
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 16 June 2022
Sec. Cardiovascular Therapeutics
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.892588
Coronary artery disease is a serious threat to human health. More and more evidences indicate chronic inflammatory plays a key role in the development of this disease. Inflammation markers are gradually used in the diagnosis and treatment. Although the treatment of coronary heart disease with colchicine is still controversial, more and more studies showed that patients can benefit from this medicine. In this review, we discuss and summarize colchicine on essential pharmacology, anti-inflammatory mechanism of action, and the most important and recent clinical studies. According to these literatures, colchicine possibly will possibly become a new valuable and cheap medicine for the treatment of coronary artery disease.
Coronary artery disease (CAD) is a pathological process characterized by the accumulation of atherosclerotic plaques in epicardial arteries (1, 2). Lifestyle adjustment, drug therapy, and invasive therapy can change this process. Despite the availability of safe and effective low-density lipoprotein (LDL)-lowering therapy and antiplatelet therapy, the incidence of cardiovascular disease has been increasing (3). In recent viewpoint, arteriosclerosis is not only caused by lipid accumulation but also a chronic inflammatory reaction. Some studies showed that more than 50% of cardiovascular events may be attributed partly to inflammation (4–6). The recent consensus published by the European Society of Cardiology (ESC) confirmed that inflammation is an important factor in atherosclerosis (2). The CANTOS study was the first large-scale clinical study to confirm that anti-inflammatory treatment could reduce cardiovascular events, providing definite evidence for the inflammation hypothesis of arteriosclerosis with a reduction from baseline in interleukin (IL)-6 (Figure 1). But canakinumab is limited in the cardiovascular field because of its high price and causing serious infections (7). However, the CIRT study showed methotrexate did not decrease the level of IL-1β, IL-6, or C reactive protein (CRP) and did not reduce cardiovascular events (8). Colchicine, one of the oldest drugs, is widely used for acute gout, familial mediterranean fever, and pericarditis, Behcet’s disease (9). Over the past decades, with advances in the knowledge of cytoskeletal microtubules (MT) and anti-inflammatory effects of colchicine, low-dose colchicine (0.5–1.0 mg/daily) was increasingly administered for the therapy of cardiovascular diseases such as CAD, postoperative atrial fibrillation (in cardiac surgery), cardiac hypertrophy-associated heart failure (10). What’s more, in an animal experiment, colchicine improved left ventricular (LV) function in coxsackievirus 3 (CVB3) induced myocarditis and decreased cardiac and splenic family pyrin domain-containing protein 3 (NLRP3) inflammasome activity (11). A large number of studies about colchicine make progress in the treatment of CAD (Table 1). The aim of this review is to discuss pharmacology, anti-inflammatory mechanism of action, and recent clinical studies of colchicine in CAD.
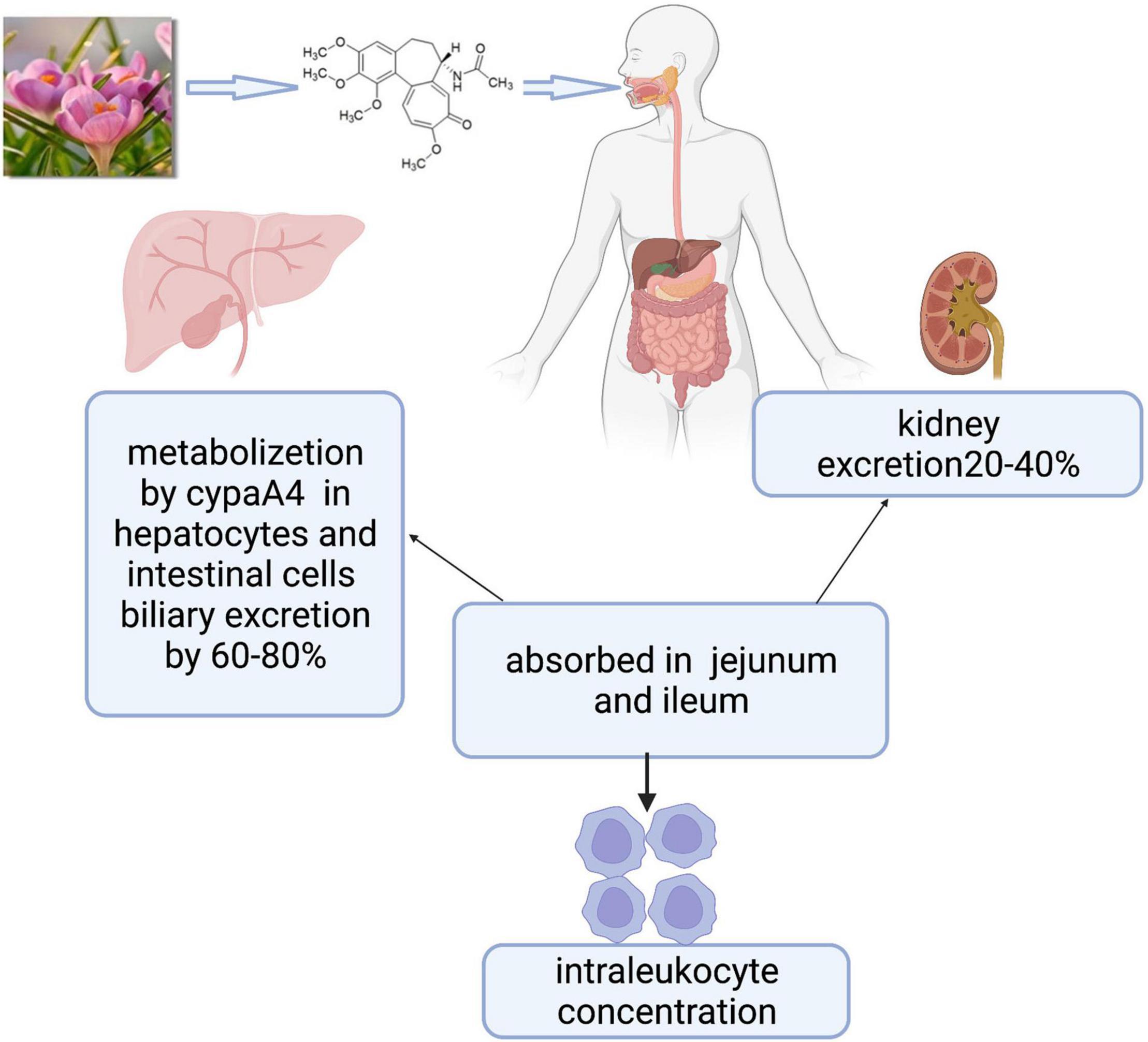
Figure 1. Colchicine is the active principle derived from colchicum autumnale plants whose formula is C22H25NO6, including three rings. Colchicine is absorbed in the jejunum and ileum, metabolized mainly by the CYP3A4 in the liver and intestine, and cleared by bile and kidney. Biological effects are mainly related to intraleukocyte concentrations.
We searched through electronic databases (MEDLINE/PubMed, EMBASE, and Google Scholar) using the search terms “colchicine” AND “cardiovascular disease” OR “coronary artery disease” OR “chronic coronary syndromes” OR “acute coronary syndromes” OR “percutaneous coronary intervention” OR “coronary artery bypass grafting.” The research was restricted to the English language.
Colchicine is an alkaloid with the formula C22H25NO6 (Figure 1), including three rings (12). The jejunum and ileum are the main sites of colchicine absorption. Following the oral route, peak plasma concentration occurs 1 h after administration, and the bioavailability can vary from 24 to 88% of the administered dose. The steady-state was reached at 8 days after the first oral administration, and the maximum anti-inflammatory effect was reached at 24–48 h. Colchicine and its metabolites are mainly cleared by kidney and bile and the elimination half-life ranged from 20 to 40 h. Pharmacokinetic/pharmacodynamic studies show that the biological effects of colchicine are related to intraleukocyte concentrations instead of plasma concentrations. Colchicine preferentially binds to three proteins including tubulin, cytochrome p3a4 (CYP3A4), and P-glycoprotein (13). The tubulin prevents persistently the fusion of autophagic vacuoles with lysosomes in neurons, bone marrow, and muscle, resulting in the risk of damage to these organ systems, especially in patients with liver and/or renal insufficiency. CYP3A4 and P-glycoprotein largely contribute to affecting colchicine metabolism and colchicine’s drug-drug interactions. CYP3A4 exists in hepatocytes and intestinal cells and metabolizes colchicine to 2- and 3-desmethylcolchicine. P-glycoprotein is an ATPase efflux pump, which exists in intestinal cells, liver cells, kidney cells, and the blood-brain barrier; it can affect the absorption of colchicine from the gastrointestinal tract. The prescribed dose, kidney or liver disease, and nature of adjunctive medication can trigger the risk of severe drug-drug interactions of colchicine. The combination with P-glycoprotein inhibitors (e.g., cyclosporine, calcium channel blockers, and ranolazine) or CYP3A4 inhibitors (e.g., clarithromycin, itraconazole, fluoxetine, ketoconazole, nefazodone, and cimetidine), makes colchicine metabolism disorders, further leads to concentration of colchicine increase, and finally happens drug poisoning (13, 14).
Colchicine was originally extracted from the autumn crocus as an anti-inflammatory drug and used by the ancient Greeks and Egyptians (15–17). The anti-inflammatory mechanism of colchicine mainly includes the following aspects. (1) Colchicine binds with high affinity to the specific domain of β-tubulin, resulting in (i) inhibition of tubulin assembly into MT, (ii) disassembly of preformed MT, and/or (iii) inhibition of membrane-bound tubulin sensitive cellular processes (10, 18), Through the mechanisms, different functions of MT are impaired such as amoeboid movements of leucocytes, exocytosis, and phagocytosis, separation of chromosome pairs during mitosis (19). Smooth muscle cells (SMC) secretion and other cellular atherogenic phenomena can be inhibited by colchicine through its anti-microtubule effect (18, 20), and multiple inflammatory factors are down-regulated by the effect of MT (17). (2) Colchicine can suppress NLRP3 inflammasome being present in myeloid cells (Figure 2), including monocytes, neutrophils, and eosinophils or its downstream inflammatory cytokine activation (21). Highly mature inflammatory cytokines IL-1β and IL-18 produced from NLRP3 lead to the progression of atherosclerotic plaques and even shedding of plaques (22, 23). In a large number of trials, NLRP3 expression and downstream cytokines in CAD were significantly higher compared with normal controls (24, 25). Blocking NLRP3 inflammasome not only inhibits oxidized LDL and cholesterol crystal-induced foam cell formation (26) but also has a good influence on ischemia/reperfusion injury, bringing about smaller infarct size and less fibrosis in mice (27). (3) High-sensitivity C-reactive protein (hs-CRP) as a downstream important effector of NLRP3 inflammasome is produced in hepatic through regulation by interleukin 6 (IL-6) (28). Colchicine can decrease significantly hs-CRP which is closely related to arteriosclerosis (29, 30). In the CANTOS study, the reduction of hs-CRP in the treatment group obviously increased cardiovascular benefits and reduced cardiovascular and total mortality (7). Nidorf et al. demonstrated that colchicine can effectively decrease hs-CRP in patients with chronic coronary syndrome (CCS) (31). Measurement of hs-CRP has been shown to independently predict future cardiovascular events in patients with CCS (32), and those with acute coronary syndrome (ACS) (33). (4) Expression of surface markers of platelet activity can be reduced and leukocyte-platelet aggregation can be inhibited by colchicine, which is a major contributor to atherothrombosis (34). Recent data showed that colchicine has antiplatelet activity (35, 36). (5) Colchicine treatment significantly reduces the expression of key inflammatory-related miRNA (37). (6) Colchicine also affects endothelial function (17). Colchicine improves endothelial function through its anti-inflammatory effect in CAD patients with leukocyte activation and reduces the adhesion of leukocytes to endothelium (16, 29). (7) In addition, the synthesis of prostaglandin E2, leukotriene B4, TNF-A, and TxA2, as well as the activity of COX-2 can be inhibited by Colchicine. Even at low doses, it inhibits inflammation by reducing the expression of E and P-selectin and weakens the adhesion of polymorphonuclear neutrophils (PMN) to the endothelium and inhibits neutrophil migration (16, 17).
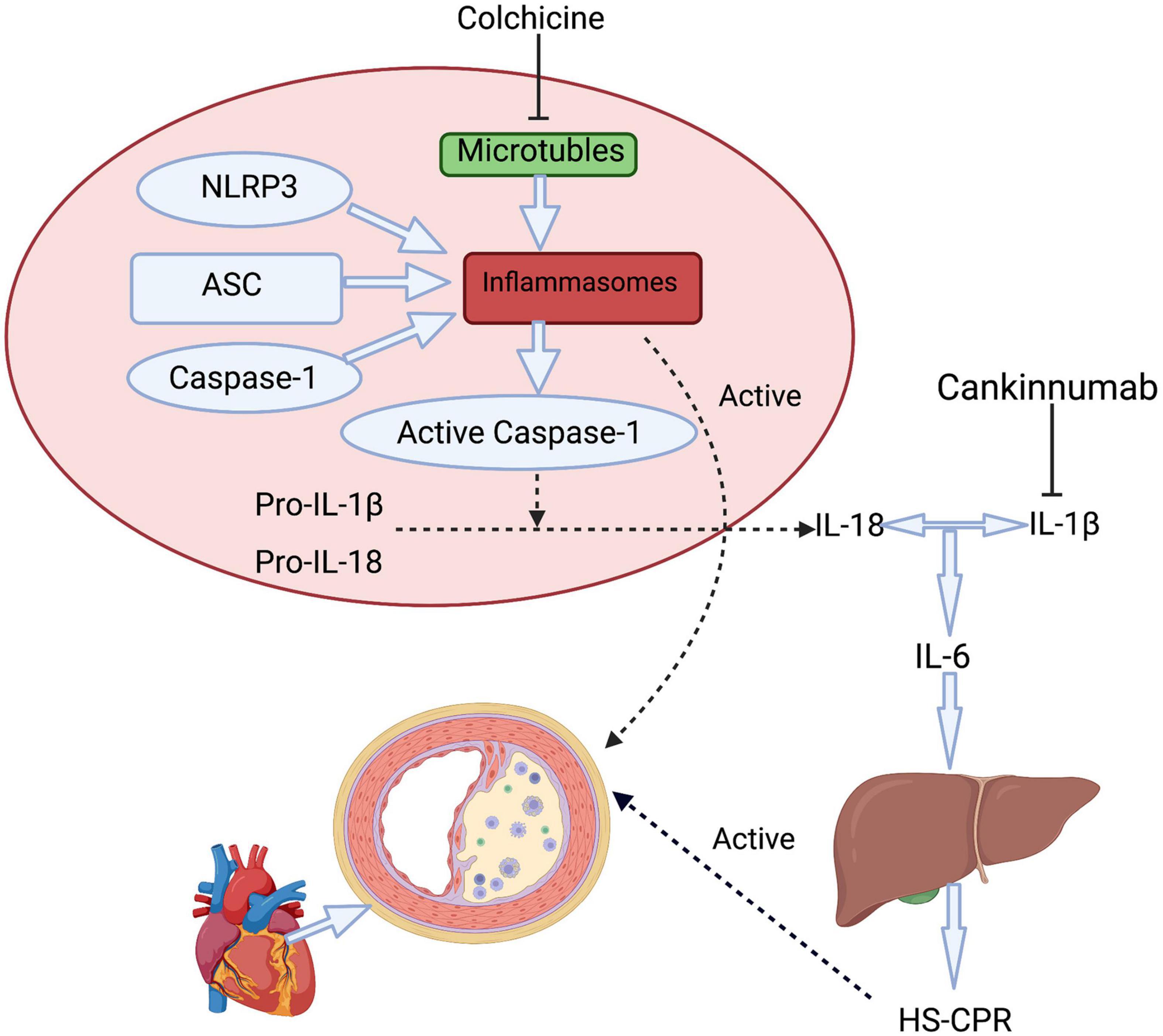
Figure 2. A simplified approach to inflammasome activation pathways includes therapy mechanisms of colchicine and canakinumab. Colchicine affects the NLRP3 inflammasome by inhibiting the function of microtubules, further resulting decrease in the downstream inflammatory factors include IL-1β, IL-6, and HS-CRP. Canakinumab prevents IL-1β and further reduces the release of IL-6 from various types of cells. hs- CRP is produced in hepatic through regulation by IL-6. ASC, apoptosis-associated speck-like protein containing a CARD; NLRP3, NLR family pyrin domain containing 3; IL, interleukin; hs- CRP, High-sensitivity C-reactive protein.
Chronic coronary syndrome is usually characterized by a reversible myocardial supply-demand mismatch. It is associated with ischemia or hypoxia, usually induced by exercise, emotion, or other stress, and occasionally occurs spontaneously (2). Despite routine treatment with antiplatelet and statins, patients are still at high risk for cardiovascular events. These treatments do not interfere with inflammation. A retrospective study matched 501 users of colchicine for gout with an equal number of non-users for a median follow-up of 16.5 months. Cardiovascular (CV) events occurred in 28 patients of 501 (5.6%) who received colchicine and 82 patients of 501 (16.4%) assigned no colchicine (hazard ratio [HR] 0.51, 95% confidence interval [CI] 0.30–0.88, p = 0.016) and a 73% reduction in all-cause mortality (HR 0.55, 95% CI: 0.35–0.85, p = 0.007) (38). Another retrospective study included 1288 gout patients, which were divided into Colchicine (n = 576) group and no colchicine (n = 712) group. Prevalence of (myocardial infarction) MI occurred in 1.2% of patients who received colchicine and 2.6% of patients assigned no colchicine (relative risk [RR]:0.46, p = 0.03). Fewer deaths and lower CRP levels appeared in the colchicine group, although without statistical significance (39). The LoDoCo trial showed that low-dose colchicine reduced the risk of plaque instability, thereby improving clinical outcomes in patients with CCS. The primary outcome was observed in 15 patients of 282 (5.3%) among users and 40 patients of 501 (16.0%) assigned among non-users (HR: 0.33; 95% CI: 0.18–0.59, p = 0.001). A low dose of colchicine in combination with other standard secondary prevention therapies occurred the prevention of cardiovascular events for patients with CCS (40). Recently, the LoDoCo2 study, published in the New England Journal, further confirmed the role of colchicine in the treatment of CCS, which matched 2762 users with 2760 non-users for a median of 28.6 months. The primary outcome which included cardiovascular death, spontaneous myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization occurred in 187 patients (5.3%) who received colchicine and 264 patients (9.6%) who did not receive colchicine (HR, 0.69; 95% CI: 0.57–0.83, P < 0.001). A key secondary endpoint event occurred in 115 patients (4.2%) who received colchicine and 157 patients (5.7%) assigned no colchicine (HR, 0.72; 95% CI: 0.57–0.92, P = 0.007). Secondary endpoints were tested in a hierarchical manner. Notably, the incidence of death from non-cardiovascular was higher in the colchicine group than placebo group (incidence, 0.7 vs. 0.5 events/100 person-years; HR, 1.51; 95% CI: 0.99–2.31) (41). Both LoDoCo and LoDoCo2 include a large number of patients, Colchicine can be an important supplement for secondary prevention of CCS. Some meta-analyses have shown that the reduction of inflammatory factors and plaque stabilization can be achieved by oral colchicine (42, 43). In general, many trials have confirmed the effectiveness of colchicine in patients with CCS. The ongoing DRC-04 study will demonstrate the effectiveness and safety of colchicine on patients with diabetes mellitus type 2 (T2DM) and CCS (Table 2).
Acute coronary syndrome was defined as symptoms of acute myocardial ischemia associated with either elevated troponin or ECG changes. The current treatments for ACS mainly include antiplatelet, anticoagulation, blood lipid regulation, and operations, such as percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) (44, 45). Even with the above treatments, mortality and morbidity in patients with ACS still keep increasing. Inflammation is involved in all stages of atherosclerosis, including plaque formation, progression, instability, and rupture. Anti-inflammatory treatment may be an effective breakthrough for ACS. In an animal trial, Colchicine can significantly reduce infarct area and cardiac fibrosis in mice, and improve hemodynamic parameters (46). Another animal trial showed the anti-inflammatory mechanism of colchicine can protect the myocardium in the rat MI model (47). In the above studies, a unique dose of colchicine by limitation of the inflammation at the early stage of MI decreased myocardial injuries, which was manifested as a reduction in infarct size and T troponin level at 24 h. More importantly, colchicine improved long-term cardiac remodeling by improving hemodynamic parameters and reducing myocardial fibrosis (46, 47). These animal studies provide some evidences for colchicine treatment of ACS. Deftereos et al. have demonstrated in a double-blind that a 5-day course of treatment combined with colchicine and PCI led to a reduction in infarct size in 151 patients with STEMI (48). In this trial, Colchicine significantly decreased circulating levels of CK-MB, troponin measured 72 h post-MI, and infarction volume measured using cardiac MRI compared to placebo (48). The COLCOT clinical trial including 4745 patients followed for a median of 22.6 months. The primary endpoint including death from cardiovascular causes, resuscitated cardiac arrest, myocardial infarction, stroke, or urgent hospitalization for angina leading to coronary revascularization occurred in 5.5% of the patients who received colchicine, as compared with 7.1% in the placebo group (HR, 0.77; 95% CI: 0.61–0.96, P = 0.02). The hazard ratios were 0.84 (95% CI: 0.46–1.52) for death from cardiovascular causes, 0.83 (95% CI: 0.25–2.73) for resuscitated cardiac arrest, 0.91 (95% CI: 0.68–1.21) for myocardial infarction, 0.26 (95% CI: 0.10–0.70) for stroke, and 0.50 (95% CI: 0.31–0.81) for urgent hospitalization for angina leading to coronary revascularization. The result showed the primary end point, stroke, urgent hospitalization for angina leading to coronary revascularization was significant lower in the colchicine group compared with the placebo group (49). The COPS randomized clinical trial recruited a total of 795 patients with ACS, 396 in colchicine group and 399 in the placebo group at 1 year of follow-up. The result showed that standard treatment with colchicine had no significant effect on cardiovascular outcomes and occurred a higher rate of mortality, such as 24 events in the colchicine group and 38 events in the placebo group (P = 0.09, log-rank). The rate of total death in the colchicine group was higher (8 versus 1; P = 0.017 log-rank), particularly, non-cardiovascular death in the colchicine group (5 versus 0; P = 0.024 log-rank). However, if the composite endpoint used only cardiovascular death rather than total death (cardiovascular death, stroke, ACS, and urgent revascularization) over the 12-month follow-up, the group of colchicine had a lower event rate (5.0% versus 9.5%; HR, 0.51 [95% CI: 0.29–0.89], P = 0.019). What’s more, at 400 days, there was a statistically significant difference between groups for the primary outcome (ACS, stroke, death, and urgent revascularization), with 24 events in the colchicine group compared with 41 events in the placebo group (P = 0.047, log-rank test) (50). Although COPS had negative results during 12 months of follow-up, we need to take into account the limitations of this trial, such as the insufficient sample size, low proportion of women, and limited follow-up time. When making a further analysis, firstly, the dose of colchicine was high in the COPS trial during the first month, and negative results may be caused by this factor. Secondly, the primary outcome at 12 months and 400 days were contradictory. It cannot be ruled out that the benefit further increases as the duration of colchicine administration is prolonged. Therefore, colchicine in the treatment of ACS is worth long-term observation. Although colchicine does not get consistent results in the treatment of ACS, many trials have been added to confirm the treatment of ACS with colchicine. We will see the results of more clinical studies about ACS in the future, including COACS, COCOMO-ACS, COLCARDIO-ACS, CADENCE, COLD-MI, COLOCT, etc.
Percutaneous coronary intervention is the most important way to treat CAD. The processes of PCI including wire injury, microdissections at the site of balloon inflations, and vascular trauma due to high-pressure balloon inflations can further aggravate plaque instability and inflammation (2, 44). Therefore, many scholars take special attention to the curative effect of controlling inflammation on PCI patients. Early studies on colchicine mainly focused on stenosis after PCI. The first study on the use of colchicine in PCI patients was published in 1992, including 197 patents, which found that colchicine 0.6 mg BD did not reduce vascular stenosis (51). A prospective, randomized clinical trial published in 2013 included 196 diabetic patients who received bare-metal stents and proved patients who received a 6-month course of 0.5 mg BD with colchicine can benefit. The angiographic in-stent restenosis rate occurred in 16% of the patients who received colchicine and 33% of patients assigned no colchicine (odds ratio [OR]: 0.38, 95% CI: 0.18–0.79, p = 0.007) (35). COLCHICINE-PCI randomized clinical trial recruited a total of 400 patients with 206 assigned to the colchicine group and 194 to the placebo group. The colchicine group was scheduled for acute preprocedural oral administration of colchicine, and the other group received an oral placebo. The composite outcome of death, non-fatal myocardial infarction, and target vessel revascularization at 30 days (11.7% versus 12.9%, P = 0.82), and the outcome of PCI-related MI (2.9% versus 4.7%, P = 0.49) did not differ between colchicine and placebo groups (52). There is very little information on the use of colchicine in CABG. Giannopoulos et al. demonstrated in a prospective, double-blind trial about CABG including 59 patients with 30 assigned to the colchicine group and 29 to the placebo group that treatment with colchicine led to a reduction in increases of hsTnT and CK-MB (53). In the trial, the median area under curve (AUC) for hsTnT was 20,363 pg h/ml (13,891–31,661) in the colchicine group versus 40,755 pg h/ml (20,868–79,176) in controls (p = 0.002). AUC for CK-MB was 1,586 ng h/ml (1,159–2,073) in the and colchicine group 2,552 ng h/ml (1,564–4,791) in control (p = 0.003). In general, a short perioperative colchicine administration can reduce the elevation of hsTnT and CK-MB compared with placebo (53). The result was partly attributed to the anti-inflammation of colchicine. At present, considering the number of trials and patients involved were too small, and the results were inconsistent, more trials are needed to take part. Ongoing clinical trials of colchicine in PCI include COVERT-MI, CLEAR-SYNERGY, ORCA, etc.
0.5–1.0 mg of colchicine per day has proven relatively safe, as evidenced in many studies. However, it is noteworthy that patients with severe kidney or hepatic disease have been expelled from most of these trials. If patients have severe renal or liver function damage, the safety of colchicine decreases and even causes fatal toxicity. The therapeutic window of colchicine is narrow (54). Colchicine is toxic at doses greater than 0.1 mg/kg and lethal at 0.8 mg/kg (13). A meta-analysis focusing on adverse events on colchicine for the treatment of cardiovascular diseases demonstrated the occurrence of adverse events with colchicine was reported in 15.3 vs. 13.9% of patients [RR 1.26, 95% CI: 0.96–1.64, P = 0.09] (55). However, most patients can tolerate a low dose of colchicine well over the long term. The most common colchicine-related adverse events are gastrointestinal discomforts including nausea, vomiting, diarrhea, and abdominal discomfort. Other adverse events include myotoxicity, hepatic event, hematology event, cutaneous event, and infectious event (54). In particular, colchicine may increase the incidence of pneumonia. In COLCOT trial, Pneumonia as a serious adverse event occurred in 0.9% of the patients who received colchicine and 0.4% of patients assigned no colchicine (P = 0.03) (49). In another study, which included 24,410 patients, the overall incidence rates of pneumonia in the colchicine group were 18.6 per 1,000 person-years as compared with 12.6 per 1,000 person-years in non-colchicine group. Pneumonia was higher in the colchicine group [adjusted HR, 1.42; 95% CI: 1.32–1.53; P < 0.05]. The risk of pneumonia is also closely related to the cumulative dose and duration of use (56). Increasing the rate of infection may be attributed to the suppression of immunity. In addition, the increase in non-cardiovascular death also deserves attention. The LoDoCo2, COPS have mentioned that colchicine may increase the rate of non-cardiovascular death. Attention should also be paid to the combination of colchicine and other drugs such as CYP3A4 inhibitors, P-glycoprotein inhibitors, or statins. When combined with drug CYP3A4 inhibitors or P-glycoprotein inhibitors, the concentration of colchicine is increased, which leads to an increase in adverse events. Combination use of statins increases with may increase the risk of myopathy (54). Although colchicine is relatively safe, considering its narrow therapeutic range and interactions with other drugs, we need to pay attention to its adverse reactions, especially in patients with impaired liver and kidney function. When considering the symptoms of an adverse reaction, the clinician first confirms whether the adverse reaction is caused by colchicine. Blood concentration monitoring can provide more scientific clues. Once established, dosage reduction or withdrawal should be actively considered.
At present, Anti-inflammatory treatment for CAD is getting more and more attention but there are also many controversies. This article will discuss the following aspects. Firstly, the author considers it is highly possible that colchicine in the treatment of CAD will be written into guidelines, but it is too early to draw a conclusion at present. The results of five trials (LoDoCo, LoDoCo2, Deftereos et al., COLCOT, Spyridon Deftereos et al.) were positive and two trials [JAMES H. O’KEEFE, et al., COLCHICINE-PCI trail] were negative. The result of the COPS trial was special, with a negative result at 12 months of follow-up and a positive result at 400 days of follow-up. When we further analyze, the weight of positive results is larger than negative. A meta-analysis including LoDoCo2, COPS, COLCOT, Spyridon Deftereo et al., and LoDoCo trials indicated that the risk of major adverse cardiovascular events (MACE) can be reduced by low-dose colchicine (57). Furtherly analyzing the grouping situation, the results of colchicine in CCS are relatively consistent. However, the results of ACS and PCI with colchicine are not consistent. Many reasons should be considered for this phenomenon. To begin with, compared with CCS, the treatment process of ACS and PCI is more complex, with higher risk and worse prognosis. What is more, many factors affect the prognosis of ACS and PCI, including the condition of patients, timeliness of the visit, quality of medication administration, and quality of the surgeon. Therefore, the weight of anti-inflammatory therapy in the whole process is relatively small, which may be an important reason for the inconsistent outcome. Because of these reasons, enthusiasm for colchicine in the treatment of ACS and PCI is growing. Secondly, inflammatory markers for assessing and treating CAD are unclear, unlike blood lipids, especially low-density lipoproteins, which can be used as indicators for monitoring the effectiveness of statins. Although many inflammatory indicators have been mentioned, such as hs-CRP, IL-1β, IL-18, and IL-6. White blood cell was even considered to be an independent predictor of MACE in a prospective study (58). But the sensitivity and specificity of these inflammation indicators are controversial. Among the inflammation biomarkers, hs-CRP is the most widely studied. In CANTOS study, the reduction of hs-CPR is parallel to the reduction in cardiovascular events (7). However, in COLCHICINE-PCI trial, colchicine could prevent a rise of hs-CRP during an acute injury, but which could not reduce PCI-related myocardial injury or 30-day major cardiovascular events (52). In the small subgroup of COLCOT trial, a large (>65%) reduction in the C-reactive protein level occurred over the first 6 months after myocardial infarction in colchicine and control groups, but the difference between the changes in the groups was not significant (49). At present, routine assessment of hs-CRP levels does not be advised as part of risk assessment for cardiovascular prevention by the European Society of Cardiology guidelines (59), while the American College of Cardiology guidelines suggest aggressive testing for hs-CRP levels (60). Inflammation indicators need more data to confirm. Finally, based on the common etiology and pathophysiological grounds of arteriosclerotic diseases, whether colchicine can prevent and treat cerebrovascular disease and peripheral arterial disease (PAD) is also worth looking forward to. A systematic review and meta-analysis including four randomized controlled trials (RCTs) and one retrospective cohort study confirmed that patients with stroke may benefit from colchicine (61). Unfortunately, clinical trials of colchicine for peripheral artery disease are few. The Ongoing CONVINCE study with high-quality randomized data will prove colchicine for secondary prevention after stroke is effective and safety (62). An ongoing LEADER-PAD study will provide some information on PAD. At present, many results of colchicine for CAD are positive and significant. The official recommendation of colchicine for CAD by cardiovascular guidelines in the future is worth anticipating. What’s more, the effect of colchicine on cerebrovascular diseases and PAD is hopeful.
Due to the high morbidity and mortality of CAD, new approaches are urgently needed. Current evidences show that inflammation is a key component in the pathogenesis of atherosclerosis. Inflammation indicators such as IL-6 and hs-CRP have been paid much attention to. Anti-inflammatory therapy may become one of the effective methods for the treatment of CAD. Colchicine effectively reduces the incidence of cardiovascular events through an anti-inflammatory mechanism. What is more, colchicine is cheap and relatively safe. At the same time, we need to care about the adverse reactions and drug interactions, in particular, colchicine may be associated with increased rates of pneumonia and non-cardiovascular death. It is highly probable that the inhibition of inflammation will become the important cornerstone of CAD treatment.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This study was supported by the Liaoning Province Central Guiding Local Science and Technology Foundation (No. 2019JH6/10400005) and the National Natural Science Foundation of China (No. 8217021483).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. (2019) 74:e177–232.
2. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. (2020) 41:407–77.
3. Behn A, Ur E. The obesity epidemic and its cardiovascular consequences. Curr Opin Cardiol. (2006) 21:353–60.
4. Moustafa Sherif HR, Niccoli G, Scalone G. TCT-599 Not all plaque ruptures are born equal: an optical coherence tomography study. J Am Coll Cardiol. (2016) 68:B243. doi: 10.1093/ehjci/jew208
5. Lawler PR, Bhatt DL, Godoy LC, Luscher TF, Bonow RO, Verma S, et al. Targeting cardiovascular inflammation: next steps in clinical translation. Eur Heart J. (2021) 42:113–31. doi: 10.1093/eurheartj/ehaa099
6. Bouabdallaoui N, Tardif JC. Initiation of low-dose colchicine early after myocardial infarction. Eur Heart J. (2021) 42:2798–9. doi: 10.1093/eurheartj/ehab038
7. Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J. (2018) 39:3499–507. doi: 10.1093/eurheartj/ehy310
8. Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med. (2019) 380:752–62. doi: 10.1056/NEJMoa1809798
9. Karamanou M, Tsoucalas G, Pantos K, Androutsos G. Isolating colchicine in 19th century: an old drug revisited. Curr Pharm Des. (2018) 24:654–8. doi: 10.2174/1381612824666180115105850
10. Chaldakov GN. Colchicine, a microtubule-disassembling drug, in the therapy of cardiovascular diseases. Cell Biol Int. (2018) 42:1079–84. doi: 10.1002/cbin.10988
11. Pappritz K, Lin J, El-Shafeey M, Fechner H, Kuhl U, Alogna A, et al. Colchicine prevents disease progression in viral myocarditis via modulating the NLRP3 inflammasome in the cardiosplenic axis. ESC Heart Fail. (2022) 9:925–41. doi: 10.1002/ehf2.13845
12. Bhattacharyya B, Howard R, Maity SN, Brossi A, Sharma PN, Wolff J. B ring regulation of colchicine binding kinetics and fluorescence. Proc Natl Acad Sci USA. (1986) 83:2052–5. doi: 10.1073/pnas.83.7.2052
14. Villa Zapata L, Hansten PD, Horn JR, Boyce RD, Gephart S, Subbian V, et al. Evidence of clinically meaningful drug-drug interaction with concomitant use of colchicine and clarithromycin. Drug Saf. (2020) 43:661–8. doi: 10.1007/s40264-020-00930-7
15. Dalbeth N, Lauterio TJ, Wolfe HR. Mechanism of action of colchicine in the treatment of gout. Clin Ther. (2014) 36:1465–79. doi: 10.1016/j.clinthera.2014.07.017
16. Cronstein BN, Molad Y, Reibman J, Balakhane E, Levin RI, Weissmann G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest. (1995) 96:994–1002. doi: 10.1172/JCI118147
17. Leung YY, Yao Hui LL, Kraus VB. Colchicine–Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. (2015) 45:341–50. doi: 10.1016/j.semarthrit.2015.06.013
18. Chaldakov GN. Colchicine, inflammation and fibrosis in cardiovascular disease- Merging three classical tales. Biomed Rev. (2017) 28:110–5.
19. Molad Y. Update on colchicine and its mechanism of action. Curr Rheumatol Rep. (2002) 4:252–6. doi: 10.1007/s11926-002-0073-2
20. Chaldakov GN. Antitubulins - A New Therapeutic Approach for Atherosclerosis? Atherocslerosis. (1982) 44:385–90. doi: 10.1016/0021-9150(82)90013-2
21. Silvis MJM, Fiolet ATL, Opstal TSJ, Dekker M, Suquilanda D, Zivkovic M, et al. Colchicine reduces extracellular vesicle NLRP3 inflammasome protein levels in chronic coronary disease: a LoDoCo2 biomarker substudy. Atherosclerosis. (2021) 334:93–100. doi: 10.1016/j.atherosclerosis.2021.08.005
22. Grebe A, Hoss F, Latz E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ Res. (2018) 122:1722–40. doi: 10.1161/CIRCRESAHA.118.311362
23. Mallat Z, Corbaz A, Scoazec A, Besnard S, Leseche G, Chvatchko Y, et al. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. (2001) 104:1598–603. doi: 10.1161/hc3901.096721
24. Wang L, Qu P, Zhao J, Chang Y. NLRP3 and downstream cytokine expression elevated in the monocytes of patients with coronary artery disease. Arch Med Sci. (2014) 10:791–800. doi: 10.5114/aoms.2014.44871
25. Altaf A, Qu P, Zhao Y, Wang H, Lou D, Niu N. NLRP3 inflammasome in peripheral blood monocytes of acute coronary syndrome patients and its relationship with statins. Coron Artery Dis. (2015) 26:409–21. doi: 10.1097/MCA.0000000000000255
26. Fernando S, Schwarz N, Williamson A, Toledo D, Zareh J, Di Bartolo B, et al. Anti-inflammatory effects of colchicine on oxidised low-density lipoproteins and cholesterol crystal-induced macrophage activation in vitro. Heart Lung Circ. (2017) 26:S69–70.
27. Fujisue K, Sugamura K, Kurokawa H, Matsubara J, Ishii M, Izumiya Y, et al. Colchicine improves survival, left ventricular remodeling, and chronic cardiac function after acute myocardial infarction. Circ J. (2017) 81:1174–82. doi: 10.1253/circj.CJ-16-0949
28. Libby P. Inflammation in atherosclerosis-no longer a theory. Clin Chem. (2021) 67:131–42. doi: 10.1093/clinchem/hvaa275
29. Kajikawa M, Higashi Y, Tomiyama H, Maruhashi T, Kurisu S, Kihara Y, et al. Effect of short-term colchicine treatment on endothelial function in patients with coronary artery disease. Int J Cardiol. (2019) 281:35–9. doi: 10.1016/j.ijcard.2019.01.054
30. Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res. (2016) 118:145–56. doi: 10.1161/CIRCRESAHA.115.306656
31. Nidorf M, Thompson PL. Effect of colchicine (0.5 mg twice daily) on high-sensitivity C-reactive protein independent of aspirin and atorvastatin in patients with stable coronary artery disease. Am J Cardiol. (2007) 99:805–7. doi: 10.1016/j.amjcard.2006.10.039
32. Albert MA, Danielson E, Rifai N, Ridker PM, Investigators P. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. (2001) 286:64–70. doi: 10.1001/jama.286.1.64
33. Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. (2001) 344:1959–65.
34. Furman MI, Barnard MR, Krueger LA, Fox ML, Shilale EA, Lessard DM, et al. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol. (2001) 38:1002–6. doi: 10.1016/s0735-1097(01)01485-1
35. Deftereos S, Giannopoulos G, Raisakis K, Kossyvakis C, Kaoukis A, Panagopoulou V, et al. Colchicine treatment for the prevention of bare-metal stent restenosis in diabetic patients. J Am Coll Cardiol. (2013) 61:1679–85. doi: 10.1016/j.jacc.2013.01.055
36. Pennings GJ, Reddel CJ, Traini M, Campbell H, Chen V, Kritharides L. Colchicine inhibits ROS generation in response to glycoprotein VI stimulation. Sci Rep. (2021) 11:11965. doi: 10.1038/s41598-021-91409-7
37. Barraclough JY, Joglekar MV, Januszewski AS, Martinez G, Celermajer DS, Keech AC, et al. A MicroRNA Signature in Acute Coronary Syndrome Patients and Modulation by Colchicine. J Cardiovasc Pharmacol Ther. (2020) 25:444–55. doi: 10.1177/1074248420922793
38. Solomon DH, Liu CC, Kuo IH, Zak A, Kim SC. Effects of colchicine on risk of cardiovascular events and mortality among patients with gout: a cohort study using electronic medical records linked with Medicare claims. Ann Rheum Dis. (2016) 75:1674–9. doi: 10.1136/annrheumdis-2015-207984
39. Crittenden DB, Lehmann RA, Schneck L, Keenan RT, Shah B, Greenberg JD, et al. Colchicine use is associated with decreased prevalence of myocardial infarction in patients with gout. J Rheumatol. (2012) 39:1458–64. doi: 10.3899/jrheum.111533
40. Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. (2013) 61:404–10.
41. Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. (2020) 383:1838–47.
42. Andreis A, Imazio M, Piroli F, Avondo S, Casula M, Paneva E, et al. Efficacy and safety of colchicine for the prevention of major cardiovascular and cerebrovascular events in patients with coronary artery disease: a systematic review and meta-analysis on 12 869 patients. Eur J Prev Cardiol. (2021) 28:1916–25. doi: 10.1093/eurjpc/zwab045
43. Xiang Z, Yang J, Yang J, Zhang J, Fan Z, Yang C, et al. Efficacy and safety of colchicine for secondary prevention of coronary heart disease: a systematic review and meta-analysis. Intern Emerg Med. (2021) 16:487–96. doi: 10.1007/s11739-020-02606-7
44. Collet JP, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367.
45. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
46. Akodad M, Fauconnier J, Sicard P, Huet F, Blandel F, Bourret A, et al. Interest of colchicine in the treatment of acute myocardial infarct responsible for heart failure in a mouse model. Int J Cardiol. (2017) 240:347–53. doi: 10.1016/j.ijcard.2017.03.126
47. Bakhta O, Blanchard S, Guihot AL, Tamareille S, Mirebeau-Prunier D, Jeannin P, et al. Cardioprotective role of colchicine against inflammatory injury in a rat model of acute myocardial infarction. J Cardiovasc Pharmacol Ther. (2018) 23:446–55. doi: 10.1177/1074248418763611
48. Deftereos S, Giannopoulos G, Angelidis C, Alexopoulos N, Filippatos G, Papoutsidakis N, et al. Anti-inflammatory treatment with colchicine in acute myocardial infarction: a pilot study. CIRCULATION. (2015) 132:1395–403. doi: 10.1161/CIRCULATIONAHA.115.017611
49. Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. (2019) 381:2497–505. doi: 10.1056/NEJMoa1912388
50. Tong DC, Quinn S, Nasis A, Hiew C, Roberts-Thomson P, Adams H, et al. Colchicine in patients with acute coronary syndrome: the Australian COPS randomized clinical trial. Circulation. (2020) 142:1890–900.
51. O’Keefe JH, McCallister BD, Bateman TM, Kuhnlein DL, Ligon RW, Hartzler GO. Ineffectiveness of colchicine for the prevention of restenosis after coronary angioplasty. J Am Coll Cardiol. (1992) 19:1597–600. doi: 10.1016/0735-1097(92)90624-v
52. Shah B, Pillinger M, Zhong H, Cronstein B, Xia Y, Lorin JD, et al. Effects of acute colchicine administration prior to percutaneous coronary intervention: COLCHICINE-PCI randomized trial. Circ Cardiovasc Interv. (2020) 13:e008717. doi: 10.1161/CIRCINTERVENTIONS.119.008717
53. Giannopoulos G, Angelidis C, Kouritas VK, Dedeilias P, Filippatos G, Cleman MW, et al. Usefulness of colchicine to reduce perioperative myocardial damage in patients who underwent on-pump coronary artery bypass grafting. Am J Cardiol. (2015) 115:1376–81. doi: 10.1016/j.amjcard.2015.02.036
54. Finkelstein Y, Aks SE, Hutson JR, Juurlink DN, Nguyen P, Dubnov-Raz G, et al. Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol. (2010) 48:407–14. doi: 10.3109/15563650.2010.495348
55. Andreis A, Imazio M, Avondo S, Casula M, Paneva E, Piroli F, et al. Adverse events of colchicine for cardiovascular diseases: a comprehensive meta-analysis of 14 188 patients from 21 randomized controlled trials. J Cardiovasc Med. (2021) 22:637–44. doi: 10.2459/JCM.0000000000001157
56. Tsai TL, Wei JC, Wu YT, Ku YH, Lu KL, Wang YH, et al. The association between usage of colchicine and pneumonia: a nationwide, population-based cohort study. Front Pharmacol. (2019) 10:908. doi: 10.3389/fphar.2019.00908
57. Fiolet ATL, Opstal TSJ, Mosterd A, Eikelboom JW, Jolly SS, Keech AC, et al. Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials. Eur Heart J. (2021) 42:2765–75. doi: 10.1093/eurheartj/ehab115
58. Shah B, Baber U, Pocock SJ, Krucoff MW, Ariti C, Gibson CM, et al. White blood cell count and major adverse cardiovascular events after percutaneous coronary intervention in the contemporary era: insights from the PARIS study (Patterns of Non-Adherence to Anti-Platelet Regimens in Stented Patients Registry). Circ Cardiovasc Interv. (2017) 10:e004981. doi: 10.1161/CIRCINTERVENTIONS.117.004981
59. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice : the sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts). Int J Behav Med. (2017) 24:321–419.
60. Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB Sr., Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2014) 63:2935–59.
61. Khandkar C, Vaidya K, Patel S. Colchicine for stroke prevention: a systematic review and meta-analysis. Clin Ther. (2019) 41:582–90.e3.
Keywords: colchicine, inflammation, coronary artery disease, percutaneous coronary intervention, coronary artery bypass grafting
Citation: Chen T, Liu G and Yu B (2022) Colchicine for Coronary Artery Disease: A Review. Front. Cardiovasc. Med. 9:892588. doi: 10.3389/fcvm.2022.892588
Received: 09 March 2022; Accepted: 17 May 2022;
Published: 16 June 2022.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
George Nikov Chaldakov, Medical University of Varna, BulgariaCopyright © 2022 Chen, Liu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Yu, eWJkeXliZHlAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.