL-C showed a significant association with elevated CIMT (OR, 1.19; 95% CI, 1.07–1.32), but no relationship with elevated baPWV and PP.
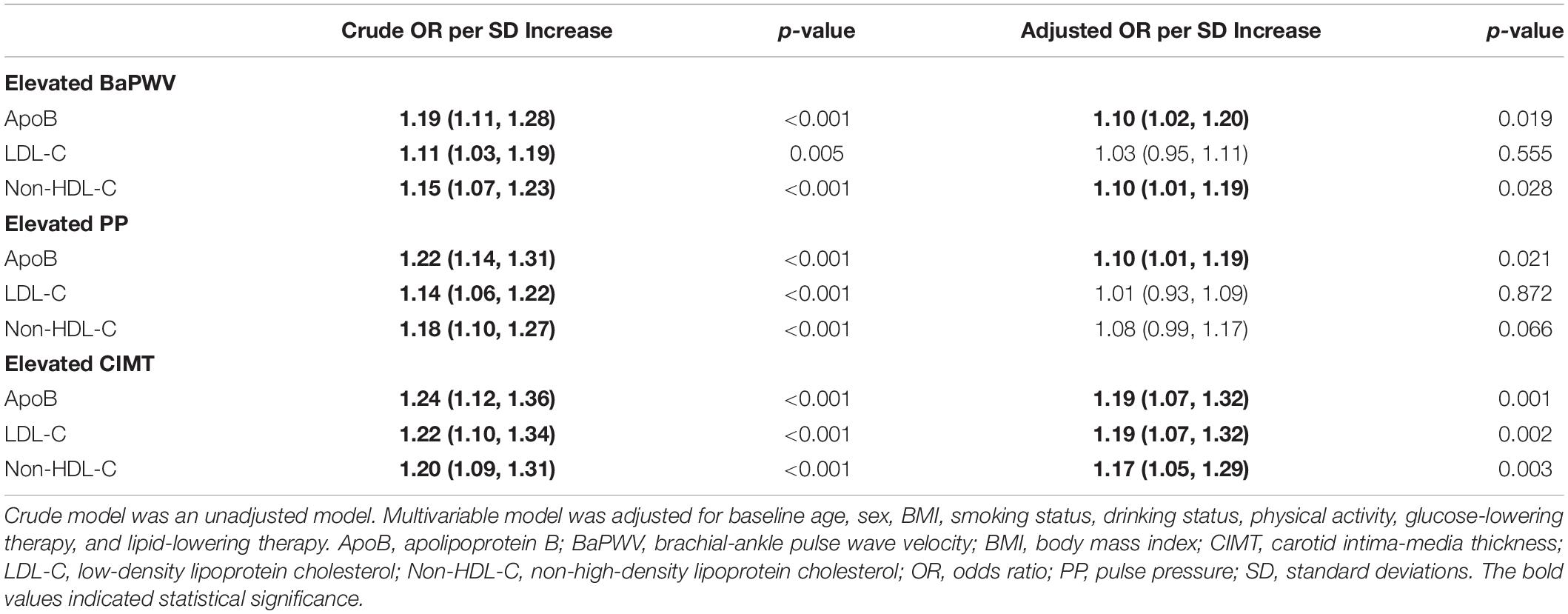
Table 2. Multivariable-adjusted odds ratios of elevated baPWV, PP, and CIMT according to per SD increase in concentrations of apoB, LDL-C, and non-HDL-C.
As the results presented in Figure 2 and Table 2, the superiority of apoB in predicting the risk of elevated baPWV or PP was subtle. There was necessary to further apply discordance analysis to explore the relative importance of apoB, non-HDL-C, and LDL-C, three highly correlated lipid traits, on investigated outcomes.
Arterial Stiffness and Elevated Carotid Intima-Media Thickness for Discordant Lipid Traits
Figures 3, 4 demonstrated the relationships of discordant vs. concordant categories of apoB, LDL-C, and non-HDL-C with the risk of arterial stiffness and elevated CIMT. Among those with low LDL-C, individuals with discordantly high apoB or non-HDL-C had ORs of 1.40 (95% CI, 1.03–1.91) and 1.56 (95% CI, 1.12–2.18) for elevated baPWV compared to those with concordantly low apoB or non-HDL-C, respectively (Figure 3A). For the same pattern of discordant lipid traits, the corresponding ORs (95% CI) for the risk of elevated PP was 1.61 (1.19–2.18) and 1.55 (1.12–2.15) (Figure 3B). Participants with discordantly low apoB and high LDL-C yielded a significant OR of 1.74 (95% CI, 1.13–2.69) for elevated CIMT compared to those with concordantly low apoB and low LDL-C (Figure 3C). Discordant apoB and non-HDL-C showed no association with risk of elevated baPWV, PP, or CIMT.
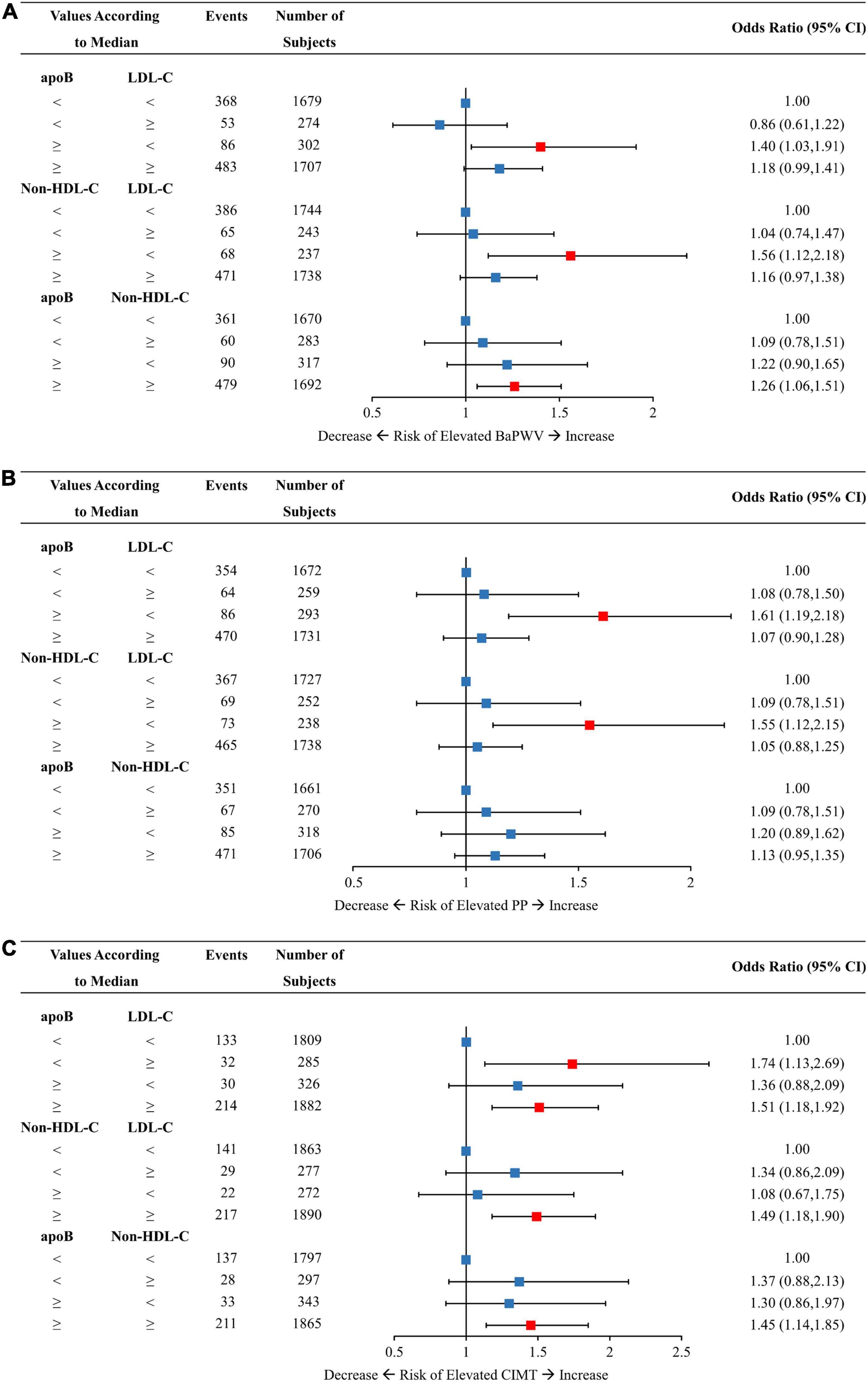
Figure 3. Multivariable-adjusted odds ratios of elevated baPWV (A), PP (B), and CIMT (C) by discordant vs. concordant categories of apoB, non-HDL-C, and LDL-C. The concordance/discordance categories are defined based on the medians of apoB, LDL-C, and non-HDL-C concentration levels in each analysis. We use the concordant group as reference: low apoB with low LDL-C, low non-HDL-C with low LDL-C, and low apoB with low non-HDL-C. Model was adjusted for baseline age, sex, BMI, smoking status, drinking status, physical activity, glucose-lowering therapy, and lipid-lowering therapy. ApoB, apolipoprotein B; Non-HDL-C, non-high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; BaPWV, brachial-ankle pulse wave velocity; PP, pulse pressure; CIMT, carotid intima-media thickness; CI, confidence interval; BMI, body mass index.
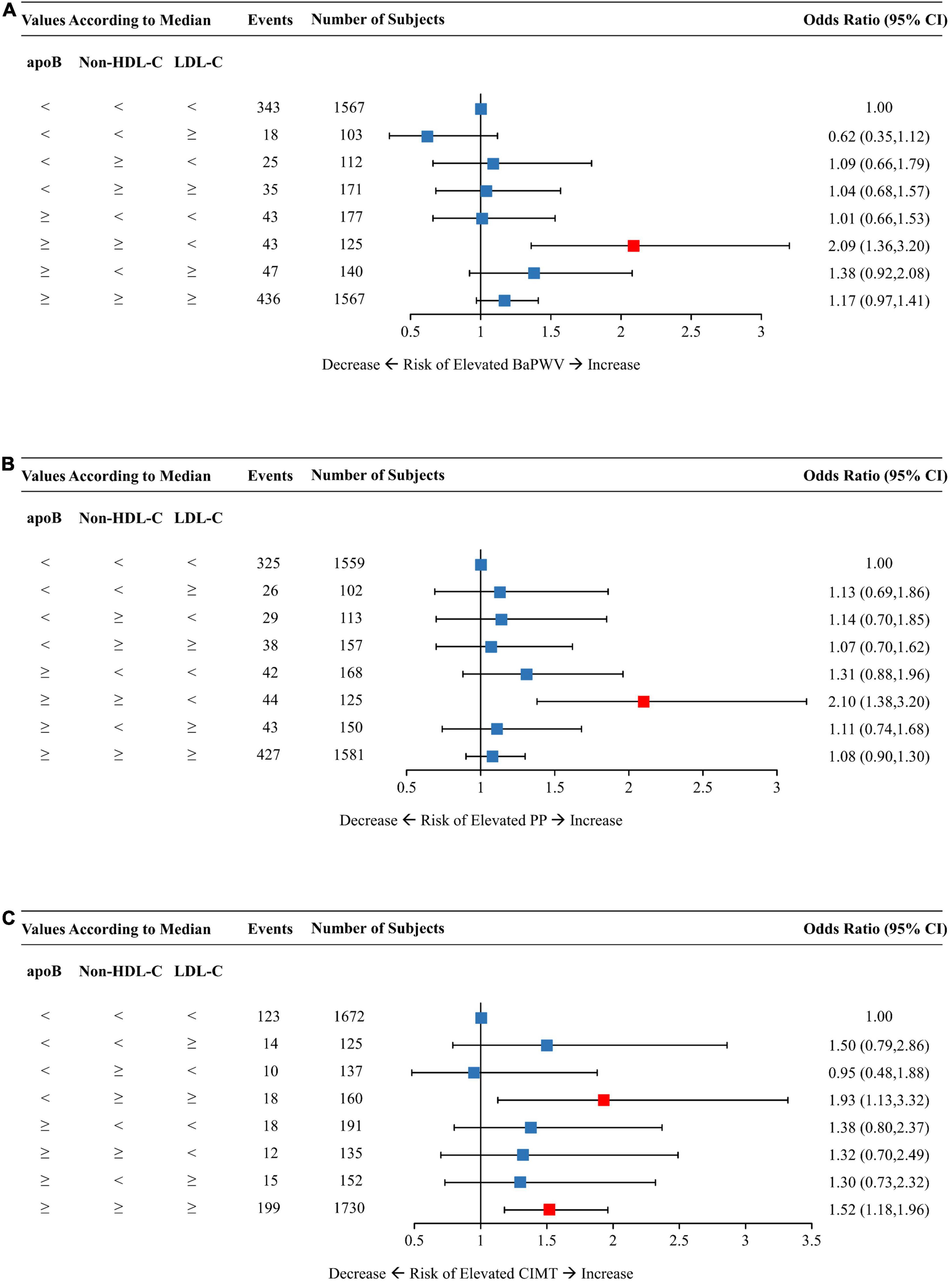
Figure 4. Multivariable-adjusted odds ratios of elevated baPWV (A), PP (B), and CIMT (C) by discordant vs. concordant categories of apoB, non-HDL-C, and LDL-C. The concordance/discordance categories are defined based on the medians of apoB, LDL-C, and non-HDL-C concentration levels in each analysis. The concordant group with apoB, LDL-C, and non-HDL-C all below the median is used as reference. Model was adjusted for baseline age, sex, BMI, smoking status, drinking status, physical activity, glucose-lowering therapy, and lipid-lowering therapy. ApoB, apolipoprotein B; Non-HDL-C, non-high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; BaPWV, brachial-ankle pulse wave velocity; PP, pulse pressure; CIMT, carotid intima-media thickness; CI, confidence interval; BMI, body mass index.
The concordant group with all three lipid traits below the medians constituted the reference group for analysis as shown in Figure 4. Individuals with low LDL-C but dual discordant apoB and non-HDL-C had an OR (95% CI) of 2.09 (1.36–3.20) for elevated baPWV (Figure 4A) and 2.10 (1.38–3.20) for elevated PP (Figure 4B). On the other hand, those with low apoB but dual discordant non-HDL-C and LDL-C yielded a 93% increased risk of elevated CIMT (Figure 4C). The concordant group with high levels of all three lipid traits had an OR of 1.52 (95% CI, 1.18–1.96) for elevated CIMT (Figure 4C).
Discussion
In this prospective cohort of middle-aged and elderly Chinese adults, we observed that apoB adequately captured the risk of new-onset elevated baPWV, elevated PP, and elevated CIMT. To the best of our knowledge, the current study is the first large-scale prospective cohort among middle-aged and elderly Chinese to evaluate the associations between discordance of apoB, non-HDL-C, and LDL-C and the risk of arterial stiffness and elevated CIMT.
The prevalence of discordance of LDL-C with either apoB or non-HDL-C reached 14.6 and 12.5%, respectively. On one hand, discordant low LDL-C with high apoB or non-HDL-C presented higher risks of elevated baPWV and elevated PP compared with concordant low LDL-C and low apoB or non-HDL-C, suggesting the risk of arterial stiffness was underestimated by LDL-C alone among such discordance populations. On the other hand, elevated CIMT had a significant association with discordant high LDL-C and low apoB. Our findings suggest that while LDL-C is a good predictor for elevated CIMT, apoB, and non-HDL-C may be better indicators for arterial stiffness risk assessment.
The putative role of apoB in the course of cardiovascular events has drawn global attention. Circulating apoB is recognized as a risk predictor of CVD through the essential role in retaining atherogenic lipid particles in the arterial wall (20). In fact, several guidelines have considered apoB as the secondary target in the management of dyslipidemia and CVD risk (21, 22). Atherosclerosis and arterial stiffness, reflected by CIMT and PWV, are early stage and risk factors of CVD (23, 24). In the cardiovascular risk in Young Finns Study, apoB assessed in early life is an indicator of adulthood CIMT and PWV development (25, 26). In addition, apoB is associated with PP, a marker of arterial stiffness (27). The findings of our study support the potential value of apoB as a predictor for elevated CIMT and arterial stiffness.
To the best of our knowledge, our study is the first large-scale prospective cohort among middle-aged and elderly Chinese to explore the relationship between discordance of apoB, non-HDL-C, and LDL-C and atherosclerosis, i.e., arterial stiffness as manifested by elevated baPWV or PP, and elevated CIMT. Previous studies have shown that elevated concentrations of apoB or non-HDL-C may better reflect subclinical CVD risk than LDL-C (28, 29). The discordance analysis, affording maximal power to compare these three highly correlated lipid traits, has been widely applied in previous research. For example, in the multicenter cohort study of young adults, high apoB and low LDL-C discordance demonstrate a 55% higher risk of midlife coronary calcification than the concordance group (30). Higher levels of apoB are accompanied by higher risk irrespective of the status of LDL-C. Our findings are consistent with reports from a cross-sectional investigation composed of 402 Northern Chinese, which reveals that groups with discordance low LDL-C and high apoB or non-HDL-C are in relation to the risk of elevated baPWV (31). The risk of arterial stiffness is more strongly influenced by apoB or non-HDL-C than by LDL-C.
The explanation behind our finding that apoB or non-HDL-C may better reflect elevated baPWV or elevated PP than LDL-C is straightforward. The cholesterol contained within apoB particles is equal to non-HDL-C, comprised of the cholesterol in LDL, VLDL, IDL, and lipoprotein(a). Discordant low LDL-C with high apoB or non-HDL-C is an approximate representation of high cholesterol contained in triglyceride-rich lipoproteins (TRLs), made up of VLDL and IDL in the fasting state. Meanwhile, direct evidence exists that cholesterol within TRLs is also accumulated in the arterial wall after uptake and that populations with high TRLs are at markedly increased risk for major CVD events independent of other risk factors (32, 33). ApoB or non-HDL-C is superior to LDL-C partially owing to the extra effect of TRLs (34).
Attention is not only limited to the cholesterol content of lipid particles but also centers on the size of lipoproteins. LDL particles, which constituted about 90% of circulating apoB-containing lipoproteins, could be counted using serum apoB (35). Contextually, the size of LDL particles may present as normal in groups with concordant LDL-C and apoB. While, lipid status of low plasma LDL-C and high apoB may occur as a result of the predominance of small, dense, cholesterol-deplete LDL (30). In a cross-sectional study, very small LDL is an important contributor to elevated baPWV (36). Besides, the LDL-C (mg/dl) to ApoB (mg/dl) ratio (LDL-C/ApoB ratio), is well recognized as a representation of the size of LDL particles (37), is associated with arterial stiffness in our study (Supplementary Table 1). We divide the population into three groups according to the LDL-C/ApoB ratio and choose the highest tertile as a reference. Participants in the lowest tertile group with a smaller size of LDL particles yield significantly increased risks of elevated baPWV (OR, 1.33; 95% CI, 1.09–1.63) and elevated PP (OR, 1.54; 95% CI, 1.27–1.88). Compared to LDL, small dense LDL shows an increased atherogenic potential owing to its high affinities for arterial proteoglycans and susceptibility to oxidative modification (38, 39). Therefore, the role of small dense LDL might be another reason why apoB and non-HDL-C are better indicators for arterial stiffness risk prediction than LDL-C.
However, our study failed to demonstrate the superiority of apoB or non-HDL-C for risk assessment on elevated CIMT. The risk may be more closely related to the mass of cholesterol within LDL particles. The development of atherosclerotic plaque probably increases in a dose-dependent manner with the retention of LDL-C (40). Hence, LDL-C retains a predominant impact on elevated CIMT.
Further detection revealed that there was no difference between apoB and non-HDL-C in predicting the risk of subclinical CVD. Our findings are consistent with those inferred in several previous reports (25, 41). Koivistoinen et al. showed that the ability of apoB and non-HDL-C to detect participants with increased risk of elevated baPWV is similar (25). ApoB is the surface structural protein of each molecule of non-HDL-C. They both are markers of apoB-containing lipoproteins and are highly correlated (42). ApoB and non-HDL-C might be equivalent in assessing subclinical cardiovascular risk.
The strengths of the study include its large sample, prospective design, the accessibility of high-quality measurement of lipid parameters, and the utility of comprehensive outcomes. Nevertheless, we take cognizance of several limitations. First, the participants in this study mainly represent the middle-aged and elderly Chinese population. There is a restriction in extrapolating the results to other age groups or ethnicity. Next, the definition of discordance is arbitrary even though using the median as the cutoff point has been applied in other large studies (10, 30). Finally, elevated baPWV, PP, and CIMT, considered the observed outcomes, are surrogate indicators of cardiovascular events and measured only at baseline and follow-up. However, many studies have reported a strong relationship between the above markers and subsequent risk of CVD (11–13). And it is credible to use such markers, collected by trained study nurses according to standard protocols although measured with no repetition, as investigated outcomes in high-quality articles (14, 19).
Conclusion
In conclusion, this prospective study showed that apoB or non-HDL-C rather than LDL-C was more strongly associated with the risk of arterial stiffness in the middle-aged and elderly population in China, but LDL-C predicted elevated CIMT well. ApoB and non-HDL-C provide utility in identifying individuals with remaining subclinical arterial stiffness burdens as LDL-C below the median. Our findings underline the importance of tackling both elevated apoB and non-HDL-C in routine clinical practice in addition to managing the LDL-C to retard or even reverse the prelude of CVD.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XJ, YB, and JL contributed to the study design and concept. XJ, YQ, and RZ analyzed the data and drafted the manuscript. LL, CH, YYZ, QC, XW, HQ, RW, and YZ contributed to data interpretation and the editing of the manuscript. MX, YX, TW, ZZ, YC, ML, and WW critically revised the manuscript for important intellectual content. All authors involved in writing and revising the manuscript and had final approval of the submitted and published versions.
Funding
This study was supported by the grants from National Natural Science Foundation of China (Grant Nos. 81870604, 81970691, 91857205, 82088102, 81930021, 81900741, and 82170819), the Shanghai Medical and Health Development Foundation (Grant No. DMRFP_I_01), the Shanghai Outstanding Academic Leaders Plan (Grant No. 20XD1422800), the Clinical Research Plan of SHDC (Grant No. SHDC2020CR3064B), and the Shanghai Science and Technology Committee (Grant Nos. 20Y11905100 and 19411964200).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the study participants for their contribution to the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.906396/full#supplementary-material
Footnotes
References
1. Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. (2017) 70:1–25. doi: 10.1016/j.jacc.2017.04.052
2. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. (2004) 364:937–52. doi: 10.1016/s0140-6736(04)17018-9
3. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. (2017) 38:2459–72. doi: 10.1093/eurheartj/ehx144
4. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. (2010) 376:1670–81. doi: 10.1016/S0140-6736(10)61350-5
5. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. (2005) 366:1267–78. doi: 10.1016/S0140-6736(05)67394-1
6. Sathiyakumar V, Park J, Quispe R, Elshazly MB, Michos ED, Banach M, et al. Impact of novel low-density lipoprotein-cholesterol assessment on the utility of secondary non-high-density Lipoprotein-C and Apolipoprotein B targets in selected worldwide dyslipidemia guidelines. Circulation. (2018) 138:244–54. doi: 10.1161/CIRCULATIONAHA.117.032463
7. Barter PJ, Ballantyne CM, Carmena R, Castro Cabezas M, Chapman MJ, Couture P, et al. Apo B versus cholesterol in estimating cardiovascular risk and in guiding therapy: report of the thirty-person/ten-country panel. J Intern Med. (2006) 259:247–58. doi: 10.1111/j.1365-2796.2006.01616.x
8. McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. (2008) 372:224–33. doi: 10.1016/S0140-6736(08)61076-4
9. Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. (2005) 294:326–33. doi: 10.1001/jama.294.3.326
10. Johannesen CDL, Mortensen MB, Langsted A, Nordestgaard BG. Apolipoprotein B and Non-HDL cholesterol better reflect residual risk than LDL cholesterol in statin-treated patients. J Am Coll Cardiol. (2021) 77:1439–50. doi: 10.1016/j.jacc.2021.01.027
11. Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension. (2017) 69:1045–52. doi: 10.1161/HYPERTENSIONAHA.117.09097
12. Cao JJ, Arnold AM, Manolio TA, Polak JF, Psaty BM, Hirsch CH, et al. Association of carotid artery intima-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality: the Cardiovascular Health Study. Circulation. (2007) 116:32–8. doi: 10.1161/CIRCULATIONAHA.106.645606
13. Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation. (1999) 100:354–60. doi: 10.1161/01.cir.100.4.354
14. Lin L, Zhang J, Jiang L, Du R, Hu C, Lu J, et al. Transition of metabolic phenotypes and risk of subclinical atherosclerosis according to BMI: a prospective study. Diabetologia. (2020) 63:1312–23. doi: 10.1007/s00125-020-05116-5
15. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) 35:1381–95.
16. Zheng M, Zhang X, Chen S, Song Y, Zhao Q, Gao X, et al. Arterial stiffness preceding diabetes: a longitudinal study. Circ Res. (2020) 127:1491–8. doi: 10.1161/CIRCRESAHA.120.317950
17. Kim J, Song TJ, Song D, Lee KJ, Kim EH, Lee HS, et al. Brachial-ankle pulse wave velocity is a strong predictor for mortality in patients with acute stroke. Hypertension. (2014) 64:240–6. doi: 10.1161/HYPERTENSIONAHA.114.03304
18. McNeely MJ, McClelland RL, Bild DE, Jacobs DR Jr., Tracy RP, Cushman M, et al. The association between A1C and subclinical cardiovascular disease: the multi-ethnic study of atherosclerosis. Diabetes Care. (2009) 32:1727–33. doi: 10.2337/dc09-0074
19. Wang B, Zhao Z, Liu S, Wang S, Chen Y, Xu Y, et al. Impact of diabetes on subclinical atherosclerosis and major cardiovascular events in individuals with and without non-alcoholic fatty liver disease. Diabetes Res Clin Pract. (2021) 177:108873. doi: 10.1016/j.diabres.2021.108873
20. Borén J, Olin K, Lee I, Chait A, Wight TN, Innerarity TL. Identification of the principal proteoglycan-binding site in LDL. A single-point mutation in apo-B100 severely affects proteoglycan interaction without affecting LDL receptor binding. J Clin Invest. (1998) 101:2658–64. doi: 10.1172/JCI2265
21. Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. (2011) 32:1769–818.
22. O’Malley PG, Arnold MJ, Kelley C, Spacek L, Buelt A, Natarajan S, et al. Management of dyslipidemia for cardiovascular disease risk reduction: synopsis of the 2020 Updated U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guideline. Ann Intern Med. (2020) 173:822–9. doi: 10.7326/M20-4648
23. Willeit P, Tschiderer L, Allara E, Reuber K, Seekircher L, Gao L, et al. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: meta-analysis of 119 clinical trials involving 100 667 patients. Circulation. (2020) 142:621–42. doi: 10.1161/CIRCULATIONAHA.120.046361
24. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. (2010) 55:1318–27. doi: 10.1016/j.jacc.2009.10.061
25. Koivistoinen T, Hutri-Kähönen N, Juonala M, Kööbi T, Aatola H, Lehtimäki T, et al. Apolipoprotein B is related to arterial pulse wave velocity in young adults: the Cardiovascular Risk in Young Finns Study. Atherosclerosis. (2011) 214:220–4. doi: 10.1016/j.atherosclerosis.2010.10.037
26. Juonala M, Viikari JS, Kähönen M, Solakivi T, Helenius H, Jula A, et al. Childhood levels of serum apolipoproteins B and A-I predict carotid intima-media thickness and brachial endothelial function in adulthood: the cardiovascular risk in young Finns study. J Am Coll Cardiol. (2008) 52:293–9. doi: 10.1016/j.jacc.2008.03.054
27. Pei WD, Sun YH, Liu Q, Zheng WY, Zhang J, Zhang CY, et al. Associations of apolipoprotein B with pulse pressure and glucose in Chinese families with familial combined hyperlipidemia. Int J Cardiol. (2007) 115:293–6. doi: 10.1016/j.ijcard.2006.03.005
28. Martin SS, Qasim AN, Mehta NN, Wolfe M, Terembula K, Schwartz S, et al. Apolipoprotein B but not LDL cholesterol is associated with coronary artery calcification in type 2 diabetic whites. Diabetes. (2009) 58:1887–92. doi: 10.2337/db08-1794
29. Holewijn S, den Heijer M, Swinkels DW, Stalenhoef AF, de Graaf J. Apolipoprotein B, non-HDL cholesterol and LDL cholesterol for identifying individuals at increased cardiovascular risk. J Intern Med. (2010) 268:567–77. doi: 10.1111/j.1365-2796.2010.02277.x
30. Wilkins JT, Li RC, Sniderman A, Chan C, Lloyd-Jones DM. Discordance between apolipoprotein B and LDL-Cholesterol in young adults predicts coronary artery calcification: the CARDIA study. J Am Coll Cardiol. (2016) 67:193–201. doi: 10.1016/j.jacc.2015.10.055
31. Qu G, Zhang Z, Zhu H. Discordance between apolipoprotein B or non-HDL-cholesterol and LDL-cholesterol in middle-aged and elderly Chinese patients predicts arterial stiffness. Lipids Health Dis. (2021) 20:80. doi: 10.1186/s12944-021-01509-6
32. Nordestgaard BG, Wootton R, Lewis B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler Thromb Vasc Biol. (1995) 15:534–42. doi: 10.1161/01.atv.15.4.534
33. Duran EK, Aday AW, Cook NR, Buring JE, Ridker PM, Pradhan AD. Triglyceride-rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J Am Coll Cardiol. (2020) 75:2122–35. doi: 10.1016/j.jacc.2020.02.059
34. Castañer O, Pintó X, Subirana I, Amor AJ, Ros E, Hernáez A, et al. Remnant cholesterol, not LDL Cholesterol, is associated with incident cardiovascular disease. J Am Coll Cardiol. (2020) 76:2712–24. doi: 10.1016/j.jacc.2020.10.008
35. Carr SS, Hooper AJ, Sullivan DR, Burnett JR. Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment. Pathology. (2019) 51:148–54. doi: 10.1016/j.pathol.2018.11.006
36. Takahashi R, Imamura A, Yoshikane M, Suzuki M, Murakami R, Cheng XW, et al. Very small low-density lipoprotein cholesterol level is a determinant of arterial stiffness in men with impaired glucose metabolism. J Atheroscler Thromb. (2010) 17:1282–9. doi: 10.5551/jat.5272
37. Hirano T, Ito Y, Yoshino G. Measurement of small dense low-density lipoprotein particles. J Atheroscler Thromb. (2005) 12:67–72. doi: 10.5551/jat.12.67
38. Galeano NF, Al-Haideri M, Keyserman F, Rumsey SC, Deckelbaum RJ. Small dense low density lipoprotein has increased affinity for LDL receptor-independent cell surface binding sites: a potential mechanism for increased atherogenicity. J Lipid Res. (1998) 39:1263–73. doi: 10.1016/s0022-2275(20)32551-7
39. Chait A, Brazg RL, Tribble DL, Krauss RM. Susceptibility of small, dense, low-density lipoproteins to oxidative modification in subjects with the atherogenic lipoprotein phenotype, pattern B. Am J Med. (1993) 94:350–6. doi: 10.1016/0002-9343(93)90144-e
40. Goldstein JL, Brown MS. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. (2015) 161:161–72. doi: 10.1016/j.cell.2015.01.036
41. Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. (2009) 302:1993–2000. doi: 10.1001/jama.2009.1619
42. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
Keywords: discordance, apolipoprotein B, non-high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, arterial stiffness, carotid intima-media thickness
Citation: Jia X, Qi Y, Zheng R, Lin L, Hu C, Zhu Y, Cao Q, Wu X, Qi H, Wei R, Zhang Y, Xu M, Xu Y, Wang T, Zhao Z, Chen Y, Li M, Wang W, Bi Y and Lu J (2022) Discordance of Apolipoprotein B, Non-HDL-Cholesterol, and LDL-Cholesterol Predicts Risk of Increased Arterial Stiffness and Elevated Carotid Intima-Media Thickness in Middle-Aged and Elderly Chinese Adults. Front. Cardiovasc. Med. 9:906396. doi: 10.3389/fcvm.2022.906396
Received: 28 March 2022; Accepted: 21 April 2022;
Published: 18 May 2022.
Edited by:
Ichiro Sakuma, Hokko Memorial Hospital, JapanReviewed by:
Yasutaka Takeda, Asahikawa Medical University, JapanTatsuya Sato, Sapporo Medical University, Japan
Copyright © 2022 Jia, Qi, Zheng, Lin, Hu, Zhu, Cao, Wu, Qi, Wei, Zhang, Xu, Xu, Wang, Zhao, Chen, Li, Wang, Bi and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jieli Lu, amllbGlsdUBob3RtYWlsLmNvbQ==; Yufang Bi, YnlmMTA3ODRAcmpoLmNvbS5jbg==
†These authors have contributed equally to this work and share first authorship
 Xiaojing Jia
Xiaojing Jia Yan Qi1,2†
Yan Qi1,2† Ruizhi Zheng
Ruizhi Zheng Lin Lin
Lin Lin Chunyan Hu
Chunyan Hu Qiuyu Cao
Qiuyu Cao Yi Zhang
Yi Zhang Min Xu
Min Xu Tiange Wang
Tiange Wang Zhiyun Zhao
Zhiyun Zhao Mian Li
Mian Li Weiqing Wang
Weiqing Wang Yufang Bi
Yufang Bi Jieli Lu
Jieli Lu