- 1Research Institute of the McGill University Health Center, Montreal, QC, Canada
- 2Department of Food and Drug, University of Parma, Parma, Italy
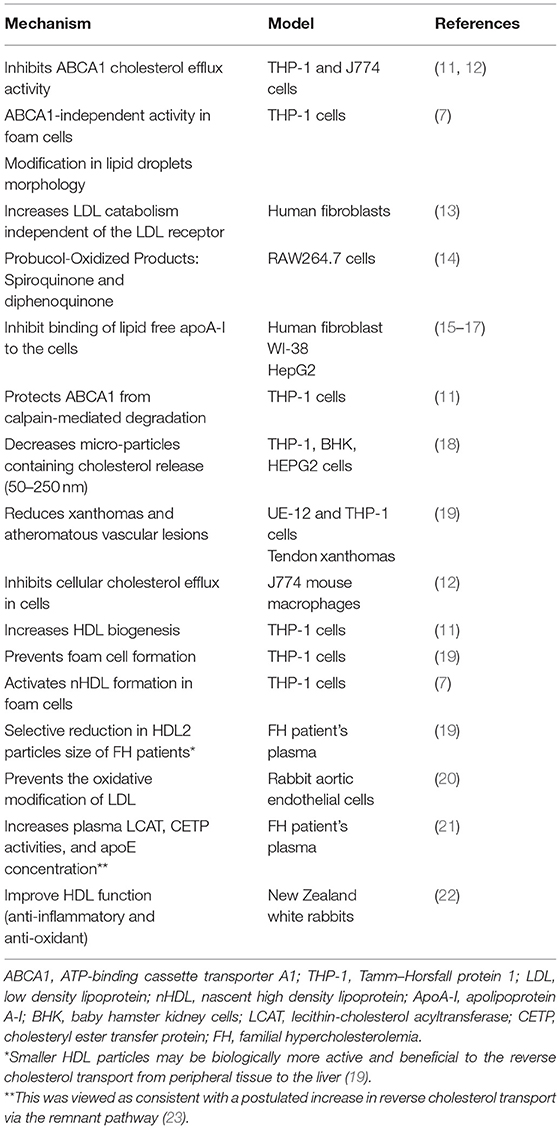
Since the 1970's, high density lipoprotein (HDL) has been an active research topic due to epidemiological studies, such as the Framingham study and many others, that established the inverse association between HDL cholesterol (HDL-C) levels and the prevalence of coronary heart disease (CHD) (1). The deceptive results of HDL-based therapies (inhibitors of cholesteryl ester transfer protein, niacin, and apolipoprotein A-I (apoA-I) infusion therapies), and Mendelian randomization approaches do not fully support a causal association between HDL-C and cardiovascular protection (2). Thus, the role of HDL in health and disease is more complex than anticipated (3). Consequently, there has been a paradigm shift in the study of HDL as a therapeutic target, from the measurement of HDL concentration to the evaluation of HDL function (i.e., cholesterol efflux capacity) (4). Growing evidence proposes that cardiovascular morbid conditions alter the HDL composition and roles transforming it from healthy and functional into pro-atherogenic and dysfunctional (5). A key player in the HDL metabolic pathway that has been substantially explored by various agonists is the ATP-binding cassette transporter A1 (ABCA1) defined as the rate-limiting factor in the formation of HDL (6). This transporter mediates cellular cholesterol and phospholipid removal to generate nascent HDL (nHDL). The most extensively studied function of HDL is the ability to promote net cellular cholesterol efflux. However, the regulation of ABCA1 receptor expression is complex and poorly understood and the physiological and clinical relevance of such a treatment remains uncertain. In the current issue of BBA Advances, we report the findings of our cellular studies on a new mechanism in foam cell macrophages that is ABCA1-independent, and revealed through the use of probucol (7). Although clinical trials were stopped (8), probucol is still being investigated for its effect on the inhibition of atherosclerosis initiation in vitro and in animal models. Of interest, probucol trials still ongoing suggest potential benefits on CHD on top of conventional therapy (9). Basically, probucol is known to act as an ABCA1 inhibitor (10), although the method of addition of probucol to cells or animals may explain some of the differences observed in the inhibitory activity. We show that probucol treated THP-1 foam cells are still able to induce the release of cholesterol-containing small nHDL particles with a diameter of more than 7 nm in an ABCA1-independent manner. In support, we demonstrate that ABCA1 expression is the same in non-foam and foam cells, despite different efflux levels. Quantitative data show that probucol only partially inhibits the transfer of cholesterol into nHDL particles. Interestingly, the release of these probucol-nHDL were active in HDL biogenesis, supporting the contention that these particles are potentially atheroprotective, especially when macrophage-derived cholesterol is involved (Table 1). Indeed, the ABCA1-independent activity influencing the total accessible plasma membrane cholesterol level that remains in foam cells is consistent with the concept that lipids within nHDL originate from specific domains in the plasma membrane. A previous study by Yamamoto et al. demonstrated that probucol enhanced the release of cholesterol from foam cells but with no description of ABCA1's role (11, 14–16, 19). Despite a paradox surrounding the lipid lowering effect of probucol (Table 1), these findings align with data supporting the potential antiatherogenic role of probucol. Indeed, previous studies indicated positive effects of probucol on atherosclerosis treatments in vitro and in vivo (19, 24, 25), however some clinical data indicated negative effects of probucol (26). In addition, a role of probucol was observed in reducing micro-particles release mainly those rich in cholesterol with size range from various cell lines (50–250 nm) (Table 1) (18). Use of probucol unveiled a novel and specific pathway in foam cells where functional cholesterol efflux and formation of nHDL is enhanced in the absence of ABCA1 activity. This activity was not observed in non-foam cells. Moreover, probucol incorporation significantly influences lipid droplet morphology and size (7). This is relevant to lipids droplets roles in mammalian innate immunity, triglyceride synthesis, and mitochondrial dynamics (27, 28). These observations will clarify the mechanisms by which HDL can be protective especially in foam cells. However, the physiological and clinical importance of such approaches remains to elucidated, and substantial additional preclinical work will be required. Exploring new HDL generating pathways that enhance cholesterol efflux is a prospect of a completely novel strategy to raising plasma HDL concentration for CHD prevention that might succeed where other approaches have failed. However, because of the unsatisfactory track record of HDL-based therapies, further research is imperative before renewing our enthusiasm for HDL as a target for therapy. Despite the fact that we have not the ability of probucol to enhance an ABCA1-independent pathway, we suggest the possibility to use probucol as a tool to probe intracellular cholesterol trafficking to inhibit ABCA1. Overall, this may provide substantial evidence for a revised model of cholesterol trafficking in macrophages foam cells. In our opinion this is a new argument in HDL metabolism among cardiovascular researchers if probucol has clinical significance. There is compelling purposes to believe that this old controversial medication has much more to offer than previously known.
Author Contributions
AH conceptualized, wrote, edited, and revised the manuscript. AR, RK, and EF edited and revised the manuscript. All authors approved the submitted version.
Funding
AH received support by Postdoctoral Fellowships from the Canadian Institutes of Health Research (CIHR, RN408399 – 430975).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham study. Am J Med. (1977) 62:707–14. doi: 10.1016/0002-9343(77)90874-9
2. Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, et al. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. J Am Med Assoc. (2008) 299:2524–32. doi: 10.1001/jama.299.21.2524
3. Ndrepepa G. High-density lipoprotein: a double-edged sword in cardiovascular physiology and pathophysiology. J Lab Precision Med. (2021) 6:32. doi: 10.21037/jlpm-21-32
4. Sacks FM, Jensen MK. From high-density lipoprotein cholesterol to measurements of function: prospects for the development of tests for high-density lipoprotein functionality in cardiovascular disease. Arterioscler Thromb Vasc Biol. (2018) 38:487–99. doi: 10.1161/ATVBAHA.117.307025
5. Chiesa ST, Charakida M. High-density lipoprotein function and dysfunction in health and disease. Cardiovasc Drugs Ther. (2019) 33:207–19. doi: 10.1007/s10557-018-06846-w
6. Bielicki JK. ABCA1 agonist peptides for the treatment of disease. Curr Opin Lipidol. (2016) 27:40–6. doi: 10.1097/MOL.0000000000000258
7. Hafiane A, Pisaturo A, Ronca A, Incerti M, Kiss RS, Favari E. Probucol treatment is associated with an ABCA1-independent mechanism of cholesterol efflux to lipid poor apolipoproteins from foam cell macrophages. BBA Adv. (2021) 1:100003. doi: 10.1016/j.bbadva.2021.100003
8. Miida T, Seino U, Miyazaki O, Hanyu O, Hirayama S, Saito T, et al. Probucol markedly reduces HDL phospholipids and elevated prebeta1-HDL without delayed conversion into alpha-migrating HDL: putative role of angiopoietin-like protein 3 in probucol-induced HDL remodeling. Atherosclerosis. (2008) 200:329–35. doi: 10.1016/j.atherosclerosis.2007.12.031
9. Yamashita S, Arai H, Bujo H, Masuda D, Ohama T, Ishibashi T, et al. Probucol trial for secondary prevention of atherosclerotic events in patients with coronary heart disease (prospective). J Atheroscler Thromb. (2021) 28:103–23. doi: 10.5551/jat.55327
10. Yamamoto S, Tanigawa H, Li X, Komaru Y, Billheimer JT, Rader DJ. Pharmacologic suppression of hepatic ATP-binding cassette transporter 1 activity in mice reduces high-density lipoprotein cholesterol levels but promotes reverse cholesterol transport. Circulation. (2011) 124:1382–90. doi: 10.1161/CIRCULATIONAHA.110.009704
11. Arakawa R, Tsujita M, Iwamoto N, Ito-Ohsumi C, Lu R, Wu CA, et al. Pharmacological inhibition of ABCA1 degradation increases HDL biogenesis and exhibits antiatherogenesis. J Lipid Res. (2009) 50:2299–305. doi: 10.1194/jlr.M900122-JLR200
12. Favari E, Zanotti I, Zimetti F, Ronda N, Bernini F, Rothblat GH. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler Thromb Vasc Biol. (2004) 24:2345–50. doi: 10.1161/01.ATV.0000148706.15947.8a
13. Basu SK, Goldstein JL, Anderson GW, Brown MS. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci USA. (1976) 73:3178–82. doi: 10.1073/pnas.73.9.3178
14. Yakushiji E, Ayaori M, Nishida T, Shiotani K, Takiguchi S, Nakaya K, et al. Probucol-oxidized products, spiroquinone and diphenoquinone, promote reverse cholesterol transport in mice. Arterioscler Thromb Vasc Biol. (2016) 36:591–7. doi: 10.1161/ATVBAHA.115.306376
15. Tsujita M, Wu CA, Abe-Dohmae S, Usui S, Okazaki M, Yokoyama S. On the hepatic mechanism of HDL assembly by the ABCA1/apoA-I pathway. J Lipid Res. (2005) 46:154–62. doi: 10.1194/jlr.M400402-JLR200
16. Wu CA, Tsujita M, Hayashi M, Yokoyama S. Probucol inactivates ABCA1 in the plasma membrane with respect to its mediation of apolipoprotein binding and high density lipoprotein assembly and to its proteolytic degradation. J Biol Chem. (2004) 279:30168–74. doi: 10.1074/jbc.M403765200
17. Tsujita M, Yokoyama S. Selective inhibition of free apolipoprotein-mediated cellular lipid efflux by probucol. Biochemistry. (1996) 35:13011–20. doi: 10.1021/bi960734h
18. Hafiane A, Genest J. ATP binding cassette A1 (ABCA1) mediates microparticle formation during high-density lipoprotein (HDL) biogenesis. Atherosclerosis. (2017) 257:90–9. doi: 10.1016/j.atherosclerosis.2017.01.013
19. Yamamoto A, Hara H, Takaichi S, Wakasugi J, Tomikawa M. Effect of probucol on macrophages, leading to regression of xanthomas and atheromatous vascular lesions. Am J Cardiol. (1988) 62:31b–6b. doi: 10.1016/S0002-9149(88)80048-1
20. Parthasarathy S, Young SG, Witztum JL, Pittman RC, Steinberg D. Probucol inhibits oxidative modification of low density lipoprotein. J Clin Invest. (1986) 77:641–4. doi: 10.1172/JCI112349
21. Adlouni A, El Messal M, Saïle R, Parra H, Fruchart J, Ghalim N. Probucol promotes reverse cholesterol transport in heterozygous familial hypercholesterolemia. Effects on apolipoprotein AI-containing lipoprotein particles. Atherosclerosis. (2000) 152:433–40. doi: 10.1016/S0021-9150(99)00493-1
22. Zhong J-K, Guo Z-G, Li C, Wang Z-K, Lai W-Y, Tu Y. Probucol alleviates atherosclerosis and improves high density lipoprotein function. Lipids Health Dis. (2011) 10:210. doi: 10.1186/1476-511X-10-210
23. McPherson R, Hogue M, Milne RW, Tall AR, Marcel YL. Increase in plasma cholesteryl ester transfer protein during probucol treatment. Relation to changes in high density lipoprotein composition. Arteriosclerosis Thrombosis. (1991) 11:476–81. doi: 10.1161/01.ATV.11.3.476
24. Yamamoto A, Takaichi S, Hara H, Nishikawa O, Yokoyama S, Yamamura T, et al. Probucol prevents lipid storage in macrophages. Atherosclerosis. (1986) 62:209–17. doi: 10.1016/0021-9150(86)90095-X
25. Kita T, Nagano Y, Yokode M, Ishii K, Kume N, Ooshima A, et al. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci USA. (1987) 84:5928–31. doi: 10.1073/pnas.84.16.5928
26. Yokoyama S, Yamamoto A, Kurasawa T. A little more information about aggravation of probucol-induced HDL-reduction by clofibrate. Atherosclerosis. (1988) 70:179–81. doi: 10.1016/0021-9150(88)90114-1
27. Bosch M, Sánchez-Álvarez M, Fajardo A, Kapetanovic R, Steiner B, Dutra F, et al. Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science. (2020) 370:aay8085. doi: 10.1126/science.aay8085
Keywords: probucol, cellular cholesterol efflux, atherosclerosis, foam cells/macrophages, HDL
Citation: Hafiane A, Ronca A, Kiss RS and Favari E (2022) High Density Lipoprotein-Based Therapeutics: Novel Mechanism of Probucol in Foam Cells. Front. Cardiovasc. Med. 9:895031. doi: 10.3389/fcvm.2022.895031
Received: 12 March 2022; Accepted: 29 March 2022;
Published: 26 April 2022.
Edited by:
Wilfried Le Goff, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Maki Tsujita, Nagoya City University, JapanCopyright © 2022 Hafiane, Ronca, Kiss and Favari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anouar Hafiane, YW5vdWFyLmhhZmlhbmVAbWFpbC5tY2dpbGwuY2E=
 Anouar Hafiane
Anouar Hafiane Annalisa Ronca2
Annalisa Ronca2 Robert S. Kiss
Robert S. Kiss Elda Favari
Elda Favari