- 1Department of Cardiology, Clinic Augustinum Munich, Munich, Germany
- 2Department of Cardiology, Munich University Clinic, Ludwig-Maximilians-University, Munich, Germany
- 3Division of Cardiovascular Imaging, Department of Radiology and Radiological Science, Medical University of South Carolina, Charleston, SC, United States
- 4Department of Cardiology, Vascular Medicine and Pneumology, Gesundheitszentrum Rhein-Neckar Hospital Weinheim, Weinheim, Germany
- 5Cardiac Imaging Center Weinheim, Hector Foundation, Weinheim, Germany
by Yamaura, H., Otsuka, K., Ishikawa, H., Shirasawa, K., Fukuda, D., and Kasayuki, N. (2022). Front. Cardiovasc. Med. 9:824470. doi: 10.3389/fcvm.2022.824470
Over the last 2 decades, coronary CT angiography (CCTA) has emerged as a cornerstone with a class I recommendation for the assessment of coronary artery disease (CAD) (1–3). CCTA allows for combined anatomical and morphological, and if required functional assessment of CAD. In addition, the multicentric, randomized SCOT Heart trial previously demonstrated a significantly lower rate of cardiovascular death or non-fatal myocardial infarction at 5 years of follow-up, in patients who received a primary CCTA vs. standard care strategy, with similar rates of coronary revascularization procedures in both arms (4). In the same direction, a subsection analysis of this trial demonstrated that low attenuation, non-calcified plaque burden is the most robust predictor of future fatal and non-fatal myocardial infarction, beyond calcium score and coronary artery stenosis (5). In addition, a recently published multicentric, randomized trial further strengthens the applicability of a primary CCTA strategy in patients with chronic coronary syndromes, since it resulted in significant lower procedure-related complications such as myocardial infarction and stroke with simultaneously similar rates of major adverse cardiovascular events during long-term follow-up, compared to a primary invasive strategy (6).
Detailed plaque quantification and characterization with the identification of so called “high-risk” plaque features like low-attenuation plaque (LAP) or the “Napkin ring sign” as markers of vulnerable plaques has demonstrated incremental value to identify patients at risk for future cardiovascular events (7, 8). A growing body of evidence supports the value of epicardial adipose tissue (EAT) assessment as a surrogate marker of coronary artery inflammation in addition to the evaluation of coronary plaque extent and morphology (9). Thus, previous studies showed that EAT quantification is closely associated with atherosclerotic plaque burden and the presence of obstructive CAD (10). In addition, EAT recently exhibited incremental value for the prediction of future cardiovascular events compared to conventional risk score models, thus representing a valuable imaging biomarker for the risk stratification of such patients (11).
In the current issue of Frontiers in Cardiovascular Medicine—Cardiovascular Imaging Otsuka and colleagues (12) retrospectively assessed the ability of CCTA-derived low-attenuation non-calcified plaque burden to predict cardiovascular outcomes. In addition, Otsuka et al. investigated the impact of epicardial adipose tissue volume (EAV) on low-attenuation plaque burden in the same patient cohort. Overall, 376 symptomatic patients without known CAD (57% male, 65 ± 13 yrs. old, 22% with diabetes mellitus) with clinical indication for CCTA were analyzed. Most patients were not on cardiac medications, including lipid-lowering therapy and platelet inhibitions at the time of the baseline CCTA (only 5 and 26% on treatment with aspirin and statins, respectively). EAV and coronary plaque burden were assessed using commercially available software tools. All quantitative measures exhibited reasonable reproducibility.
Among the 376 patients included in the trial, the primary endpoint was observed in 15 patients during a mean follow-up of 2.2 ± 0.9 yrs., including death in 2, acute coronary syndromes in 6 and urgent revascularization in seven patients, respectively. Low-attenuation plaque burden was highly predictive for future cardiac events, independent of traditional CAD risk scores, coronary calcification, coronary stenosis severity and EAV (HR 3.05, 95%CI 1.09–8.54, p = 0.033 by adjusted Cox regression analysis). Thus, most patients who reached the primary endpoint, were in the highest low-attenuation plaque burden quartile (Q4). EAV on the other hand, was not predictive for future clinical endpoints but was associated with low-attenuation plaque burden (r = 0.386, p < 0.001). Thus, both EAV ≥125 ml and calcium score ≥218.3 were associated with the presence of the highest low-attenuation plaque burden quartile (Q4).
The report of the study by Otsuka et al. (12) needs to be considered in the context of the current cardiovascular risk assessment approaches. Despite continuous advances in the diagnosis of CHD, sudden death and acute ischemic syndromes continue to be leading causes of morbidity and mortality (13), whereas almost 50% of patients with a sudden cardiac death does not experience limiting clinical symptoms before (14). The results presented by Otsuka et al. agree with previous reports, demonstrating the value of coronary plaque burden and composition for the prediction of future cardiovascular events (5, 10, 15). In addition, the association of EAV with low-attenuation plaque burden could be nicely shown. EAT is the fat tissue located between the visceral pericardium and the myocardium and is widely recognized having numerous exocrine and paracrine effects by the secretion of bioactive substances called adipokines. Both EAT as a whole and EAT within a radial distance from the outer wall of coronary vessels, the so called pericoronary adipose tissue (PCAT) (16), exhibit complex bidirectional interactions with the underlying vascular wall. Thus, dysfunctional EAT and PCAT are involved in the production of proinflammatory adipokines, which may cause the activation of inflammatory pathways via paracrine or vasocrine effects, resulting in endothelial dysfunction, increase coagulability and vasoconstriction, smooth muscle cell proliferation and ultimately atherogenesis progression (17). Importantly, EAT and PCAT inflammation were shown to be associated with CAD progression and adverse cardiovascular events in several clinical studies (18–21). Importantly, in contrast to other imaging modalities, such as echocardiography and cardiac magnetic resonance (CMR), CCTA can visualize and precisely quantify both EAT, PCAT and coronary plaque composition and burden within a single examination, as illustrated in Figure 1 (9).
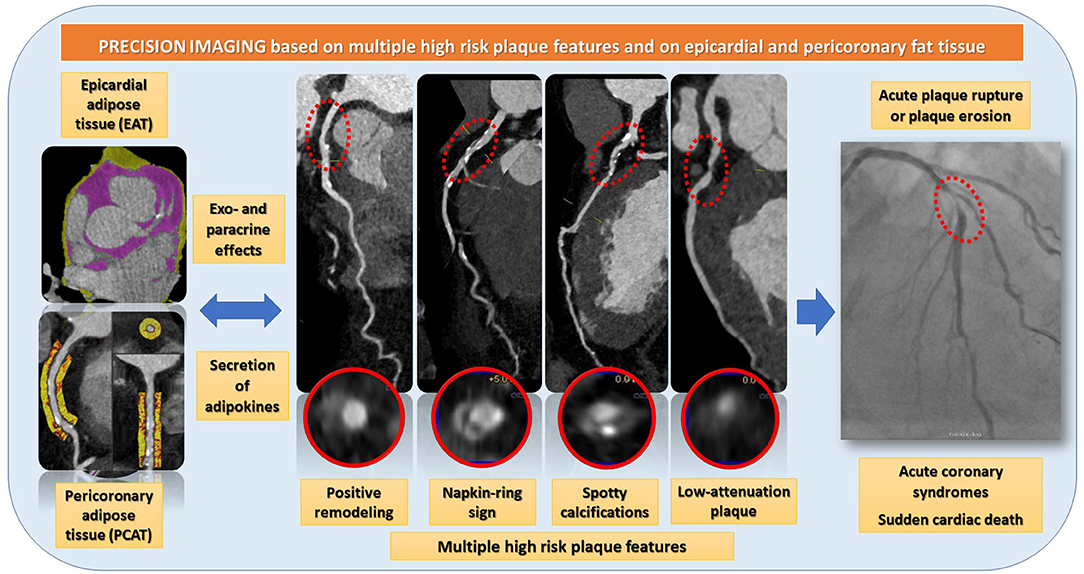
Figure 1. CCTA can visualize and precisely quantify both EAT, PCAT and coronary plaque composition and burden within a single examination. EAT and PCAT are associated with high-risk plaque features, such as positive remodeling, the napkin ring sign, spotty calcifications, and low-attenuation plaques. Such high-risk plaques are rupture prone and therefor potential precursors of acute coronary syndromes.
Some limitations need to be considered when interpreting the results of the present study. From a technical point of view, the assessment of plaque burden and EAV was not purely automatic, so that manual corrections were necessary when using semiautomated plaque and EAV quantification algorithms. This represents a time-consuming process, hampering the translation of such quantification approaches in the clinical realm. This limitation, however, may be overcome by the implementation of artificial intelligence and machine-learning algorithms for fully automated atherosclerotic plaque quantification and characterization derived from CCTA data together with incorporation of “big data” from electronic health records for personalized decision-making and risk prediction (22). Notably, the authors only included low attenuation plaque burden as a central marker of in their analysis, whereas other high risk plaque features, such as the “napkin-ring sign,” spotty calcifications, and the positive vessel remodeling index were not considered. Such a ‘multi-feature' approach may improve the predictive value of CCTA and may be the way to go in future studies. In addition, biochemical markers, such as cardiac troponins, which were previously shown to be associated with plaque composition and cardiovascular outcomes (23, 24) were not analyzed in the present study, which is a limitation. Furthermore, the study was performed retrospectively, and the number of cardiac events was relatively small, especially when focusing on hard cardiac events, such as death and myocardial infarction. Thus, larger studies are warranted to further elucidate the relationship between EAT, PCAT and multiple high risk plaque features for the prediction of cardiovascular outcomes.
Despite these limitations, the study by Otsuka et al. (12) provides excellent evidence that EAV is associated with low-attenuation plaque burden, which in turn is the most robust variable for the prediction of future adverse cardiovascular events. In the era of precision medicine, quantification assessment of such parameters may help identifying patients at high risk for future cardiovascular events, tailoring therapeutic and preventions strategies in such individuals. Health care systems will also definitely need to consider adapting reimbursement strategies in this context, providing incentives for the broader use of CCTA over the much more costly invasive procedures in individuals who develop cardiovascular complications.
Disclosure
CT receives honoraria for speaking and consulting from HeartFlow Inc. and Siemens Healthineers. GK receives institutional research grants from Siemens Healthineers.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pontone G, Rossi A, Guglielmo M, Dweck MR, Gaemperli O, Nieman K, et al. Clinical applications of cardiac computed tomography: a consensus paper of the European Association of Cardiovascular Imaging-part II. Eur Heart J Cardiovasc Imaging. (2022) 23:e136–61. doi: 10.1093/ehjci/jeab293
2. Narula J, Chandrashekhar Y, Ahmadi A, Abbara S, Berman DS, Blankstein R, et al. SCCT 2021 expert consensus document on coronary computed tomographic angiography: a report of the society of cardiovascular computed tomography. J Cardiovasc Comput Tomogr. (2021) 15:192–217. doi: 10.1016/j.jcct.2020.11.001
3. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. (2020) 41:407–77. doi: 10.1093/eurheartj/ehz425
4. Investigators S-H, Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. (2018) 379:924–33. doi: 10.1056/NEJMoa1805971
5. Williams MC, Kwiecinski J, Doris M, McElhinney P, D'Souza MS, Cadet S, et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART trial (Scottish Computed Tomography of the HEART). Circulation. (2020) 141:1452–62. doi: 10.1161/CIRCULATIONAHA.120.049840
6. Group DT, Maurovich-Horvat P, Bosserdt M, Kofoed KF, Rieckmann N, Benedek T, et al. CT or invasive coronary angiography in stable chest pain. N Engl J Med. (2022). doi: 10.1056/NEJMoa2200963. [Epub ahead of print].
7. Shaw LJ, Blankstein R, Bax JJ, Ferencik M, Bittencourt MS, Min JK, et al. Society of cardiovascular computed tomography / North American society of cardiovascular imaging - expert consensus document on coronary CT imaging of atherosclerotic plaque. J Cardiovasc Comput Tomogr. (2021) 15:93–109. doi: 10.1016/j.jcct.2020.11.002
8. Velangi PS, Maharaj V, Athwal SS, Bartos JA, Markowitz J, Duval S, et al. Computed tomography coronary plaque characteristics predict ischemia detected by invasive fractional flow reserve. J Thorac Imaging. (2021) 36:360–6. doi: 10.1097/RTI.0000000000000543
9. Guglielmo M, Lin A, Dey D, Baggiano A, Fusini L, Muscogiuri G, et al. Epicardial fat and coronary artery disease: role of cardiac imaging. Atherosclerosis. (2021) 321:30–8. doi: 10.1016/j.atherosclerosis.2021.02.008
10. Gitsioudis G, Schmahl C, Missiou A, Voss A, Schussler A, Abdel-Aty H, et al. Epicardial adipose tissue is associated with plaque burden and composition and provides incremental value for the prediction of cardiac outcome. A clinical cardiac computed tomography angiography study. PLoS ONE. (2016) 11:e0155120. doi: 10.1371/journal.pone.0155120
11. Brandt V, Bekeredjian R, Schoepf UJ, Varga-Szemes A, Emrich T, Aquino GJ, et al. Prognostic value of epicardial adipose tissue volume in combination with coronary plaque and flow assessment for the prediction of major adverse cardiac events. Eur J Radiol. (2022) 148:110157. doi: 10.1016/j.ejrad.2022.110157
12. Yamaura H, Otsuka K, Ishikawa H, Shirasawa K, Fukuda D, Kasayuki N. Determinants of non-calcified low-attenuation coronary plaque burden in patients without known coronary artery disease: A coronary ct angiography study. Front Cardiovasc Med. (2022) 9. doi: 10.3389/fcvm.2022.824470 [Epub ahead of print].
13. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. (2022) 145:e153–639. doi: 10.1161/CIR.0000000000001052
14. Marijon E, Uy-Evanado A, Dumas F, Karam N, Reinier K, Teodorescu C, et al. Warning symptoms are associated with survival from sudden cardiac arrest. Ann Intern Med. (2016) 164:23–9. doi: 10.7326/M14-2342
15. Yamamoto H, Kihara Y, Kitagawa T, Ohashi N, Kunita E, Iwanaga Y, et al. Coronary plaque characteristics in computed tomography and 2-year outcomes: the PREDICT study. J Cardiovasc Comput Tomogr. (2018) 12:436–43. doi: 10.1016/j.jcct.2018.07.001
16. Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. (2017) 9:eaal2658. doi: 10.1126/scitranslmed.aal2658
17. Mancio J, Azevedo D, Saraiva F, Azevedo AI, Pires-Morais G, Leite-Moreira A, et al. Epicardial adipose tissue volume assessed by computed tomography and coronary artery disease: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. (2018) 19:490–7. doi: 10.1093/ehjci/jex314
18. Zhou J, Chen Y, Zhang Y, Wang H, Tan Y, Liu Y, et al. Epicardial fat volume improves the prediction of obstructive coronary artery disease above traditional risk factors and coronary calcium score. Circ Cardiovasc Imaging. (2019) 12:e008002. doi: 10.1161/CIRCIMAGING.118.008002
19. Goeller M, Achenbach S, Marwan M, Doris MK, Cadet S, Commandeur F, et al. Epicardial adipose tissue density and volume are related to subclinical atherosclerosis, inflammation and major adverse cardiac events in asymptomatic subjects. J Cardiovasc Comput Tomogr. (2018) 12:67–73. doi: 10.1016/j.jcct.2017.11.007
20. Goeller M, Tamarappoo BK, Kwan AC, Cadet S, Commandeur F, Razipour A, et al. Relationship between changes in pericoronary adipose tissue attenuation and coronary plaque burden quantified from coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging. (2019) 20:636–43. doi: 10.1093/ehjci/jez013
21. Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. (2018) 392:929–39. doi: 10.1016/S0140-6736(18)31114-0
22. Al'Aref SJ, Anchouche K, Singh G, Slomka PJ, Kolli KK, Kumar A, et al. Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. Eur Heart J. (2019) 40:1975–86. doi: 10.1093/eurheartj/ehy404
23. Gitsioudis G, Schussler A, Nagy E, Maurovich-Horvat P, Buss SJ, Voss A, et al. Combined assessment of high-sensitivity troponin T and noninvasive coronary plaque composition for the prediction of cardiac outcomes. Radiology. (2015) 276:73–81. doi: 10.1148/radiol.15141110
Keywords: epicardial fat tissue, pericoronary adipose tissue attenuation, low-attenuation plaque, high-risk plaque features, cardiac outcomes, troponin
Citation: Tesche C, Giesen A and Korosoglou G (2022) Commentary: Plaque Features and Epicardial Fat Volume for Cardiovascular Risk Assessment—A Key Role With Cardiac Computed Tomography? Front. Cardiovasc. Med. 9:896321. doi: 10.3389/fcvm.2022.896321
Received: 14 March 2022; Accepted: 11 April 2022;
Published: 29 April 2022.
Edited by:
Damiano Caruso, Sapienza University of Rome, ItalyReviewed by:
Domenico De Santis, Sapienza University of Rome, ItalyCopyright © 2022 Tesche, Giesen and Korosoglou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Grigorios Korosoglou, Z2tvcm9zb2dsb3VAaG90bWFpbC5jb20=
 Christian Tesche
Christian Tesche Alexander Giesen4,5
Alexander Giesen4,5 Grigorios Korosoglou
Grigorios Korosoglou