- 1Department of Clinical Pharmacy, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt
- 2Department of Cardiothoracic Surgery, Cardiothoracic Surgery Academy, Ain Shams University, Cairo, Egypt
Background: Despite warfarin therapy had been used for decades for patients with mechanical mitral valve prostheses (MMVPs), serious and life-threatening complications are still reported worldwide with a significant economic burden. This study is aimed at assessing the clinical and the cost-effectiveness of adopting pharmacist-managed warfarin therapy (PMWT) services for optimizing warfarin treatment in Egypt.
Methods: A prospective randomized trial in which 59 patients with MMVPs were randomly assigned to receive the PMWT services or the standard care and followed up for 1 year. The primary outcome was percentage time in the therapeutic range (TTR). For the cost-effectiveness analysis, a Markov cohort process model with nine mutually exclusive health states was developed from a medical provider’s perspective. A lifetime horizon was applied. All costs and outcomes were discounted at 3.5% annually.
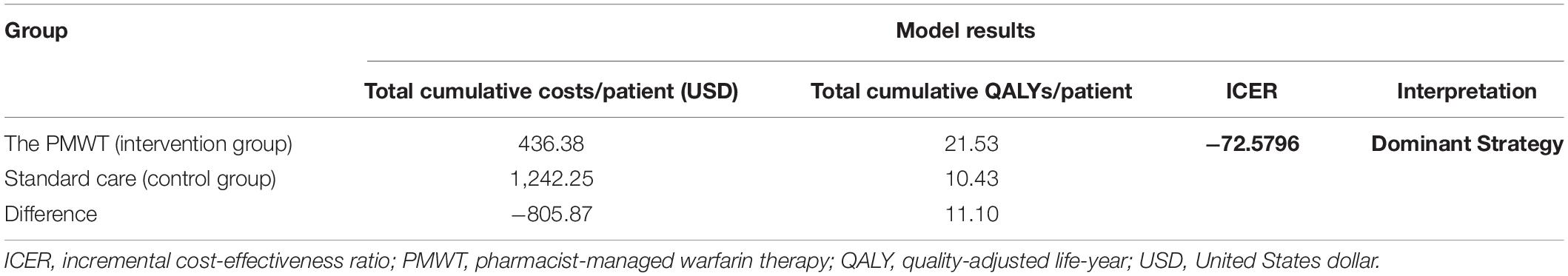
Results: The study results revealed a significantly higher median TTR in the intervention group as compared to the control group; 96.8% [interquartile range (IQR) 77.9–100%] vs. 73.1% (52.7–95.1%), respectively, p = 0.008. A significant association between standard care and poor anticoagulation control (p = 0.021) was demonstrated by the multivariate regression analysis. For the cost-effectiveness analysis, the total cumulative quality-adjusted life-years (QALYs) and total costs per patient were 21.53 and 10.43; 436.38 and 1,242.25 United States dollar (USD) in the intervention and the control groups, respectively, with an incremental cost-effectiveness ratio (ICER) of −72.5796 for the intervention group.
Conclusion: The PMWT strategy was proven to provide a significantly better anticoagulation control and to be a cost-saving approach in Egyptian patients with MMVPs. Nevertheless, the dominance of this strategy is sustained by maintaining the therapeutic International Normalized Ratio (INR) control within the recommended range. Our findings will benefit Egyptian policy-makers who may seek novel health strategies for better resource allocation.
Clinical Trial Registration: [ClinicalTrials.gov], identifier [NCT04409613].
Introduction
Rheumatic heart disease (RHD) is the most prevalent heart condition in children and adolescents who are aged 25 or below. So far, RHD deems endemic in many low- and middle-income countries among vulnerable groups (1, 2). The highest RHD deaths were reported from Egypt, Yemen, Pakistan, Afghanistan, and Iran, which count for 80% of the total death rates for the Eastern Mediterranean Region (3). Almost 20% of patients with RHD become afflicted by congestive heart failure that requires valve surgery within 5–10 years (4). The surgical replacement of a heart valve with a mechanical prosthetic one aims to restore heart function; however, the implantation of a mechanical valve is an absolute indication for lifelong oral anticoagulation therapy to avert the risk of potential complications, such as bleeding, thromboembolic events, prosthetic valve endocarditis, and dysfunction. Moreover, the risk of extensive anticoagulation, i.e., to effectively avoid thromboembolic events, has to be weighed against another risk of bleeding complications (5, 6).
Vitamin K antagonists (VKAs) are the unique recommended option for oral anticoagulation in patients with mechanical valves (7). Among VKAs, warfarin is the most frequently prescribed drug that had been used for decades for those patients. However, it is still challenging to maintain a safe and efficient treatment (8). Major bleeding (which can be life-threatening), intracranial bleeding, and fatal bleeding are observed in 2–5, 0.2–0.4, and 0.5–1.0%, respectively, with patients on warfarin per year (9). What is more, warfarin therapy is fraught with several inherent problems, such as great diversity in dosing, delayed onset of action along with prolonged effect after discontinuation, a wide range of serious interactions, and a narrow therapeutic index. Collectively, all these problems designate warfarin as a “high alert medication” that calls for extraordinary caution and care (10–14).
There is growing evidence that the management of anticoagulation by experienced pharmacists can lead to better outcomes as compared to standard care. This is backed by data from multiple studies that reported improved clinical outcomes with the pharmacist-managed warfarin therapy (PMWT) services, such as significantly better International Normalized Ratio (INR) control, and significant reductions in rates of emergency department visits, hospitalizations, hospital length of stay, and hospital readmission rates. In consequence, cost savings have been well demonstrated in studies from different sides of the world due to decreased rates of both adverse events and complications, with subsequent impact on medical and non-medical costs (12, 15–21). The objective of this trial-based economic evaluation study was to evaluate the clinical effectiveness and the cost-effectiveness of the PMWT services for outpatients with mechanical mitral valve prostheses (MMVPs) in an Egyptian University Teaching Hospital setting.
Patients and Methods
Study Design and Participants
This was a prospective randomized controlled study in which patients with MMVPs were recruited from the outpatients’ anticoagulation clinic at the Cardiothoracic Surgery Academy, Ain Shams University, Cairo, Egypt and were randomly assigned to either the control group, who received standard medical care or the intervention group, who received the PMWT services. Patients were considered for eligibility in the study if they had met the following inclusion criteria: (1) men or women between 18 and 70 years of age, (2) post-mitral valve surgery patients with MMVPs, and (3) patients with a prescription of warfarin. Although pregnant patients, patients with double or aortic valve replacement surgery, patients with biological prostheses, and patients with congenital blood disorders were excluded from the study.
Methods
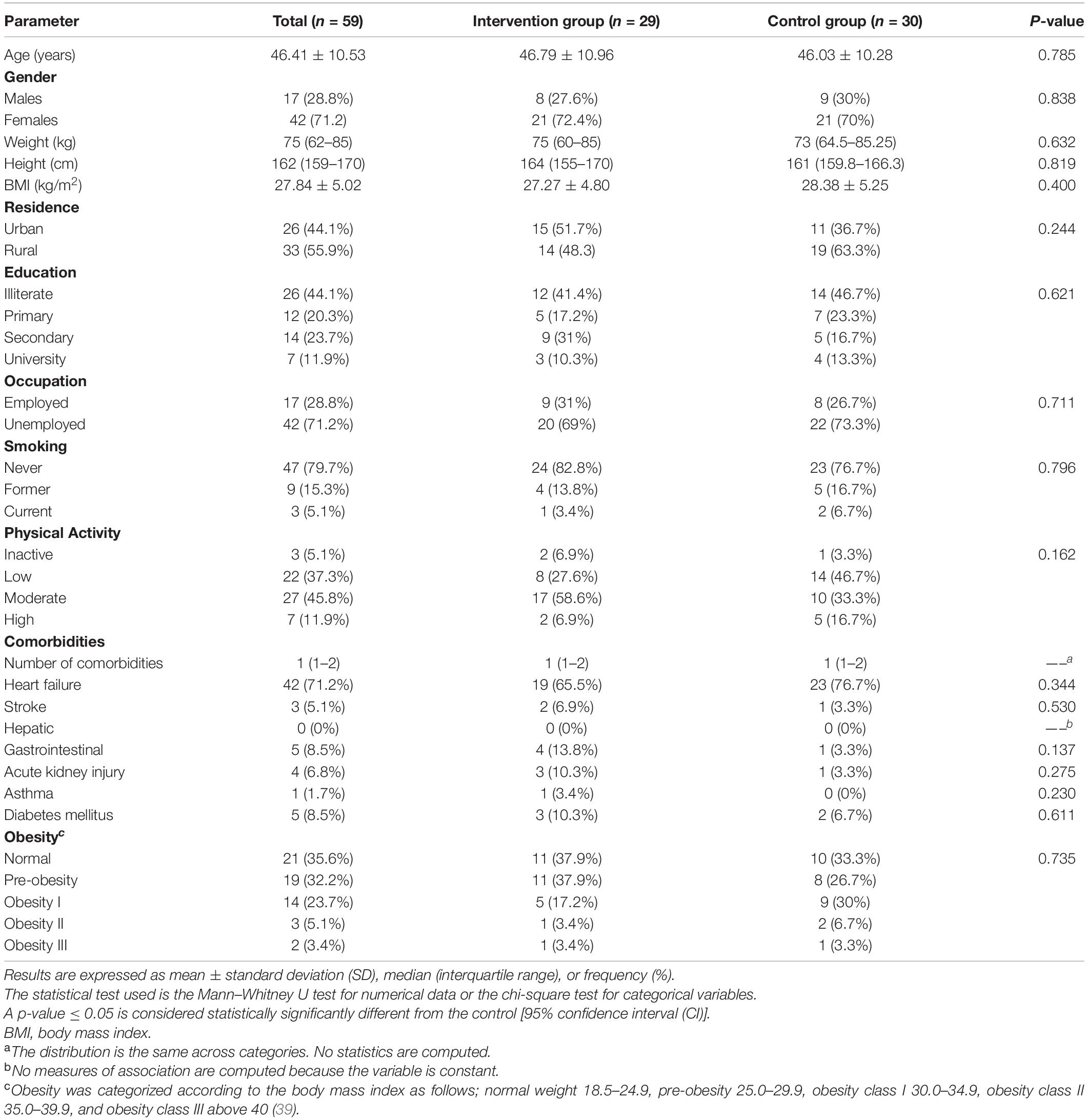
Starting 1 February 2020, a total of 107 patients were screened for eligibility in the study, of which, 48 patients were excluded, as they did not meet the inclusion criteria. Thus, a total of 59 patients were included and randomly allocated to either the intervention (patients who received the PMWT, n = 29) or the control (patients who received the standard medical care, n = 30) groups [a Consolidated Standards of Reporting Trial (CONSORT) flow diagram is provided in the Supplementary Figure 1]. During the first patient visit, demographic data were collected which included name, age, gender, weight, height, education, occupation, residence, and lifestyle habits (smoking and level of physical activity). Along with these data, previous and current medications, comorbidities, and INR test results were also collected. Eligible patients in both groups were followed up for 1 year.
Standard Medical Care
Standard medical care included the documentation of INR test results, and providing the patients with instructions regarding warfarin dose, regimen, any dose modification, and frequency of INR testing. All these services were provided by the anticoagulation clinic staff (which consisted of both cardiologists and nurses).
Pharmacist-Managed Warfarin Therapy Services
The PMWT services included two main pillars, which are patients’ education and counseling and patients’ follow-up. Patients in the intervention group (or their caregivers) received an educational session, which was aimed to ensure that the patient understands the risks, the required precautions, and the necessity for frequent monitoring. Education involves assessing the patient’s understanding of his health problems, the ability to take warfarin correctly, and attitudes toward any warfarin-related complications.
Multiple methods were applied during this session, such as asking open-ended questions to evaluate the patient’s understanding and subsequently, to decide what information is needed to be provided. Visual aids were also used to promote the patient’s comprehension. Therefore, the information was presented as an educational leaflet (Supplementary Figure 2) and explained to the patient in simple language. The information provided in the leaflet included the drug’s brand and generic name, purpose, anticipated onset, frequency of dosing, important precautions, lifestyle modifications, common side effects and what to do if they occur, the importance of regular monitoring and proper adherence, and the need to inform provider if the patient is planning to become pregnant, before any procedure or hospitalization and before starting any new drug. At subsequent visits, this information was briefly recalled to refresh the patient’s information.
Patients were followed up from February 2020 to February 2021. They were regularly assessed through INR value recording, warfarin dose, and any warfarin-related problems, with further dose readjustment recommendations according to the patient’s INR test result. Signs of warfarin overdose were also reported. The time interval for measuring INR levels was typically 1 month. However, this was not constant on a practical basis and the time interval between successive patient visits was primarily based on INR test results.
Primary End Point
The primary end point was the percentage time in therapeutic range (TTR), i.e., the proportion of time spent within the target INR range (22), and was calculated by the Rosendaal method (23). TTR below 65% was considered to be associated with poor anticoagulation control as per the 2020 National Institute for Health and Care Excellence (NICE) recommendations (24). An INR range of 2.5–3.5 was recommended as per the 2020 American College of Cardiology/American Heart Association Guideline for the Management of Patients With Valvular Heart Disease (7).
Ethical Approval
This study was performed following the principles of the Declaration of Helsinki (25). Ethical approval was granted by the Research Ethics Committee at the Faculty of Pharmacy, Ain Shams University, Cairo, Egypt (ENREC–ASU, 2019-99). Informed consent was obtained from each participant without any obligation to complete the study if they did not want to.
Sample Size Calculation and Statistical Methods
Reference to Falamić et al. (8), in which the median TTR was significantly higher in the group that received PMWT services than the standard care (93% vs. 31.2%, a difference of 61.8%), a total of 36 patients (18 per group) were required. The sample size was calculated using Stata/ES 14.2 software for windows. IBM SPSS program (version 20) was used to perform the statistical analysis. All graphs were done using Microsoft Excel 2016. All p-values were two-sided, and the difference was considered statistically significant if the p-value was ≤ 0.05 [95% confidence intervals (CI); detailed sample size calculation and statistical methods are provided in Supplementary Figure 3].
Economic Evaluation
Overview
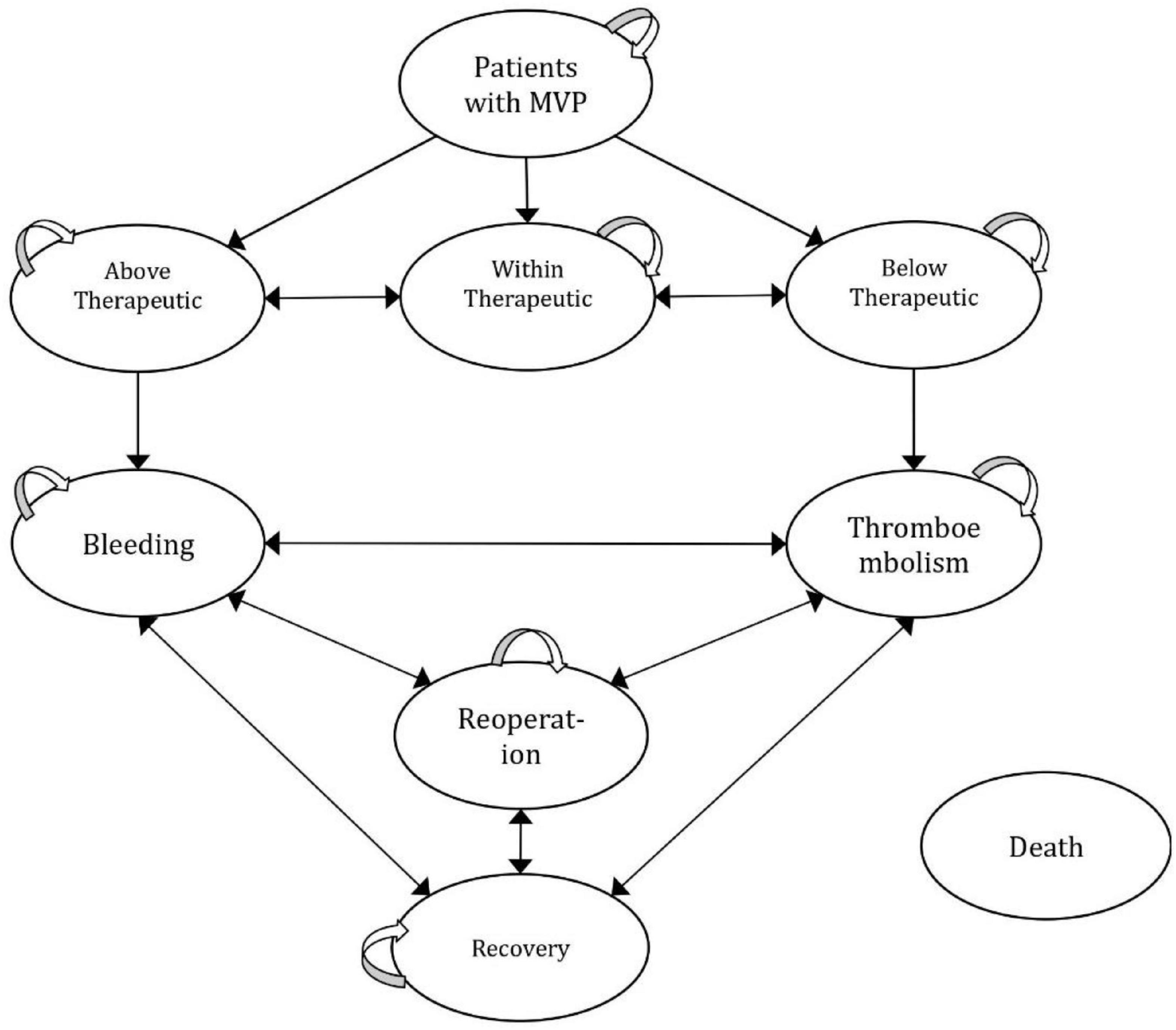
Figure 1 represents a half-cycle-corrected Markov cohort process model, which is developed to reflect the real practice of patient management in Egypt and risks that change repeatedly over time, with nine mutually exclusive health states (25). The model structure and inputs were verified by clinical experts and a lifetime horizon was selected to reflect the long-term consequences. A 1-month cycle length was adopted. The model was populated with relevance to a medical provider’s perspective. All costs and outcomes were discounted at 3.5% annually (26). The analysis was performed using Microsoft Excel 2016.
Model Inputs and the Likelihood of Events
Patients entered the Markov model by either receiving the PMWT services or the standard care. The health states included in the model were “within therapeutic range,” “below therapeutic range,” and “above therapeutic range,” which were defined as INR values below 2.5, within 2.5–3.5, and above 3.5, respectively (27). The “bleeding” state was defined by a major bleeding event that required hospitalization or blood transfusion; the “thromboembolism (TE)” state was defined by the occurrence of a thromboembolic event with morbidity that included ischemic stroke/transient ischemic attack, deep vein thrombosis, and pulmonary embolism; the “reoperation” state was defined as patients who underwent reoperation; and the “recovery” state was defined as the patients recovered after an event; and death, which was defined as death from any cause (27, 28).
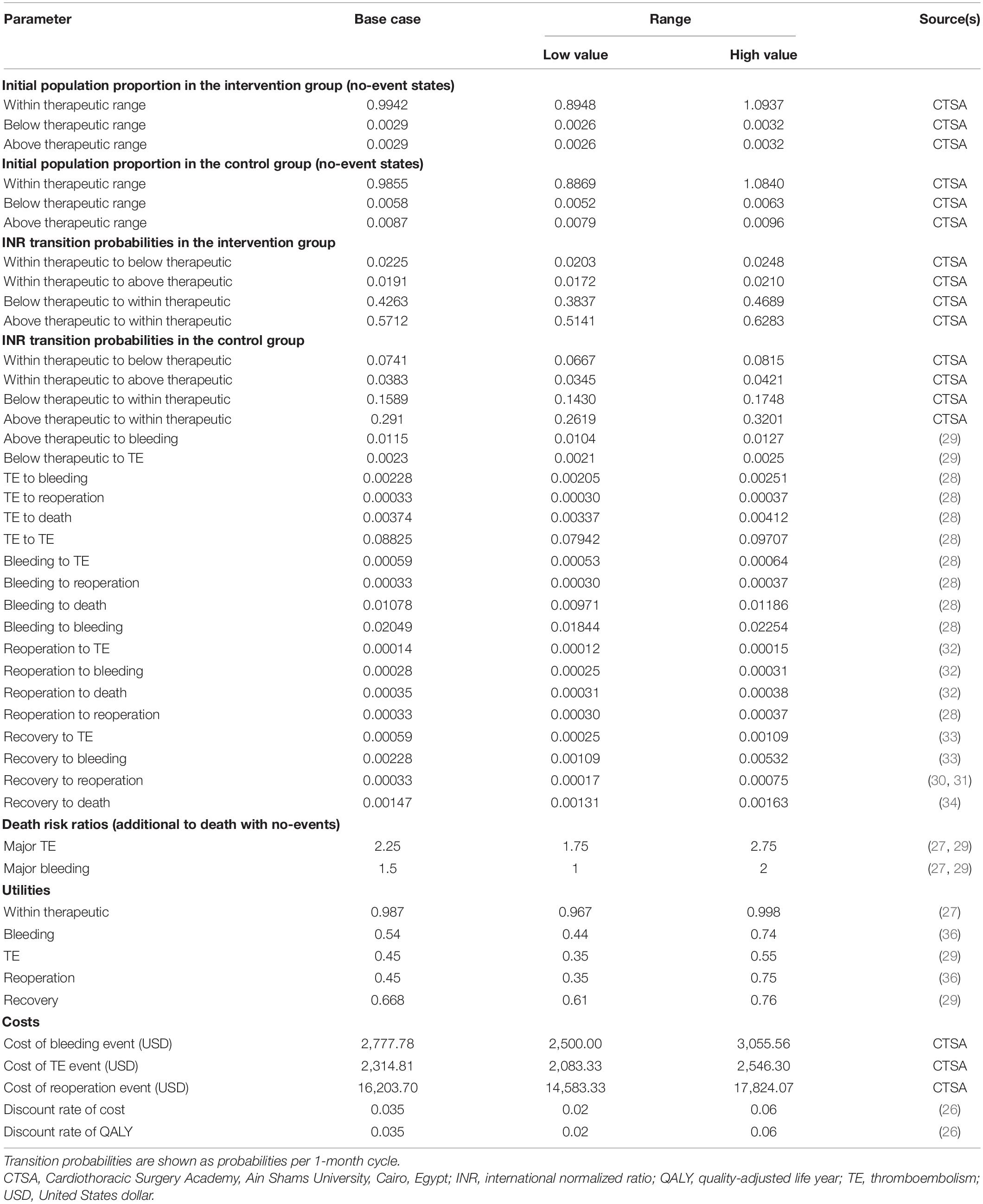
The transition probabilities used in the model are shown in Table 1. To reflect the Egyptian population, the cohort that entered the model was transitioned to within, below, or above therapeutic range states based on probabilities derived from the Cardiothoracic Surgery Academy, Cairo, Egypt. With every cycle, the patient could remain in the current state or transit between below, within, and above the therapeutic range back and forth. Afterward, the patients could stay in the no-event state or experience the following events: bleeding, TE, reoperation, or death from any cause. The model accounts for the risk of mortality at all states of any warfarin-associated events. Death risk ratios from major TE or bleeding events (i.e., additional to death from any cause) were also added to the model.
Due to the lack of local data, we used the probabilities of developing major TE and bleeding events, and death risk ratios after major TE or bleeding events, using published pooled data from several sources (29). The risk for reoperation was obtained from two clinical trials that included 394 and 211 patients with mitral valve replacement (MVR) and patients who were randomized to receive either biological or mechanical valves (30, 31). The probabilities of bleeding, TE, or death with reoperation were derived from a published review that compared the results of 106 patients who underwent repeat MVR with 562 control patients who underwent primary replacement using a computerized database. These risks were calculated using a multivariate logistic regression model to predict associated events or mortality (32). The risks of both major bleeding and TE events after recovery were derived from a large study that included a total of 1,608 patients who were followed for 6,475 patient-years to determine the incidence of complications of oral anticoagulation therapy in patients with mechanical valves (33). The risk of mortality after a recovery state was developed from a retrospective review of 671 patients’ hospital records over 9 years (34). The transition probabilities from the recovery state to reoperation, bleeding, and TE were derived from a previous decision analysis that was modeled on Egyptian patients and assumed that these probabilities are the same as those for patients in the reoperation state (28).
Outcomes
The outcomes were measured in terms of quality-adjusted life-years (QALYs) for both groups. This generic measurement is commonly used as a summary measure for economic evaluations, which combines the impact on both the quality and quantity of life into a single parameter (35). The utilities within the therapeutic range, bleeding, reoperation, recovery, and TE states incorporated in the model were obtained from different published studies (27, 29, 36).
Costs
From a medical provider’s perspective, only direct medical care costs were taken into account, i.e., the costs of bleeding, reoperation, and TE events. All cost inputs were obtained from the databases of the Cardiothoracic Surgery Academy, Ain Shams University, Cairo, Egypt. A macro-costing approach was applied. The costs of INR testing and warfarin were not included since these costs are not covered by the medical provider. We assumed no difference in resource use between the two strategies. Conversion of the local Egyptian currency to United States dollar (USD) was performed using the purchasing power parity rate. All costs were calculated in USD for the financial year of 2019 (37).
Sensitivity Analysis
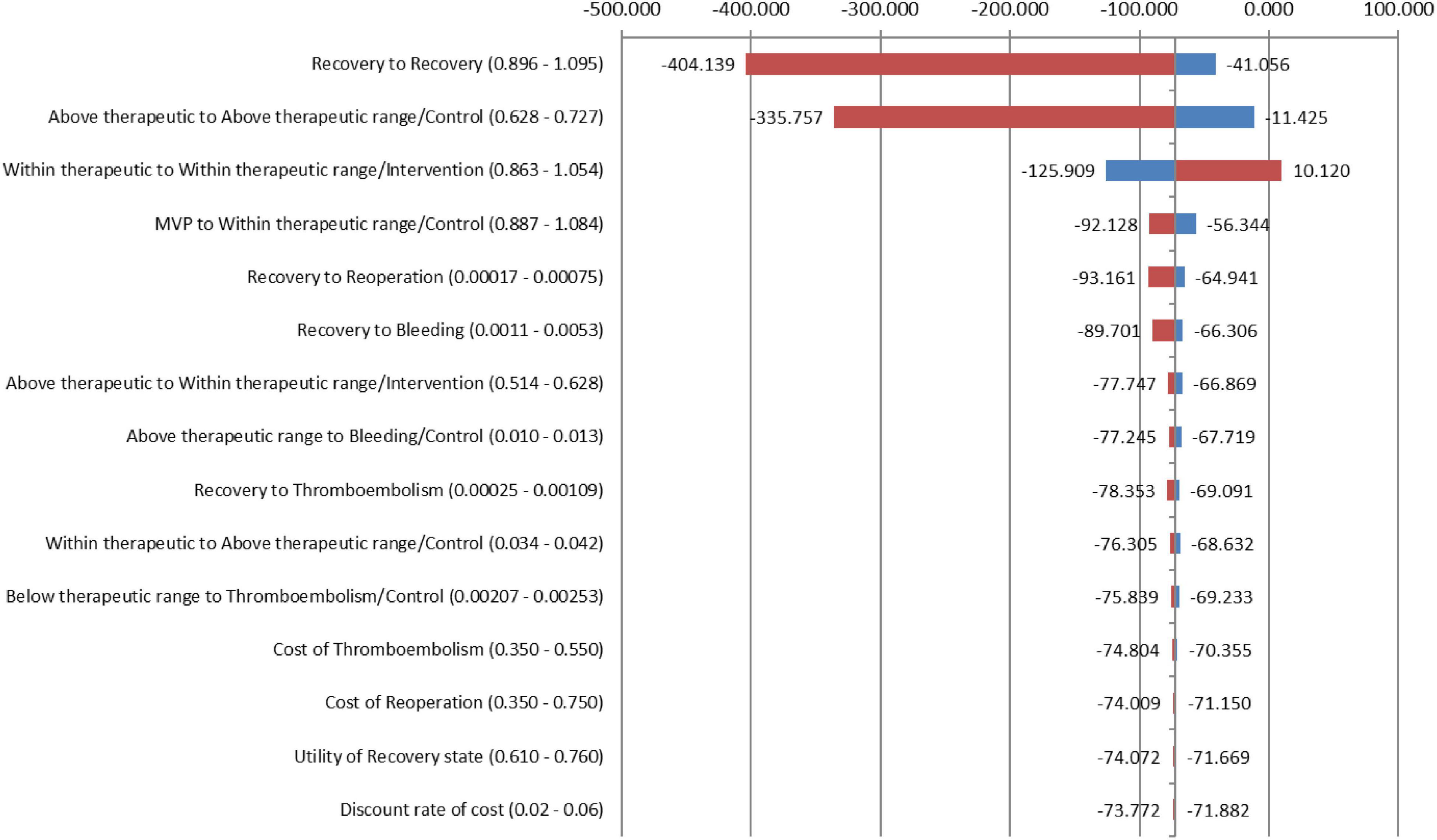
To test the robustness of our model results across variations in input estimates, one-way sensitivity analyses were performed as recommended by the Consolidated Health Economic Evaluation Reporting Standards (CHEERS): The International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Task Force report (38). The stability of the model was tested for all estimates of clinical parameters, utilities of health states, costs, and discount rates. All inputs were varied across upper and lower limits for each parameter. These parameters were delineated by CIs from the literature or reasonable ranges that were determined based on different published sources. Microsoft Excel 2016 was used to perform all analyses.
Results
Study Population
A total of 59 patients were included in the study, of which, 29 patients (49.2%) were included in the intervention group and 30 patients (50.8%) in the control group. Detailed demographic and clinical characteristics of the study participants are illustrated in Table 2. There was no statistically significant difference between both groups in terms of baseline characteristics.
Time in Therapeutic Range
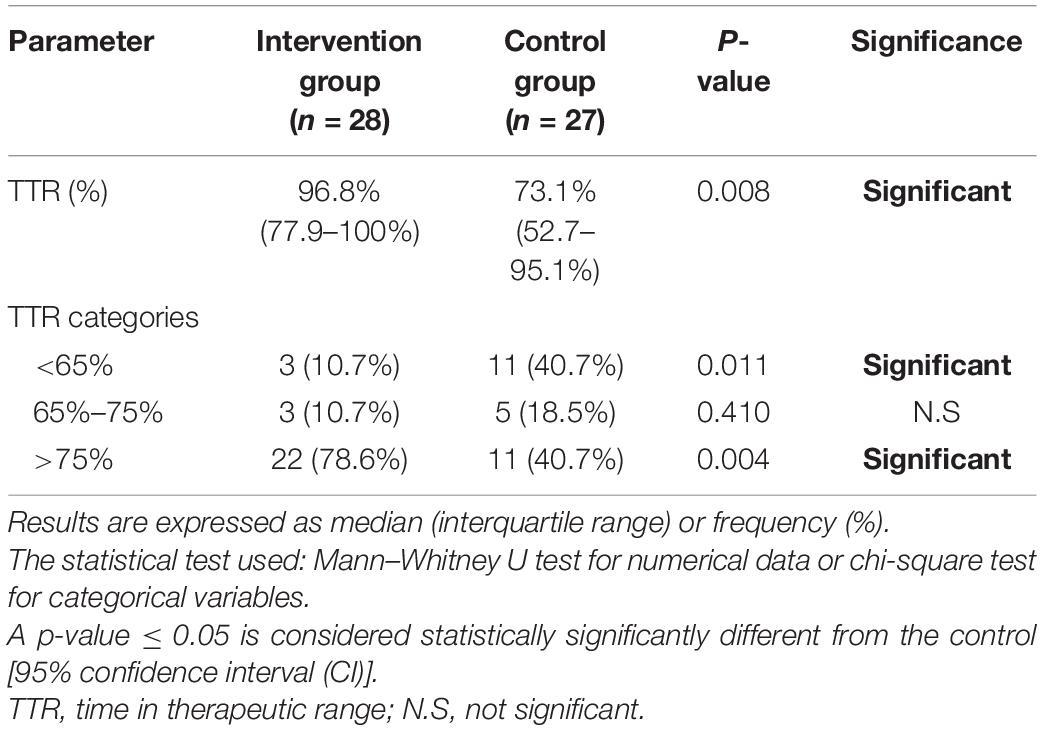
By the end of the follow-up period, the median TTR was significantly higher in the intervention group than in the control group (p = 0.008) indicating that patients who received the PMWT services spent significantly more time in the therapeutic range. For TTR categories, the percentage of patients with poor INR control, i.e., TTR < 65%, was also significantly higher in the control group (p = 0.011). On the other hand, PMWT services were associated with a significantly higher percentage of patients with controlled TTR > 75% as compared to the control group (p = 0.004). TTR-related outcomes are illustrated in Table 3 (additional figures are illustrated in Supplementary Figures 4, 5). Red spots, bleeding gums, and nose bleeding were decreased in patients who received the PMWT services as compared to the control group (7.1% vs. 13.8%, 3.4% vs. 6.9%, and 6.9% vs. 10.3%, respectively); however, the differences did not reach statistical significance (p > 0.05).
The Association Between Standard Care and Poor Quality of Anticoagulation (TTR < 65%): A Multivariate Logistic Regression Analysis
The results of the multivariate logistic regression analysis (Table 4) show that receiving the standard care is significantly associated with poor INR control (dependent variable is TTR < 65%), with an odds ratio (OR) of 6.53 [95% CI (1.33–32.19)], and p = 0.021. These results were obtained while controlling other covariates, namely, age, body mass index (BMI), number of comorbidities, heart failure, stroke, and diabetes (a forest plot diagram is illustrated in Supplementary Figure 6).
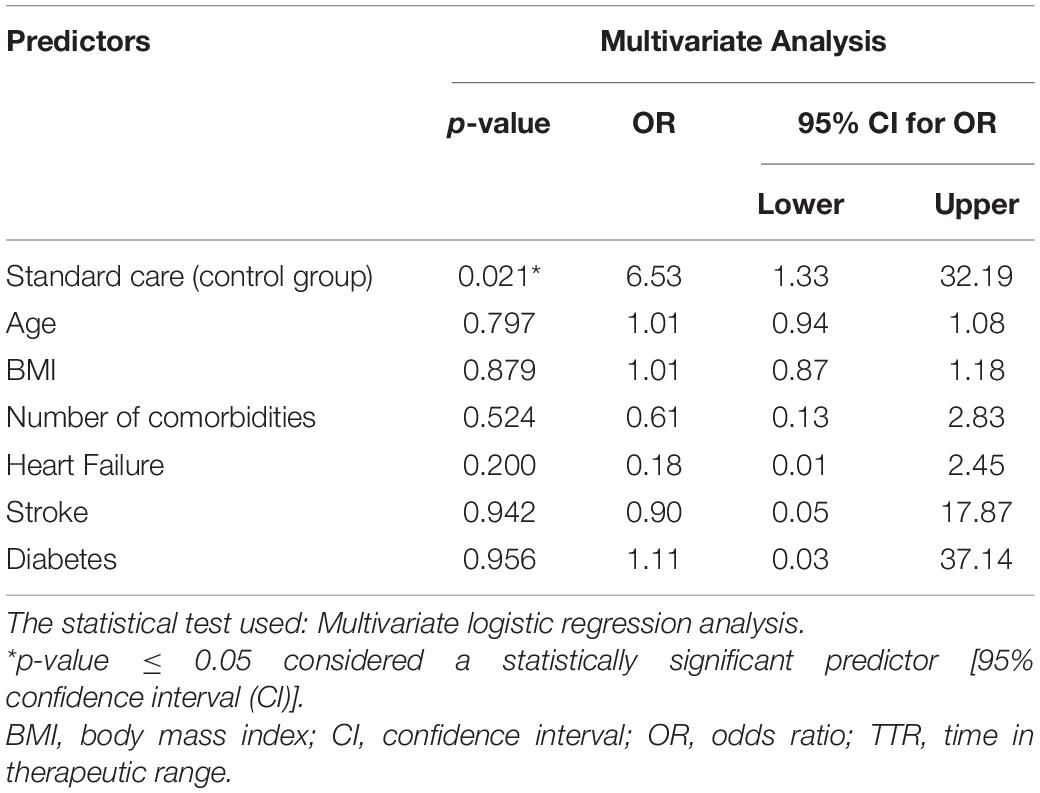
Table 4. Risk of poor quality of anticoagulation (TTR < 65%) associated with standard care: a multivariate logistic regression analysis.
Results of the Cost-Effectiveness Analysis
From the medical provider’s perspective, the total cumulative QALY per patient for the PMWT group was 21.53 when compared with 10.43 for the standard care group. The total cumulative costs per patient for the PMWT and the standard care groups were 436.38 and 1,242.25 USD, respectively. These results yielded an incremental cost-effectiveness ratio (ICER) of − 72.5796 for the intervention group. Thus, the strategy of the PMWT services was dominant, i.e., a cost-saving strategy (Table 5). For the one-way sensitivity analyses, the PMWT strategy would become very cost-effective [based on the threshold stated by the World Health Organization (WHO); 3 × GDP/capita] rather than being cost-saving by a 10% reduction in the probability of remaining within the therapeutic range for the intervention group. When all other inputs were altered between the low and high values, no effects on the resultant ICER values were found. None of the extreme values of any parameter changed the results to be not cost-effective (Figure 2).
Discussion
This study was a trial-based cost-effectiveness evaluation for adopting the PMWT services to patients with MMVPs on warfarin, which revealed that this strategy is cost-saving in the Egyptian setting. To the best of our knowledge, this is the first study to provide this evidence in Egypt. We believe that this evidence could be used to target further quality improvement efforts, which can ultimately enhance both clinical outcomes and cost-saving attempts in an era when a better allocation of scarce resources is at the precedence of healthcare policy initiatives.
As has been shown, the PMWT services have demonstrated a substantial improvement in patients’ anticoagulation control as compared to standard care. This was confirmed by the significantly higher median TTR recorded with the PMWT services (96.8% in the intervention group vs. 73.1% in the control group, p = 0.008), which indicates a significant increase in time spent under the target INR range. Our study results are in accordance with the results of a recent systematic review that was conducted by Entezari-Maleki et al. (40). They studied a total of 4 randomized controlled trials (RCTs) and 20 non-RCT studies with total included patients 11,607 to compare the potential benefit of PMWT as compared to usual medical care. Their results revealed significant improvement in TTR (72.1% vs. 56.7%; p = 0.013) in favor of the PMWT. Moreover, significant differences were reported in major bleeding events, thromboembolic events, hospitalization, emergency department visits in favor of the PMWT as compared to usual medical care (0.6% vs. 1.7%, p < 0.001; 0.6% vs. 2.9%, p < 0.001; 3% vs. 10%, p < 0.001; and 7.9% vs. 23.9%; p < 0.0001, respectively).
Another study was conducted by Marcatto et al. (11) to evaluate the impact of pharmacist’s warfarin management in 268 patients with poor quality of anticoagulation (TTR < 50%). They applied a different design by comparing the retrospective data for the included patients with data obtained prospectively with the same patients after assigning them to receive the pharmacist’s warfarin management services. The investigators reported a statistically significant increase in TTR after 4 and 12 weeks as compared to basal TTR. Furthermore, the mean TTR 1 year before (retrospective phase) was significantly lower than the TTR reported after 12 weeks of pharmacist-driven treatment (prospective phase; p < 0.001). Similarly, Dib et al. (41) conducted a study to evaluate the impact of the first pharmacist-managed anticoagulation clinic in the eastern province of Saudi Arabia. The authors reported that the total percentage of INR within the target range was 59% vs. 48% with the pharmacist-managed clinic vs. the traditional care, respectively. In contrast, Wu et al. (42) evaluated the impact of pharmacist interventions on INR control after MVR during the warfarin initiation phase and demonstrated a numerically higher TTR in the PMWT group as compared to the conventional group, however, the difference did not reach the statistical significance.
In this work, the results of the multivariate logistic regression analysis revealed that standard care was significantly associated with poor quality of anticoagulation; OR 6.53, 95% CI [1.33–32.19], p = 0.021. These results were in line with the results reported by Falamic et al. (8). They reported that the pharmacist’s intervention was significantly associated with good quality of anticoagulation control (dependent variable TTR ≥ 65%); OR 77.84, 95% CI [8.25–734.14], p < 0.001. Our study results are consistent with the results reported by Wu et al. (42). They revealed that PMWT was associated with achieving therapeutic INR at discharge by the multivariate regression analysis [OR 3.14, 95% CI (1.08–9.14)] and was inversely associated with achieving INRs above the target range during admission [OR 0.21, 95% CI (0.05–0.82)].
In the current study, the results of the cost-effectiveness analysis demonstrated that the PMWT strategy was dominant (i.e., less costly and more effective) as compared to standard care from the medical provider’s perspective. This finding was similar to the results from previous studies conducted in other countries, such as the United States and Thailand, which strongly suggests that this intervention is cost-effective across different types of healthcare systems (15, 27, 43, 44).
In Egypt, RHD has been considered a national healthcare problem since the rate of misdiagnosis of rheumatic fever remains high. Moreover, the mitral valve is the most afflicted site among Egyptian patients (95.2% out of all valvular afflictions). In response, the Egyptian Ministry of Health has established the national RHD prevention and control program in 2006. The program was projected to save 1.7 billion USD, which represents the cost of valve replacement surgeries required for the number of RHD cases if they are neglected (45). In line with these efforts, we believe that our study results advocate for adopting a simple strategy, the PMWT strategy, that ultimately ends up with massive cost savings and better allocation of the scarce healthcare resources in Egypt. Moreover, our study was conducted from the medical provider’s perspective; however, we believe that the adoption of these services will be even more cost-saving from the societal perspective.
This study included several strong points as it incorporated real-life data to demonstrate the clinical effectiveness of the PMWT services as compared to the actual care experienced by patients in day-to-day practice in Egypt. Moreover, this model is the first one in Egypt that incorporated transitions over time through INR changes and translated this in terms of increased risks of developing warfarin-associated events. Additionally, the parameters used in our analysis were derived from large RCTs (30, 31). In the used model, we explicitly accounted for uncertainties of the epidemiologic parameters, relative risks, and quality of life by using ranges and CIs based on published sources.
Our study has several limitations. We did not compare the time to achieve therapeutic INR in both arms. We believe that larger studies are needed to investigate this outcome that is strongly believed to endorse not only the clinical effectiveness of the PMWT but also the cost savings associated with this approach. In our economic model, we assumed that the rate of TE on reoperation is identical to that for patients in the recovery state due to the lack of reliable data. In this context, it is worth mentioning that the incorporation of several simplifying assumptions is considered a weak point for the model; however, this was overcome by the sensitivity analyses that adopted wide ranges for parameter values. In addition, some input parameters were derived from studies that were conducted on different populations due to a lack of local data. However, these parameters did not show any significant impact on the results in the one-way sensitivity analyses. Another additional limitation, we adopted the medical provider’s perspective. Thus, we did not include the loss of productivity costs and the costs incurred by the patient (costs of the drug and the INR testing) as an out-of-pocket cost.
Conclusion
The pharmacist-managed warfarin therapy strategy was proven to provide a significantly better anticoagulation control and to be a cost-saving approach in Egyptian patients with MMVPs. Nevertheless, the dominance of this strategy is sustained by maintaining the therapeutic INR control within the recommended range. Our findings will benefit Egyptian policy-makers who may seek novel health strategies for better resource allocation.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee at Faculty of Pharmacy, Ain Shams University, Cairo, Egypt (ENREC–ASU. 2019-99). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
RB and IA performed the practical part at the anticoagulation clinic. RB performed the statistical and economic analysis and wrote the manuscript. SF and NS revised the manuscript. SF, IA, and NS supervised the project. All authors designed the research and read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the staff of the anticoagulation clinic at the Cardiothoracic Surgery Academy, Ain Shams University, Cairo, Egypt for their great effort and support in recruiting and following up with the patients. We also thank Dr. Hussien Abdelgawad for his support in the sample size calculation for this work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.889197/full#supplementary-material
References
1. Watkins DA, Beaton AZ, Carapetis JR, Karthikeyan G, Mayosi BM, Wyber R, et al. Rheumatic heart disease worldwide: JACC scientific expert panel. J Am Coll Cardiol. (2018) 72:1397–416. doi: 10.1016/j.jacc.2018.06.063
2. World Health Organization. Rheumatic Heart Disease. (2021). Available online at: https://www.who.int/health-topics/rheumatic-heart-disease#tab=tab_1 (accessed Mar 28, 2021).
3. Abul-Fadl A, Mourad M, Ghamrawy A, Sarhan A. Trends in deaths from rheumatic heart disease in the Eastern Mediterranean region: burden and challenges. J Cardiovasc Dev Dis. (2018) 5:32. doi: 10.3390/jcdd5020032
4. Sorour KA. Rheumatic heart disease in Egypt: gloomy past and promising future. Egypt Heart J. (2014) 66:139–42. doi: 10.1016/j.ehj.2013.12.083
5. Pastori D, Lip GYH, Poli D, Antonucci E, Rubino L, Menichelli D, et al. Determinants of low-quality warfarin anticoagulation in patients with mechanical prosthetic heart valves. The nationwide PLECTRUM study. Br J Haematol. (2020) 190:588–93. doi: 10.1111/bjh.16528
6. Misawa Y. Valve-related complications after mechanical heart valve implantation. Surg Today. (2015) 45:1205–9. doi: 10.1007/s00595-014-1104-0
7. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2021) 143:e72–227. doi: 10.1161/CIR.0000000000000923
8. Falamić S, Lucijanić M, Hadžiabdić MO, Marušić S, Bačić Vrca V. Pharmacist’s interventions improve time in therapeutic range of elderly rural patients on warfarin therapy: a randomized trial. Int J Clin Pharm. (2018) 40:1078–85. doi: 10.1007/s11096-018-0691-z
9. Catterall F, Ames PRJ, Isles C. Warfarin in patients with mechanical heart valves. BMJ. (2020) 371:m3956. doi: 10.1136/bmj.m3956
10. El Ghousain HE, Thomas M, Varghese SJ, Hegazi MO, Kumar R. Long term oral anticoagulant therapy with warfarin: experience with local patient population in Kuwait. Indian J Hematol Blood Transfus. (2014) 30:111–9. doi: 10.1007/s12288-012-0223-2
11. Marcatto LR, Sacilotto L, Tavares LC, Facin M, Olivetti N, Strunz CMC, et al. Pharmaceutical care increases time in therapeutic range of patients with poor quality of anticoagulation with warfarin. Front Pharmacol. (2018) 9:1052. doi: 10.3389/fphar.2018.01052
12. Hawes E. Patient education on oral anticoagulation. Pharmacy. (2018) 6:34. doi: 10.3390/pharmacy6020034
13. Magro L, Arzenton E, Leone R, Stano MG, Vezzaro M, Rudolph A, et al. Identifying and characterizing serious adverse drug reactions associated with drug-drug interactions in a spontaneous reporting database. Front Pharmacol. (2021) 11:622862. doi: 10.3389/fphar.2020.622862
14. Jiang S, He Q, Yan J, Zhao L, Zheng Y, Chen P, et al. Evaluation of a pharmacist-led remote warfarin management model using a smartphone application (Yixing) in improving patients’ knowledge and outcomes of anticoagulation therapy. Front Pharmacol. (2021) 12:677943. doi: 10.3389/fphar.2021.677943
15. Saokaew S, Permsuwan U, Chaiyakunapruk N, Nathisuwan S, Sukonthasarn A, Jeanpeerapong N. Cost-effectiveness of pharmacist-participated warfarin therapy management in Thailand. Thromb Res. (2013) 132:437–43. doi: 10.1016/j.thromres.2013.08.019
16. Thanimalai S, Shafie AA, Ahmad Hassali MA, Sinnadurai J. Cost-effectiveness of warfarin medication therapy adherence clinic versus usual medical clinic at Kuala Lumpur hospital. Value Health Reg Issues. (2018) 15:1534–41. doi: 10.1016/j.vhri.2017.05.006
17. Aidit S, Soh YC, Yap CS, Khan TM, Neoh CF, Shaharuddin S, et al. Effect of standardized warfarin treatment protocol on anticoagulant effect: comparison of a warfarin medication therapy adherence clinic with usual medical care. Front Pharmacol. (2017) 8:637. doi: 10.3389/fphar.2017.00637
18. Marcatto LR, Sacilotto L, Tavares LC, Souza DSP, Olivetti N, Strunz CMC, et al. Evaluation of the long-term impact on quality after the end of pharmacist-driven warfarin therapy management in patients with poor quality of anticoagulation therapy. Front Pharmacol. (2020) 11:1056. doi: 10.3389/fphar.2020.01056
19. Mostafa LS, Sabri NA, El-Anwar AM, Shaheen SM. Evaluation of pharmacist-led educational interventions to reduce medication errors in emergency hospitals: a new insight into patient care. J Public Health (Bangkok). (2019) 42:169–74. doi: 10.1093/pubmed/fdy216
20. Levesque AA, Lewin AR, Rimsans J, Sylvester KW, Coakley L, Melanson F, et al. Development of multidisciplinary anticoagulation management guidelines for patients receiving durable mechanical circulatory support. Clin Appl Thromb. (2019) 25:107602961983736. doi: 10.1177/1076029619837362
21. Bishop MA, Streiff MB, Ensor CR, Tedford RJ, Russell SD, Ross PA. Pharmacist-managed international normalized ratio patient self-testing is associated with increased time in therapeutic range in patients with left ventricular assist devices at an academic medical center. ASAIO J. (2014) 60:193–8. doi: 10.1097/MAT.0000000000000047
22. Clarkesmith DE, Pattison HM, Lip GYH, Lane DA. Educational intervention improves anticoagulation control in atrial fibrillation patients: the TREAT randomised trial. PLoS One. (2013) 8:e74037. doi: 10.1371/journal.pone.0074037
23. Rosendaal FR, Cannegieter SC, Van der Meer FJM, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. (1993) 69:236–9. doi: 10.1055/s-0038-1651587
24. Roberts M, Rollason J, Warren S. Improving Patients Time in Range on Warfarin, NICE Shared Learning. (2020). Available online at: https://www.nice.org.uk/sharedlearning/improving-patients-time-in-range-on-warfarin (accessed Jun 09, 2021).
25. Komorowski M, Raffa J “Markov models and cost effectiveness analysis: applications in medical research,” in Secondary Analysis of Electronic Health Records. (Cham: Springer) (2016). doi: 10.1007/978-3-319-43742-2_24
26. Elsisi GH, Kaló Z, Eldessouki R, Ragab S, Elshalakani AMRM, Abaza S, et al. Recommendations for reporting pharmacoeconomic evaluations in Egypt. Value Health Reg Issues. (2013) 2:319–27. doi: 10.1016/j.vhri.2013.06.014
27. Chang JY, Wang CC, Kang HC, Shen LJ, Huang CF. Cost-effectiveness of the pharmacist-assisted warfarin monitoring program at a medical center in Taiwan. Int J Qual Health Care. (2017) 29:817–25. doi: 10.1093/intqhc/mzx109
28. Elsisi GH, Eldessouki R, Kaló Z, Elmazar MM, Taha AS, Awad BF, et al. Cost-effectiveness of the combined use of warfarin and low-dose aspirin versus warfarin alone in Egyptian patients with aortic valve replacements: a markov model. Value Health Reg Issues. (2014) 4:24–30. doi: 10.1016/j.vhri.2014.06.004
29. Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al. Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anticoagulation therapy: a systematic review and economic modelling. Health Technol Assess. (2007) 11:iii–iv, ix–66. doi: 10.3310/hta11380
30. Bloomfield P, Wheatley DJ, Prescott RJ, Miller HC. Twelve-year comparison of a Bjork–Shiley mechanical heart valve with porcine bioprostheses. N Engl J Med. (1991) 324:573–9. doi: 10.1056/nejm199102283240901
31. Hammermeister KE, Sethi GK, Henderson WG, Oprian C, Kim T, Rahimtoola S. A comparison of outcomes in men 11 years after heart-valve replacement with a mechanical valve or bioprosthesis. N Engl J Med. (1993) 328:1289–96. doi: 10.1056/nejm199305063281801
32. Potter DD, Sundt TM III, Zehr KJ, Dearani JA, Daly RC, Mullany CJ, et al. Risk of repeat mitral valve replacement for failed mitral valve prostheses. Ann Thorac Surg. (2004) 78:67–72; discussion 67–72. doi: 10.1016/j.athoracsur.2004.02.014
33. Cannegieter SC, Rosendaal FR, Wintzen AR, van der Meer FJM, Vandenbroucke JP, Briet E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. (1995) 333:11–7. doi: 10.1056/nejm199507063330103
34. Jones JM, O’kane H, Gladstone DJ, Sarsam MA, Campalani G, MacGowan SW, et al. Repeat heart valve surgery: risk factors for operative mortality. J Thorac Cardiovasc Surg. (2001) 122:913–8. doi: 10.1067/mtc.2001.116470
35. Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. (2010) 96:5–21. doi: 10.1093/bmb/ldq033
36. Regier DA, Sunderji R, Lynd LD, Gin K, Marra CA. Cost-effectiveness of self-managed versus physician-managed oral anticoagulation therapy. CMAJ. (2006) 174:1847–52. doi: 10.1503/cmaj.051104
37. World Bank. PPP Conversion Factor, GDP (LCU Per International $) – Egypt, Arab Rep. (2021). Available online at: https://data.worldbank.org/indicator/PA.NUS.PPP?locations=EG (accessed Jun 17, 2021).
38. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS)-explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. (2013) 16:231–50. doi: 10.1016/j.jval.2013.02.002
39. WHO. Body Mass Index – BMI. (2021). Available online at: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed May 15, 2021).
40. Entezari-Maleki T, Dousti S, Hamishehkar H, Gholami K. A systematic review on comparing 2 common models for management of warfarin therapy; pharmacist-led service versus usual medical care. J Clin Pharmacol. (2016) 56:24–38. doi: 10.1002/jcph.576
41. Dib JG, Mohammed K, Momattin HI, Alshehri AM. Implementation of pharmacist-managed anticoagulation clinic in a Saudi Arabian health center. Hosp Pharm. (2014) 49:260–8. doi: 10.1310/hpj4903-260
42. Wu C-W, Wu C-C, Chen C-H, Lin S-Y, Hsu R-B, Huang C-F. The impact of pharmacist-managed service on warfarin therapy in patients after mechanical valve replacement. Int J Clin Pract. (2022) 2022:1–6. doi: 10.1155/2022/1617135
43. You JHS, Chan FWH, Wong RSM, Cheng G. Cost-effectiveness of two models of management for patients on chronic warfarin therapy – a Markov model analysis. Thromb Haemost. (2003) 90:1106–11. doi: 10.1160/th03-06-0367
44. Sullivan PW, Arant TW, Ellis SL, Ulrich H. The cost effectiveness of anticoagulation management services for patients with atrial fibrillation and at high risk of stroke in the US. Pharmacoeconomics. (2006) 24:1021–33. doi: 10.2165/00019053-200624100-00009
Keywords: cost-effectiveness, pharmacist intervention, mechanical mitral valve, warfarin, time in therapeutic range, Egypt
Citation: Batran RA, Sabri NA, Ali I and Fahmy SF (2022) Cost-Effectiveness of the Pharmacist-Managed Warfarin Therapy vs. Standard Care for Patients With Mechanical Mitral Valve Prostheses: An Egyptian Healthcare Perspective. Front. Cardiovasc. Med. 9:889197. doi: 10.3389/fcvm.2022.889197
Received: 03 March 2022; Accepted: 30 May 2022;
Published: 13 July 2022.
Edited by:
Kamal Sharma, B. J. Medical College and Civil Hospital, IndiaReviewed by:
Komal Shah, Indian Institute of Public Health Gandhinagar (IIPHG), IndiaHardik Dineshbhai Desai, Gujarat Adani Institute of Medical Sciences, India
Prashant Vazirani, Dr. Jivraj Mehta Health Care, India
Copyright © 2022 Batran, Sabri, Ali and Fahmy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nagwa Ali Sabri, bmFnd2Euc2FicmlAcGhhcm1hLmFzdS5lZHUuZWc=, orcid.org/0000-0002-2611-4853
 Radwa Ahmed Batran
Radwa Ahmed Batran Nagwa Ali Sabri
Nagwa Ali Sabri Ihab Ali2
Ihab Ali2 Sarah Farid Fahmy
Sarah Farid Fahmy