- 1Department of Cardiology, Einstein Medical Center, Philadelphia, PA, United States
- 2Department of Medicine, The Wright Center for Graduate Medical Education, Scranton, PA, United States
Cardiovascular disease, in particular ischemic heart disease is a major cause of morbidity and mortality worldwide. Primary aldosteronism is the leading cause of secondary hypertension, yet commonly under diagnosed, and represents a major preventable risk factor. In contrast to historical teaching, recent studies have shown that excess aldosterone production is associated with increased burden of ischemic heart disease disproportionate to the effects caused by hypertension alone. Aldosterone through its genomic and non-genomic actions exerts various detrimental cardiovascular changes contributing to this elevated risk. Recognition of primary hyperaldosteronism and understanding the distinctive pathophysiology of ischemic heart disease in primary aldosteronism is crucial to develop strategies to improve outcomes.
Introduction
Cardiovascular diseases (CVDs), consisting of ischemic heart disease, heart failure, peripheral arterial disease, and several other cardiac and vascular conditions, constitute the leading cause of global mortality and are a major contributor to reduced quality of life (1). Amongst CVDs, ischemic heart disease (IHD) is the main global cause of death, accounting for more than 9 million deaths in 2016 according to the World Health Organization (WHO) estimates. Although IHD rates are decreasing globally, risk factor prevalence is rising (2). Hypertension is a major modifiable risk factor for ischemic heart disease with nearly 1.39 billion adults affected worldwide in 2010 (3). It is estimated that risk of a fatal coronary event doubles with an increase in systolic blood pressure of 20 mm Hg or each 10-mm Hg increase in diastolic blood pressure (4). Aldosterone, a steroid hormone secreted by the adrenal gland, is an important regulator of blood pressure and primary hypersecretion of this hormone leads to dysregulation of homeostatic control mechanisms. Moreover, normotensive individuals with higher plasma aldosterone levels within physiological range are at an increased risk of subsequent rise in blood pressure and development of incident hypertension (5). The effect of excess production of aldosterone has traditionally been conceptualized as a result of its action on renal collecting duct principal cells, via mineralocorticoid receptors (MR), to induce sodium and water reabsorption and potassium excretion clinically culminating in hypertension associated with low or low-normal serum potassium levels. Historically, primary aldosteronism was considered an uncommon disease, however, with advances in diagnostic technology, primary aldosteronism (PA) is now identified as the most common endocrinological cause of secondary hypertension, with aldosterone producing adenoma (APA) and bilateral adrenal hyperplasia (BAH, idiopathic hyperaldosteronism) accounting for vast majority of cases (6). Recent evidence has linked primary aldosteronism with increased cardiovascular morbidity and mortality, out of proportion to the adverse effects caused solely by elevated blood pressure, underscoring its pleiotropic effect and multifaceted role in cardiovascular pathophysiology (7–9). In this review, we aim to focus on the prevalence and pathophysiologic mechanisms of IHD in primary aldosteronism.
Prevalence of Ischemic Heart Disease in Primary Aldosteronism
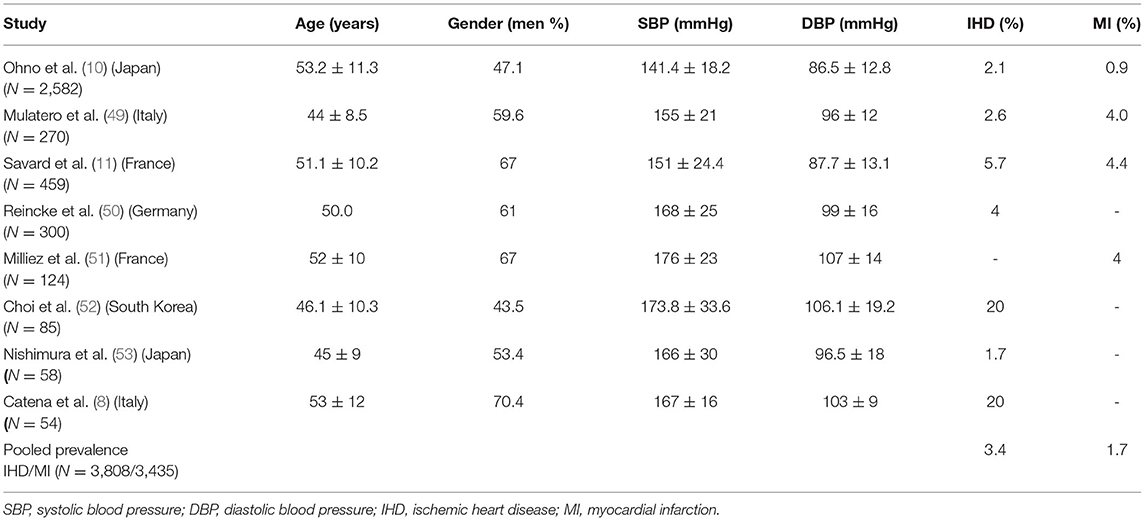
The true prevalence of PA is underestimated, with current studies reporting a prevalence of 3–13% in primary care setting and up to 30% in referral centers (6). The prevalence of IHD and MI in patients with primary aldosteronism is variable depending on the study design and diagnostic criteria ranging from 1.7 to 20% and 0.9 to 4.4%, respectively. The pooled prevalence of IHD and MI in PA were estimated to be 3.4 and 1.7% respectively (Table 1). Various epidemiological studies clearly demonstrate that individuals with primary aldosteronism experience higher burden of IHD compared to individuals with essential hypertension with comparable demographic and cardiovascular risk factor profile (8, 10, 11). Individuals with PA presenting with unilateral subtype, or plasma aldosterone concentration ≥125 pg/ml are at a greater risk of CVD (10). It has also been observed that hypokalemic variant of primary aldosteronism is associated with excessive burden of ischemic heart disease compared to normokalaemia variant, possibly due to effects of higher concentration of aldosterone exposure (12). Additionally, patients with primary hyperaldosteronism are more likely to have experienced an ischemic cardiovascular complication (non-fatal myocardial infarction or angina) at the time of diagnosis of PA than otherwise similar patients with essential hypertension (11). These data should draw the clinician's attention to broaden the scope to suspect PA, especially when severity of IHD or CVD morbidity is considered out of proportion to that effectuated by essential hypertension.
Pathophysiology of Ischemic Heart Disease in Primary Aldosteronism
IHD occurs due to an imbalance between myocardial blood supply and myocardial demand, either at rest or exertion. Atherosclerosis is the major pathophysiological process involved in development of IHD. IHD can be silent, termed as silent myocardial ischemia, or manifest clinically either as acute coronary syndrome, secondary to plaque rupture and coronary thrombosis, or as stable angina, due to fixed narrowing of the coronary arteries from plaque buildup in the vessel wall and luminal narrowing. Conventional risk factors for IHD include increased age, hypertension, diabetes mellitus, smoking, hyperlipidemia, and family history of IHD. Excess aldosterone not only adversely affects the vasculature and cardiac muscle, but also influences cardiovascular risk factors via various biochemical pathways, uniquely contributing to the development of IHD (Figure 1).
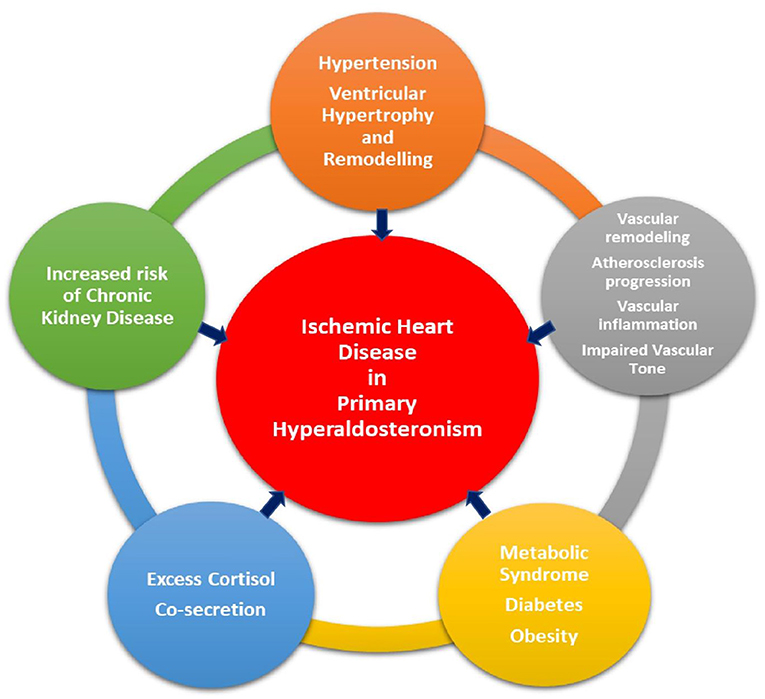
Figure 1. Pathways through which primary aldosteronism plays a role in the development of ischemic heart disease.
Effect of Hyperaldosteronism on Blood Pressure and Left Ventricle
It is well known that elevated aldosterone levels raise blood pressure via its genomic action mediated through mineralocorticoid receptor (MR) to absorb sodium and water in the renal collecting ducts causing volume expansion. Additionally, hyperaldosteronism increases blood pressure mediated via its harmful effect on vascular remodeling. Hypertension due to PA is often undiagnosed and chronic exposure to elevated blood pressure, results in compensatory left ventricular (LV) hypertrophy. In addition, long term exposure of cardiac myocyte to elevated aldosterone levels leads to myocyte hypertrophy by increasing myocardial expression of cardiotrophin-1 (CT-1), a cytokine that induces expression of myosin light chain and skeletal α-actin and enhances myosin light chain phosphorylation (13). In addition, exposure to aldosterone causes increased mRNA levels of α- and β-myosin heavy chain via activation of mineralocorticoid receptors (MRs), extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase and protein kinase C-α (14). Remodeling of left ventricle in PA not only occurs via cardiac myocyte hypertrophy but also simultaneously via cardiac fibrosis through chronic inflammation, and dysregulation of extra cellular matrix metabolism (15). This results in stiffening of the LV with subsequent elevation in LV end-diastolic pressure (LVEDP). High LVEDP compromises diastolic coronary blood flow (CBF) by decreasing the coronary perfusion pressure (CPP) leading to decreased myocardial oxygen supply (16, 17). A combination of increased LV mass and diminished diastolic CBF causes supply-demand mismatch resulting in myocardial ischemia.
Effect of Hyperaldosteronism on Vasculature
Elevated serum aldosterone levels in PA patients lead to detrimental effects on the endothelium via genomic and nongenomic mechanisms via mineralocorticoid receptor-dependent and independent manners. Excess generation of reactive oxygen species (ROS) via increased NADPH oxidase production, decreased endothelial expression of G6PD, and mitochondrial ROS generation in the electron transport chain and release result in oxidative stress and amplify vascular inflammation. These processes are thought to be mediated via MR-independent (via extracellular-signal-regulated kinase (ERK) 1/2, c-Jun N-terminal kinase (JNK) and GPER pathways) and MR-dependent pathways. Aldosterone also increases the expressions of adhesion molecules, namely ICAM1 and VCAM-1, and inflammatory markers, such as COX-2 and MCP-1 in the endothelium, which induces monocytes and macrophage infiltration. The infiltrated monocyte-derived macrophages which are rich in NADPH oxidase further augment the generation of ROS and worsen vascular inflammation. The infiltrated macrophages ingest oxidized low-density lipoproteins and become foam cells, which potentiate the formation of atherosclerotic plaque. Aldosterone promotes inflammatory plaque formation via placental growth factor (PlGF) which binds to VEGF type 1 receptors on endothelial and inflammatory cells, and further promotes vascular smooth cell proliferation and monocyte chemotaxis, which are fundamental processes of atherosclerosis. Aldosterone also increases the formation of vasoconstrictors and decreases production and bioavailability of nitric oxide causing impairment of vascular relaxation. Aldosterone via MR-dependent pathways in the endothelium and vascular smooth muscle cells induces vascular fibrosis contributing to vascular stiffness and remodeling (18, 19).
Effect of Hyperaldosteronism on Obesity, Diabetes, and Metabolic Syndrome
Diabetes is a well-established risk factor for IHD and is considered a coronary artery disease equivalent (20). PA is linked to increased risk of diabetes and metabolic syndrome. Clustering of hypertension, abdominal obesity, dyslipidemia and impaired glucose metabolism, is more commonly encountered in PA patients than individuals with essential hypertension (21, 22). Hyperaldosteronism is thought to cause increase in fat mass through mineralocorticoid receptor activation in adipocytes, which in turn induce excess aldosterone production through the actions of adipocytokines (CTRP1, leptin, and resistin) and activation of the sympathetic nervous system, which turns on the renin-angiotensin-aldosterone system, thereby creating a vicious cycle (23, 24). Deranged glucose metabolism occurs through aldosterone mediated impaired insulin sensitivity in skeletal muscle and adipose tissue via the MR receptor, and impaired insulin secretion, albeit the underlying mechanisms leading to decreased insulin secretion are poorly understood (25). In addition, blockade of MR has shown to improve coronary flow reserve on cardiac PET scan among individuals with type 2 diabetes without clinical evidence of ischemic heart disease, suggesting that excess MR activation in diabetes contributes to coronary microvascular dysfunction (26).
Effect of Hyperaldosteronism on Kidneys
Primary aldosteronism causes renal dysfunction, independent of blood pressure, by inducing renal fibrosis, vascular remodeling, and podocyte injury via MR stimulation from excess aldosterone production (27). Chronic kidney disease is an independent risk factor for development of ischemic heart disease (28). Renal dysfunction increases oxidative stress, imparts endothelial dysfunction, and promotes systemic inflammation which accelerates atherosclerosis.
Role of Hypercortisolism in Primary Hyperaldosteronism
Individuals with PA frequently have excess cortisol co-secretion, which can further worsen cardio-metabolic risk through their synergistic effects (29, 30). Cortisol is normally converted to an inactive metabolite, cortisone, by the action of 11β-hydroxysteroid dehydrogenase type 2(11β-HSD2). However, in hypercortisolism the activity of this enzyme is decreased, albeit due to unclear reasons (31). Loss of 11β-HSD2 has been shown to promote atherogenesis via activation of MR stimulating pro-inflammatory processes in the endothelium of knock-out murine models (32). Likewise, individuals with hypercortisolism demonstrate an increased burden of coronary calcification and noncalcified coronary plaque. Additionally, hypercortisolism promotes a prothrombotic state (33). This phenotype of PA and glucocorticoid co-secretion underscores the importance of additional screening for hypercortisolism due to therapeutic and prognostic implications (34).
Role of Aldosterone Excess Post-acute Myocardial Infarction
After acute myocardial infarction (MI) circulating levels of serum aldosterone are elevated as a consequence of neurohormonal activation (35). Hyperaldosteronism after acute MI effectuates a myriad of maladaptive changes in the post-MI heart which increase morbidity and mortality (36, 37). Post-MI hyperaldosteronism contributes to ventricular remodeling that involves both infarcted and non-infarcted zones, which at a cellular level occurs through myocyte apoptosis, myocyte hypertrophy, macrophage/monocyte infiltration, and collagen deposition via fibroblast activation and proliferation. Excess aldosterone also induces endothelial dysfunction by reducing nitric oxide generation and increasing formation of reactive oxygen species (38). Moreover, elevated aldosterone leads to electrical remodeling, lengthened action potential duration, increase in Ca2+ current (ICa) and a decrease in K+ transient outward current (Ito), even before morphological remodeling occurs, creating a pro-arrhythmogenic milieu (39).
The Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) showed beneficial effect of MR antagonists when utilized in the early post-MI period, namely by decreasing the incidence of sudden cardiac death and heart failure hospitalizations (40). Current guidelines recommend treatment with MR antagonists in patients with acute MI with ejection fraction <40% and clinical heart failure or diabetes (41, 42). Given the unfavorable effects of hyperaldosteronism in the post-MI setting, and the positive impact of aldosterone antagonists among patients with post MI systolic heart failure, the role aldosterone antagonists in improving outcomes in post-MI patients without systolic heart failure has garnered incredible clinical interest. A recent pilot study showed MR antagonists when initiated prior to reperfusion in STEMI patients resulted in improvement in ventricular remodeling at the end of 3 months, however, no impact on reducing MI size was seen (43). Despite abundant preclinical and mechanistic data supporting the concept, no clinically meaningful benefit, i.e., reduction in overall or cardiovascular mortality, ventricular arrhythmia, or rehospitalization, with use of MR antagonists in early post-MI patients without evidence of heart failure has been demonstrated in major prospective randomized clinical trials (44, 45). This underscores the need for more adequately powered prospective randomized trials evaluating the safety and efficacy of MRA administration in early post-MI patients without evidence of HF.
Effect of Primary Aldosteronism Treatment on Ischemic Heart Disease
If diagnosed, patients with PA can be offered targeted treatment, either in the form of unilateral adrenalectomy for APA, or mineralocorticoid receptor antagonists, typically used for BAH, and sometimes for APA who are unable or unwilling to undergo adrenalectomy. Despite the presence of increased cardiovascular morbidity in PA patients at the time of diagnosis, administration of appropriate treatment results in improved cardiovascular outcome, when the effects of excess aldosterone are permanently removed. Younger age and shorter duration of hypertension independently predict beneficial cardiovascular outcomes, underscoring the importance of a timely correction of this disorder (8). Surgical adrenalectomy appears to be superior in mitigating adverse cardiovascular events compared to medical therapy in unilateral PA (46). PA patients on MR antagonist therapy with unsuppressed plasma renin activity (PRA) ≥ 1 ng/ml/h, a marker of effective MR blockade, seem to have comparable cardiovascular outcomes to those with essential hypertension. In contrast, patients with suppressed PRA <1 ng/ml/h experience poorer cardiovascular outcomes despite similar blood pressure control (47). Future prospective studies are necessary to determine treatment approaches in patients with PA to optimize cardiovascular outcomes. Given the reversal of this increased cardiovascular risk through therapy, a robust effort to diagnose and effectively treat PA, undoubtedly reduces health costs and improves quality of life (48).
Conclusion
Primary aldosteronism is common, with true prevalence expected to be higher than current estimates. Furthermore, it carries a significantly worse cardiovascular prognosis compared to individuals with essential hypertension. Early detection of this entity could not only improve outcomes for patients but also potentially be cost saving for the healthcare system.
Author Contributions
SP and CR made substantial contribution to the article design and conception of the work, contributed to the acquisition of data, and drafting and editing of the manuscript. AA and SP made critical revisions. All authors read and approved the final manuscript.
Funding
This work was funded by Einstein Medical Center, Philadelphia.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mensah GA, Roth GA, Fuster V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J Am Coll Cardiol. (2019) 74:2529-32. doi: 10.1016/j.jacc.2019.10.009
2. Nowbar AN, Gitto M, Howard JP, Francis DP, Al-Lamee R. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. (2019) 12:e005375. doi: 10.1161/CIRCOUTCOMES.118.005375
3. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. (2016) 134:441-50. doi: 10.1161/CIRCULATIONAHA.115.018912
4. Rosendorff C, Lackland DT, Allison M, Aronow WS, Black HR, Blumenthal RS, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Circulation. (2015) 131:e435-70. doi: 10.1161/CIR.0000000000000207
5. Vasan R., Evans C, Larson Mg, et al. Serum aldosterone and the insidence of hypertension in nonhypertensive persons. N Engl Med. (2004) 351:33–42. doi: 10.1056/NEJMoa033263
6. Libianto R, Fuller PJ, Young MJ, Yang J. Primary aldosteronism is a public health issue: challenges and opportunities. J Hum Hypertens. (2020) 34:478–86. doi: 10.1038/s41371-020-0336-2
7. Xanthakis V, Vasan RS. Aldosterone and the risk of hypertension. Curr Hypertens Rep. (2013) 15:102–7. doi: 10.1007/s11906-013-0330-y
8. Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Int Med. (2008) 168:80–5. doi: 10.1001/archinternmed.2007.33
9. Wu X, Yu J, Tian H. Cardiovascular risk in primary aldosteronism: a systematic review and meta-analysis. Medicine. (2019) 98:e15985. doi: 10.1097/MD.0000000000015985
10. Ohno Y, Sone M, Inagaki N, Yamasaki T, Ogawa O, Takeda Y, et al. Prevalence of cardiovascular disease and its risk factors in primary aldosteronism: a multicenter study in Japan. Hypertension. (2018) 71:530–7. doi: 10.1161/HYPERTENSIONAHA.117.10263
11. Savard S, Amar L, Plouin P-F, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. (2013) 62:331–6. doi: 10.1161/HYPERTENSIONAHA.113.01060
12. Born-Frontsberg E, Reincke M, Rump L, Hahner S, Diederich S, Lorenz R, et al. Participants of the German conn's registry. cardiovascular and cerebrovascular comorbidities of hypokalemic and normokalemic primary aldosteronism: results of the German Conn's Registry. J Clin Endocrinol Metab. (2009) 94:1125–30. doi: 10.1210/jc.2008-2116
13. López-Andrés N, Martin-Fernandez B, Rossignol P, Zannad F, Lahera V, Fortuno MA, et al. A role for cardiotrophin-1 in myocardial remodeling induced by aldosterone. Am J Physiol Heart Circul Physiol. (2011) 301:H2372-82. doi: 10.1152/ajpheart.00283.2011
14. Okoshi M, Yan X, Okoshi K, Nakayama M, Schuldt A. O′ Connell Td, et al. Aldosterone directly stimulates cardiac myocyte hypertrophy. J Card Fail. (2004) 10:511–8. doi: 10.1016/j.cardfail.2004.03.002
15. Tsai C-H, Pan C-T, Chang Y-Y, Chen Z-W, Wu V-C, Hung C-S, et al. Left ventricular remodeling and dysfunction in primary aldosteronism. J Hum Hypertens. (2021) 35:131–47. doi: 10.1038/s41371-020-00426-y
16. Nguyen T, Do H, Pham T, Vu LT, Zuin M, Rigatelli G. Left ventricular dysfunction causing ischemia in patients with patent coronary arteries. Perfusion. (2018) 33:115–22. doi: 10.1177/0267659117727826
17. Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. (2000) 102:470–9. doi: 10.1161/01.CIR.102.4.470
18. Chen Z-W, Tsai C-H, Pan C-T, Chou C-H, Liao C-W, Hung C-S, et al. Endothelial dysfunction in primary aldosteronism. Int J Mol Sci. (2019) 20:5214. doi: 10.3390/ijms20205214
19. Chrissobolis S. Vascular consequences of aldosterone excess and mineralocorticoid receptor antagonism. Curr Hypertens Rev. (2017) 13:46–56. doi: 10.2174/1573402113666170228151402
20. Hajar R. Diabetes as “coronary artery disease risk equivalent”: a historical perspective. Heart Views. (2017) 18:34. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_37_17
21. Monticone S, D'Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. (2018) 6:41–50. doi: 10.1016/S2213-8587(17)30319-4
22. Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. (2006) 91:454–9. doi: 10.1210/jc.2005-1733
23. Urbanet R, Nguyen Dinh Cat A, Feraco A, Venteclef N, El Mogrhabi S, Sierra-Ramos C, et al. Adipocyte mineralocorticoid receptor activation leads to metabolic syndrome and induction of prostaglandin D2 synthase. Hypertension. (2015) 66:149–57. doi: 10.1161/HYPERTENSIONAHA.114.04981
24. Ohno Y, Sone M, Inagaki N, Yamasaki T, Ogawa O, Takeda Y, et al. Obesity as a key factor underlying idiopathic hyperaldosteronism. J Clin Endocrinol Metab. (2018) 103:4456–64. doi: 10.1210/jc.2018-00866
25. Luther JM. Effects of aldosterone on insulin sensitivity and secretion. Steroids. (2014) 91:54–60. doi: 10.1016/j.steroids.2014.08.016
26. Garg R, Rao AD, Baimas-George M, Hurwitz S, Foster C, Shah RV, et al. Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes. (2015) 64:236–42. doi: 10.2337/db14-0670
27. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. (2018) 72:658–66. doi: 10.1161/HYPERTENSIONAHA.118.11568
28. Sarnak M. American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. (2003) 42:1050–65. doi: 10.1161/01.HYP.0000102971.85504.7c
29. Gerards J, Heinrich DA, Adolf C, Meisinger C, Rathmann W, Sturm L, et al. Impaired glucose metabolism in primary aldosteronism is associated with cortisol cosecretion. J Clin Endocrinol Metab. (2019) 104:3192–202. doi: 10.1210/jc.2019-00299
30. Adolf C, Köhler A, Franke A, Lang K, Riester A, Löw A, et al. Cortisol excess in patients with primary aldosteronism impacts left ventricular hypertrophy. J Clin Endocrinol Metab. (2018) 103:4543–52. doi: 10.1210/jc.2018-00617
31. Stewart PM, Walker BR, Holder G, O'Halloran D, Shackleton C. 11 Beta-hydroxysteroid dehydrogenase activity in cushing's syndrome: explaining the mineralocorticoid excess state of the ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metabol. (1995) 80:3617–20. doi: 10.1210/jcem.80.12.8530609
32. Deuchar GA, McLean D, Hadoke PW, Brownstein DG, Webb DJ, Mullins JJ, et al. 11β-hydroxysteroid dehydrogenase type 2 deficiency accelerates atherogenesis and causes proinflammatory changes in the endothelium in Apoe–/– mice. Endocrinology. (2011) 152:236–46. doi: 10.1210/en.2010-0925
33. Neary NM, Booker OJ, Abel BS, Matta JR, Muldoon N, Sinaii N, et al. Hypercortisolism is associated with increased coronary arterial atherosclerosis: analysis of noninvasive coronary angiography using multidetector computerized tomography. J Clin Endocrinol Metab. (2013) 98:2045–52. doi: 10.1210/jc.2012-3754
34. Peng K-Y, Liao H-W, Chan C-K, Lin W-C, Yang S-Y, Tsai Y-C, et al. Presence of subclinical hypercortisolism in clinical aldosterone-producing adenomas predicts lower clinical success. Hypertension. (2020) 76:1537–44. doi: 10.1161/HYPERTENSIONAHA.120.15328
35. Udell JA, Morrow DA, Braunwald E, Swedberg K, Bode C, Rifai N, et al. Inhibition of the renin-angiotensin system reduces the rise in serum aldosterone in acute coronary syndrome patients with preserved left ventricular function: observations from the avant garde-timi 43 trial. Clin Chem. (2013) 59:959–67. doi: 10.1373/clinchem.2012.199729
36. Mignano A, Pitruzzella V, Arnone G, Arnone MT, Rotolo A, Assennato P, et al. Prognostic role of aldosterone in patients with acute coronary syndrome: short and medium term follow-up. J Cardiovasc Med. (2014) 15:27–32. doi: 10.2459/JCM.0b013e328364129c
37. Beygui F, Collet J-P, Benoliel J-J, Vignolles N, Dumaine R, Barthélémy O, et al. High plasma aldosterone levels on admission are associated with death in patients presenting with acute st-elevation myocardial infarction. Circulation. (2006) 114:2604–10. doi: 10.1161/CIRCULATIONAHA.106.634626
38. Cohn JN, Colucci W. Cardiovascular effects of aldosterone and post–acute myocardial infarction pathophysiology. Am J Cardiol. (2006) 97:4–12. doi: 10.1016/j.amjcard.2006.03.004
39. Perrier E, Kerfant B-G, Lalevee N, Bideaux P, Rossier MF, Richard S, et al. Mineralocorticoid receptor antagonism prevents the electrical remodeling that precedes cellular hypertrophy after myocardial infarction. Circulation. (2004) 110:776–83. doi: 10.1161/01.CIR.0000138973.55605.38
40. Pitt B. Eplerenone post-acute myocardial infarction heart failure efficacy and survival study investigators: eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. (2003) 348:1309–21. doi: 10.1056/NEJMoa030207
41. Amsterdam EA, Wenger NK, Brindis RG, Casey Jr DE, Ganiats TG, Holmes DR Jr, et al. 2014 Aha/Acc guideline for the management of patients with non–st-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. (2014) 130:2354–94. doi: 10.1161/CIR.0000000000000133
42. O'gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, De Lemos JA, et al. 2013 Accf/Aha Guideline for the Management of St-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2013) 61:e78-140. doi: 10.1161/CIR.0b013e3182742c84
43. Bulluck H, Fröhlich GM, Nicholas JM, Mohdnazri S, Gamma R, Davies J, et al. Mineralocorticoid receptor antagonist pre-treatment and early post-treatment to minimize reperfusion injury after st-elevation myocardial infarction: the minimize stemi trial. Am Heart J. (2019) 211:60–7. doi: 10.1016/j.ahj.2019.02.005
44. Beygui F, Cayla G, Roule V, Roubille F, Delarche N, Silvain J, et al. Early aldosterone blockade in acute myocardial infarction: the albatross randomized clinical trial. J Am Coll Cardiol. (2016) 67:1917–27. doi: 10.1016/j.jacc.2016.02.033
45. Montalescot G, Pitt B, Lopez de Sa E, Hamm CW, Flather M, Verheugt F, et al. Early eplerenone treatment in patients with acute st-elevation myocardial infarction without heart failure: the randomized double-blind reminder study. Eur Heart J. (2014) 35:2295–302. doi: 10.1093/eurheartj/ehu164
46. Huang W-C, Chen Y-Y, Lin Y-H, Chueh JS. Composite cardiovascular outcomes in patients with primary aldosteronism undergoing medical versus surgical treatment: a meta-analysis. Front Endocrinol. (2021) 12:644260. doi: 10.3389/fendo.2021.644260
47. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. (2018) 6:51–9. doi: 10.1016/S2213-8587(17)30367-4
48. Lubitz CC, Economopoulos KP, Sy S, Johanson C, Kunzel HE, Reincke M, et al. Cost-effectiveness of screening for primary aldosteronism and subtype diagnosis in the resistant hypertensive patients. Circulation. (2015) 8:621–30. doi: 10.1161/CIRCOUTCOMES.115.002002
49. Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, et al. Long-term cardio-and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. (2013) 98:4826–33. doi: 10.1210/jc.2013-2805
50. Reincke M, Fischer E, Gerum S, Merkle K, Schulz S, Pallauf A, et al. Observational study mortality in treated primary aldosteronism: the German Conn's Registry. Hypertension. (2012) 60:618–24. doi: 10.1161/HYPERTENSIONAHA.112.197111
51. Milliez P, Girerd X, Plouin P-F, Blacher J, Safar ME, Mourad J-J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. (2005) 45:1243–8. doi: 10.1016/j.jacc.2005.01.015
52. Kim SH, Ahn JH, Hong HC, Choi HY, Kim YJ, Kim NH, et al. Changes in the clinical manifestations of primary aldosteronism. Korean J Int Med. (2014) 29:217. doi: 10.3904/kjim.2014.29.2.217
Keywords: primary hyperaldosteronism (PA), ischemic heart disease, atherosclerosis, secondary hypertension, coronary artery disease
Citation: Patil S, Rojulpote C and Amanullah A (2022) Primary Aldosteronism and Ischemic Heart Disease. Front. Cardiovasc. Med. 9:882330. doi: 10.3389/fcvm.2022.882330
Received: 23 February 2022; Accepted: 14 April 2022;
Published: 23 May 2022.
Edited by:
Carmine Pizzi, Università di Bologna, ItalyReviewed by:
Valentina Vicennati, University of Bologna, ItalyGuido Di Dalmazi, University of Bologna, Italy
Copyright © 2022 Patil, Rojulpote and Amanullah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shivaraj Patil, cGF0aWxzaGlAZWluc3RlaW4uZWR1
 Shivaraj Patil
Shivaraj Patil Chaitanya Rojulpote2
Chaitanya Rojulpote2 Aman Amanullah
Aman Amanullah