
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 03 June 2022
Sec. Cardio-Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.903323
This article is part of the Research Topic HF2Cancer: Exploring bidirectional interaction between cardiovascular diseases and cancer View all 18 articles
 Jinping Si1†
Jinping Si1† Xinxin Zhang1†
Xinxin Zhang1† Na Chen1†
Na Chen1† Fangfang Sun2
Fangfang Sun2 Ping Du1
Ping Du1 Zhiyong Li3
Zhiyong Li3 Di Tian3
Di Tian3 Xiuli Sun4
Xiuli Sun4 Guozhen Sun4
Guozhen Sun4 Tao Cong5*
Tao Cong5* Xuemei Du2*
Xuemei Du2* Ying Liu1*
Ying Liu1*Background: Eosinophilic leukemia (EL) is a rare, serious and potentially life-threatening condition characterized by the overproduction of eosinophils leading to tissue eosinophilic infiltration and damage. Although multiple organ systems may be involved, progressive eosinophilic myocarditis (EM) is the most common cause of morbidity and mortality. Early diagnosis and follow-up surveillance combined with multimodal imaging are crucial for appropriate treatment of EM.
Case Summary: It’s a rare case of EL with EM and intracardiac thrombus in a 59-year-old patient who presented with asthenia for 3 weeks. Full blood count analysis indicated significant eosinophilia. Bone marrow aspirate revealed dysplastic eosinophilia and a FIP1L1-PDGFRA fusion gene (4q12) was detected, confirming EL. Echocardiography revealed EM with intracardiac thrombus. This was later confirmed by cardiac magnetic resonance imaging. The patient was commenced on imatinib and prednisolone and good clinical response was obtained. Through 18F-FAPI PET/CT imaging, we obtained in vivo visualization of fibroblast activation changes in the early stage of cardiac structure remodeling. With anti-fibrotic therapy after heart failure, the patient achieved a good clinical response.
Conclusion: This case demonstrates in vivo visualization of fibroblast activation after EM. Multimodality imaging can provide early diagnosis and may guide tailored antifibrotic therapy in early stage of EM.
Eosinophilic myocarditis (EL) is a neoplastic condition with persistent eosinophilia as the main hematological abnormality and with the eosinophils being part of the neoplastic clone (1). These eosinophils have a clonal molecular genetic abnormality that excludes PDGFRA, PDGFRB, and FGFR1 rearrangements and PCM1-JAK2, ETV6-JAK2, and BCR-JAK2 fusion genes, especially a FIP1L1–PDGFRA fusion gene resulting from a cryptic deletion of part of the long arm of chromosome 4 (2). Reducing eosinophil levels both in peripheral blood and tissue, preventing end-organ damage and avoiding adverse thrombotic events are the three primary goals for the management of EL (3). EM, a common cardiac complication of EL and the major cause of morbidity and mortality which can occur in about 40–50% of patients (4), is an inflammatory disorder of the myocardium characterized by eosinophilic infiltration (5). EM usually undergoes acute necrotic, thrombotic, and fibrotic stages, which may overlap. Early diagnosis and follow-up surveillance combined with multimodal imaging are crucial for appropriate treatment of EM.
A 56-year-old male presented with asthenia for 3 weeks. He had no food or drug allergies, no laboratory evidence of parasite, fungal or virus infection. Vital signs were normal with blood pressure of 130/80 mmHg, heart rate of 80 beats/min and respiratory rate of 12 breaths/min on presentation. Complete blood examination revealed hemoglobin 95 g/dL, total leukocyte count 27.74 × 109/L with an absolute eosinophil count of 12.94 × 109/L, accounting for 59.8% of cells, platelet count 55 × 109/L, elevated N-terminal pro-brain natriuretic peptide (NT-proBNP) level (604.6 ng/L, normal range <450 ng/L) and C-reactive protein (11 mg/L, reference: <8 mg/L). High Sensitivity Cardiac Troponin I (hs-cTnI) level was within the normal range. Other laboratory results were unremarkable, including anti-nuclear antibody screen. The electrocardiogram (ECG) revealed sinus rhythm (Figure 1A). Neither coronary computed tomographic angiography nor encephalic computed tomography showed significant lesions. Echocardiography demonstrated slightly increased chamber size, normal left ventricular systolic function (left ventricular ejection fraction of 57%), and abnormal diastolic function of grade III. There was a large, heterogeneous cardiac mass (39 mm × 22 mm, arrow) in the left ventricle (LV). The most probable diagnosis was Löffler’s endocarditis (LE; cardiac involvement in hypereosinophilia) with intracardiac thrombus (Figures 2A,B). A cardiac magnetic resonance (CMR) short-axis image showed a curved line of late gadolinium enhancement (LGE) located subendocardial in the apical-middle heart of LV. The arc-shaped unenhanced area in the left ventricular cavity and the medical history were consistent with eosinophilic endocarditis with subendocardial thrombosis (3) (Figure 3). Because of the drastically increased eosinophils, we suspected a myeloproliferative disorder. We did a bone marrow examinations including aspiration cytology, biopsy, cytogenetic, and gene rearrangement analysis. While waiting for the results of the examinations, on day 3 of his admission, the patient complained of progressive development of chest pain. The electrocardiographic examination showed a new ST segment depression with T-wave inversion in precordial leads (Figure 1B). Laboratory findings demonstrated an elevated hs-cTnI and NT-proBNP of 2.72 and 1,789 ng/L, respectively, indicating ongoing myocardial damage. After treatment with nitrates to dilate the coronary arteries, the chest pain disappeared. As the symptoms were related to EM, the cause might be coronary artery spasm or a small thromboembolism obstructing the microcirculation, but the patient refused to undergo coronary angiography. Subsequently, bone marrow aspirate revealed dysplastic eosinophilia and eosinophilic promyelocytes. A normal karyotype (46, XY) was present and a FIP1L1-PDGFRA fusion gene (4q12) was detected. The marrow B- and T-cell receptor rearrangement analysis and flow cytometry showed no B- or T-cell clone, confirming EL. The patient was treated with glucocorticoid. The initial medication prescribed was intravenous methylprednisolone (60 mg per day for 3 days and then 40 mg per day for 4 days), then which was switched to oral prednisolone (50 mg per day), with a gradual tapering of the doses and imatinib mesylate (a tyrosine kinase inhibitor) of 100 mg/day was commenced. After only 4 days on imatinib and prednisolone, and the patient’s eosinophil cell levels returned to normal (the lowest level was 0.01 × 109/L). After diagnosis of intracardiac thrombus, he was initially treated with low-molecular-weight heparin (1 mg/kg/dose q12 h) for 7 days. The heparin was gradually switched to warfarin. Warfarin was adjusted to a PT INR of 2–3. Before discharge, the hs-cTnI and NT-proBNP levels had significantly decreased to 0.032 and 617.8 ng/L, respectively. Furthermore, an ECG showed the normalization of ST segment and T wave (Figure 1C).
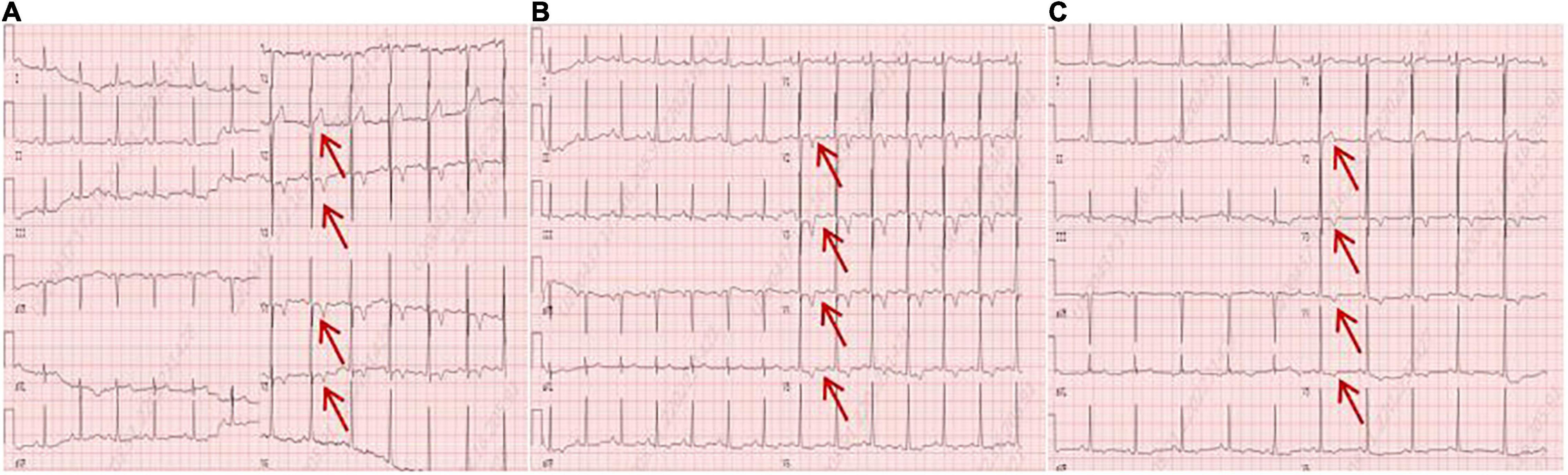
Figure 1. (A) An electrocardiogram on admission. (B) An electrocardiogram revealed a new ST segment depression with T-wave inversion in the precordial leads (arrows). (C) An electrocardiogram before discharge showed the normalization of ST segment and T wave (arrows).
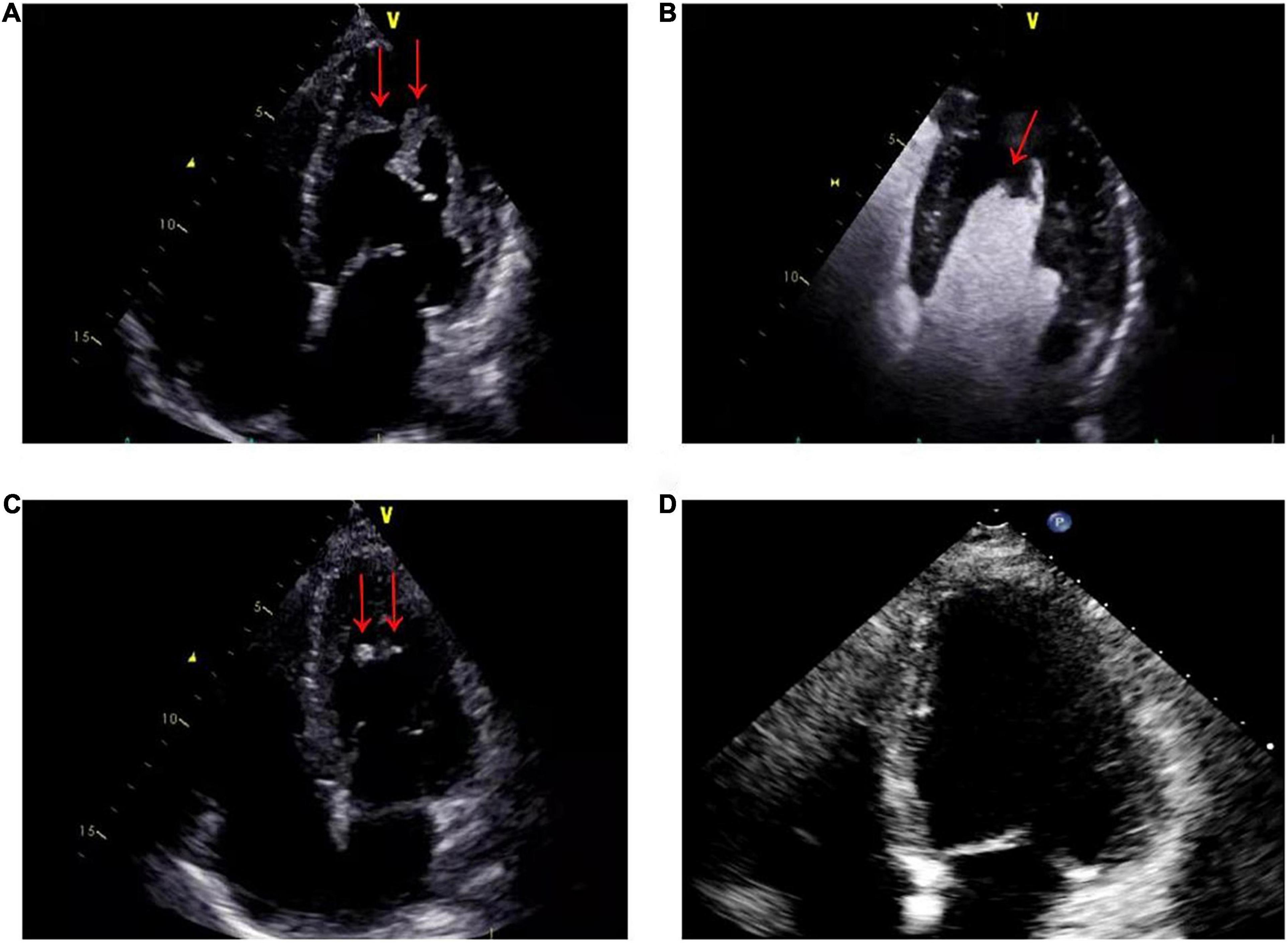
Figure 2. (A) On admission, echocardiography showed that the apex of the left ventricle was filled with a thrombotic mass measuring 3.9 cm × 2.2 cm (arrows). (B) On admission, echocardiographic view after venous injection of an ultrasound contrast agent: thrombus (arrow) at left ventricle apex. (C) Two months after therapy, echocardiography showed a marked reduction in the size of the mass, with a thrombotic mass measuring 6 mm × 13 mm (arrows). (D) Six months after therapy, echocardiography showed that left ventricular thrombus had resolved.
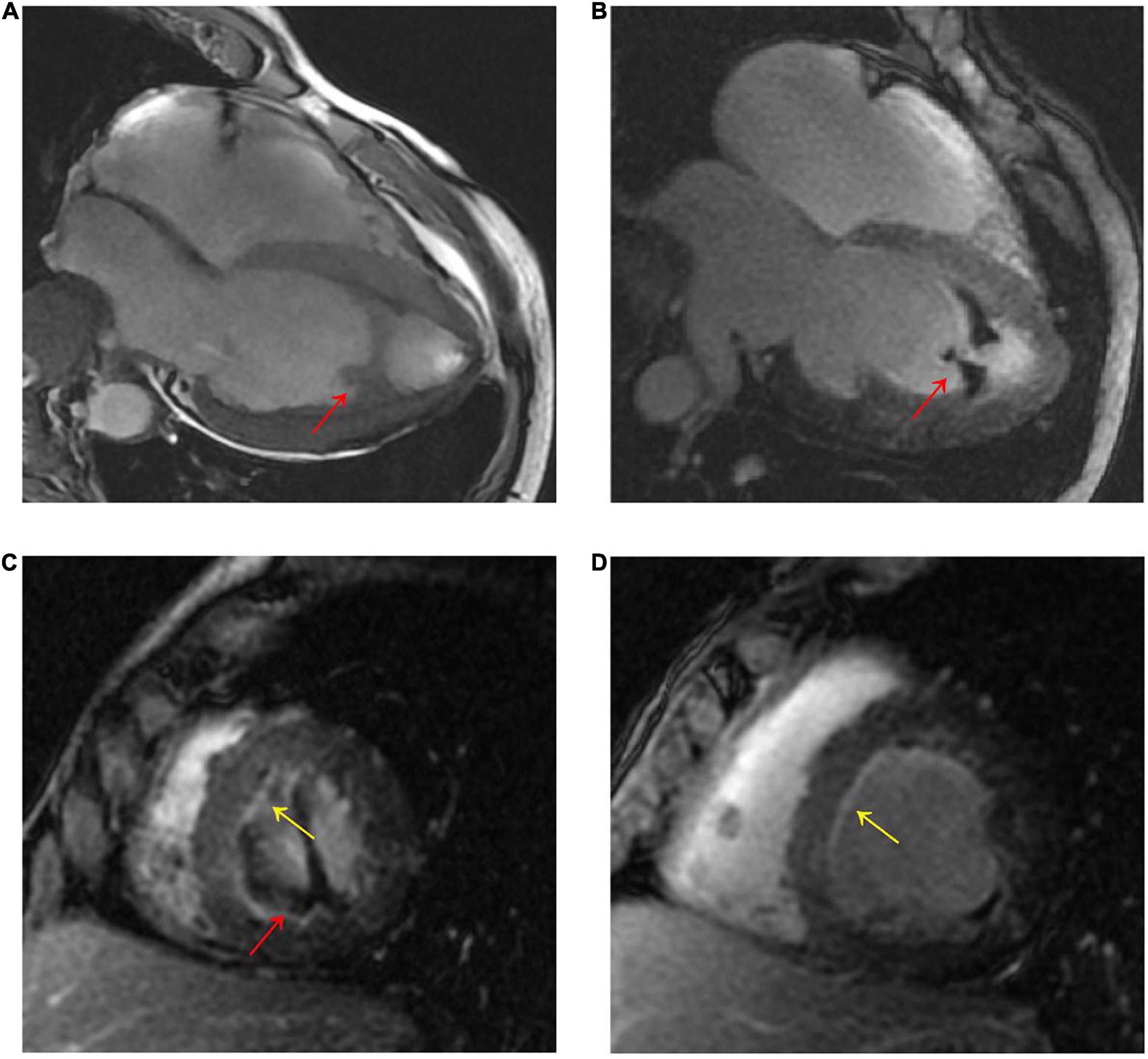
Figure 3. (A) CMR four-chamber cine sequence exhibited arc-like region in the LV apex (red arrow). (B–D), Late gadolinium enhancement imaging showed LV apex arc-like unenhanced region (red arrow), and diffuse subendocardial enhancement in LV mid- to apical parts (yellow arrow). The patient was diagnosed with Loeffler’s endocarditis based on the typical clinical and imaging features.
The patient underwent twice 18F-FAPI PET/CT for the visualization of cardiac fibroblast activation during treatment. Before discharge, on whole-body 18F-FAPI PET imaging, heterogeneously increased accumulations of 18F-FAPI were seen in endomyocardial. No other suspected fibrosis with 18F-FAPI accumulation was found (Figures 4A,B). Given the presence of myocardial fibrosis, he was treated for myocardial fibrosis and heart failure with angiotensin receptor-neprilysin inhibitor (ARNI), β-blockers and spironolactone. Good clinical response was obtained and after 2 months of treatment, follow-up PET/CT revealed that accumulations of 18F-FAPI in endomyocardial were significantly reduced (Figures 4C,D).
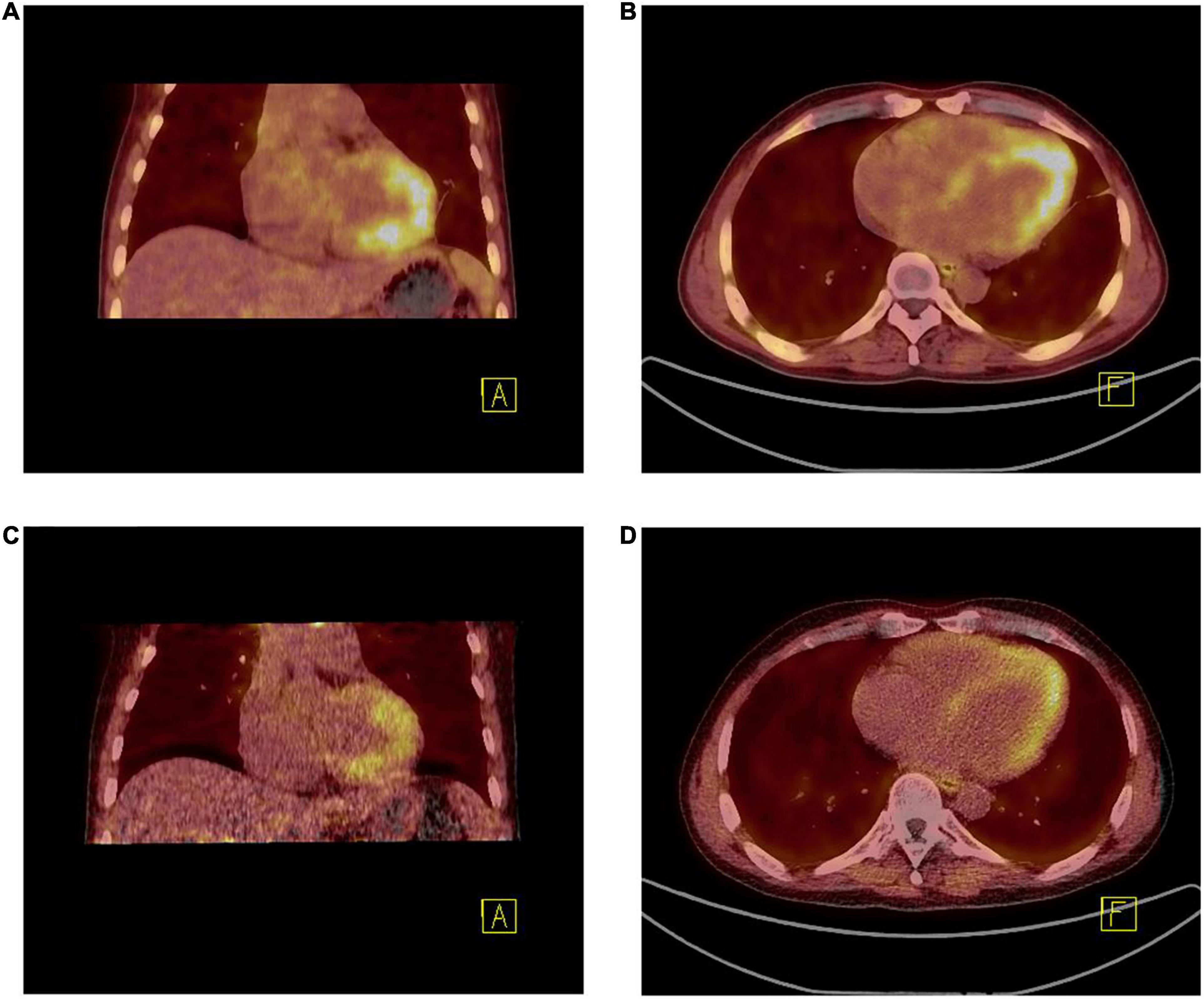
Figure 4. (A,B) 18F-FAPI PET/CT fused image demonstrated the uptake [10.10 (SUVmax), 8.75 (SUVpeak), and 1.92 (SUVmean)] was much higher than that after 2 months of treatment (C,D) [8.40 (SUVmax), 6.91 (SUVpeak), and 1.32 (SUVmean)].
The patient was closely followed up in both cardiology and hematology outpatient clinics over a course of 2 months. The eosinophil counts remained within the normal range, when the dose of prednisolone was gradually tapered to 5 mg per day. Peripheral blood examination gave the following results: hemoglobin, 124 g/dL; total leukocyte count 8.66 × 109/L with 6.71% eosinophils; and platelet count, 177 × 109/L. While remaining on warfarin, imatinib mesylate, prednisolone and heart failure therapy, he had no specific symptoms on evaluation in the outpatient clinic. Follow-up echocardiogram revealed normal left ventricular size and rapid regression of the left ventricular mass (6 mm × 13 mm, arrow) (Figure 2C).
During a 6-month follow-up, there was no recurrence of hypereosinophilia. The disease remained clinically stable. Echocardiogram showed that left ventricular thrombus had resolved (Figure 2D).
The diagnostic features of EL include (1) persistent proliferation of eosinophil precursors and (2) marked eosinophilia in peripheral blood (eosinophils >1.5 × 109/L) and bone marrow. Eosinophils play a role in host defense and immune regulation by secreting intracytoplasmic granules containing cytotoxic molecules, cytokines and chemokines, which can lead to end-organ injury. EM is a common cardiac complication of hypereosinophilia. The pathophysiology of EM progresses through three stages, namely acute necrotic stage, thrombotic stage and fibrotic stage, which may overlap (6). The prognosis for EM is often poor, with a 5-year mortality rate of up to 50% (7). Besides, endomyocardial exhibits various cardiac manifestations depending on the severity and extent of the endomyocardial damage caused by eosinophilic infiltration (8). Therefore, EM often leads to the development of heart failure. Treatment of EM to prevent further deterioration includes halting of eosinophilic infiltration, counteraction of inflammation reaction, and prevention of myocardial fibrosis/intracardiac thrombus formation, in addition to symptomatic treatment of heart failure. If left untreated, serious complications such as valvular regurgitation or restrictive cardiomyopathy may occur at an advanced stage of EM. However, early and proper diagnosis is particularly challenging due to the subclinical presentation and the lack of characteristic signs in early stages of disease. In our case, the EM was most likely in the second or early third stage due to the presence of LV thrombus and fibroblast activation; echocardiography and PET-CT follow-up revealed improved LV function and suppressed fibroblast activation suggesting that the prompt treatment was protective against permanent cardiac dysfunction.
In this report, we describe a case of EL with EM and LV thrombus. Our case may offer several valuable clinical lessons. Imatinib therapy is indicated in all patients with either a chronic myeloid leukemia or a myeloproliferative disorder with PDGFRB and PDGFRA abnormalities (9). Corticosteroids are used as a cornerstone of myocarditis treatment, but its mechanism of action is not fully understood. It is thought that corticosteroids effectively inhibit the proliferative, developmental maturation pathway of eosinophils, thereby promoting redistribution of peripheral blood eosinophils (10). So a very effective treatment with imatinib and corticosteroids was possible in this case with FIP1L1–PDGFRA fusion gene, and early treatment with imatinib and corticosteroids did show favorable results. In the case described, the patient developed chest pain during treatment, elevated leukocytosis, eosinophilia, hs-cTnI, and NT-proBNP were evident and this was explained by infiltration of eosinophils into the myocardium. This infiltration leads to mitochondrial dysfunction, myocardial cell damage and necrosis through the release of toxic granules, cationic proteins, pro-inflammatory cytokines and oxygen free radicals. Additionally, eosinophil infiltration can lead to coronary vasculitis and the release of cytokines that might cause coronary artery spasm (11). Therefore, in our case with chest pain, pronounced eosinophilia levels, and normal coronary computed tomography angiography, the diagnosis of EM should be considered. In some individuals, EM masquerades as acute coronary syndrome, with severe chest pain, electrocardiographic changes, and elevated serum levels of troponins (12). If patients who have hypereosinophilia presenting as acute coronary syndrome only receive stent implantation and anti-angina pectoris, coronary spasm will occur repeatedly and may even lead to sudden death. However, if the glucocorticoid or immunosuppressor is administered their condition will be improved (13, 14). In addition to first-line therapy (corticosteroids), early and aggressive anticoagulation therapy shall be ensured (15). While there is no standard recommendation for the use of anticoagulation for EL in the presence of thrombosis, anticoagulation should be considered in patients at high risk (i.e., with evidence of intracardiac thrombosis, deep venous thrombosis or recurrent thromboembolic events) with a view to treating this potentially fatal complication (16). In our case, after anticoagulation treatment, the patient achieved good therapeutic response.
CMR allows the visualization of myocardial inflammation, mural thrombus and fibrosis, with high sensitivity, specificity and accuracy, and the differentiation of inflammation from fibrosis based on the enhancement on LGE imaging (8, 17). EM is often associated with diffuse or patchy subendocardial LGE, which was shown in our patient. However, CMR is not specific for assessing early cardiac structural remodeling.
The newly developed PET tracer FAPI specifically targets fibroblast activation protein, which allows in vivo visualization of fibroblast activation during an early stage of cardiac structural remodeling (18). Currently, there are no other imaging studies of human fibroblast activation using this novel biosignal for EM. Given the limited treatment approach in an irreversible stage, earlier diagnosis and therapeutic intervention in EM progression is essential. Although endomyocardial biopsy (EMB) is the cornerstone of EM histological diagnosis, it is an invasive procedure and inflammatory cell infiltration is heterogeneous distributed, resulting in low diagnostic accuracy (19). Additionally, EMB is not indicated in all patients with suspected EM (5). In our case, because the patient remained in good condition after starting corticosteroid therapy, biopsy was not performed, and the presence of left ventricular thrombosis was also an influencing factor. PET/CT imaging can safely provide valuable information about the location, extent, and pattern of endomyocardial fibrosis. Moreover, excessive fibrosis development is suggested to cause a progressive decline in ventricular diastolic and systolic function, which may lead to the development of chronic heart failure (20). Our case illustrates how EM due to EL could cause endomyocardial fibrosis, diastolic dysfunction, and thrombus (21). The FAPI uptake peaks on day 6 following ischemia (22), in the present case, the patient underwent FAPI imaging 16 days after chest pain, and the FAPI uptake area also did not conform to the patterns of myocardial ischemia. Thus, we excluded myocardial ischemia and considered that the FAPI imaging changes were caused by EM. Affected myocardium showed a partial to complete match between tracer uptake and confirmed lesion by contrast echocardiography and CMR. Pathologic features of EM fibrosis were identified. As fibroblast activation protein expression is low in most normal organs, it provides an interesting target for PET imaging to assess the severity of fibrosis (23). 18F-FAPI may represents a highly promising, novel biosignal for monitoring short-term structural remodeling processes and guiding tailored antifibrotic therapy in hypereosinophilia (24, 25). In our case, 18F-FAPI PET/CT, CMR and Echocardiography together present synergies that go beyond the limitations that each examination presents separately.
In conclusion, this case illustrates how EM due to EL could cause endomyocardial fibrosis, diastolic dysfunction, and thrombus. Advances in diagnostic imaging techniques may aid in the diagnosis of EM. Multimodality imaging can provide early diagnosis and potentially guide tailored antifibrotic therapy in EM.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the First Affiliated Hospital of Dalian Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant for the publication of this case report.
YL, XD, and TC contributed to the conception of the case report. JS and XZ contributed to the management of the patient and manuscript writing. NC contributed to the manuscript preparation and clinical data collection. FS, ZL, and DT contributed to the imageological examination. PD, GS, and XS contributed to the eosinophilic leukemia, eosinophilic myocarditis, and intracardiac thrombus treatment of the patient. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Nos. U1908209 and 82170385).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Morsia E, Reichard K, Pardanani A, Tefferi A, Gangat N. WHO defined chronic eosinophilic leukemia, not otherwise specified (CEL, NOS): a contemporary series from the Mayo Clinic. Am J Hematol. (2020) 95:E172–4. doi: 10.1002/ajh.25811
2. Shomali W, Gotlib J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am J Hematol. (2019) 94:1149–67. doi: 10.1002/ajh.25617
3. Kuang FL. Approach to patients with eosinophilia. Med Clin North Am. (2020) 104:1–14. doi: 10.1016/j.mcna.2019.08.005
4. Al Ali AM, Straatman LP, Allard MF, Ignaszewski AP. Eosinophilic myocarditis: case series and review of literature. Can J Cardiol. (2006) 22:1233–7. doi: 10.1016/s0828-282x(06)70965-5
5. Kuchynka P, Palecek T, Masek M, Cerny V, Lambert L, Vitkova I, et al. Current diagnostic and therapeutic aspects of eosinophilic myocarditis. Biomed Res Int. (2016) 2016:2829583. doi: 10.1155/2016/2829583
6. Mankad R, Bonnichsen C, Mankad S. Hypereosinophilic syndrome: cardiac diagnosis and management. Heart. (2016) 102:100–6. doi: 10.1136/heartjnl-2015-307959
7. Li H, Dai Z, Wang B, Huang W. A case report of eosinophilic myocarditis and a review of the relevant literature. BMC Cardiovasc Disord. (2015) 15:15. doi: 10.1186/s12872-015-0003-7
8. Kleinfeldt T, Nienaber CA, Kische S, Akin I, Turan RG, Korber T, et al. Cardiac manifestation of the hypereosinophilic syndrome: new insights. Clin Res Cardiol. (2010) 99:419–27. doi: 10.1007/s00392-010-0144-8
9. Bain BJ. Relationship between idiopathic hypereosinophilic syndrome, eosinophilic leukemia, and systemic mastocytosis. Am J Hematol. (2004) 77:82–5. doi: 10.1002/ajh.20088
10. Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J. (2009) 30:1995–2002. doi: 10.1093/eurheartj/ehp249
11. Sable-Fourtassou R, Cohen P, Mahr A, Pagnoux C, Mouthon L, Jayne D, et al. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann Intern Med. (2005) 143:632–8. doi: 10.7326/0003-4819-143-9-200511010-00006
12. Tran N, Kwok CS, Bennett S, Ratib K, Heatlie G, Phan T. Idiopathic eosinophilic myocarditis presenting with features of an acute coronary syndrome. Echo Res Pract. (2020) 7:K1–6. doi: 10.1530/ERP-19-0044
13. Desai N, Morkhandikar S, Sahay R, Jijina F, Patil P. Myeloproliferative hypereosinophilic syndrome presenting as cardiac failure and response to imatinib. Am J Ther. (2014) 21:e35–7. doi: 10.1097/MJT.0b013e3182491df1
14. Kumar AN, Sathyanarayanan V, Devi VL, Rajkumar NN, Das U, Dutt S, et al. FIP1L1-PDGFRA-positive chronic eosinophilic leukemia: a low-burden disease with dramatic response to imatinib - a report of 5 cases from South India. Turk J Haematol. (2014) 31:56–60. doi: 10.4274/Tjh.2013.0086
15. Zhuang Q, Zheng ZY, Mao W, Dong BL, Yang BS, Ni GB, et al. Right ventricular apical obstruction in a patient with hypereosinophilia: loffler endocarditis. Heart Lung. (2015) 44:165–9. doi: 10.1016/j.hrtlng.2014.11.003
16. Simon HU, Klion A. Therapeutic approaches to patients with hypereosinophilic syndromes. Semin Hematol. (2012) 49:160–70. doi: 10.1053/j.seminhematol.2012.01.002
17. Lagan J, Schmitt M, Miller CA. Clinical applications of multi-parametric CMR in myocarditis and systemic inflammatory diseases. Int J Cardiovasc Imaging. (2018) 34:35–54. doi: 10.1007/s10554-017-1063-9
18. Kessler L, Kupusovic J, Ferdinandus J, Hirmas N, Umutlu L, Zarrad F, et al. Visualization of fibroblast activation after myocardial infarction using 68Ga-FAPI PET. Clin Nucl Med. (2021) 46:807–13. doi: 10.1097/RLU.0000000000003745
19. Chow LH, Radio SJ, Sears TD, McManus BM. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol. (1989) 14:915–20. doi: 10.1016/0735-1097(89)90465-8
20. Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. (2000) 101:2981–8. doi: 10.1161/01.cir.101.25.2981
21. Gotlib J. World Health Organization-defined eosinophilic disorders: 2014 update on diagnosis, risk stratification, and management. Am J Hematol. (2014) 89:325–37. doi: 10.1002/ajh.23664
22. Varasteh Z, Mohanta S, Robu S, Braeuer M, Li Y, Omidvari N, et al. Molecular imaging of fibroblast activity after myocardial infarction using a (68)Ga-labeled fibroblast activation protein inhibitor, FAPI-04. J Nucl Med. (2019) 60:1743–9. doi: 10.2967/jnumed.119.226993
23. Gascard P, Tlsty TD. Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes Dev. (2016) 30:1002–19. doi: 10.1101/gad.279737.116
24. Nattel S. How does fibrosis promote atrial fibrillation persistence: in silico findings, clinical observations, and experimental data. Cardiovasc Res. (2016) 110:295–7. doi: 10.1093/cvr/cvw092
Keywords: 18F-FAPI, eosinophilic leukemia, thrombotic, fibroblast activation protein, PET/CT
Citation: Si J, Zhang X, Chen N, Sun F, Du P, Li Z, Tian D, Sun X, Sun G, Cong T, Du X and Liu Y (2022) Case Report: Multimodal Imaging Guides the Management of an Eosinophilic Leukemia Patient With Eosinophilic Myocarditis and Intracardiac Thrombus. Front. Cardiovasc. Med. 9:903323. doi: 10.3389/fcvm.2022.903323
Received: 24 March 2022; Accepted: 17 May 2022;
Published: 03 June 2022.
Edited by:
Canan G. Nebigil, INSERM U1260 Nanomedicine Régénératrice (RNM), FranceReviewed by:
Daniela Di Lisi, Azienda Ospedaliera Universitaria Policlinico Paolo Giaccone, ItalyCopyright © 2022 Si, Zhang, Chen, Sun, Du, Li, Tian, Sun, Sun, Cong, Du and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Cong, Y29uZ3RhbzE5NzVAMTYzLmNvbQ==; Xuemei Du, ZHV4bTkxN0AxNjMuY29t; Ying Liu, eWluZ2xpdS5tZWRAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.