- 1Department of Surgical Intensive Care Unit, Huaqiao University Affiliated Strait Hospital, Quanzhou, China
- 2Department of General Surgery, Huaqiao University Affiliated Strait Hospital, Quanzhou, China
- 3School of Medicine, Huaqiao University, Quanzhou, China
- 4Department of Burn, Huaqiao University Affiliated Strait Hospital, Quanzhou, China
- 5Key Laboratory of Intelligent Computing and Information Processing, Quanzhou Normal University, Quanzhou, China
Background: Severely burned children are at high risk of secondary intraabdominal hypertension and abdominal compartment syndrome (ACS). ACS is a life-threatening condition with high mortality and requires an effective, minimally invasive treatment to improve the prognosis when the condition is refractory to conventional therapy.
Case presentation: A 4.5-year-old girl was admitted to our hospital 30 h after a severe burn injury. Her symptoms of burn shock were relieved after fluid resuscitation. However, her bloating was aggravated, and ACS developed on Day 5, manifesting as tachycardia, hypoxemia, shock, and oliguria. Invasive mechanical ventilation, vasopressors, and percutaneous catheter drainage were applied in addition to medical treatments (such as gastrointestinal decompression, diuresis, sedation, and neuromuscular blockade). These treatments did not improve the patient's condition until she received continuous renal replacement therapy. Subsequently, her vital signs and laboratory data improved, which were accompanied by decreased intra-abdominal pressure, and she was discharged after nutrition support, antibiotic therapy, and skin grafting.
Conclusion: ACS can occur in severely burned children, leading to rapid deterioration of cardiopulmonary function. Patients who fail to respond to conventional medical management should be considered for continuous renal replacement therapy.
Case Presentation
A 4.5-year-old girl was transferred to the emergency department of our tertiary care center, with burns covering 40% of her body surface area from boiling water. She received no intravenous fluid administration within 30 h post-scalding and complained of tachycardia, dizziness, weakness, and oliguria. Her physical examination at admission showed that her blood pressure was 98/73 mmHg, her body temperature was 37.3°C, her pulse rate was 164 beats per minute, and her respiration rate was 25 breaths per minute. The pulse oxygen oximeter read 95% on room air. The patient presented with clammy extremities and an increased capillary refill time. While receiving appropriate first aid and wound assessment, she was resuscitated immediately using lactated ringer's injection based on a potential diagnosis of burn shock from her focused history taking, signs, and symptoms. Then, the patient was quicikly and gently transferred to the burn center of our hospital for further treatment.
Laboratory investigations revealed a serum creatinine level of 97.3 μmol/L and an arterial blood serum lactate level of 5.2 mmol/L. These data indicated acute kidney injury induced by hypovolemic shock and confirmed the fluid resuscitation requirement. After resuscitation to correct dangerous deficits in accordance with the Parkland Formula, the patient's vital signs, mental status, capillary refill time, and serum creatinine level improved, and reached a target of 0.5–1.0 ml/kg/h−1 of urine output, indicating adequate fluid resuscitation. Then, the fluid rates were adjusted accordingly based on the monitoring results. The patient's condition improved as expected within the first 2 days. When the patient showed signs of bloating on the 3rd day, a nasogastric tube was inserted, and gastrointestinal prokinetic agents were administered to prevent abdominal over distension. During the aggravation of bloating on the 4th day, the volume of fluid administration was increased in accordance with the trend of decreased urine output. The daily fluid balances are summarized in Figure 1. On the 5th day of hospitalization, she developed hypoxemia, tachypnoea, hypotension, and oliguria. Blood pressure was 75/53 mmHg, body temperature was 37.2°C, pulse rate was 155 beats per minute, and respiration rate was 45 breaths per minute. The PaO2/FiO2 ratio was 126 mmHg, with 10 L/min oxygen flow delivered by nasal cannula. Her intra-abdominal pressure (IAP) increased to and remained above 15 mmHg as measured from the intrabladder pressure. The central venous pressure increased to a level above 14 cmH2O, the extravascular lung water index increased to >10 ml/kg, and the B-type natriuretic peptide level was >35,000 pg/ml. The urine output was <0.3 ml/kg·h−1, with serum creatinine at 81 μmol/L. The patient was unresponsive to furosemide. These findings indicated the development of secondary abdominal compartment syndrome (ACS), coupled with refractory fluid overload. The patient was intubated and mechanically ventilated immediately, and norepinephrine (1.6 μg/kg·min−1) was administered to maintain mean arterial pressure above 70 mmHg. A neuromuscular blocking agent (cisatracurium besylate) was administered upon sedation and analgesia to improve thoracic and abdominal wall compliance. To manage the increased IAP, a percutaneous catheter was inserted into the abdominal cavity for drainage with ultrasound guidance, and 1,100 ml of fluid was drained within 28 h. The IAP decreased to 11 mmHg, but the clinical condition did not improve. Then, continuous renal replacement therapy (CRRT) was performed with an ultrafiltration flow rate of 20–25 ml/kg·h−1. After 40 h of hemofiltration, 5,080 ml of fluid was removed in total. IAP declined to 7 mmHg immediately and then dropped to 5 mmHg. The patient's vital signs subsequently stabilized, the B-type natriuretic peptide level decreased to 5,204 pg/ml, and urine output increased to 1.3 ml/kg·h−1. CRRT was terminated on Day 7, and mechanical ventilation was weaned on Day 8. The trends of the laboratory tests and vital parameters are presented in Figure 2. After recovering from ACS, the patient continued to improve under routine enteral nutrition support and antibiotic therapy. No significant infections were observed. Skin grafting was performed on Day 17. The patient fully recovered and was discharged from the hospital 34 days after her admission.
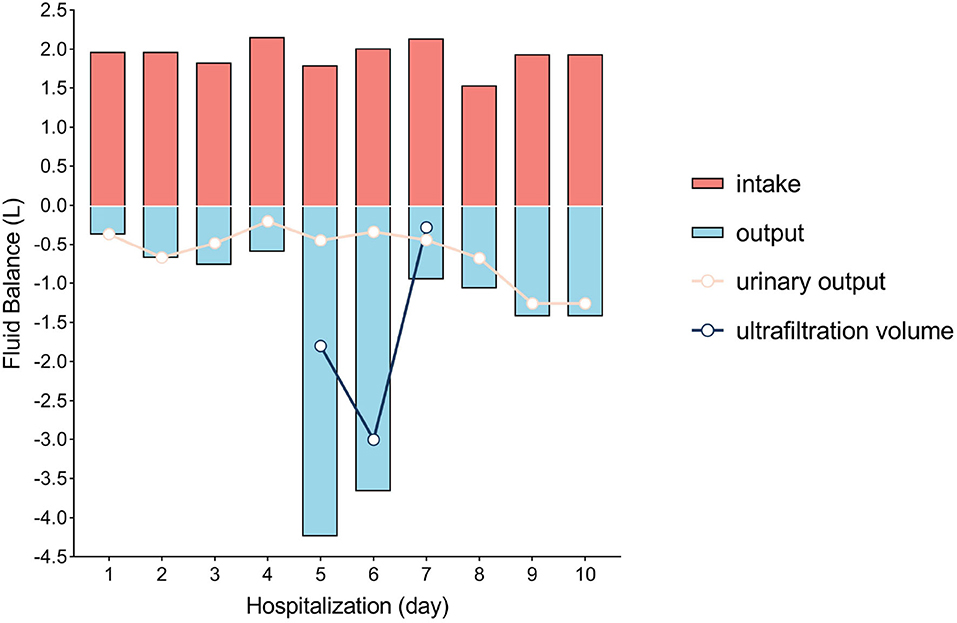
Figure 1. Fluid balance in the first 10 days of hospitalization. Day 1 was defined as the time between hospital admission and the next morning (14 h).
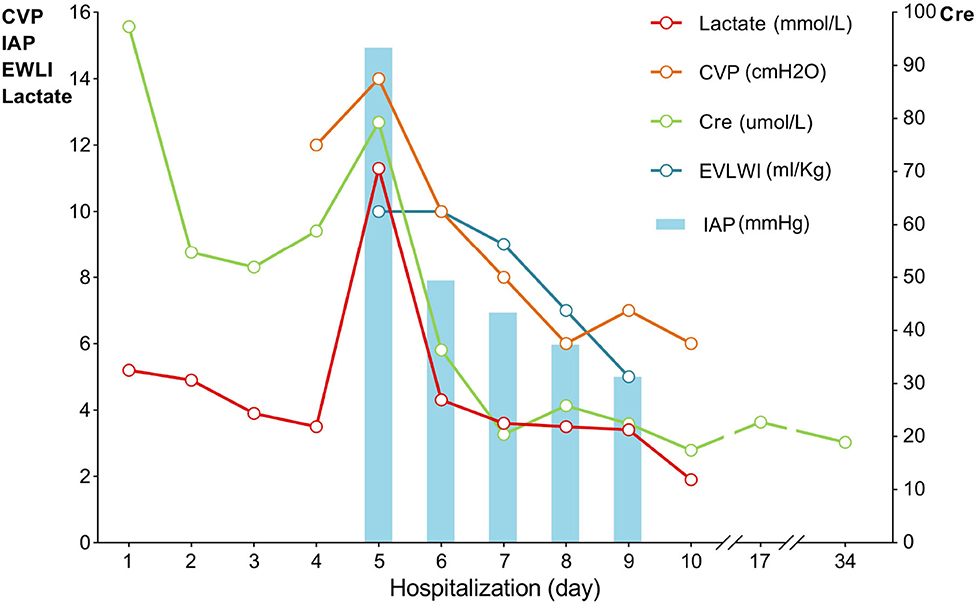
Figure 2. Trends of vital parameters during hospitalization. CVP, central venous pressure; IAP, intra-abdominal pressure; EVLWI, extravascular lung water index; Lac, lactate, Cre, serum creatinine.
Discussion
ACS in children is defined as a sustained elevated IAP (>10 mmHg) associated with new-onset or worsening organ dysfunction (1). Secondary ACS occurs in the absence of injury or disease in the abdominal or pelvic area. A study showed that 10–30% of patients with a burn injury, covering more than 20% of the total body surface, develop secondary ACS, and the mortality ranges from 40 to 100% (2–6).
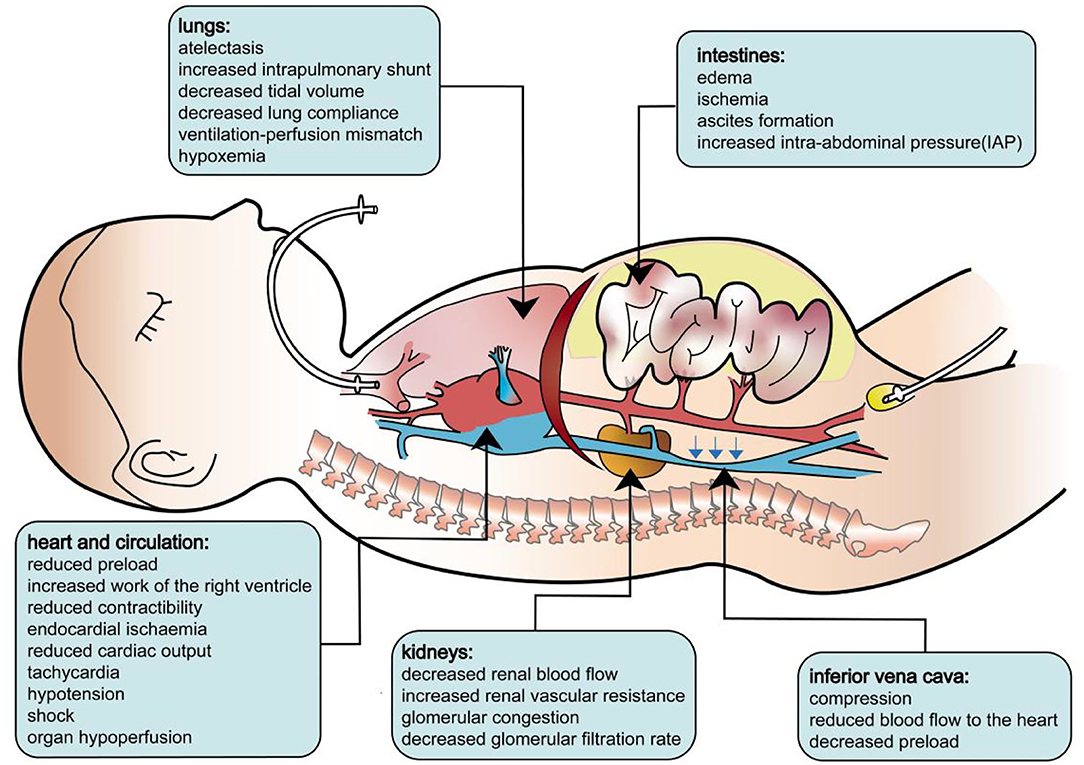
The pathophysiology of secondary ACS is identical to that of primary ACS. As the IAP increases, cardiac output is reduced as a result of decreased central venous return and a consequently diminished right ventricular end-diastolic volume. Initially, high IAP increases systemic vascular resistance, and a “normal” blood pressure may be observed. Paradoxically, intracardiac filling pressures, such as pulmonary artery occlusion pressure and central venous pressure, typically increase with a rising IAP despite the reduced venous return and cardiac output. Then, the increased after load will undermine the contractibility of the cardiac muscle, tampering with the cardiac output. In clinical settings, this will manifest as tachycardia and shock. Beyond the heart, ACS also affects the lungs, kidneys, and other organs. During ACS, the diaphragm shifts cranially, leading to lower respiratory compliance, which increases the effort needed for breathing and the mismatch of perfusion and ventilation. Patients will present with elevated peak pressures, a decreased P/F ratio, hypoxemia, hypercarbia, and atelectasis. IAH may significantly compress the kidney and diminish renal perfusion. Studies have shown that acute renal dysfunction may develop even at relatively low levels of IAP. Renal dysfunction presents as oliguria, progressing to anuria due to a reduced glomerular filtration rate. In addition, ACS also leads to mesenteric, gastrointestinal, and neurological complications secondary to decreased cardiac output and direct compression from IAH (7–10). The pathophysiology of ACS is illustrated in Figure 3.
The mechanism of secondary ACS can be related to visceral, peritoneal, and retroperitoneal edema induced by inflammation or fluid resuscitation (11), which are commonly reported in burned children (12). Burn injury is characterized by a hypermetabolic response with physiological, catabolic, and immune effects. Burn areas larger than 15% of the total body surface will lead to a systemic inflammatory response, resulting in disruption of the endothelial glycocalyx, as well as alterations in the structure and function of the extracellular matrix (13–15). This will increase vascular permeability and promote the leakage of plasma fluid to the extracellular space and the interstitial compartment (16). Delayed or insufficient fluid resuscitation may lead to burn shock, poor tissue perfusion, multiple organ dysfunction, and death (17); moreover, fluid overload also results in edema associated with organ dysfunction (as depicted in Figure 4). However, commonly applied fluid resuscitation strategies may be complicated by swelling of the viscera due to inflammation and resuscitation per se. Excessive fluid resuscitation, typically using too much crystalloid, may lead to ACS and pulmonary edema (18). This is known as the concept of “fluid creep,” (19) which is reported in 30–90% of severely burned patients (20). Therefore, aggressive fluid resuscitation must be balanced against the possibility of “fluid creep”-induced secondary ACS. The life-threatening ACS in this patient could have resulted from delayed fluid resuscitation, severe burns, and fluid overload.
ACS in burn patients usually develops with three particular events: ~4 days post-injury, following a surgical procedure, and during a period of sepsis. In our case, the child developed ACS 5 days following the burn injury. It is important to know that a reduced urine output or a raised serum lactate may not be because of hypovolemia, IAH could also be a potential cause. Early detection of IAH allows physicians to intervene before the development of ACS. A routine fluid responsiveness assessment [such as passive leg-raising technique (21) and measurement of the inferior vena cava diameters by ultrasound (22)] is helpful for detecting hypovolemia. However, this would become complex and challenging in the advent of ACS. In patients with persistent oliguria and negative fluid responsiveness, IAP should be measured, especially in the aforementioned three circumstances. Current methods of IAP measurement and their reliability have been comprehensively reviewed (1). In brief, bladder pressure (at the end of expiration) is recommended for patients who require ongoing monitoring. In addition, ultrasound may be a useful tool for identifying compression and evaluating bowel movement and abdominal and bowel contents, but its reliability and feasibility in the diagnosis of ACS require further investigation.
Once the diagnosis of ACS is established, medical management should focus on three key areas: management of intraluminal contents, management of the abdominal wall, and management of systemic fluid balance. A comprehensive review of these medical management strategies has been published previously (1). When medical management fails, further advanced management should be considered (1, 23). In general, emergency decompressive surgery is considered but has high morbidity and mortality (24). Some evidence has shown the effectiveness of percutaneous drainage in burned patients with ACS, and this procedure is supported by the World Society of Abdominal Compartment Syndrome (1). In our case, ultrasound-guided percutaneous catheter drainage was successfully performed, with a significant decrease in IAP from 15 to 11 mmHg. However, the clinical condition of the patient did not improve.
CRRT is commonly used for critically ill patients with acute renal failure, fluid overload, and sepsis. In adult burned patients, the effectiveness of CRRT has been reported in reversing septic shock and improving acute renal failure (25). However, its effect on pediatric burned patients is unknown. To the best of our knowledge, this is the first case of the use of CRRT in a pediatric burned patient with ACS. In our case, the patient was prescribed a dose of 20–25 ml/kg·h−1 ultrafiltration for 40 h. The excessive fluid was successfully removed, accompanied by a decrease in B-type natriuretic peptide levels, and ACS was reversed. Our case showed that, in pediatric burned patients, CRRT can be beneficial by effectively removing inflammatory mediators, excessive fluid, and accumulated metabolic products while minimizing the effect on hemodynamics. Compared to decompressive laparotomy, CRRT provides a less invasive and promising measure for secondary ACS in severely burned children.
Conclusion
In severely burned children, secondary ACS can develop after a few days of fluid resuscitation, which requires routine IAP monitoring in these patients. In patients with refractory ACS, CRRT could be considered when other medical treatments fail. This report highlights the role of the interprofessional team in managing severely burned patients.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Ethics Research Committee of Huaqiao University Affiliated Strait Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author Contributions
SD and CH designed the research. JS, RL, and SH performed the research. JZ, ZP, RS, JC, and YA collected clinical data. JS, XZ, and HL analyzed the data. JS was responsible for patient treatment and drafted the manuscript. CZ and JH reviewed and revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by Science and Technology Program of Quanzhou (Grant Nos. 2021CT0010, 2019C080R, and 2021N003S).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank all of the doctors, nurses, technicians, and the patient involved at the participating center for their dedication to the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.904400/full#supplementary-material
References
1. Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the world society of the abdominal compartment syndrome. Intensive Care Med. (2013) 39:1190–206. doi: 10.1007/s00134-013-2906-z
2. Wise R, Jacobs J, Pilate S, Jacobs A, Peeters Y, Vandervelden S, et al. Incidence and prognosis of intra-abdominal hypertension and abdominal compartment syndrome in severely burned patients: pilot study and review of the literature. Anaesthesiol Intensive Ther. (2016) 48:95–109. doi: 10.5603/AIT.a2015.0083
3. Strang SG, Van Lieshout EM, Breederveld RS, Van Waes OJ. A systematic review on intra-abdominal pressure in severely burned patients. Burns. (2014) 40:9–16. doi: 10.1016/j.burns.2013.07.001
4. Ramirez JI, Sen S, Palmieri TL, Greenhalgh DG. Timing of laparotomy and closure in burn patients with abdominal compartment syndrome: effects on survival. J Am Coll Surg. (2018) 226:1175–80. doi: 10.1016/j.jamcollsurg.2018.03.032
5. Steinau G, Kaussen T, Bolten B, Schachtrupp A, Neumann UP, Conze J, et al. Abdominal compartment syndrome in childhood: diagnosis, therapy and survival rate. Pediatr Surg Int. (2011) 27:399–405. doi: 10.1007/s00383-010-2808-x
6. Ejike JC, Mathur M, Moores DC. Abdominal compartment syndrome: focus on the children. Am Surg. (2011) 77 (Suppl. 1):S72–7.
7. Mohmand H, Goldfarb S. Renal dysfunction associated with intra-abdominal hypertension and the abdominal compartment syndrome. J Am Soc Nephrol. (2011) 22:615–21. doi: 10.1681/ASN.2010121222
8. De Waele JJ, De Laet I, Malbrain ML. Understanding abdominal compartment syndrome. Intensive Care Med. (2016) 42:1068–70. doi: 10.1007/s00134-015-4089-2
9. De Waele JJ, De Laet I, Kirkpatrick AW, Hoste E. Intra-abdominal hypertension and abdominal compartment syndrome. Am J Kidney Dis. (2011) 57:159–69. doi: 10.1053/j.ajkd.2010.08.034
10. Dalfino L, Tullo L, Donadio I, Malcangi V, Brienza N. Intra-abdominal hypertension and acute renal failure in critically ill patients. Intensive Care Med. (2008) 34:707–13. doi: 10.1007/s00134-007-0969-4
11. Ball CG, Kirkpatrick AW, McBeth P. The secondary abdominal compartment syndrome: not just another post-traumatic complication. Can J Surg. (2008) 51:399–405.
12. Greenhalgh DG, Warden GD. The importance of intra-abdominal pressure measurements in burned children. J Trauma. (1994) 36:685–90. doi: 10.1097/00005373-199405000-00015
13. Fernández-Sarmiento J, Salazar-Peláez LM, Carcillo JA. The endothelial glycocalyx: a fundamental determinant of vascular permeability in sepsis. Pediatr Crit Care Med. (2020) 21:e291–300. doi: 10.1097/PCC.0000000000002266
14. Joffre J, Hellman J, Ince C, Ait-Oufella H. Endothelial responses in sepsis. Am J Respir Crit Care Med. (2020) 202:361–70. doi: 10.1164/rccm.201910-1911TR
15. Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. (2014) 10:37–47. doi: 10.1038/nrneph.2013.232
16. Williams FN, Herndon DN, Jeschke MG. The hypermetabolic response to burn injury and interventions to modify this response. Clin Plast Surg. (2009) 36:583–96. doi: 10.1016/j.cps.2009.05.001
17. Schulman CI, King DR. Pediatric fluid resuscitation after thermal injury. J Craniofac Surg. (2008) 19:910–2. doi: 10.1097/SCS.0b013e318175b566
18. Zak AL, Harrington DT, Barillo DJ, Lawlor DF, Shirani KZ, Goodwin CW. Acute respiratory failure that complicates the resuscitation of pediatric patients with scald injuries. J Burn Care Rehabil. (1999) 20:391–9. doi: 10.1097/00004630-199909000-00011
19. Saffle JI. The phenomenon of “fluid creep” in acute burn resuscitation. J Burn Care Res. (2007) 28:382–95. doi: 10.1097/BCR.0B013E318053D3A1
20. Saffle JR. Fluid creep and over-resuscitation. Crit Care Clin. (2016) 32:587–98. doi: 10.1016/j.ccc.2016.06.007
21. Monnet X, Marik P, Teboul JL. Passive leg raising for predicting fluid responsiveness: a systematic review and meta-analysis. Intensive Care Med. (2016) 42:1935–47. doi: 10.1007/s00134-015-4134-1
22. Orso D, Paoli I, Piani T, Cilenti FL, Cristiani L, Guglielmo N. Accuracy of ultrasonographic measurements of inferior vena cava to determine fluid responsiveness: a systematic review and meta-analysis. J Intensive Care Med. (2020) 35:354–63. doi: 10.1177/0885066617752308
23. De Laet IE, Malbrain MLNG, De Waele JJ. A clinician's guide to management of intra-abdominal hypertension and abdominal compartment syndrome in critically ill patients. Crit Care. (2020) 24:97. doi: 10.1186/s13054-020-2782-1
24. di Natale A, Moehrlen U, Neeser HR, Zweifel N, Meuli M, Mauracher AA, et al. Abdominal compartment syndrome and decompressive laparotomy in children: a 9-year single-center experience. Pediatr Surg Int. (2020) 36:513–21. doi: 10.1007/s00383-020-04632-0
25. Chung KK, Coates EC, Smith DJ Jr, Karlnoski RA, Hickerson WL, Arnold-Ross AL, et al. Randomized controlled evaluation of high-volume hemofiltration in adult burn patients with Septic shoCk and acUte kidnEy injury (RESCUE) investigators. High-volume hemofiltration in adult burn patients with septic shock and acute kidney injury: a multicenter randomized controlled trial. Crit Care. (2017) 21:289. doi: 10.1186/s13054-017-1878-8
Keywords: tachycardia, hypoxemia, shock, abdominal compartment syndrome, pediatric, severe burns, continuous renal replacement therapy
Citation: Shi J, Huang C, Zheng J, Ai Y, Liu H, Pan Z, Chen J, Shang R, Zhang X, Dong S, Lin R, Huang S, Huang J and Zhang C (2022) Case Report: Tachycardia, Hypoxemia and Shock in a Severely Burned Pediatric Patient. Front. Cardiovasc. Med. 9:904400. doi: 10.3389/fcvm.2022.904400
Received: 25 March 2022; Accepted: 19 May 2022;
Published: 16 June 2022.
Edited by:
Ruizheng Shi, Central South University, ChinaReviewed by:
Zhonghua Shi, Academic Medical Center, NetherlandsJunping Tian, Capital Medical University, China
Copyright © 2022 Shi, Huang, Zheng, Ai, Liu, Pan, Chen, Shang, Zhang, Dong, Lin, Huang, Huang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianlong Huang, cm9ib3RpY3MmI3gwMDA0MDtxenRjLmVkdS5jbg==; Chenghua Zhang, emNoMTgwJiN4MDAwNDA7MjYzLm5ldA==
†These authors have contributed equally to this work
 Jianshe Shi
Jianshe Shi Chuheng Huang1†
Chuheng Huang1† Jianlong Huang
Jianlong Huang