- 1First Department of Arrhythmia, National Institute of Cardiology, Warsaw, Poland
- 2Second Department of Arrhythmia, National Institute of Cardiology, Warsaw, Poland
- 3Department of Cardiology, Congenital Heart Diseases and Electrotherapy, Silesian Center of Heart Disease, Zabrze, Poland
- 4Cardiology Department, University and Polytechnic Hospital la Fe, Valencia, Spain
- 5Section of Arrhythmias, Department of Cardiology, Clinical Sciences, Skåne University Hospital, Lund University, Lund, Sweden
- 6Bakken Research Center, Medtronic plc, Maastricht, Netherlands
- 7Queen Elisabeth Hospital, Birmingham University, Birmingham, United Kingdom
- 8Aston Medical School, Aston Medical Research Institute, Aston University, Birmingham, United Kingdom
The aim of the SYNSEQ (Left Ventricular Synchronous vs. Sequential MultiSpot Pacing for CRT) study was to evaluate the acute hemodynamic response (AHR) of simultaneous (3P-MPP syn) or sequential (3P-MPP seq) multi-3-point-left-ventricular (LV) pacing vs. single point pacing (SPP) in a group of patients at risk of a suboptimal response to cardiac resynchronization therapy (CRT). Twenty five patients with myocardial scar or QRS ≤ 150 or the absence of LBBB (age: 66 ± 12 years, QRS: 159 ± 12 ms, NYHA class II/III, LVEF ≤ 35%) underwent acute hemodynamic assessment by LV + dP/dtmax with a variety of LV pacing configurations at an optimized AV delay. The change in LV + dP/dt max (%ΔLV + dP/dt max) with 3P-MPP syn (15.6%, 95% CI: 8.8%-22.5%) was neither statistically significantly different to 3P-MPP seq (11.8%, 95% CI: 7.6-16.0%) nor to SPP basal (11.5%, 95% CI:7.1-15.9%) or SPP mid (12.2%, 95% CI:7.9-16.5%), but higher than SPP apical (10.6%, 95% CI:5.3-15.9%, p = 0.03). AHR (defined as a %ΔLV + dP/dt max ≥ 10%) varied between pacing configurations: 36% (9/25) for SPP apical, 44% (11/25) for SPP basal, 54% (13/24) for SPP mid, 56% (14/25) for 3P-MPP syn and 48% (11/23) for 3P-MPP seq.Fifteen patients (15/25, 60%) had an AHR in at least one pacing configuration. AHR was observed in 10/13 (77%) patients with a LBBB but only in 5/12 (42%) patients with a non-LBBB (p = 0.11). To conclude, simultaneous or sequential multipoint pacing compared to single point pacing did not improve the acute hemodynamic effect in a suboptimal CRT response population.
Clinical Trial Registration: ClinicalTrials.gov, identifier: NCT02914457.
Introduction
Cardiac resynchronization therapy (CRT) has transformed the treatment of patients with heart failure, impaired left ventricular (LV) function and a wide QRS complex (1). It is well accepted, however, that the response to CRT delivered using bipolar and unipolar leads is variable. Quadripolar LV leads are associated with higher implant success rates, lower rates of re-interventions for LV lead displacement or phrenic nerve stimulation (2–4) and better clinical outcomes (3, 4).
Intuitively, the wide LV activation front provided by simultaneous, multipoint pacing (MPPsyn) could achieve a more rapid and uniform LV activation than single point pacing (SPP). A better acute hemodynamic response (AHR) to CRT with MPP compared to SPP has been reported by some studies (5, 6), but not others (7, 8). It has also been shown that MPP confers a better LV reverse remodeling response to CRT compared to SPP (9). With respect to clinical outcomes, some studies showed a superiority of MPP over SPP (2), but this was not supported by a recent randomized, controlled trial (10). Physiologically, sequential MPP from apex to base (MPPseq) could also provide a physiological pattern of LV activation (11, 12). In this respect, a favorable response to CRT delivered using apical LV pacing is consistent with the notion that CRT, delivered using LV sequential activation from apex to base may be more physiological and therefore, more advantageous (13–15).
Response to CRT still raises many questions and there is a large population of subjects in which CRT brings moderate or even no benefit (16). Ischemic cardiomyopathy (17, 18), a relatively narrow QRS complex (19) and an non-LBBB morphology are associated with a higher risk of incomplete or poor/absent clinical improvement due to CRT (“sub-response”) (20). In this experimental, interventional study, we compare the acute hemodynamic effect in presumed sub-responders to CRT delivered using SPP as well as 3-point, simultaneous (3P-MPPsyn) or sequential (3P-MPPseq) MPP pacing. Recent data show that acute hemodynamic response measured by LV dP/dtmax is correlated with better clinical outcome and reverse remodeling, expressed as reduction of LVESV and LVEF improvement (21). Therefore, our work is part of the search for more effective resynchronizing stimulation techniques in a “sub-response” group. At the same time it offers new perspectives on this topic.
Methods
Study Design
The SYNSEQ (Left Ventricular Synchronous versus Sequential MultiSpot Pacing for CRT) study (NCT02914457) was an acute hemodynamic study with prospective enrolment, conducted across five European centers. All patients provided written informed consent. The study was approved by the Local Ethics Committees and complied with the Declaration of Helsinki.
Study Population
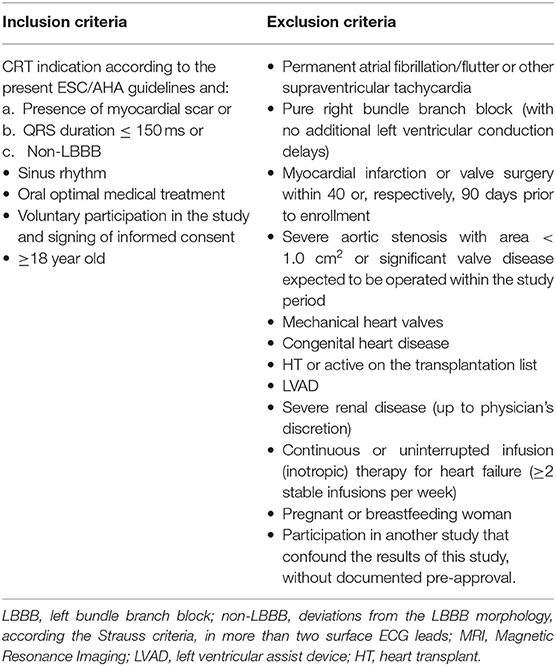
LBBB morphology on ECG was defined using the Strauss criteria (22). Patients diagnosed with LBBB with QRS > 150 ms together with absence of scar or patients having pure RBBB were not allowed in the study. Deviations from the above morphology in more than two surface ECG leads were classified as non-LBBB. ECG morphology was assessed independently by two blinded investigators. The etiology of heart failure was confirmed on basis of clinical history, and the echocardiographic examination. In addition, transmural/subendocardial myocardial scar was accessed by late-gadolinium enhancement cardiac magnetic resonance (23). All inclusion and exclusion criteria are listed in Table 1. This specific population was chosen based upon the (a) the relatively low-response acutely and chronic and therefore represent an opportunity for an experimental LV stimulation model, and (b) that typical-LBBB patients with relatively wide QRS and no scar do in general respond very well to conventional CRT-therapy.
Lead Implantation
This was undertaken using standard transvenous techniques with cephalic, axillary or subclavian access. Right atrial and right ventricular leads were first deployed into typical locations (preferred right atrium appendage if possibly and right ventricular apex or low septum, respectively), followed by deployment of a quadripolar LV lead within the vein chosen by implanters, who were instructed to deploy the LV lead tip as apical as possible within the vein of choice (an example of lead placement is shown in Figure 1). If the apex could not be reached with a transvenous LV lead, a 0.14” pacing wire (VisionWire, Biotronik, Berlin) was used for apical pacing. Apical position was defined by 30° RAO fluoroscopy as the lowest quartile in the longitudinal direction and was achieved in 100% of the patients. Acceptable LV lead position was either lateral or posterolateral (Figure 1).
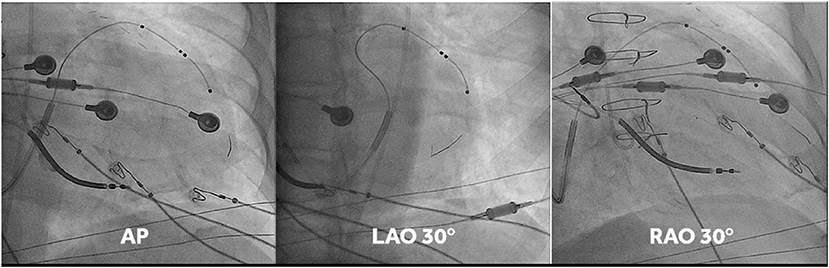
Figure 1. Fluoro-images at AP, LAO 30° and RAO 30° displaying position of the different CRT leads. Note that in this case the vision wire administered through the lumen of the quadripolar was used to obtain true apical position. MPP was delivered on the vision wire, and on the distal and most proximal electrodes of the short bipole of the quadripolar lead.
Lead Positions
Anteroposterior, left anterior oblique (30°) and right anterior oblique (30°) fluoroscopic views were used to localize lead positions, as previously described (12). Briefly, the position of pacing poles was determined by measuring the distance from the coronary sinus to the apex, using 30° right anterior oblique fluoroscopic view. The circumferential position over the LV free wall was determined using the o'clock method, assuming that the anterior interventricular vein was at a 12 o'clock position and the inferior vein at a 6 o'clock position. Thus, the LV pacing pole position (basal, mid, and apical) refers to the subtended myocardial segments, rather than the position of the pacing poles on the lead.
Pacing Protocol
The acute hemodynamic study was undertaken during implantation of a CRT device. The CRT implantation was performed as per standard practice after completion of the acute study. Four external pacemakers (Medtronic Model 5388, Medtronic, MN), synchronized by a central master pacemaker (Analyzer Medtronic 2290, Medtronic, MN) and a custom-made switch box, were used for each pacing site to ensure capture. The atrial channel of the central master was used for right atrial pacing. Throughout the acute study, cardiac electrograms, surface ECGs, invasive arterial blood pressure (femoral artery) and LV pressure (MicroCath Millar instruments, TX, USA) were acquired with a 32-channel recording system (Porti TMSi, Oldenzaal, Netherlands) and recorded on a laptop computer using customized software. Beat-to-beat raw signals were visualized and checked in real time to ensure appropriate signal quality and to confirm capture. Experimental lead configurations and atrioventricular (AV) delay settings were digitally annotated for off-line analysis.
The reference for calculation of %ΔLV + dP/dtmax was AAI pacing 10 bpm above the intrinsic rate. For AV optimization, LV + dP/dtmax was measured at five different AV delays, namely the AV delay determined by the CardioSync algorithm (Medtronic, MN) and AV delays of ±30 and ±60 ms around this AV delay. All measurements were repeated 4 times over 20 beats for each pacing configuration and AV delay, interspersed with AAI pacing, to minimize sampling error (24). The inter-ventricular (VV) pacing delay was set to zero for all configurations except for the 3-P MPPseq (VV-delay = 20ms between LVapex and LVmid and between LVmid and LVbasal). The tested LV pacing configurations were RV and SPPapex, RV and SPPmid, RV and SPPbasal, RV and 3P-MPPsyn, RV and MPPseq. For analysis, up to eight beats prior and eight beats immediately after each pacing change from a pacing configuration to AAI pacing were used to calculate percentage change in LV + dP/dtmax.
Hemodynamic Endpoint
Acute hemodynamic effect (AHE) was assessed as the percentage change in LV + dP/dtmax (%ΔLV + dP/dtmax) from pacing on to pacing off (AAI). The acute hemodynamic response (AHR) was defined as ≥10% increase in the acute hemodynamic effect (%ΔLV + dP/dtmax).
Data Analysis
Beat-to-beat LV intraventricular pressure, 12 lead surface ECG and endocardial (RA, RV, and LV) electrograms were acquired simultaneously using a 32-channel physiological recording system (Porti, TMSi, Twente, The Netherlands). Data analysis was undertaken offline. The Raschlab v0.3.0 software package (Raphael Schneider, Medtronic Inc.) was used for data review and annotation. Non-captured beats and ventricular ectopic beats plus the subsequent two beats were identified visually and excluded from further analyses. The dataset was then converted to Matlab (The Mathworks Inc., Massachusetts) compatible format for further analysis.
The AHE for each configuration was calculated with the median LV + dP/dtmax for up to eight cardiac beats before and after the experimental transition from pacing on to pacing off. We then calculated ΔLV + dP/dtmax for each of the eight transitions.
The paced QRS duration was measured from the ventricular pacing spike to the end of the QRS complex in surface ECGs. The Q-LV interval was defined as the interval from the onset of the intrinsic QRS on the surface ECG to the first large positive or negative peak of the LV electrogram. Q-LV-timing data are expressed as Q-LV/QRS. The electrical delay from RV or LV pacing spike to the different LV activations was also measured.
Statistical Analysis
Statistical analysis was performed using SAS 9.4 (SAS Institute, Cary, NC) and R (versions up to 3.6.1). Primary objectives: For comparison between pacing configurations, the following approach was performed. Firstly, the maximal average %ΔLV + dP/dtmax was calculated for each subject and each configuration by a regression analysis constructing a quadratic curve through all AV-delays (25, 26). Secondly, two-sided (except for non-inferiority which is one-sided per definition) weighted paired t-tests were performed to compare the pacing configurations to each other. Subjects were inversely weighted per comparison based on the model estimated variability of their maximal average %ΔLV + dP/dtmax for the compared configurations. Sensitivity analyses were performed comparing analysis results between a non-parametric Wilcoxon signed-rank test, unweighted t-test and weighted t-test. Two-tailed p-values smaller than 0.05 and one-tailed p-values smaller than 0.025 were considered significant. P-values are presented as two-sided unless indicated otherwise. For the comparison between 3P-MPPseq and 3P-MPPsyn, non-inferiority testing was performed using a margin of −3% and a significance level of 0.025. If non-inferiority testing was significant, a test for superiority at a significance level of 0.05 was performed. Categorical variables were compared using Fisher's exact test. Binomial sample proportions were compared to expected percentages using a one-sided Wald test to see whether one configuration was more often the best one than would be expected by chance. Secondary objectives: Linear multiple regression analysis was used to assess correlation between %ΔLV + dP/dtmax and Q-LV/QRS ratio or ΔQRS duration.
Continuous variables are expressed as mean ± SD (unless indicated otherwise). No correction for multiple testing was performed because of the exploratory nature of this study.
Results
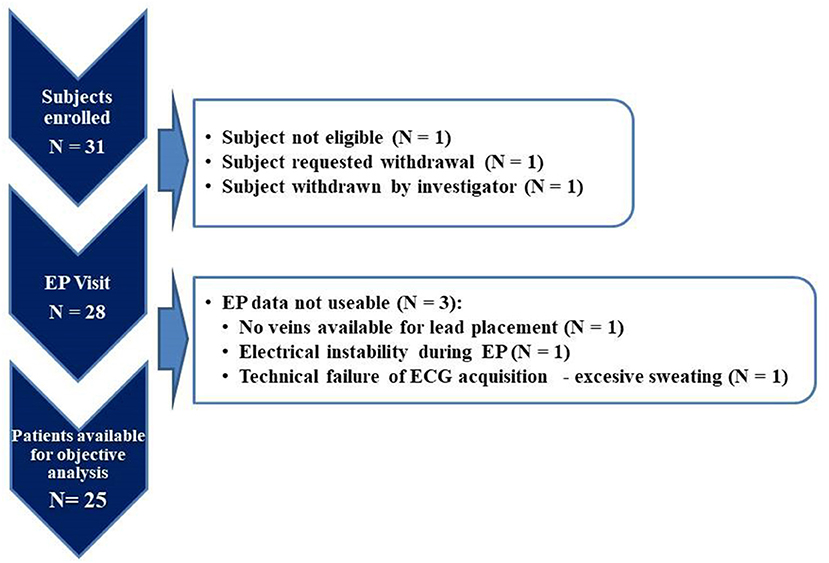
Thirty-one patients were enrolled in the study. Complete datasets were available for analysis for 25 patients (study flowchart is shown in Figure 2). For comparison of typical LBBB vs. non-LBBB the groups size was only 13 and 12 patients, respectively, indicating only a proof-of-principle (see also Limitations in the Discussion).
Baseline Characteristics of Patients With Complete Datasets
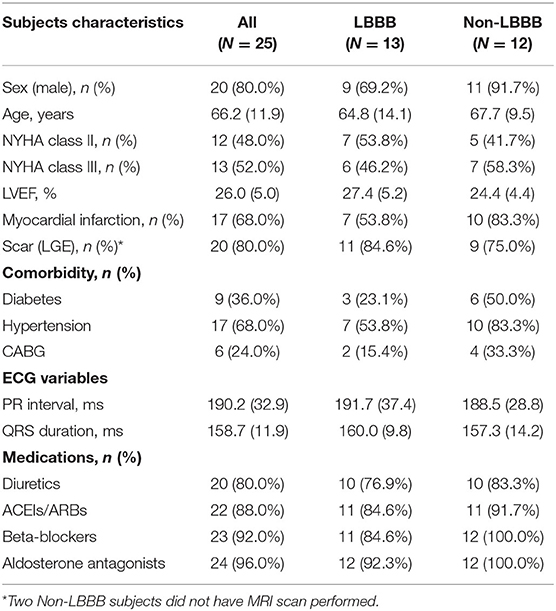
There were 25 subjects, (age: 66 ± 12 yrs [mean ± SD], 80% male), 12 of whom (12/25, 48%) showed no typical LBBB pattern on ECG. Twenty patients presented with myocardial scar (20/25, 80%), and 10 had a QRS-duration ≤ 150 (10/25, 40%). Patients received maximally tolerated pharmacological therapy for heart failure prior to the CRT implant. Patients' demographics are summarized in Table 2. No arrhythmias were induced during any of the pacing protocols. Data on the duration of the electrophysiological measurements determined by the protocol are included in Supplementary Table 1.
Effect of Simultaneous and Sequential Pacing Configurations
We observed an increase in %ΔLV + dP/dtmax for all pacing configurations at the optimized AV delay: 3P-MPPsyn (15.6%, 95% CI: 8.8-22.5%), 3P-MPPseq (11.8%, 95% CI: 7.6-16.0%), SPPbasal (11.5%, 95% CI: 7.1-15.9%), SPPmid (12.2%, 95% CI: 7.9-16.5%), and SPPapical (10.6%, 95% CI: 5.3-15.9%). Comparisons between 3P-MPPsyn and SPP configurations, 3PP-MPPseq and SPP configurations as well as between 3P-MPPsyn and 3P-MPPseq based on the weighted within-patient differences were not statistically significant except for comparison between 3P-MPPsyn and SPPapical (3.2%, 95% CI: 0.3-6.0%, p = 0.03) as well as 3P-MPPseq and SPPapical (3.3%, 95% CI: 0.3-6.4%, p = 0.04) (%ΔLV + dPdtmax boxplot at best AV-delay is shown in Figure 3). The sensitivity analysis seemed to indicate that different results between weighted t-test, unweighted t-test and Wilcoxon signed-rank test were mainly due to the weighting of individual subjects rather than strong violation of the assumption of normality for the t-tests.
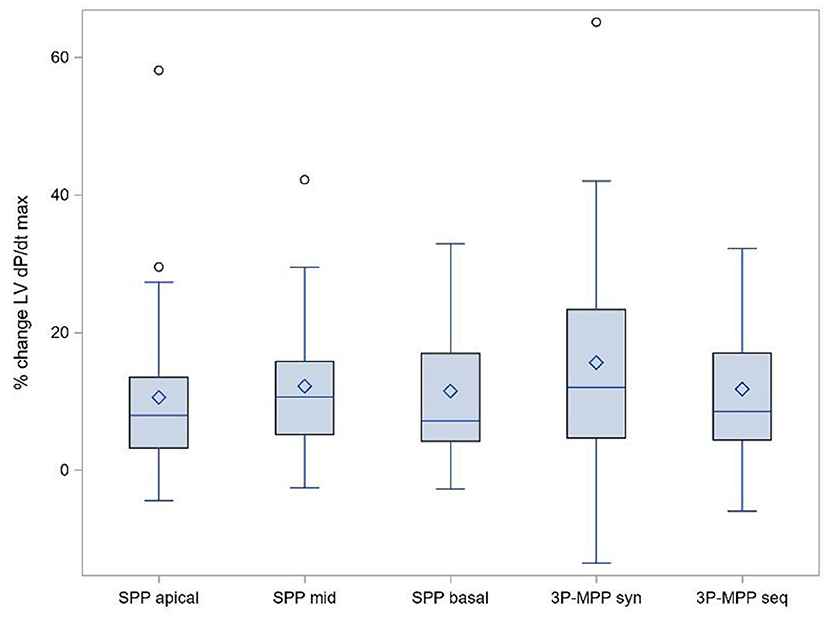
Figure 3. Primary objective: % ΔLV + dPdtmax boxplot at best AV-delay. SPP, RV-LV Single-point pacing and MPP, RV-LV Multi-point pacing. MPPseq, Sequential MPP; MPPsyn, Synchronous (simultaneous) MPP; SPPbasal,mid,apical, SPP from base, mid, apical LV electrode. Solid line depicts the median value, and boxes are 25th and 75th percentile. Whiskers represent the most extreme data point within 1.5x interquartile range from the boxes. Diamonds represent mean value, and dots are outliers.
Fifteen patients (15/25; 60%) showed an acute hemodynamic response in at least one pacing configuration. Acute hemodynamic responder rates (i.e., AHR) varied between pacing configurations: 36% (9/25) for SPPapical, 44% (11/25) for SPPbasal, 54% (13/24) for SPPmid, 56% (14/25) for 3P-MPPsyn and 48% (11/23) for 3P-MPPseq. Overall, AHR was similar for MPP configurations and SPP configurations.
Effect of LBBB Morphology
Patients had a mean QRS-duration of 158.7 ± 11.9 ms, and 52% (13/25) of patients presented with typical LBBB pattern on ECG. As shown in Figure 4, the acute hemodynamic effect (%ΔLV + dP/dtmax) trended higher for all pacing configuration in patients with a LBBB. The AHR in at least one pacing configuration was (77%, 10/13) for patients with a typical LBBB compared to patients with a non-LBBB (42%, 5/12) (p = 0.11).
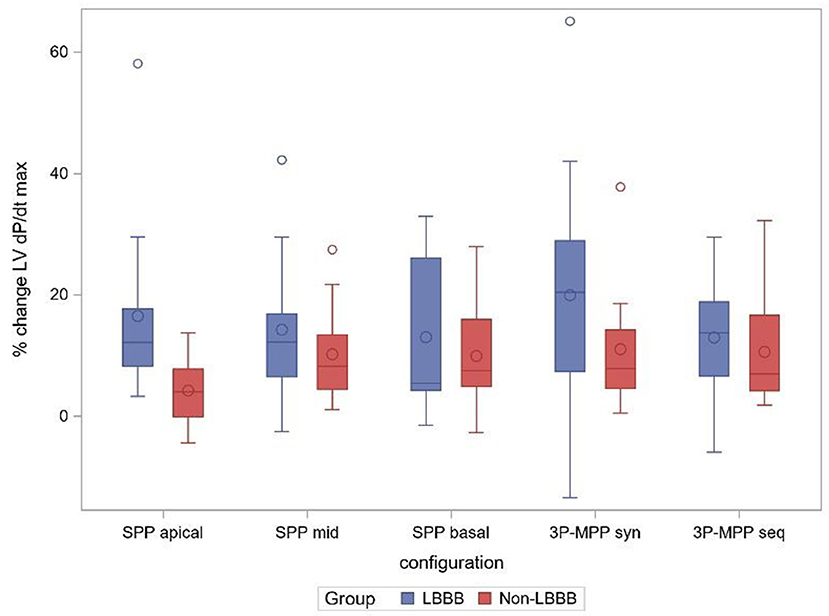
Figure 4. % change ΔLV + dPdtmax boxplot at best AV-delay in the subgroups LBBB and non-LBBB. SPP, RV-LV Single-point pacing and MPP, RV-LV Multi-point pacing. MPPseq, Sequential MPP; MPPsyn, Synchronous (simultaneous) MPP; SPPbasal,mid,apical, SPP from base, mid, apical LV electrode. Solid line depicts the median value, and boxes are 25th and 75th percentile. Whiskers represent the most extreme data point within 1.5x interquartile range from the boxes. Diamonds represent mean value, and dots are outliers.
Effect of QRS Duration
Percentage change QRS duration (%ΔQRS duration) increased by 3-9% for most pacing configurations [SPPbasal (4.9% ± 16.5), SPPmid (3.2 ± 14.9%), SPPapical (8.7% ± 18.0), and MPPseq (8.5% ± 19.7)], but decreased by 4.3% with 3P-MPPsyn (−4.3% ± 14.3). No significant correlation emerged between %ΔQRS duration and %ΔLV + dP/dtmax (N = 24, ρ = −0.28, 95% CI: −0.44-0.10).
Effect of QLV-Delay
The Q-LV/QRS timings ranged from 0.46 ± 0.21 on the apical electrode to 0.55 ± 0.23 and 0.56 ± 0.24 on the mid and basal electrode, respectively. No significant correlation was found between the Q-LV/QRS ratio and the acute hemodynamic effect (%ΔLV + dP/dtmax) for the whole study group with available data (N = 20, ρ = 0.20, 95% CI: −0.06-0.44). However, Q-LV/QRS ratio correlated more strongly with %ΔLV + dP/dtmax for patients with non-LBBB (N = 9, ρ = 0.41, 95% CI: 0.04-0.69), but not with LBBB. Q-LV/QRS ratio correlation with %ΔLV + dP/dtmax was lower (N = 11, ρ = 0.03, 95% CI: −0.33-0.37, p = 0.13) in LBBB patients for all LV electrodes.
Discussion
The current study was designed to search for potential solutions to increase the effectiveness of CRT, in a group of patients initially at risk of non- or sub-response. Factors affecting suboptimal or even non-response phenomenon are well known and have been previously described (27). They have been listed in Supplementary Table 2. Nevertheless current expert opinions (28, 29) indicate that majority of those factors might be easily modifiable and managed by systematic and methodological algorithms of care. LV lead location and LV pacing modes and types—in case of inadequate dyssynchrony correction—remain one of main reason of non-satisfying response and are challenging.
In this acute hemodynamic study, we explored whether CRT, delivered using 3P-MPPsyn or 3P-MPPseq is superior to SPP in patients who are likely sub-responders using low-variance measurement of the acute hemodynamic response (8, 30). Several findings have emerged. First, 3P-MPPsyn and 3P-MPPseq were not superior to SPP. Second, a trend toward an AHR in at least one pacing configuration was observed in patients with a typical LBBB morphology, but in less than half of patients without.
Acute Hemodynamic Response
The AHR rate for our population of patients with myocardial scar, or QRS ≤ 150 or the absence of LBBB was indeed low (~44%). This was considerably lower than the response rate of 96% (23/24) observed in the iSPOT study [in patients with CRT indication and presence of LBBB using the 4 pacing configurations and otherwise a completely comparable protocol: (8)]. Our study confirms the necessity for tailored patient selection for CRT and multipoint LV pacing as proposed by authors (15).
The range of the hemodynamic effect within an individual patient is large (data shown in Supplementary Figure 1). In this study this is especially obvious because of the small standard error for each individual patient configuration, as enforced by the specific measurement protocol applied. This allows within patient assessment, which would otherwise not be possible. In 40% of the patients we find no response (%ΔLV + dP/dtmax <10%) for any configuration (consistent hemodynamic non-responders). In 24% patients we find an acute hemodynamic response (%ΔLV + dP/dtmax ≥ 10%) independent of the configuration (consistent hemodynamic responders). And finally, in the remaining 36% patients we find an acute hemodynamic response only in some of the tested configurations. This last group is clinically the most relevant one, as choosing the right configuration will make the difference between acute hemodynamic response and non-response and thus result in reversed remodeling of LV and long-term patient benefit (31). However, identifying the LV lead position to obtain the maximal possible hemodynamic effect is beyond today's clinical practice, and new non-invasive approaches are clinically needed. QLV/QRSd was not strongly associated with acute hemodynamic response at group level (32). Optimization of the pacing configuration of CRT (with a quadripolar LV lead) is best to rely on functional assessment of cardiac function, instead of local electric delay (32).
Multi-Point Pacing
In the present study, 3P-MPPsyn was the optimal configuration in 36% of all patients which was almost statistically significantly higher than the value of 20% expected by chance (one sided p-value = 0.03). At the same time 3P-MPPsyn demonstrated the highest acute hemodynamic benefit. Moreover, 3P-MPPsyn was the optimal configuration in 47% of those 15 patients who demonstrated an AHR in at least one configuration which was significantly higher than the proportion 21% expected by chance (one sided p-value p < 0.01). This indicates that MPP appears to consistently display better hemodynamic response.
In an pressure-volume loop study of 44 patients, Pappone et al. (6) showed that the best MPP vector configuration was associated with a greater ΔLV + dP/dtmax, stroke work, stroke volume and LVEF, compared with the best SPP vector configuration. Thibault et al. also showed that MPPsyn was associated with a higher ΔLV + dP/dtmax than AAI pacing and that MPP was superior to SPP in 72% patients (33). These data however, maybe confounded by their experimental setup favoring positive outcomes in MPP attributed to multiple MPP configurations vs. one BiV setting using the distal electrode.
In the present study, 3P-MPPseq was the optimal configuration in 28% of all patients which was not significantly higher than the value of 20% expected by chance (one sided p = 0.17). 3P-MPPseq had similar mean AHE as SPPmid and SPPbasal. An acute hemodynamic effect emerged compared to SPP-apical, which must however be attributed to the relatively lesser effect of SPP-apical stimulation. In normal sinus rhythm, electrical impulses travel through the rapid conduction system from the His bundle toward the apex. Thereafter, LV activation spreads from apex to base as impulses exit the Purkinje system into the slower-conducting working myocardium (34). Accordingly, pacing at the apex would thus be expected to provide a physiological sequence of activation. Indeed, computer-modeling studies suggest that LV pacing guided by what is closest to normal activation is superior to pacing the latest activated region (35). In canine LBBB models, the highest hemodynamic response to CRT is observed with LV apical positions, rather than with basal and mid positions (36). This is consistent with our previous publication of a better hemodynamic response from LV apical pacing compared to basal LV pacing in patients with ischemic cardiomyopathy and a LBBB (37). Kandala et al. (38) showed that in patients with a LBBB a longer Q-LV in apically positioned LV leads was associated with more favorable LV reverse remodeling and better outcomes, compared to apically positioned LV leads with shorter Q-LV. Lercher et al. showed that a greater AHE (%ΔLV + dP/dtmax) could be achieved by synchronizing pacing to the earliest activated segment (39). They found that the AHR (i.e., change in systolic blood pressure) was highest when pacing from with distal to basal poles. Together, these findings suggest that mimicking physiological activation by using interpole electrical separation, from apex to base, could be beneficial. In the present study, however, no advantage of 3P-MPPseq was observed.
Collectively the two MPP configurations achieved the highest acute hemodynamic response in 16/25 (64%) patients which was significantly higher than the value (39.2%) expected by chance (one sided p < 0.01).
LBBB Morphology
Sub-analyses of both REVERSE (40) and MADIT-CRT (41) suggested a reduced benefit in patients with non-LBBB QRS morphology. In the present study, we found that a typical LBBB morphology, even in patients with a QRS ≤ 150 ms or myocardial scar, trended toward a higher AHR (albeit small sample size in the current study). This is consistent with the importance placed on LBBB morphology by clinical guidelines (42).
According to recent studies, sequential His bundle pacing (HBP) followed by left ventricular (LV) pacing [His-Optimized CRT (HOT-CRT)] improves ventricular electrical synchrony beyond BiV and MPP (43, 44). In Vijayaraman et al. study (43) clinical response in HOT—CRT patients was also observed in CRT non-responders and non-LBBB patients. Similarly, Jastrzebski et al. (45) showed the best effect of electrical resynchronization and a higher percentage of clinical improvement in the left bundle branch area pacing—optimized CRT (LOT—CRT) group. On the other hand, Senes at al. (46) showed a better ECG effect in patients with HBP or HOT-CRT, but no clinical improvement compared to the conventional BIV pacing. However, large and randomized trials we needed.
Electrical Evaluation
A metanalysis of individual patient data from randomized, controlled trials suggested that the survival benefit from CRT starts at a QRS > 140 ms, with less clear benefit between 120 and 140 ms (47). We found that QRS duration increased by 3-9% in most pacing protocols, with the exception of 3P-MPPsyn, which led to a reduction. As in other studies (32, 48) we have observed no correlation between intrinsic QRS duration and ΔLV + dP/dtmax nor between ΔQRS duration and ΔLV + dP/dtmax. In this study, Q-LV/QRS were lower (0.46-0.56) than observed in patients with LBBB and greater QRS durations (typically around 0.80) (8). In this respect, a low Q-LV/QRS has been shown to relate to worse clinical outcomes (48, 49).
Clinical Implications
This study shows that even in patients with a reduced likelihood of response to, a typical LBBB morphology seems still associated with an improved acute hemodynamics. Our findings indicate that tailoring of pacing configurations (i.e., pacing electrode and optimizing the program) is required to achieve an acute hemodynamic effect in individual patients on the borderline of an clinically relevant hemodynamic response. Whilst our findings support the use of MPP as an option in some patients, it has no clear general benefit in the entire potentially predisposed group.
Limitations
The small sample size is an important limitation, especially for the comparison between LBBB and non-LBBB patients. Therefore, other group comparisons like scar and no-scar or QRS < or > than 150 ms were not performed. The current study was a relatively small, but multicenter, non-randomized study. Furthermore, only acute hemodynamic measurements were used to define the optimal CRT device setting resulting in the best CRT-response. The results observed in this study should be tested in a larger cohort including besides acute hemodynamic measurements also longer term echocardiographic and clinical outcomes (50).
Conclusions
No acute hemodynamic advantage emerged for 3P-MPPsyn or 3P-MPPseq compared to SPP pacing configuration in patients with higher likelihood of CRT sub-response, except when compared to LVapical pacing.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
Study has been approved for all participating sites by Local Ethics Committees and all participants signed informed consent forms: 1. Komisja Bioetyczna Śląskiego Uniwersytetu Medycznego w Katowicach, Katowice, Poland. 2. Eticka Komisia NUSCH Pod Krasnou Horkou 1 83348 Bratislava Slovakia (Slovak Republic). 3. Comité Ético de Investigación Clínica (Instituto de Investigación Sanitaria La Fe), Valencia, Spain. 4. West Midlands - Edgbaston Research Ethics Committee The Old Chapel Royal Standard Place Nottingham NG1 6FS. 5. Regionala Etikprövningsn ämden Afdelning 1 Sandgatan 1 22350 Lund Sweden. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The authors declare that this study received funding from Medtronic, Inc., Bakken Research Center (BRC), Maastricht, The Netherlands. The funder had the following involvement in the study: a role in study design, data collection and analysis (including statistician and database management), decision to publish, preparation of the manuscript and submission process.
Conflict of Interest
VS, FLem, and BS were employed by the company Medtronic Inc. BM and RC were an employee of Medtronic and holds Medtronic stocks. MS, AM, AS, and FLey they received fees from commercial companies.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.901267/full#supplementary-material
References
1. Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol. (2014) 64:1047–58. doi: 10.1016/j.jacc.2014.06.1178
2. Forleo GB, Mantica M, Di Biase L, Panattoni G, Della Rocca DG, Papavasileiou LP, et al. Clinical and procedural outcome of patients implanted with a quadripolar left ventricular lead: early results of a prospective multicenter study. Heart Rhythm. (2012) 9:1822–8. doi: 10.1016/j.hrthm.2012.07.021
3. Leyva F, Zegard A, Qiu T, Acquaye E, Ferrante G, Walton J, et al. Cardiac resynchronization therapy using quadripolar versus non-quadripolar left ventricular leads programmed to biventricular pacing with single-site left ventricular pacing: impact on survival and heart failure hospitalization. J Am Heart Assoc. (2017) 6:e007026. doi: 10.1161/JAHA.117.007026
4. Behar JM, Bostock J, Zhu Li AP, Chin HM, Jubb S, Lent E, et al. Cardiac Resynchronization therapy delivered via a multipolar left ventricular lead is associated with reduced mortality and elimination of phrenic nerve stimulation: long-term follow-up from a multicenter registry. J Cardiovasc Electrophysiol. (2015) 26:540–6. doi: 10.1111/jce.12625
5. Rinaldi CA, Kranig W, Leclercq C, Kacet S, Betts T, Bordachar P, et al. Acute effects of multisite left ventricular pacing on mechanical dyssynchrony in patients receiving cardiac resynchronization therapy. J Card Fail. (2013) 19:731–8. doi: 10.1016/j.cardfail.2013.10.003
6. Pappone C, Calović ž, Vicedomini G, Cuko A, McSpadden LC, Ryu K, et al. Multipoint left ventricular pacing improves acute hemodynamic response assessed with pressure-volume loops in cardiac resynchronization therapy patients. Heart Rhythm. (2014) 11:394–401. doi: 10.1016/j.hrthm.2013.11.023
7. Leyva F, Umar F, Taylor RJ, Steeds RP, Frenneaux MP. The clinical outcome of cardiac resynchronization therapy in post-surgical valvular cardiomyopathy. Europace. (2016) 18:732–8. doi: 10.1093/europace/euv287
8. Sterliński M, Sokal A, Lenarczyk R, Van Heuverswyn F, Rinaldi CA, Vanderheyden M, et al. In heart failure patients with left bundle branch block single lead multispot left ventricular pacing does not improve acute hemodynamic response to conventional biventricular pacing. A multicenter prospective, interventional, non-randomized study. PLoS ONE. (2016) 11:e0154024. doi: 10.1371/journal.pone.0154024
9. Pappone C, Calović Ž, Vicedomini G, Cuko A, McSpadden LC, Ryu K, et al. Multipoint left ventricular pacing in a single coronary sinus branch improves mid-term echocardiographic and clinical response to cardiac resynchronization therapy. J Cardiovasc Electrophysiol. (2015) 26:58–63. doi: 10.1111/jce.12513
10. Leclercq C, Burri H, Curnis A, Delnoy PP, Rinaldi CA, Sperzel J, et al. Cardiac resynchronization therapy non-responder to responder conversion rate in the more response to cardiac resynchronization therapy with MultiPoint Pacing (MORE-CRT MPP) study: results from Phase I. Eur Heart J. (2019) 40:2979–87. doi: 10.1093/eurheartj/ehz109
11. Vanagt WY, Prinzen FW, Delhaas T. Reversal of pacing-induced heart failure by left ventricular apical pacing. N Engl J Med. (2007) 357:2637–8. doi: 10.1056/NEJMc072317
12. Leyva F, Zegard A, Taylor RJ, Foley PWX, Umar F, Patel K, et al. Long-term outcomes of cardiac resynchronization therapy using apical versus nonapical left ventricular pacing. J Am Heart Assoc. (2018) 7:e008508. doi: 10.1161/JAHA.117.008508
13. Rüssel IK, Götte MJ, Bronzwaer JG, Knaapen P, Paulus WJ, van Rossum AC. Left ventricular torsion: an expanding role in the analysis of myocardial dysfunction. JACC Cardiovasc Imaging. (2009) 2:648–55. doi: 10.1016/j.jcmg.2009.03.001
14. Pedrizzetti G, La Canna G, Alfieri O, Tonti G. The vortex–an early predictor of cardiovascular outcome? Nat Rev Cardiol. (2014) 11:545–53. doi: 10.1038/nrcardio.2014.75
15. Leyva F, Zegard A, Umar F, Taylor RJ, Acquaye E, Gubran C, et al. Long-term clinical outcomes of cardiac resynchronization therapy with or without defibrillation: impact of the aetiology of cardiomyopathy. Europace. (2018) 20:1804–12. doi: 10.1093/europace/eux357
16. Świerżyńska E, Mitkowski P, Zakrzewska-Koperska J, Orȩziak A, Baranowski R, Bilińska M, Sterliński M. Spatial separation of left and right ventricular leads adjusted to the left ventricular end-diastolic dimension does not affect the change of the paced QRS complex duration in resynchronization therapy. Kardiol Pol. (2020) 78:1159–61. doi: 10.33963/KP.15595
17. Ginks MR, Shetty AK, Lambiase PD, Duckett SG, Bostock J, Peacock JL, et al. Benefits of endocardial and multisite pacing are dependent on the type of left ventricular electric activation pattern and presence of ischemic heart disease: insights from electroanatomic mapping. Circ Arrhythm Electrophysiol. (2012) 5:889–97. doi: 10.1161/CIRCEP.111.967505
18. Hussain MA, Bhamra-Ariza P, Jacques A, Wilkinson P, Odemuyiwa O, Fluck D, et al. Benefits of a quadripolar left ventricular lead in patients undergoing cardiac resynchronization therapy with underlying myocardial scar. Pacing Clin Electrophysiol. (2013) 36:e45–47. doi: 10.1111/j.1540-8159.2011.03065.x
19. Bordachar P, Ploux S, Ritter P. Three left ventricular leads required for improved haemodynamic and clinical status of a patient with very severe heart failure and a narrow QRS duration. Europace. (2011) 13:439. doi: 10.1093/europace/euq370
20. Henin M, Ragy H, Mannion J, David S, Refila B, Boles U. Indications of cardiac resynchronization in non-left bundle branch block: clinical review of available evidence. Cardiol Res. (2020) 11:1–8. doi: 10.14740/cr989
21. Sohal M, Hamid S, Perego G, Della Bella P, Adhya S, Paisey J, et al. A multicenter prospective randomized controlled trial of cardiac resynchronization therapy guided by invasive dP/dt. Heart Rhythm. (2021) 2:19–27. doi: 10.1016/j.hroo.2021.01.005
22. Strauss DG, Selvester RH. The QRS complex–a biomarker that “images” the heart: QRS scores to quantify myocardial scar in the presence of normal and abnormal ventricular conduction. J Electrocardiol. (2009) 42:85–96. doi: 10.1016/j.jelectrocard.2008.07.011
23. McCrohon JA, Moon JCC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJS, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. (2003) 108:54–9. doi: 10.1161/01.CIR.0000078641.19365.4C
24. Stegemann B, Francis DP. Atrioventricular and interventricular delay optimization and response quantification in biventricular pacing: arrival of reliable clinical algorithms and research protocols, and how to distinguish them from unreliable counterparts. Europace. (2012) 14:1679–83. doi: 10.1093/europace/eus242
25. Whinnett ZI, Davies JE, Willson K, Manisty CH, Chow AW, Foale RA, et al. Haemodynamic effects of changes in atrioventricular and interventricular delay in cardiac resynchronisation therapy show a consistent pattern: analysis of shape, magnitude and relative importance of atrioventricular and interventricular delay. Heart. (2006) 92:1628–34. doi: 10.1136/hrt.2005.080721
26. Whinnett ZI, Francis DP, Denis A, Willson K, Pascale P, van Geldorp I, et al. Comparison of different invasive hemodynamic methods for AV delay optimization in patients with cardiac resynchronization therapy: implications for clinical trial design and clinical practice. Int J Cardiol. (2013) 168:2228–37. doi: 10.1016/j.ijcard.2013.01.216
27. Mullens W, Nijst P. Leadless left ventricular pacing: another step toward improved CRT response. J Am Coll Cardiol. (2017) 69:2130–3. doi: 10.1016/j.jacc.2017.03.534
28. Mullens W, Auricchio A, Martens P, Witte K, Cowie MR, Delgado V, et al. Optimized implementation of cardiac resynchronization therapy: a call for action for referral and optimization of care: a joint position statement from the Heart Failure Association (HFA), European Heart Rhythm Association (EHRA), and European Association of Cardiovascular Imaging (EACVI) of the European Society of Cardiology. Eur J Heart Fail. (2020) 22:2349–69. doi: 10.1002/ejhf.2046
29. Puvrez A, Duchenne J, Gorcsan J, Marwick TH, Smiseth OA, Voigt JU. Why mechanical dyssynchrony remains relevant to cardiac resynchronization therapy. Letter regarding the article 'Optimized implementation of cardiac resynchronization therapy: a call for action for referral and optimization of care: a joint position statement from the Heart Failure Association (HFA), European Heart Rhythm Association (EHRA), and European Association of Cardiovascular Imaging (EACVI) of the European Society of Cardiology'. Eur J Heart Fail. (2021) 23:843–4. doi: 10.1002/ejhf.2150
30. Shun-Shin MJ, Miyazawa AA, Keene D, Sterliński M, Sokal A, Van Heuverswyn F, et al. How to deliver personalized cardiac resynchronization therapy through the precise measurement of the acute hemodynamic response: Insights from the iSpot trial. J Cardiovasc Electrophysiol. (2019) 30:1610–9. doi: 10.1111/jce.14001
31. Duckett SG, Ginks M, Shetty AK, Bostock J, Gill JS, Hamid S, et al. Invasive acute hemodynamic response to guide left ventricular lead implantation predicts chronic remodeling in patients undergoing cardiac resynchronization therapy. J Am Coll Cardiol. (2011) 58:1128–36. doi: 10.1016/j.jacc.2011.04.042
32. van Everdingen WM, Zweerink A, Cramer MJ, Doevendans PA, Nguyên UC, van Rossum AC, et al. Can we use the intrinsic left ventricular delay (QLV) to optimize the pacing configuration for cardiac resynchronization therapy with a quadripolar left ventricular lead? Circ Arrhythm Electrophysiol. (2018) 11:e005912. doi: 10.1161/CIRCEP.117.005912
33. Thibault B, Dubuc M, Khairy P, Guerra PG, Macle L, Rivard L, et al. Acute haemodynamic comparison of multisite and biventricular pacing with a quadripolar left ventricular lead. Europace. (2013) 15:984–91. doi: 10.1093/europace/eus435
34. Durrer D, van Dam RT, Freud GE, Janse MJ, Meijler FL, Arzbaecher RC. Total excitation of the isolated human heart. Circulation. (1970) 41:899–912. doi: 10.1161/01.CIR.41.6.899
35. Pluijmert M, Bovendeerd PH, Lumens J, Vernooy K, Prinzen FW, Delhaas T. New insights from a computational model on the relation between pacing site and CRT response. Europace. (2016) 18(suppl 4):iv94–103. doi: 10.1093/europace/euw355
36. van Deursen C, van Geldorp IE, Rademakers LM, van Hunnik A, Kuiper M, Klersy C, et al. Left ventricular endocardial pacing improves resynchronization therapy in canine left bundle-branch hearts. Circ Arrhythm Electrophysiol. (2009) 2:580–7. doi: 10.1161/CIRCEP.108.846022
37. Umar F, Taylor RJ, Stegemann B, Marshall H, Flannigan S, Lencioni M, et al. Haemodynamic effects of cardiac resynchronization therapy using single-vein, three-pole, multipoint left ventricular pacing in patients with ischaemic cardiomyopathy and a left ventricular free wall scar: the MAESTRO study. Europace. (2016) 18:1227–34. doi: 10.1093/europace/euv396
38. Kandala J, Upadhyay GA, Altman RK, Bose A, Heist EK, Mela T, et al. Electrical delay in apically positioned left ventricular leads and clinical outcome after cardiac resynchronization therapy. J Cardiovasc Electrophysiol. (2013) 24:182–7. doi: 10.1111/j.1540-8167.2012.02428.x
39. Lercher P, Lunati M, Rordorf R, Landolina M, Badie N, Qu F, et al. Long-term reverse remodeling by cardiac resynchronization therapy with MultiPoint Pacing: a feasibility study of noninvasive hemodynamics-guided device programming. Heart Rhythm. (2018) 15:1766–74. doi: 10.1016/j.hrthm.2018.06.032
40. Gold MR, Thebault C, Linde C, Abraham WT, Gerritse B, Ghio S, et al. Effect of QRS duration and morphology on cardiac resynchronization therapy outcomes in mild heart failure: results from the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study. Circulation. (2012) 126:822–9. doi: 10.1161/CIRCULATIONAHA.112.097709
41. Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, Brown M, et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT). Circulation. (2011) 123:1061–72. doi: 10.1161/CIRCULATIONAHA.110.960898
42. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. (2021) 42:3427–520. doi: 10.1093/eurheartj/ehab364
43. Vijayaraman P, Herweg B, Ellenbogen KA, Gajek J. His-optimized cardiac resynchronization therapy to maximize electrical resynchronization: a feasibility study. Circ Arrhythm Electrophysiol. (2019) 12:e006934. doi: 10.1161/CIRCEP.118.006934
44. Zweerink A, Zubarev S, Bakelants E, Potyagaylo D, Stettler C, Chmelevsky M, et al. His-optimized cardiac resynchronization therapy with ventricular fusion pacing for electrical resynchronization in heart failure. JACC Clin Electrophysiol. (2021) 7:881–92. doi: 10.1016/j.jacep.2020.11.029
45. Jastrzebski M, Moskal P, Huybrechts W, Curila K, Sreekumar P, Rademakers LM, et al. Left bundle branch-optimized cardiac resynchronization therapy (LOT-CRT): Results from an international LBBAP collaborative study group. Heart Rhythm. (2022) 19:13–21. doi: 10.1016/j.hrthm.2021.07.057
46. Senes J, Mascia G, Bottoni N, Oddone D, Donateo P, Grimaldi T, et al. Is His-optimized superior to conventional cardiac resynchronization therapy in improving heart failure? Results from a propensity-matched study. Pacing Clin Electrophysiol. (2021) 44:1532–9. doi: 10.1111/pace.14336
47. Cleland JG, Abraham WT, Linde C, Gold MR, Young JB, Claude Daubert J, et al. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J. (2013) 34:3547–56. doi: 10.1093/eurheartj/eht290
48. Engels EB, Mafi-Rad M, van Stipdonk AM, Vernooy K, Prinzen FW. Why QRS Duration should be replaced by better measures of electrical activation to improve patient selection for cardiac resynchronization therapy. J Cardiovasc Transl Res. (2016) 9:257–65. doi: 10.1007/s12265-016-9693-1
49. Gold MR, Birgersdotter-Green U, Singh JP, Ellenbogen KA, Yu Y, Meyer TE, et al. The relationship between ventricular electrical delay and left ventricular remodelling with cardiac resynchronization therapy. Eur Heart J. (2011) 32:2516–24. doi: 10.1093/eurheartj/ehr329
Keywords: heart failure, biventricular pacing, quadripolar lead for left ventricle pacing, multipoint pacing, acute hemodynamic effect, cardiac resynchronization therapy
Citation: Sterliński M, Zakrzewska-Koperska J, Maciąg A, Sokal A, Osca-Asensi J, Wang L, Spyropoulou V, Maus B, Lemme F, Okafor O, Stegemann B, Cornelussen R and Leyva F (2022) Acute Hemodynamic Effects of Simultaneous and Sequential Multi-Point Pacing in Heart Failure Patients With an Expected Higher Rate of Sub-response to Cardiac Resynchronization Therapy: Results of Multicenter SYNSEQ Study. Front. Cardiovasc. Med. 9:901267. doi: 10.3389/fcvm.2022.901267
Received: 21 March 2022; Accepted: 19 April 2022;
Published: 12 May 2022.
Edited by:
Laurens F. Tops, Leiden University Medical Center, NetherlandsReviewed by:
Bert Vandenberk, University of Calgary, CanadaGiuseppe Mascia, San Martino Hospital (IRCCS), Italy
Copyright © 2022 Sterliński, Zakrzewska-Koperska, Maciąg, Sokal, Osca-Asensi, Wang, Spyropoulou, Maus, Lemme, Okafor, Stegemann, Cornelussen and Leyva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joanna Zakrzewska-Koperska, jzakrzewska@ikard.pl
†These authors have contributed equally to this work and share first authorship
 Maciej Sterliński
Maciej Sterliński