
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 20 June 2022
Sec. Atherosclerosis and Vascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.904250
Background and Purpose: Multiple guidelines suggest the ω-3 polyunsaturated fatty acids (ω-3 PUFAs) help to prevent major vascular events of coronary heart disease (CHD), but the data on large trials of ω-3 fatty acids are controversial. We reviewed the available evidence to determine the effect of ω-3 PUFAs on coronary atherosclerosis.
Materials and Methods: Literature were from online databases. Randomized controlled trials (RCTs) or observational studies were acceptable. Quantitative data synthesis was conducted using R version 4.1.2. Each outcome was calculated using standardized mean difference (SMD) in a random-effect model. Sensitivity analysis was conducted for each outcome. A total of 21 RCTs and 1 observational study with 2,277 participants were included.
Results: Meta-analysis indicated a benefit of ω-3 PUFAs on coronary atherosclerosis, namely, (1) ω-3 PUFAs can reduce the atherosclerotic plaque volume (SMD −0.18; 95% CI −0.31 to −0.05); (2) ω-3 PUFAs can help reduce the loss of the diameter of the narrowest segments of coronary arteries in patients with CHD (SMD 0.29; 95% CI, 0.05–0.53); (3) ω-3 PUFAs do not have significant effect on volume of lipid plaque in coronary arteries (SMD −1.18; 95% CI −2.95 to 0.58), volume of fiber plaque (SMD 0.26; 95% CI −0.81 to 1.33), and calcified plaque (SMD 0.17; 95% CI −0.55 to 0.89); and (4) ω-3 PUFAs had no significant effect on endothelial inflammatory factors in peripheral blood.
Conclusions: We confirmed that ω-3 PUFAs benefit patients with CHD by reducing the progression of coronary atherosclerosis. We indicated that the benefits were not caused by reducing endothelial inflammations of coronary arteries.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021285139, identifier: CRD42021285139.
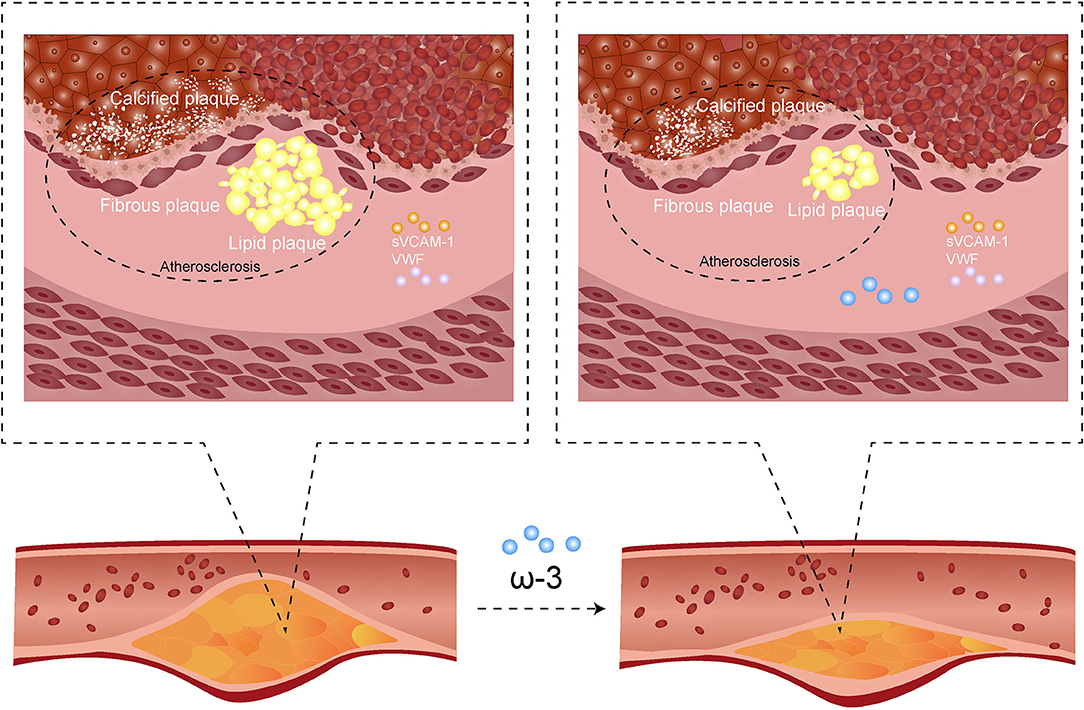
Graphical Abstract. In order to increase the exposure of our work, we made a graphical abstract: ω-3 PUFAs (ω-3 polyunsaturated fatty acid) reduce coronary atherosclerotic plaques growth and relieve the lesions process of the most stenotic segment of the coronary artery. However, the effects on all main compositions of atherosclerotic plaques (fiberboard, calcified plaque, and lipid plaque) are not statistically significant. Serum soluble vascular cell adhesion molecule-1 (sVCAM-1) level and activation percentage of von Willebrand factor (VWF%) are not significantly affected. The mechanism of ω-3 PUFAs on coronary atherosclerosis may need further studies.
Omega-3 polyunsaturated fatty acids (ω-3 PUFAs) are a class of PUFAs with the first unsaturated double bond between the third and fourth carbon atoms from the methyl end. ω-3 PUFAs have various effects on the cardiovascular system and can provide multiple health benefits for the cardiovascular system (1, 2) via various mechanisms, such as reduction in blood lipids and inflammatory cell aggregation (3–10), and the reduction of triglycerides in the blood is thought to be the main benefit (11). Multiple studies have investigated the association between ω-3 PUFAs supplementation and less coronary atherosclerotic “high-risk” plaque. In fact, atherosclerotic plaques can be mainly classified into four different types, namely, calcified tissue, necrotic or soft tissue, fibrous tissue, and mixed tissue (12, 13). In recent years, many studies tried to determine whether ω-3 PUFAs have a protective effect on coronary atherosclerosis in patients with coronary heart disease (CHD). However, the results among different studies were controversial. For example, Balk et al. (14) reported that ω-3 PUFAs showed prevented effect on restenosis after percutaneous transluminal coronary intervention (PCI). In contrast, Laake et al. (15) suggested that ω-3 PUFAs had no significant clinical effect on CHD. Previous meta-analyses only investigated the relation between ω-3 PUFAs supplementation and major vascular events of CHD. However, there is a lack of information focusing on the effect of ω-3 PUFAs on the pathology of plaques and inflammation level. Therefore, this meta-analysis systematically summarized and analyzed the effects of ω-3 PUFAs on coronary atherosclerosis and inflammation level of vascular endothelial.
This meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (16) and Cochrane's guidelines (17). It has been registered in the international Prospective Register Of Systematic Reviews (CRD42021285139). The review protocol can be accessed at https://www.crd.york.ac.uk/PROSPERO/. We strictly followed the protocol while conducting this review. All the included studies were confirmed to be free of ethical and moral concerns. Data for the review were analyzed anonymously. So, the need for consent was waived by the ethics committee.
Three reviewers searched articles from Embase database, Web of Science, PubMed, Cochrane Library, Clinical Trial, and China National Knowledge Infrastructure (CNKI) (published from February 1979 to September 2021) for published randomized clinical trials (RCTs) and controlled clinical trials, aiming to assess the effect of ω-3 PUFAs on coronary atherosclerosis in patients with CHD. We used atherosclerotic plaques, ω-3 polyunsaturated fatty acids, vessels, blood, fibroatheroma, and coronary heart diseases as the keywords for the literature search.
The eligibility criteria were (1) interventions for RCTs should be eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) at a total dose of no <1 g/day, as recommended to patients with CHD (18) since ω-3 PUFAs are mainly represented by EPA and DHA (19–21). (2) The subjects for coronary imaging should be patients diagnosed with CHD or those with a high risk of CHD determined by the original clinical researchers. The subjects should not include patients with multiple complications; for example, severe diabetes, heart failure, arrhythmia, hypertrophic cardiomyopathy, and cardiac syndrome X and kidney disease would be excluded. (3) All the data needed can be extracted from the research article. (4) Death cases were excluded from endpoint assessment. (5) We included only studies that all the follow-up work completed to ensure the whole data was available. (6) The number of follow-up patients should not be <15, which means the data could be calculated by Cohen's d effect size. (7) Studies on endothelial cell markers could include healthy individuals but with no platelet dysfunction. Studies of bed quality, those with inadequate sample size, incomplete follow-up works, or studies that did not meet the criteria were excluded. Disagreement in literature identifications was reported to another reviewer. The search strategy is provided in Figure 1; Supplementary Appendix 1.
The primary outcomes were (1) the volume increases in coronary atherosclerotic plaques and (2) reduction in the diameter of the narrowest segments of the coronary arteries. The secondary outcomes consisted of endothelial inflammatory factor levels in the peripheral blood [including activation of von Willebrand factor (VWF%) and content of soluble vascular cell adhesion molecule 1 (sVCAM-1)] and compositions of plaques (fiber plaque, lipid plaque, and calcified plaque).
Following the inclusion and exclusion criteria, three researchers (ZG, XY, DZ) screened, selected, and extracted data from studies independently. All the data should be extracted directly from the articles, in the tables or text. Data from images were not contained in this review. Any disagreement was reported to an experienced doctor (HS) to decide whether to include the literature. One reviewer (ZG) conducted data synthesis for all trials. Screening and selection of included trials are shown in a flow diagram (Figure 1).
Three authors (GZ, YXC, and ZDW) independently extracted clinical variables and outcome data using the retrieval format of population, intervention, control, and outcomes (PICO), i.e., (1) basic information, including publishing year, authors, country, and journal; (2) population: age, gender, complications, country, body mass, history of heart surgery, history of other medications use like stains, and blood lipid levels. We excluded studies with (3) interventions: placebos, type of ω-3 PUFAs, treatment duration of ω-3 PUFAs. (4) Outcome: volume of coronary atherosclerotic plaques and their compositions, loss of diameter of the narrowest segments of the coronary arteries, the content of sVCAM-1, and VWF%. (5) Designs: intergroup or intragroup RCT.
All included trials were assessed for risk of bias using the following quality scales: (1) bias arising from the randomization process; (2) bias due to deviations from intended interventions; (3) bias due to missing outcome data; (4) bias in measurement of the outcome; and (5) bias in selection of the reported result. Each potential source of bias was graded into three levels, namely, “low,” “some concerns,” and “high.” The quality assessments were performed using R software (version 4.1.1) under the guidance of the Cochrane Handbook (17) and RoB2 (22), the revised Cochrane risk-of-bias tool for randomized trials.
Heterogeneity among studies was estimated using Cochran's Q test and quantified by the I2 statistic. Each outcome was calculated using a standardized mean difference (SMD) in a random effect model. I2 values of 25, 50, and 75% were thought to indicate a low, moderate, or high heterogeneity (23). We used Cohen's d as the effect for p-values and 95% confidence intervals. Statistical significance was determined using a two-sided α < 0.05. Funnel plots and peter's tests were employed to estimate publication bias. Meta-regression analyses were used to detect possible sources of heterogeneity. Potential publication biases were further determined by the function “trim and fill.” Publication bias was considered significant when the trimmed result led to an inconsistent conclusion. Sensitivity analysis was performed to determine heterogeneity and individual studies' influence on overall estimates. Studies were serially excluded using the function “metainf” for the sensitivity analyses. The included studies in each group were excluded one by one in the method. All data analyses were conducted using R version 4.1.2, using the package “meta” (R Project for Statistical Computing) (R Core Team. R: a language and environment for statistical computing. Vienna R Foundation for Statistical Computing; 2019. https://www.R-project.org).
The results are described based on “Per-Protocol analyses” (24). For the studies available, we adjusted for factors, such as age and body mass index. For outcomes with significant heterogeneity, meta-regression analyses were used to detect possible sources of heterogeneity. We mainly focused on the average age of the population and the duration of follow-up, which were believed to be major factors affecting the effects of medicines.
A flow diagram of this systematic review is shown in Figure 1. Studies that had not met the inclusion criteria mentioned above were excluded. The literature search yielded 3,879 studies, of which 22 studies (24–45) with 2,277 participants were included in this systematic review and meta-analysis (Table 1). Of the 22 included studies, 21 (24–31, 33–45) were RCTs and 1 was an observational study (32) (Table 1). All follow-up work was completed when studies were identified.
Of the 22 studies, 2 (9.09%) were at a high risk of bias, 4 (18.18%) were at “some concerns,” and 16 (72.73%) were at low risk of bias (Figure 2). Studies with one domain of high risk and only one domain of “some concerns” were thought to be at “some concerns.” Studies with one “high-risk” domain and two or more domains of “some concerns” were thought to be at “high risk.” Studies with two or more domains of “high risk” were excluded. A comprehensive assessment of the included studies suggests the present evidence of well credibility.
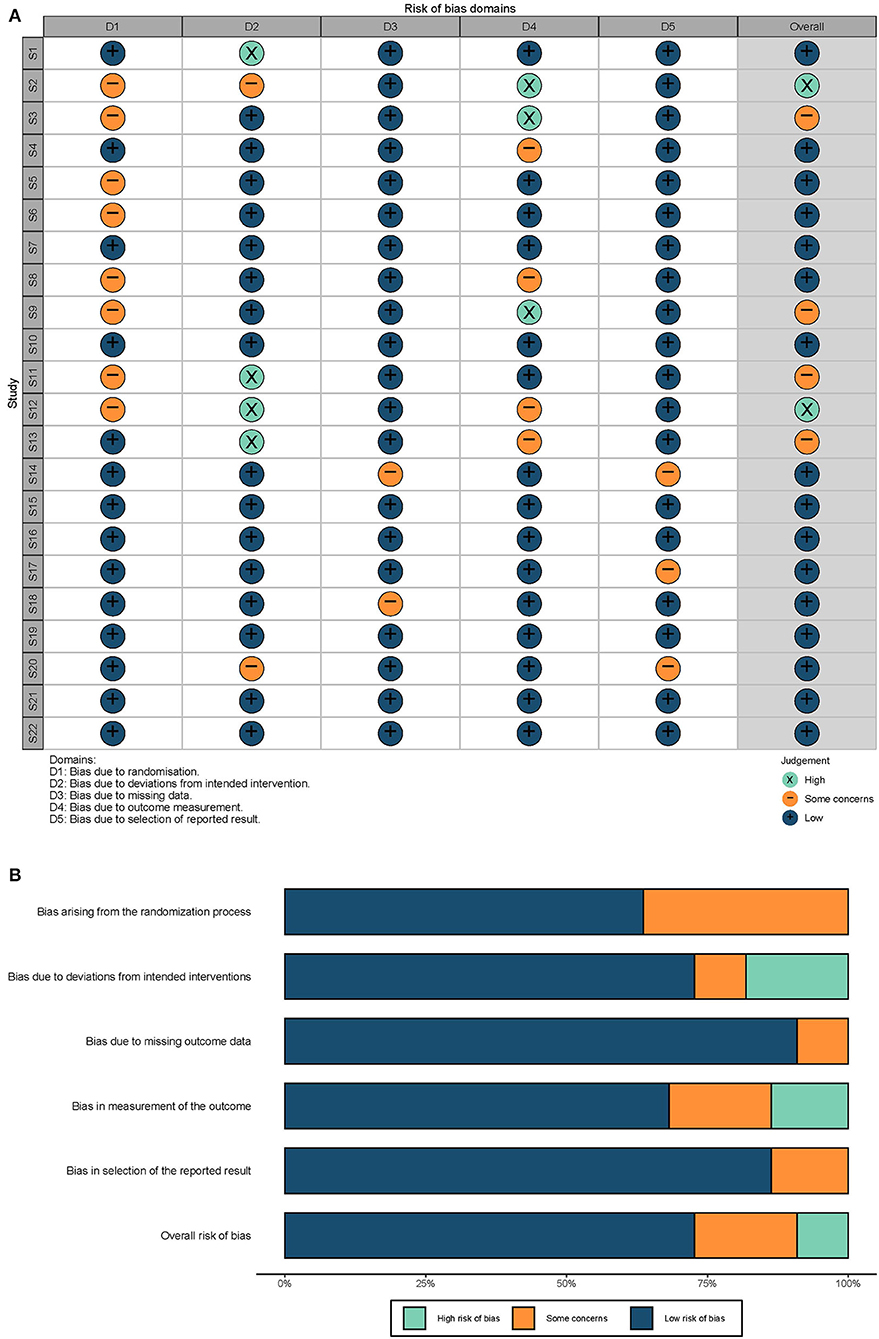
Figure 2. Quality evaluations. (A) Risk biases of each study. S1–S22: 22 studies included in this review, serially. The overall risk was evaluated based on the criteria described previously. (B) Summary of risk biases. The length of each color represents the proportion of the risk.
The mean age of individuals included in the studies was over 50 years old except for one (40), if characteristics were available. All available studies enrolled mainly male participants. Placebos was used in 19 studies (24, 25, 27, 29, 31–45) as the controls, and dietary interventions were used in 3 (26, 28, 30) studies. One observational study (32) used EPA index of 1.9 as the basis for dividing observation and control group. Plaque volume in related studies was measured using imaging techniques, including coronary computed tomographic angiography (24, 35, 38), optical coherence tomography (33, 34), and intravascular ultrasound (25, 32, 38, 41, 44). Characteristics of the included studies were extracted by one of us (GZ), which are described in a table (studies are shown in Table 1). As shown in each forest plots, three studies were conducted in England (26, 28, 31), one in Korea (25), one in Canada (27), five in Japan (32–34, 41, 44), five in Norway (30, 36, 39, 40, 43), one in Iran (29), one in Germany (42), one in Finland (38), three in Israel (24, 35, 37) and one conducted in Finland and the United States (45).
A total of seven trials (24, 25, 32, 35, 38, 41, 44) with 947 participants investigated the correlation between ω-3 PUFAs and sizes of coronary atherosclerotic plaques. It was shown that ω-3 PUFAs could reduce the atherosclerotic plaque volume (SMD −0.18; 95% CI −0.31 to −0.05), with a low heterogeneity (I2 = 44%) (Figure 3). Omega-3 PUFAs' effect was significantly protective on atherosclerotic plaques when Niki's et al. (44) study or Ahn's et al. (25) study was excluded, same as shown in the sensitivity analysis (Supplementary Figure S1.1). Heterogeneity did not reduce significantly when studies were excluded serially (Supplementary Figure S1.2). We further explored whether the effect of ω-3 PUFAs supplementation on plaque volume was dose-related. The linear regression showed that there was no significant correlation between the dose of ω-3 PUFAs supplementation and plaque volume change (R2 = 0.09, p = 0.29), same as shown in Supplementary Figures S1.3, S1.4.
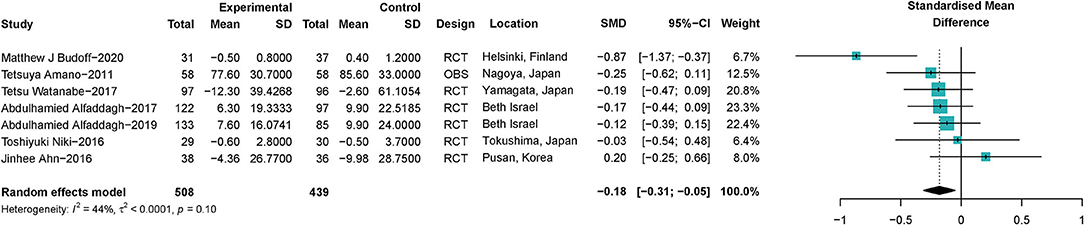
Figure 3. Forest plots of associations of ω-3 PUFAs supplementation and coronary atherosclerotic plaques. Point sizes are an inverse function of the precision of the estimates, and bars correspond to 95% CIs. Data are calculated using a random-effect model. RCT, randomized controlled trial; OBS, observational study.
A total of 936 lesions samples from 462 patients with CHD were included to investigate the association between ω-3 PUFAs and stenosis of the coronary artery, in which 233 patients with CHD received ω-3 PUFAs supplements, and 229 patients received placebos. One study (45) provided data on both EPA and DHA, which we took into account. The pooled data showed that ω-3 PUFAs could help reduce the loss of the diameter of the narrowest segments of CA in patients with CHD (SMD 0.29; 95% Cl 0.05–0.53), with a moderate heterogeneity (I2 = 69%) (Figure 4). ω-3 PUFAs' effect became not significant on the most stenotic segment of the coronary artery when the study Erkkilä et al. (45) was excluded, as shown in Supplementary Figure S2.1. Heterogeneity became 0% when Sacks' et al. (37) study was excluded (Supplementary Figure S2.2).
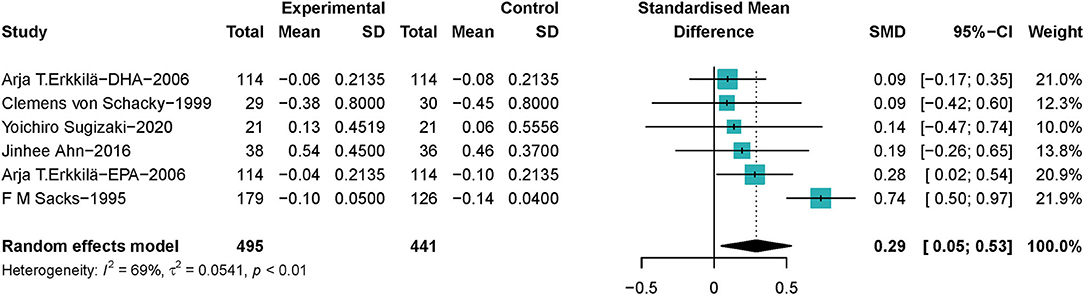
Figure 4. Forest plots of associations of ω-3 PUFAs and most stenotic segment of the coronary artery. Point sizes are an inverse function of the precision of the estimates, and bars correspond to 95% CIs. Data are calculated using a random-effect model.
As shown in Figure 5, seven studies (24, 32, 33, 35, 38, 41, 44) with a total of 935 patients with CHD reported the effect of ω-3 PUFAs on the volume change of lipid plaque in coronary arteries (SMD −1.18; 95% CI −2.95 to 0.58) with a significant heterogeneity (I2 = 94%). The outcome was stable when studies were excluded serially (Supplementary Figure S3.1). Heterogeneity reduced significantly when Niki's (44) study was excluded (Supplementary Figure S3.2). For Amano's study, we only chose EPA to represent ω-3 PUFAs, which was observed to be significantly different between the acute coronary syndrome (ACS) and non-ACS groups in Amano's participants. It was believed that the conclusions drawn in this way may be more clinically meaningful. Associations between ω-3 PUFAs and volume of fiber plaque were reported by six studies (24, 32, 35, 38, 41, 44) (SMD 0.26; 95% CI −0.81 to 1.33), with a significant heterogeneity (I2 = 94%), same as shown in Figure 5. Both the estimates and heterogeneities were stable when studies were excluded serially (Supplementary Figures S4.1, S4.2). As shown in Figure 5, six studies (24, 32, 35, 38, 41, 44) with a total of 873 patients with CHD reported the effect of ω-3 PUFAs on the volume change of calcified plaque in coronary arteries (SMD 0.17; 95% CI −0.55 to 0.89) with a significant heterogeneity (I2 = 90%). The exclusion of Niki's et al. (44) study led to both a significant prospective effect of ω-3 PUFAs on calcified plaque volume and a very low heterogeneity (Supplementary Figures S5.1, S5.2).
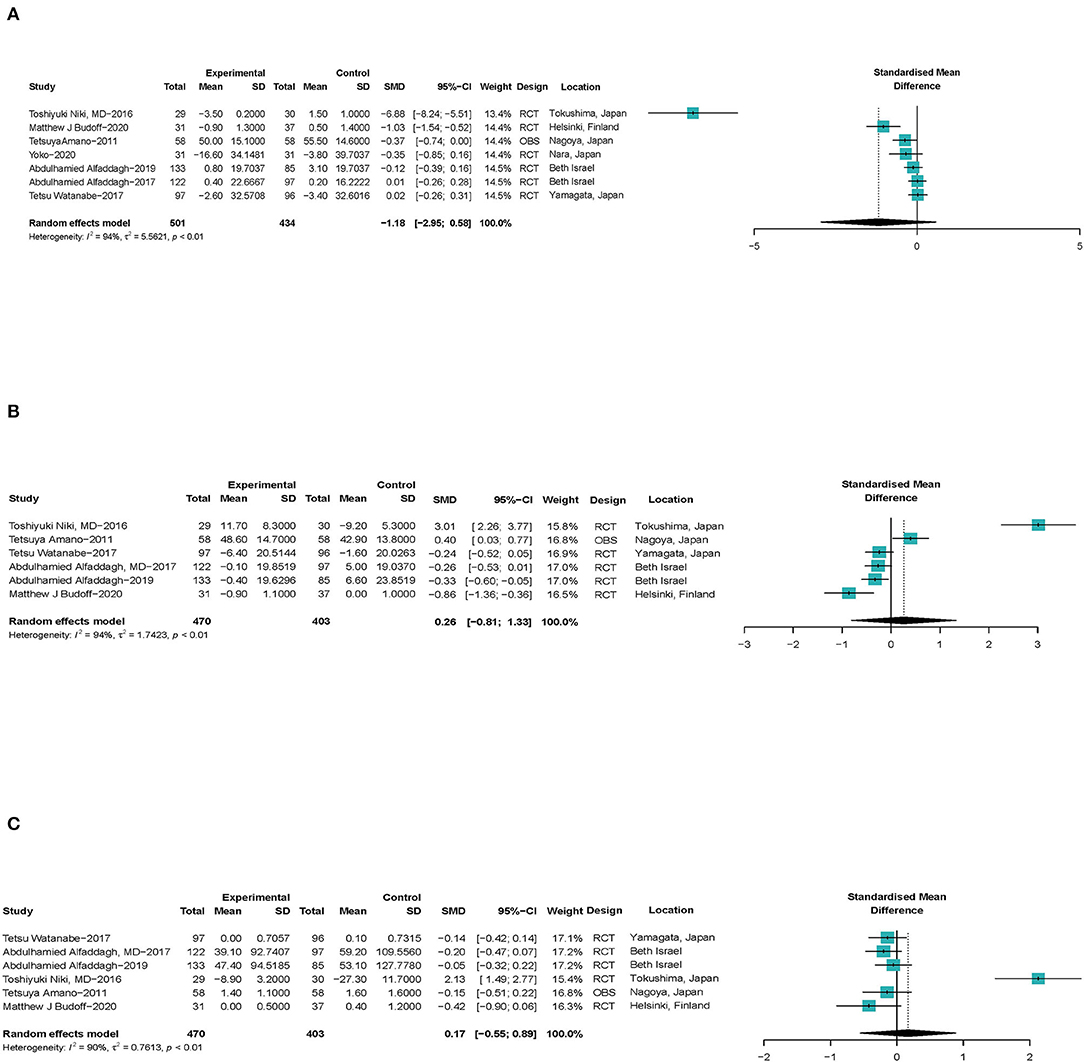
Figure 5. Forest plots of associations of ω-3 PUFAs and coronary atherosclerotic plaque compositions. (A) Lipid plaque volume, (B) fiber plaque volume, and (C) calcified plaque. Point sizes are an inverse function of the precision of the estimates, and bars correspond to 95% CIs. Data are calculated using a random-effect model. RCT, randomized controlled trial; OBS, observational study.
Outcomes suggest that ω-3 PUFAs have no significant effect on the volume of atherosclerotic plaque compositions.
In eight studies (27, 29–31, 36, 39, 40, 43), a total of 740 participants were included to investigate the association between ω-3 PUFAs and sVCAM-1 level in peripheral blood. The results showed that ω-3 PUFAs had no significant effect on sVCAM-1 in peripheral blood (SMD −0.02; 95% Cl −0.28 to 0.23), with a moderate heterogeneity (I2 = 54%) (Figure 6). Outcomes were stable when studies were excluded serially, heterogeneity significantly reduced when Mirfatahi's study (29) was excluded (Supplementart Figures S6.1, S6.2).
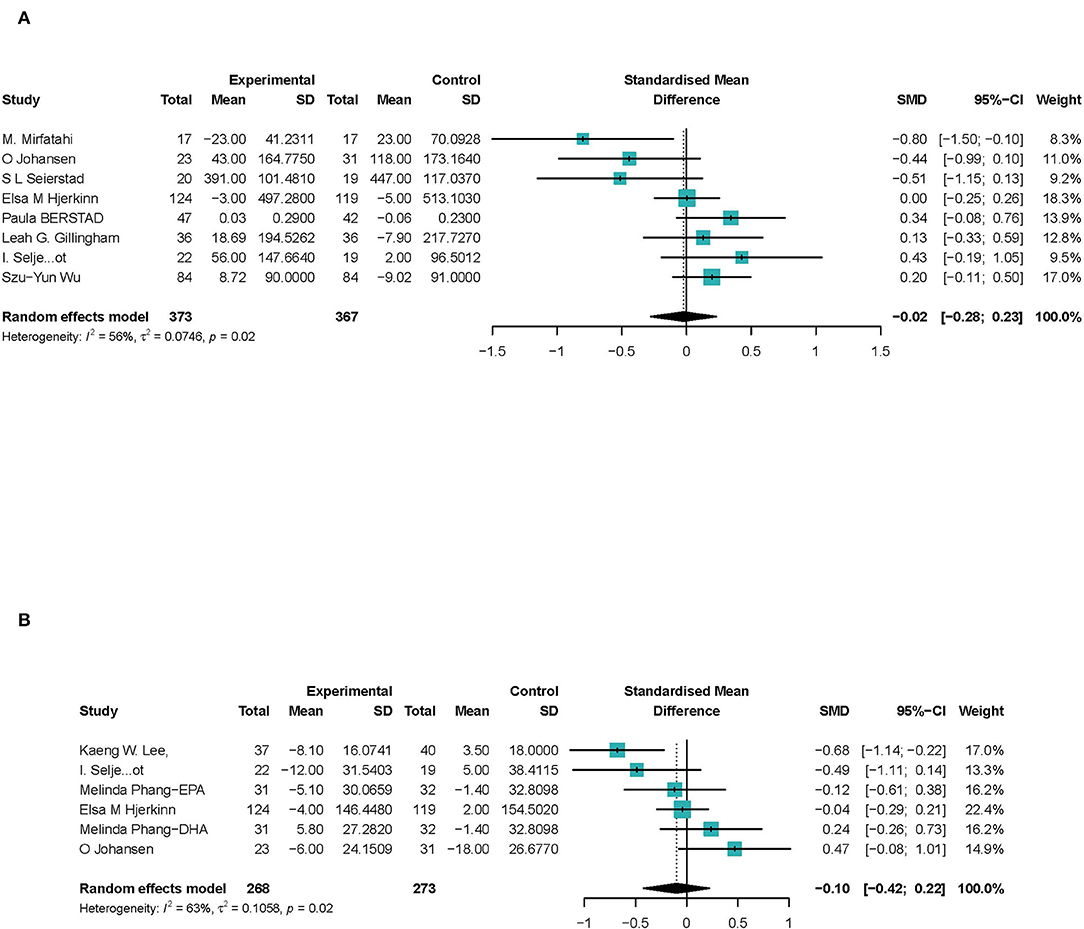
Figure 6. Forest plots of associations of ω-3 PUFAs and endothelial cell markers. (A) sVCAM-1; (B) VWF%. Point sizes are an inverse function of the precision of the estimates, and bars correspond to 95% CIs. Data are calculated using a random-effect model.
Six studies (26, 28, 36, 39, 43, 45) focus on the activation of VWF (VWF%). One study (28) reported both EPA and DHA's effect on activation of VWF, which we discussed, respectively. A total of 478 CHD participants were included, and 541 were calculated to investigate the association between ω-3 PUFAs and VWF activation in peripheral blood. The results showed that ω-3 PUFAs had no significant effect on VWF% (SMD −0.10; 95% Cl −0.42 to 0.22), with a moderate heterogeneity (I2 = 63%) (Figure 6). Estimates were stable when studies were excluded, and heterogeneity reduced significantly when Lee's et al. (26) study was excluded (Supplementary Figures S7.1, S7.2).
In summary, we suggested that ω-3 PUFAs have no significant effect on endothelial inflammatory factors in peripheral blood.
Publication bias was detected in the outcome on lipid plaque volume. The trimmed result led to a consistent conclusion, which suggested a negative publication bias, as shown in Supplementary Figures S8.1–S8.3. Publication bias was not found in other outcomes, as shown in funnel plots and Peter's tests (Supplementary Figures S9.1–S9.6, S10.1–S10.6). Pooled results of publication bias tests, sensitivity analyses, and quality assessments suggest conclusions of the present meta-analysis of high quality. The standardized mean difference, sensitivity analysis, heterogeneity and publication bias (Peter's test) for each outcome in the present review were summarized in Table 2.
The results of meta-regression failed to confirm the influence of the average age of the population or the length of follow-up on the results. The quantitative results of meta-regression are described in Supplementary Figures S11.1–S11.12. The meta-regression curves are described in Supplementary Figures S12.1–S12.12.
This study is based on 22 studies, which included 2,277 participants. The large evidence base, obtained through multiple databases, provided us with more information on the effect of ω-3 PUFAs in CHD pathology. Our data showed that ω-3 PUFAs significantly slowed the atheromatous plaque volume increases of coronary arteries.
To the best of our knowledge, this meta-analysis was the first study that summarized the effect of ω-3 PUFAs on coronary atherosclerosis and analyzed potential mechanisms. We first explored the potential effect of ω-3 PUFAs on lipid plaque, fibrous plaque, calcified plaque, and two important endothelial inflammatory factors (sVCAM-1 and VWF). Our data showed that ω-3 PUFAs had an effect on the plaque growth in coronary arteries of patients with CHD, which was consistent with other studies. Previously, many studies have proved the efficiency of ω-3 PUFAs on the prevention of major vascular events, with an unclear mechanism (46–50). We suggested that ω-3 PUFAs may slow the growth of plaque in coronary arteries and then delay the progression of atherosclerosis. The effect of ω-3 PUFAs supplementation on plaque volume was not dose-related. We indicated that the chief mechanisms may not contain ω-3 PUFAs' effect on lipid, fibrous, or calcified compositions or endothelial inflammatory factors. Moreover, previous reports had suggested that the outcomes of ω-3 PUFAs supplementation may vary by patients' prior medications, such as stains (51). However, this meta-analysis demonstrated no heterogeneity in the effects of ω-3 PUFAs on plaque size of patients with CHD with/without the use of stains. Besides, we investigated the effect of ω-3 PUFAs on the lumen of coronary arteries. The data showed that ω-3 PUFAs contribute to the changes of the lumen of coronary arteries. The full-text review of the literature showed that in a considerable number of patients, even if ω-3 PUFAs reduced the progression of some lesions, the overall lesions were still deteriorating. We suggested that atherosclerosis is an inevitable disease of aging, contributed by various pathological changes, and the effect of ω-3 PUFAs might be limited.
Several studies have reported that ω-3 PUFAs can cause changes in arterial intimamedia thickness (52, 53), which may also be associated with the changes in the lumen of coronary arteries.
In patients with CHD, sVCAM-1 is closely related to atherosclerosis lesions in the early stage (54–56), in stable and unstable angina and acute myocardial infarction, by accelerating mononucleosis accumulates into endothelial cells (57). Von Willebrand factor is another important marker for the inflammatory activity of the vascular endothelium (58–60), especially activation rate (VWF%). Receptors and cytokines that participated in the process may include G protein-coupled receptors 120, cyclooxygenase-2, induced nitric oxide synthase 2 (61), interleukin 1 beta, and interleukin 6 (62). Outcomes on VWF% and content of sVCAM-1 both suggested that ω-3 PUFAs did not reduce the inflammatory responses of peripheral vessels.
Fiber plaque is also one of the major compositions of coronary atherosclerotic plaque that consists of a large number of collagen fibers, a few elastics and proteoglycans, foam cells, extracellular lipids, and inflammatory cells (12). Lipid pool and granulation tissue reaction could only be seen in the late stage of the lesion (63). This suggests that collagen fibers are the main component of fiber plaques. The volume of collagen fibers is mainly affected by inflammatory factors and inflammatory cells (64). As mentioned earlier, inflammatory activity was not significantly affected by ω-3 PUFAs. This explains why the volume of fibrous plaques is not affected by ω-3 PUFAs. Therefore, we confirmed that the change of fiber plaque volume is not one of the main causes for the reduction of growth of atherosclerotic plaques. The formation of calcified plaques is considered to be due to the accumulation of crystalline calcium in the lipid bodies of the nucleus. Some scholars reported that sheet calcification is highly prevalent in stable plaques, while microcalcifications, punctate, and fragmented calcifications are more frequent in unstable lesions, which means calcification of different degrees may play a dual role in the stability of coronary atherosclerotic plaques (65). This suggests that the result of the current meta-analysis on calcification plaques may be due to differences in calcification in different populations.
Previous studies have demonstrated that the formation of plaques plays a key role in the pathology of CHD (66). Thrombotic lesions are composed of a fibromuscular cap overlying a large necrotic tissue core containing macrophages and lipid core. However, there exist other plaques that contain more fibrotic tissues, and these types of plaques are likely to rupture and generate thrombosis. Therefore, depending on plaque composition, coronary artery disease may predispose to stable angina or unstable angina (67). We fail to suggest whether ω-3 PUFAs benefit more to patients with stable or unstable angina.
A previous study reported the controversial role of ω-3 PUFAs on cardiovascular events in severe and lethal cardiovascular events—some reported protective effects (46–50)—while others reported no significant effects (68, 69). Our review explained the effect of ω-3 on segments of severe lesions at a more microscopic level. By summarizing the data, we found that ω-3 PUFAs reduced the lesion of the narrowest segments, which supported the former conclusion mentioned above. Studies have shown that regular intake of both EPA + DHA as supplements may bring benefits for patients with CHD through reduction of arrhythmias, endothelial dysfunction, and inflammation (70, 71). This review actually challenges the inflammatory mechanism. Safi's et al. (46) review also gave an interpretation that EPA monotherapy brings more profiles for patients with CHD than treatment with EPA + DHA, and the physiological mechanism cannot be reflected in this review.
Heterogeneity was generally moderate among the measures in this review. We analyzed the heterogeneity through sensitivity analyses and detected several potential sources. We further full-text reviewed the included studies. The possible sources of heterogeneity are summarized as follows. (1) Statistical heterogeneity: some studies use a quartile spacing table, while others use mean and standard deviation to represent the outcomes, which may generate statistical heterogeneity in the process of merger and transformation. (2) Standardized patients are rarely used in the existing interested studies, and different ethnic populations and those with comorbidities may produce heterogeneity. We conducted a sensitivity analysis for impact factors of each outcome and provided the results in Supplementary Figures S5.1–S5.10. (3) Heterogeneity may result from different designs of the studies, including the length of follow-up and the criteria of the population included in the follow-up measurement, for the reason that the progression of the lesions may vary in different populations with different duration. However, the results of meta-regression failed to confirm the influence of the average age of the population or the length of follow-up on the results. (4) The imaging methods and kits used in the evaluation of coronary atherosclerosis and the measurement of endothelial factors vary greatly.
This review has some limitations; the chief among them is the inadequacy of participants. Available studies for each outcome fail to provide a sufficient sample size of more than 5,000 totally, which was needed to produce a definitive conclusion. As most of the participants in this study were elderly people over 50 years old and male patients accounted for the majority, the conclusions obtained in this review were more applicable to elderly male patients with CHD. However, due to the age and gender tendency of CHD, we did not use age or gender ratio as a special factor to perform a subgroup analysis and bias evaluation. It was hard to explain inconsistent conclusions on total plaque volume and volume of the main plaque compositions. The weak robustness of some of the outcomes needs to be noted. There have been noticeable changes in both the result and heterogeneity of some outcomes when some studies were excluded. Hence, some conclusions may be considered more cautiously. We fail to determine the specific linear relationship between the change of total atherosclerotic plaques volume and volume of the plaque compositions because the volume change of plaque compositions cannot be judged under the current statistical standard, which may need further studies. It is worth noting that a large portion of the high-quality studies interested in the effect of ω-3 PUFAs on inflammatory response focused on healthy individuals. This prevents us from directly demonstrating the relationship between ω-3 PUFAs and coronary inflammations.
Important questions remain regarding ω-3 PUFAs and coronary atherosclerosis. Chief among them is the lack of large-scale random controlled trials or cohort studies reporting the association between ω-3 PUFAs and coronary atherosclerosis. Currently available studies only include dozens to more than 100 samples, which was thought to be unable to produce more convincing conclusions. The portion of the studies included in this review had a short follow-up period, which is also a common problem in many current clinical studies on ω-3 PUFAs. More studies with long-term follow-up are needed to confirm the effects of ω-3 PUFAs on the cardiovascular system through long-term effects on lipids or inflammatory cytokine levels. Larger-scale studies are needed to confirm the effect of ω-3 PUFAs so as to provide more credible evidence.
We confirmed that ω-3 PUFAs benefit patients with CHD by reducing the progression of coronary atherosclerosis. We indicated that the benefits may not be caused by reducing endothelial inflammations of coronary arteries.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
ZG, DZ, XX, and XY contributed to the literature search, study design and identification, and data acquisition and recording of the characteristics of studies. ZG contributed to the data analysis. DZ contributed to the data review and correction. ZG, DZ, HS, XX, and XY contributed to the data interpretation and critical revision of the manuscript. HS embellished the images and further interpreted the data. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Special thanks to professor Chao Liu, a renowned expert in evidence-based medicine and for his guidance on study design and execution and Professor Li for his outstanding contributions and constructive suggestions in the language polishing and data verification of the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.904250/full#supplementary-material
1. Writing Group for the AREDS2 Research Group, Bonds DE, Harrington M, Worrall BB, Bertoni AG, Eaton CB. Effect of long-chain ω-3 fatty acids and lutein + zeaxanthin supplements on cardiovascular outcomes: results of the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA Intern Med. (2014) 174:763–71. doi: 10.1001/jamainternmed.2014.328
2. Marchioli R, Marfisi RM, Borrelli G, Chieffo C, Franzosi MG, Levantesi G, et al. Efficacy of n-3 polyunsaturated fatty acids according to clinical characteristics of patients with recent myocardial infarction: insights from the GISSI-Prevenzione trial. J Cardiovasc Med. (2007) 8(Suppl. 1):S34–7. doi: 10.2459/01.JCM.0000289271.80180.b6
3. Skulas-Ray AC, Wilson PWF, Harris WS, Brinton EA, Kris-Etherton PM, Richter CK, et al. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. (2019) 140:e673–91. doi: 10.1161/CIR.0000000000000709
4. Rudkowska I, Caron-Dorval D, Verreault M, Couture P, Deshaies Y, Barbier O, et al. PPARalpha L162V polymorphism alters the potential of n-3 fatty acids to increase lipoprotein lipase activity. Mol Nutr Food Res. (2010) 54:543–50. doi: 10.1002/mnfr.200900085
5. Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. (2017) 45:1105–15. doi: 10.1042/BST20160474
6. Harris WS, Rambjør GS, Windsor SL, Diederich D. n-3 fatty acids and urinary excretion of nitric oxide metabolites in humans. Am J Clin Nutr. (1997) 65:459–64. doi: 10.1093/ajcn/65.2.459
7. Goua M, Mulgrew S, Frank J, Rees D, Sneddon AA, Wahle KW. Regulation of adhesion molecule expression in human endothelial and smooth muscle cells by omega-3 fatty acids and conjugated linoleic acids: involvement of the transcription factor NF-kappaB. Prostaglandins Leukot Essent Fatty Acids. (2008) 78:33–43. doi: 10.1016/j.plefa.2007.10.004
8. Preston Mason R. New insights into mechanisms of action for omega-3 fatty acids in atherothrombotic cardiovascular disease. Curr Atheroscler Rep. (2019) 21:2. doi: 10.1007/s11883-019-0762-1
9. Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. (2010) 50:50–61. doi: 10.1177/0091270009336234
10. Kaur G, Cameron-Smith D, Garg M, Sinclair AJ. Docosapentaenoic acid (22:5n-3): a review of its biological effects. Prog Lipid Res. (2011) 50:28–34. doi: 10.1016/j.plipres.2010.07.004
11. Sakamoto A, Saotome M, Iguchi K, Maekawa Y. Marine-derived omega-3 polyunsaturated fatty acids and heart failure: current understanding for basic to clinical relevance. Int J Mol Sci. (2019) 20:4025. doi: 10.3390/ijms20164025
12. Banach M, Serban C, Sahebkar A, Mikhailidis DP, Ursoniu S, Ray KK, et al. Impact of statin therapy on coronary plaque composition: a systematic review and meta-analysis of virtual histology intravascular ultrasound studies. BMC Med. (2015) 13:229. doi: 10.1186/s12916-015-0459-4
13. Vergallo R, Crea F. Atherosclerotic plaque healing. N Engl J Med. (2020) 383:846–57. doi: 10.1056/NEJMra2000317
14. Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega-3 fatty acids on coronary restenosis, intima-media thickness, and exercise tolerance: a systematic review. Atherosclerosis. (2006) 184:237–46. doi: 10.1016/j.atherosclerosis.2005.06.042
15. Laake K, Seljeflot I, Fagerland MW, Njerve IU, Arnesen H, Solheim S. Effects on serum fractalkine by diet and omega-3 fatty acid intervention: relation to clinical outcome. Mediators Inflamm. (2015) 2015:373070. doi: 10.1155/2015/373070
16. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
17. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
18. Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, et al. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the american heart association. Circulation. (2017) 135:e867–84.
19. Aucoin M, Cooley K, Knee C, Fritz H, Balneaves LG, Breau R, et al. Fish-derived omega-3 fatty acids and prostate cancer: a systematic review. Integr Cancer Ther. (2017) 16:32–62. doi: 10.1177/1534735416656052
20. Yang K, Zeng L, Bao T, Ge J. Effectiveness of omega-3 fatty acid for polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol. (2018) 16:27. doi: 10.1186/s12958-018-0346-x
21. Hu Y, Hu FB, Manson JE. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc. (2019) 8:e013543. doi: 10.1161/JAHA.119.013543
22. Lyu XZ, Sun F, Zhan SY. [Risk related to bias assessment: (4) revised cochrane risk of bias tool for cluster-randomized control trials (RoB2.0)]. Zhonghua Liu Xing Bing Xue Za Zhi. (2018) 39:240–4. doi: 10.3760/cma.j.issn.0254-6450.2018.02.020
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
24. Alfaddagh A, Elajami TK, Ashfaque H, Saleh M, Bistrian BR, Welty FK. Effect of Eicosapentaenoic and docosahexaenoic acids added to statin therapy on coronary artery plaque in patients with coronary artery disease: a randomized clinical trial. J Am Heart Assoc. (2017) 6:e006981. doi: 10.1161/JAHA.117.006981
25. Ahn J, Park SK, Park TS, Kim JH, Yun E, Kim SP, et al. Effect of n-3 polyunsaturated fatty acids on regression of coronary atherosclerosis in statin treated patients undergoing percutaneous coronary intervention. Korean Circ J. (2016) 46:481–9. doi: 10.4070/kcj.2016.46.4.481
26. Lee KW, Blann AD, Lip GY. Effects of omega-3 polyunsaturated fatty acids on plasma indices of thrombogenesis and inflammation in patients post-myocardial infarction. Thromb Res. (2006) 118:305–12. doi: 10.1016/j.thromres.2005.07.018
27. Gillingham LG, Gustafson JA, Han SY, Jassal DS, Jones PJ. High-oleic rapeseed (canola) and flaxseed oils modulate serum lipids and inflammatory biomarkers in hypercholesterolaemic subjects. Br J Nutr. (2011) 105:417–27. doi: 10.1017/S0007114510003697
28. Phang M, Scorgie FE, Seldon M, Garg ML, Lincz LF. Reduction of prothrombin and Factor V levels following supplementation with omega-3 fatty acids is sex dependent: a randomised controlled study. J Nutr Biochem. (2014) 25:997–1002. doi: 10.1016/j.jnutbio.2014.05.001
29. Mirfatahi M, Tabibi H, Nasrollahi A, Hedayati M, Taghizadeh M. Effect of flaxseed oil on serum systemic and vascular inflammation markers and oxidative stress in hemodialysis patients:a randomized controlled trial. Int Urol Nephrol. (2016) 48:1335–41. doi: 10.1007/s11255-016-1300-5
30. Berstad P, Seljeflot I, Veierød MB, Hjerkinn EM, Arnesen H, Pedersen JI. Supplementation with fish oil affects the association between very long-chain n-3 polyunsaturated fatty acids in serum non-esterified fatty acids and soluble vascular cell adhesion molecule-1. Clin Sci. (2003) 105:13–20. doi: 10.1042/CS20020349
31. Wu SY, Mayneris-Perxachs J, Lovegrove JA, Todd S, Yaqoob P. Fish-oil supplementation alters numbers of circulating endothelial progenitor cells and microparticles independently of eNOS genotype. Am J Clin Nutr. (2014) 100:1232–43. doi: 10.3945/ajcn.114.088880
32. Amano T, Matsubara T, Uetani T, Kato M, Kato B, Yoshida T, et al. Impact of omega-3 polyunsaturated fatty acids on coronary plaque instability: an integrated backscatter intravascular ultrasound study. Atherosclerosis. (2011) 218:110–6. doi: 10.1016/j.atherosclerosis.2011.05.030
33. Kita Y, Watanabe M, Kamon D, Ueda T, Soeda T, Okayama S, et al. Effects of fatty acid therapy in addition to strong statin on coronary plaques in acute coronary syndrome: an optical coherence tomography study. J Am Heart Assoc. (2020) 9:e015593. doi: 10.1161/JAHA.119.015593
34. Sugizaki Y, Otake H, Kuroda K, Kawamori H, Toba T, Nagasawa A, et al. Concomitant use of rosuvastatin and eicosapentaenoic acid significantly prevents native coronary atherosclerotic progression in patients with in-stent neoatherosclerosis. Circ J. (2020) 84:1826–36. doi: 10.1253/circj.CJ-20-0199
35. Alfaddagh A, Elajami TK, Saleh M, Mohebali D, Bistrian BR, Welty FK. An omega-3 fatty acid plasma index ≥4% prevents progression of coronary artery plaque in patients with coronary artery disease on statin treatment. Atherosclerosis. (2019) 285:153–62. doi: 10.1016/j.atherosclerosis.2019.04.213
36. Hjerkinn EM, Seljeflot I, Ellingsen I, Berstad P, Hjermann I, Sandvik L, et al. Influence of long-term intervention with dietary counseling, long-chain n-3 fatty acid supplements, or both on circulating markers of endothelial activation in men with long-standing hyperlipidemia. Am J Clin Nutr. (2005) 81:583–9. doi: 10.1093/ajcn/81.3.583
37. Sacks FM, Stone PH, Gibson CM, Silverman DI, Rosner B, Pasternak RC. Controlled trial of fish oil for regression of human coronary atherosclerosis. HARP Research Group. J Am Coll Cardiol. (1995) 25:1492–8. doi: 10.1016/0735-1097(95)00095-L
38. Tokgozoglu L, Catapano AL. Can EPA evaporate plaques. Eur Heart J. (2020) 41:3933–5. doi: 10.1093/eurheartj/ehaa750
39. Johansen O, Seljeflot I, Høstmark AT, Arnesen H. The effect of supplementation with omega-3 fatty acids on soluble markers of endothelial function in patients with coronary heart disease. Arterioscler Thromb Vasc Biol. (1999) 19:1681–6. doi: 10.1161/01.ATV.19.7.1681
40. Seierstad SL, Seljeflot I, Johansen O, Hansen R, Haugen M, Rosenlund G, et al. Dietary intake of differently fed salmon; the influence on markers of human atherosclerosis. Eur J Clin Invest. (2005) 35:52–9. doi: 10.1111/j.1365-2362.2005.01443.x
41. Watanabe T, Ando K, Daidoji H, Otaki Y, Sugawara S, Matsui M, et al. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol. (2017) 70:537–44. doi: 10.1016/j.jjcc.2017.07.007
42. von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. (1999) 130:554–62. doi: 10.7326/0003-4819-130-7-199904060-00003
43. Seljeflot I, Arnesen H, Brude IR, Nenseter MS, Drevon CA, Hjermann I. Effects of omega-3 fatty acids and/or antioxidants on endothelial cell markers. Eur J Clin Invest. (1998) 28:629–35. doi: 10.1046/j.1365-2362.1998.00336.x
44. Niki T, Wakatsuki T, Yamaguchi K, Taketani Y, Oeduka H, Kusunose K, et al. Effects of the addition of eicosapentaenoic acid to strong statin therapy on inflammatory cytokines and coronary plaque components assessed by integrated backscatter intravascular ultrasound. Circ J. (2016) 80:450–60. doi: 10.1253/circj.CJ-15-0813
45. Erkkilä AT, Matthan NR, Herrington DM, Lichtenstein AH. Higher plasma docosahexaenoic acid is associated with reduced progression of coronary atherosclerosis in women with CAD. J Lipid Res. (2006) 47:2814–9. doi: 10.1194/jlr.P600005-JLR200
46. Khan SU, Lone AN, Khan MS, Virani SS, Blumenthal RS, Nasir K, et al. Effect of omega-3 fatty acids on cardiovascular outcomes: a systematic review and meta-analysis. EClinicalMedicine. (2021) 38:100997. doi: 10.1016/j.eclinm.2021.100997
47. Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. (2002) 112:298–304. doi: 10.1016/S0002-9343(01)01114-7
48. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. (2012) 308:1024–33. doi: 10.1001/2012.jama.11374
49. Kwak SM, Myung SK, Lee YJ, Seo HG. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: a meta-analysis of randomized, double-blind, placebo-controlled trials. Arch Intern Med. (2012) 172:686–94. doi: 10.1001/archinternmed.2012.262
50. Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, et al. ω-3 Polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med. (2016) 176:1155–66. doi: 10.1001/jamainternmed.2016.2925
51. Sharma G, Martin SS, Blumenthal RS. Effects of omega-3 fatty acids on major adverse cardiovascular events: what matters most: the drug, the dose, or the placebo. JAMA. (2020) 324:2262–4. doi: 10.1001/jama.2020.22387
52. Hino A, Adachi H, Toyomasu K, Yoshida N, Enomoto M, Hiratsuka A, et al. Very long chain N-3 fatty acids intake and carotid atherosclerosis: an epidemiological study evaluated by ultrasonography. Atherosclerosis. (2004) 176:145–9. doi: 10.1016/j.atherosclerosis.2004.04.020
53. Ebbesson SO, Roman MJ, Devereux RB, Kaufman D, Fabsitz RR, Maccluer JW, et al. Consumption of omega-3 fatty acids is not associated with a reduction in carotid atherosclerosis: the Genetics of Coronary Artery Disease in Alaska Natives study. Atherosclerosis. (2008) 199:346–53. doi: 10.1016/j.atherosclerosis.2007.10.020
54. Morisaki N, Saito I, Tamura K, Tashiro J, Masuda M, Kanzaki T, et al. New indices of ischemic heart disease and aging: studies on the serum levels of soluble intercellular adhesion molecule-1 (ICAM-1) and soluble vascular cell adhesion molecule-1 (VCAM-1) in patients with hypercholesterolemia and ischemic heart disease. Atherosclerosis. (1997) 131:43–8. doi: 10.1016/S0021-9150(97)06083-8
55. Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM Jr, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. (1997) 96:4219–25. doi: 10.1161/01.CIR.96.12.4219
56. Kawakami A, Aikawa M, Alcaide P, Luscinskas FW, Libby P, Sacks FM. Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation. (2006) 114:681–7. doi: 10.1161/CIRCULATIONAHA.106.622514
57. Bossowska A, Kiersnowska-Rogowska B, Bossowski A, Galar B, Sowiński P. [Assessment of serum levels of adhesion molecules (sICAM-1, sVCAM-1, sE-selectin) in stable and unstable angina and acute myocardial infarction]. Przegl Lek. (2003) 60:445–50.
58. Willeit P, Thompson A, Aspelund T, Rumley A, Eiriksdottir G, Lowe G, et al. Hemostatic factors and risk of coronary heart disease in general populations: new prospective study and updated meta-analyses. PLoS ONE. (2013) 8:e55175. doi: 10.1371/journal.pone.0055175
59. van Schie MC, de Maat MP, Isaacs A, van Duijn CM, Deckers JW, Dippel DW, et al. Variation in the von Willebrand factor gene is associated with von Willebrand factor levels and with the risk for cardiovascular disease. Blood. (2011) 117:1393–9. doi: 10.1182/blood-2010-03-273961
60. Vischer UM. von Willebrand factor, endothelial dysfunction, and cardiovascular disease. J Thromb Haemost. (2006) 4:1186–93. doi: 10.1111/j.1538-7836.2006.01949.x
61. Innes JK, Calder PC. The Differential effects of eicosapentaenoic acid and docosahexaenoic acid on cardiometabolic risk factors: a systematic review. Int J Mol Sci. (2018) 19:532. doi: 10.3390/ijms19020532
62. Carracedo M, Artiach G, Arnardottir H, Bäck M. The resolution of inflammation through omega-3 fatty acids in atherosclerosis, intimal hyperplasia, and vascular calcification. Semin Immunopathol. (2019) 41:757–66. doi: 10.1007/s00281-019-00767-y
63. Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. (2014) 114:1852–66. doi: 10.1161/CIRCRESAHA.114.302721
64. Ueda M. [Pathology of AtheroThrombosIS (ATIS)]. Drugs. (2010) 70(Suppl. 1):3–8. doi: 10.2165/00000004-000000000-00000
65. Jinnouchi H, Sato Y, Sakamoto A, Cornelissen A, Mori M, Kawakami R, et al. Calcium deposition within coronary atherosclerotic lesion: implications for plaque stability. Atherosclerosis. (2020) 306:85–95. doi: 10.1016/j.atherosclerosis.2020.05.017
66. Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. (2005) 111:3481–8. doi: 10.1161/CIRCULATIONAHA.105.537878
67. Vergallo R, Porto I, D'Amario D, Annibali G, Galli M, Benenati S, et al. Coronary atherosclerotic phenotype and plaque healing in patients with recurrent acute coronary syndromes compared with patients with long-term clinical stability: an in vivo optical coherence tomography study. JAMA Cardiol. (2019) 4:321–9. doi: 10.1001/jamacardio.2019.0275
68. Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. (2018) 3:225–34. doi: 10.1001/jamacardio.2017.5205
69. Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. (2018) 7:CD003177. doi: 10.1002/14651858.CD003177.pub4
70. Burillo E, Martín-Fuentes P, Mateo-Gallego R, Baila-Rueda L, Cenarro A, Ros E, et al. Omega-3 fatty acids and HDL. How do they work in the prevention of cardiovascular disease. Curr Vasc Pharmacol. (2012) 10:432–41. doi: 10.2174/157016112800812845
Keywords: ω-3 polyunsaturated fatty acids, coronary atherosclerosis, endothelial inflammations, cardiovascular imaging, cardiovascular therapy
Citation: Gao Z, Zhang D, Yan X, Shi H and Xian X (2022) Effects of ω-3 Polyunsaturated Fatty Acids on Coronary Atherosclerosis and Inflammation: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 9:904250. doi: 10.3389/fcvm.2022.904250
Received: 29 March 2022; Accepted: 11 May 2022;
Published: 20 June 2022.
Edited by:
Marat V. Ezhov, Ministry of Health of the Russian Federation, RussiaReviewed by:
Tetsuro Miyazaki, Juntendo University Urayasu Hospital, JapanCopyright © 2022 Gao, Zhang, Yan, Shi and Xian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohui Xian, aGVibXV4eGhAaGVibXUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.