- 1Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Peking Union Medical College, Chinese Academy of Medical Sciences (CAMS), Beijing, China
Background: Uterine intravenous leiomyomatosis (IVL), a rare type of uterine leiomyoma, is defined by the intravascular proliferation of a histologically benign smooth muscle cell tumor. Pelvic arteriovenous fistula (AVF) is a rare vascular malformation that is most commonly congenital, post-traumatic, or iatrogenic. The link between leiomyomatosis and AVF has received little attention in the medical literature.
Results: We provide a case series of seven patients, four of whom were from our center, who had IVL complicated by a pelvic AVF. The symptoms of right heart failure were noted as swelling in the abdomen and two legs as well as a significant amount of ascites. Coil embolization of AVFs may be beneficial in minimizing bleeding during IVL surgery. A review of all accessible literature published on IVLs from 2000 to 2020 was conducted, and data were retrieved from 78 papers totaling 262 cases. Complications and recurrence were associated with pelvic mass excision and intravascular remnant tumor, respectively.
Conclusion: Intravenous leiomyomatosis combined with AVF aggravates congestion symptoms of surrounding organs. It is worth noting the uncommon combination of AVF and IVL, stressing the importance of a thorough assessment and surgical approach in IVL treatment.
Introduction
Uterine intravenous leiomyomatosis (IVL), a rare neoplasm defined by the intravascular proliferation of a histologically benign smooth muscle cell tumor, is an uncommon growth pattern of uterine leiomyoma (1). The clinical course varies depending on the severity of the condition. Vascular smooth muscle tumors can spread into the major veins and even the right atrium of the heart, obstructing blood flow and causing death (2–5).
Appropriate imaging examinations are required since IVLs could be misdiagnosed as intravascular thrombus, myxoma, or pancreatic tumors (6–8). Enhanced CT imaging can show the location, size, and full-scale extension pathway of IVL lesions, and it can be utilized in preoperative assessment (9). MRI could help with an accurate diagnosis, which is critical for deciding on a surgical plan and achieving a positive outcome (6). Echocardiography can be used to assess extension into the right atrium (10).
Pelvic arteriovenous fistula (AVF) is a rare vascular malformation that is most commonly congenital, post-traumatic, or iatrogenic (11, 12). Massive AV shunting causes high output cardiac failure. This malformation is hard to eradicate completely because of a high recurrence rate. Because of the significant hemorrhage, surgical resection is often challenging. Although transcatheter embolization has recently become the treatment of choice for pelvic AVF, full embolization to stop the shunt flow is equally challenging (11–13). The link between leiomyomatosis and AVF has only been mentioned rarely in the medical literature. Three definite cases have been recorded since the first description in 1993 (14–16). We provide four further examples of fistula associated with leiomyomatosis, review the literature on the topic, and speculate on possible pathophysiological causes for the cooccurrence.
Methods
For the literature review, PubMed, Embase, CNKI, and WanFang were utilized to conduct systematic searches of peer-reviewed literature published between 2000 and 2020. IBM SPSS was used to conduct the statistical analysis (IBM SPSS 26.0, SPSS Inc). For qualitative variables, Fisher's exact test and Pearson's chi-square test were utilized. All tests of statistical significance were two-sided, with p < 0.05.
Results
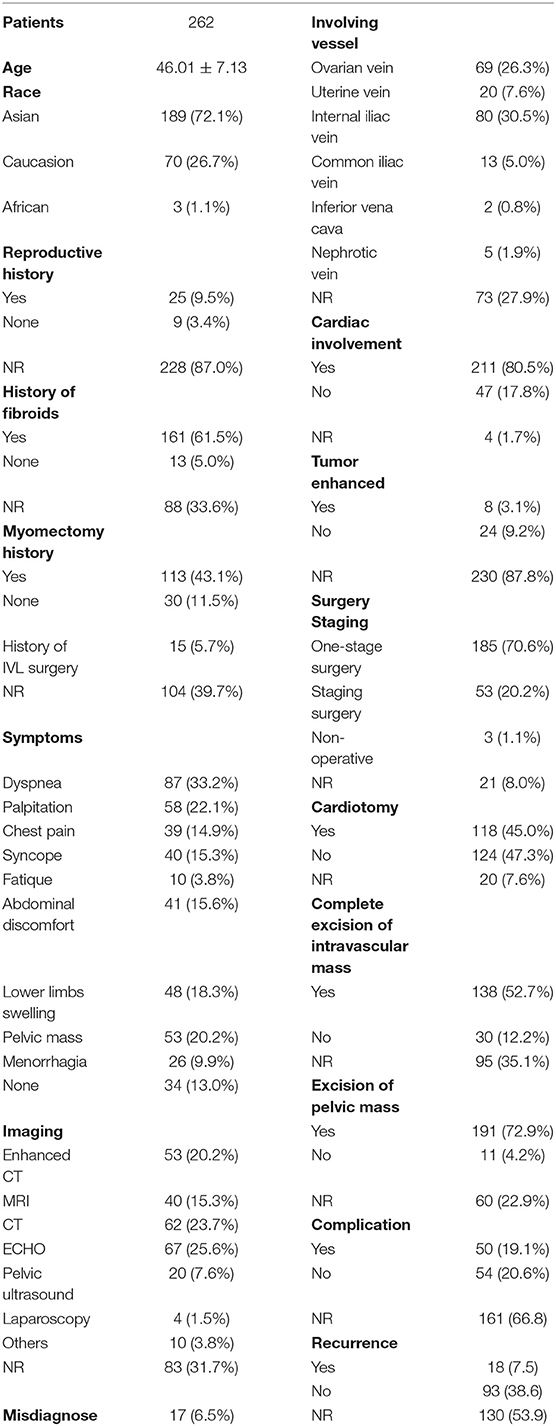
Case Series
A total of seven patients, four of whom were admitted to Peking Union Medical College Hospital from 2018 to 2020, were discovered to have IVL combined with AVF. Cases 1, 4, 5, 6, and 7 were found to have developed IVL and AVF at the same time, while in cases 2 and 3, AVF was revealed a few years after IVL surgeries.
The first patient was a 58-year-old woman who was admitted with a 2-month history of right lower limb swelling. IVL was found incidentally 7 months before. Her past medical history included leiomyoma, which was treated by total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH-BSO). Abdomen-pelvic enhanced CT showed a pelvic mass with abundant blood supply invading the right internal iliac vein, right common iliac vein, and soft tissue in the pelvis. Digital subtraction angiography (DSA) demonstrated multiple iliac AVF (Figure 1). After gynecological consultation, the patient underwent laparotomy and right common iliac venous tumor excision. A mass 2 cm in diameter and 10 cm in length was removed. However, the residual tumor within the right internal iliac vein was left untreated because of a tendency to bleed. On outpatient follow-up in 6 months, IVL recurrence was revealed by ultrasound.
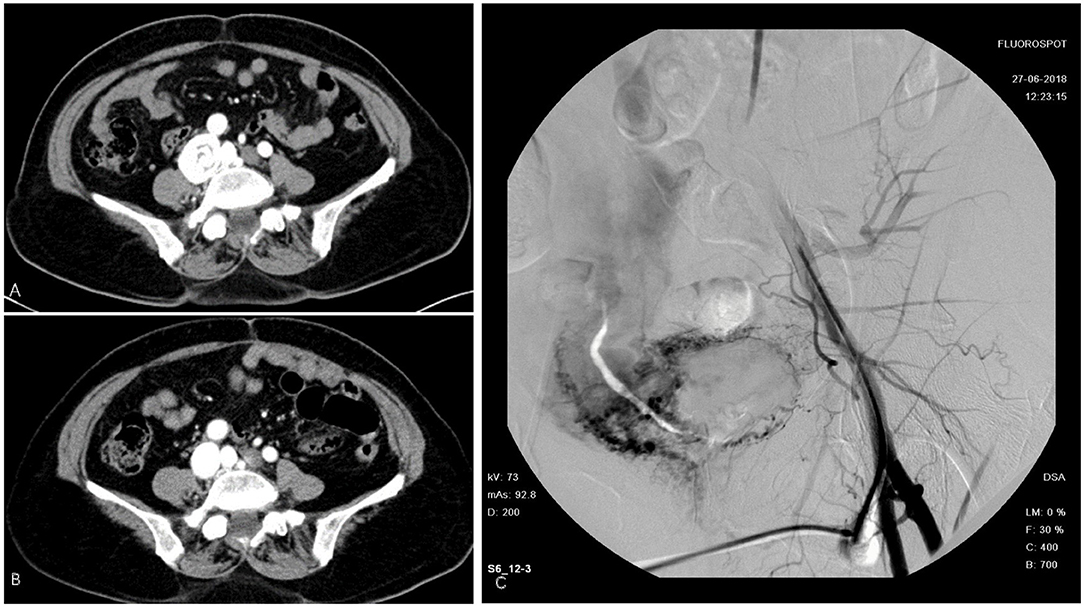
Figure 1. Intravenous leiomyomatosis (IVL) in inferior vena cava (IVC). (A) Cord like tumor in IVC. (B) Tumor removed surgically. (C) Digital subtraction angiography (DSA) demonstrated a contrast agent entering the right iliac vein and IVC through vascular malformation.
The second patient was a 49-year-old female who complained of swelling of the abdomen and two legs for 12 months. Iliac AVF was found 2 months before. Her past medical history included leiomyoma, which was treated by hysterectomy and left oophorectomy 17 years ago. She underwent vascular surgery for IVL 3 years before. Echocardiography (Figure 2) revealed enlargement of both atria and right ventricle. The arterial phase during angiography shows the enormous dilatation of both uterine and ovarian arteries to accommodate the high-volume shunting through the pelvic AVF. Coil embolization for pelvic AVF was performed successfully. On outpatient follow-up in 4 months, her condition was favorable and her heart function improved.
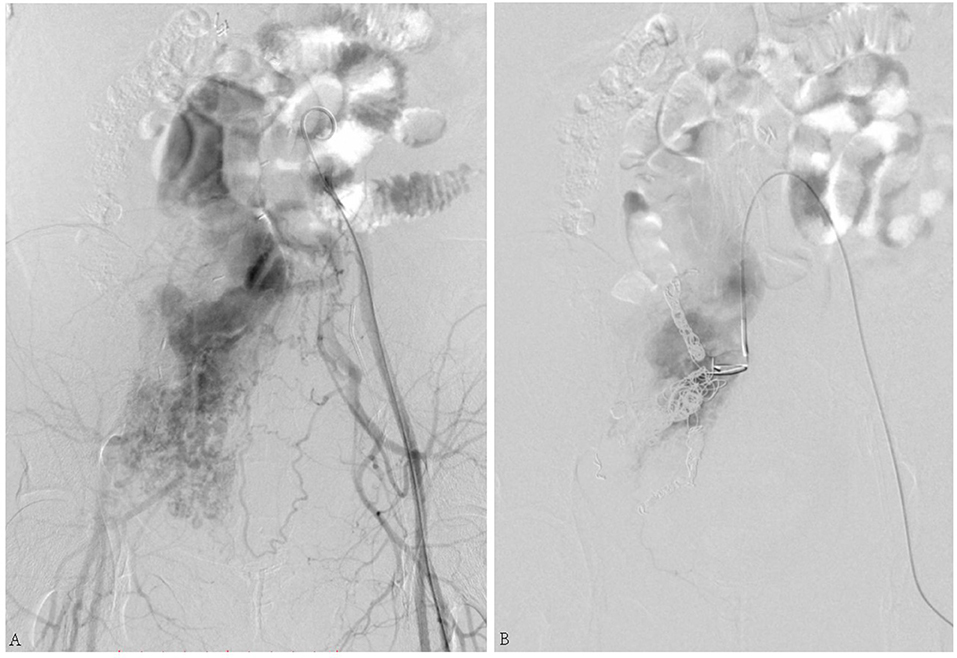
Figure 2. (A) Arteriovenous fistula (AVF) connecting the right internal iliac artery and internal iliac vein. (B) Shunt flow disappeared after coil embolization.
The third case was a 45-year-old female suffering from a large number of ascites. Her skin and mucosa were yellow. A vascular murmur could be heard in both femoral regions. The medical history was complicated by myomectomy and coil embolization for bilateral iliac aneurysms 8 years before. An ultrasound revealed enlarged liver. Pelvic AVF with high-volume shunting is obvious in angiography (Figure 3). She was treated with coil embolization. However, the iliac AVF still existed after 5 months.
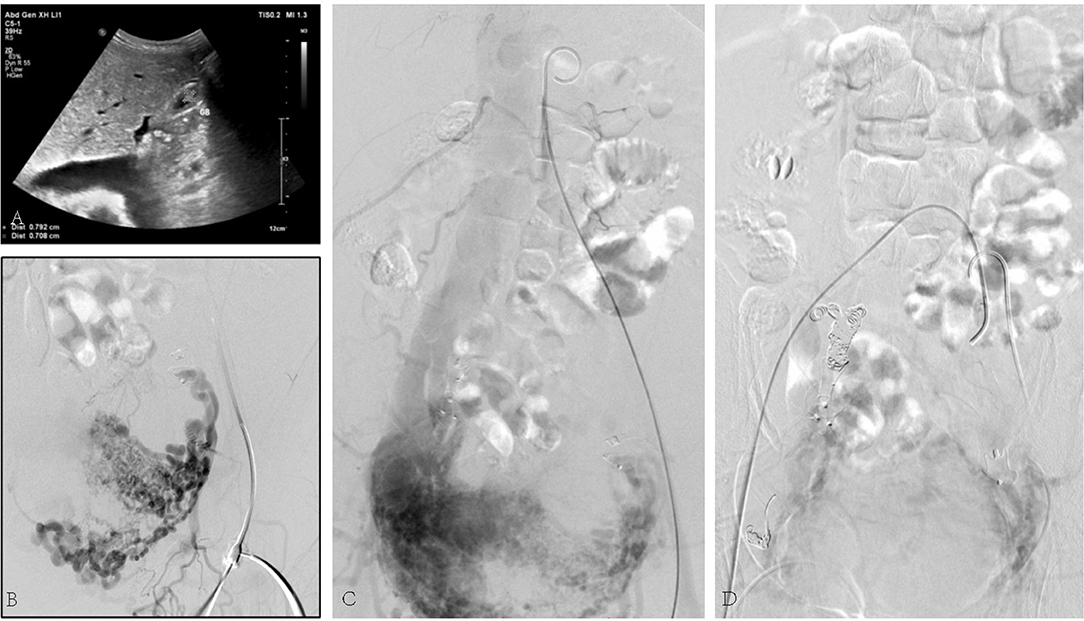
Figure 3. (A) Ultrasound revealed congested liver. (B) Arteriovenous malformation. (C) Shunt flow entering the vein. (D) Abnormal flow stopped by the placement of embolization coils.
The fourth case, 50 years old, had edema of both lower limbs, coughing, and blood-stained sputum for 3 months. She had a hysterectomy with right oophorectomy for over 7 years. Doppler ultrasound of iliac arteries and veins, echocardiography, PET-CT, and enhanced pelvic MRI were carried out (Figure 4), showing a giant tumor with enhancement in her inferior vena cava (IVC) and right atrium. The tumor was successfully extracted through IVC incision, yet the pelvic mass was not removed due to massive blood supply. Bilateral internal iliac veins were ligated to reduce the arteriovenous shunt flow. The patient recovered well.
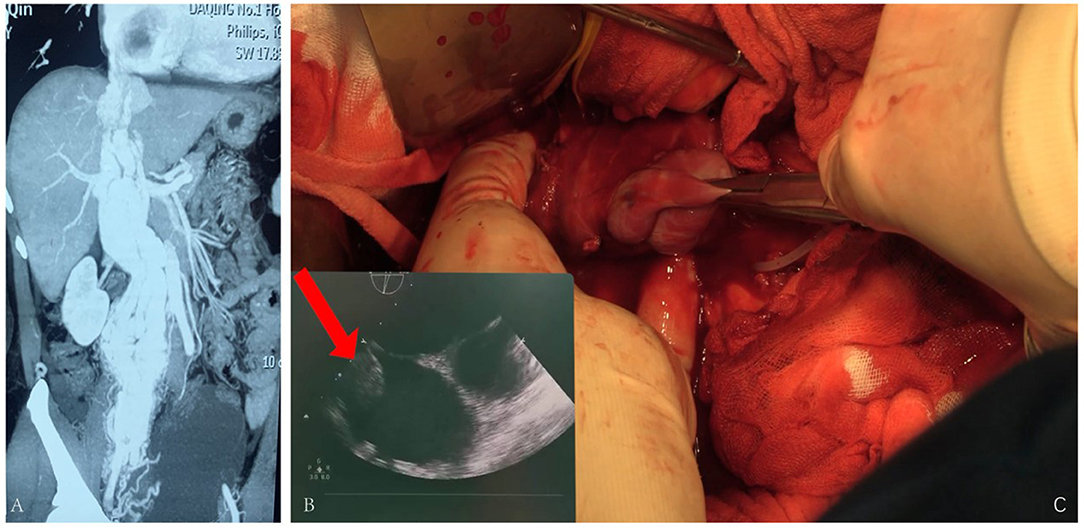
Figure 4. (A) Tumor enhanced in the arterial phase. (B) Ultrasound revealing tumor being extracted during surgery. (C) Tumor being pulled out from the IVC.
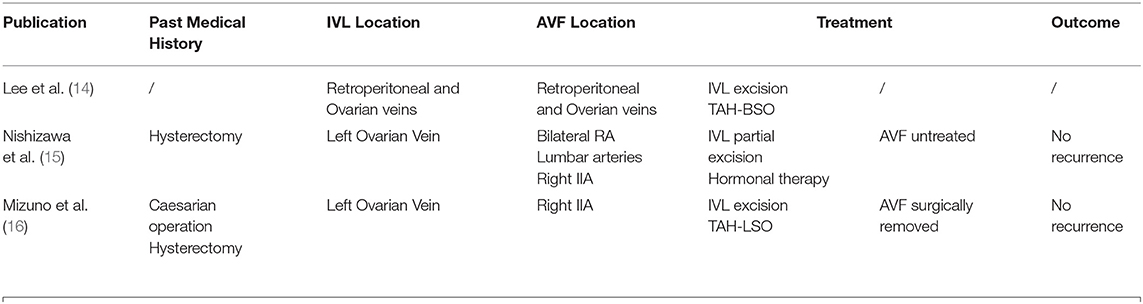
After a thorough literature search, three case reports with a combination of AVF and IVL were found (Table 1). Lee et al. reported the first case in which high-output cardiac failure was caused by the development of arteriovenous shunting within the intravenous component of the tumor. Treatment by TAH-BSO and tumor mass excision was successful. This report pointed out that AVF could be formed within the tumor of leiomyomatosis. This was also shown in a few case reports, where arteries within the tumor of IVL revealed by computed tomography angiography (CTA) are parallel with the vena cava (5, 14).
Nishizawa et al. reported the second case. The patient had received a hysterectomy, whose AVF was extensive and was involved with the intravenous tumor. Therefore, the surgery was in danger of massive hemorrhage. In the end, the tumor was only partially resected, and AVF was left untreated (15).
Mizuno et al. reported the third case of lVL associated with a pelvic AVF. The patient had received a caesarian operation and a hysterectomy. However, the patient had a separate pelvic AVF, which was not associated with IVL. Besides, the IVL was complicated by intracardiac extension. During the surgery, bleeding was hard to control due to the high venous return from the pelvic AVF at the time of removing the intravenous tumor (16). In these cases, AVF was found at the same time when IVL was diagnosed, yet few treatments were done for the AVF.
Literature Review
A total of 457 articles on IVLs were retrieved from the initial search from 2000 to 2020 (Figure 5). A total of 78 documents were chosen for complete analysis after abstract screening and full-text reviews, reporting a total of 262 cases.
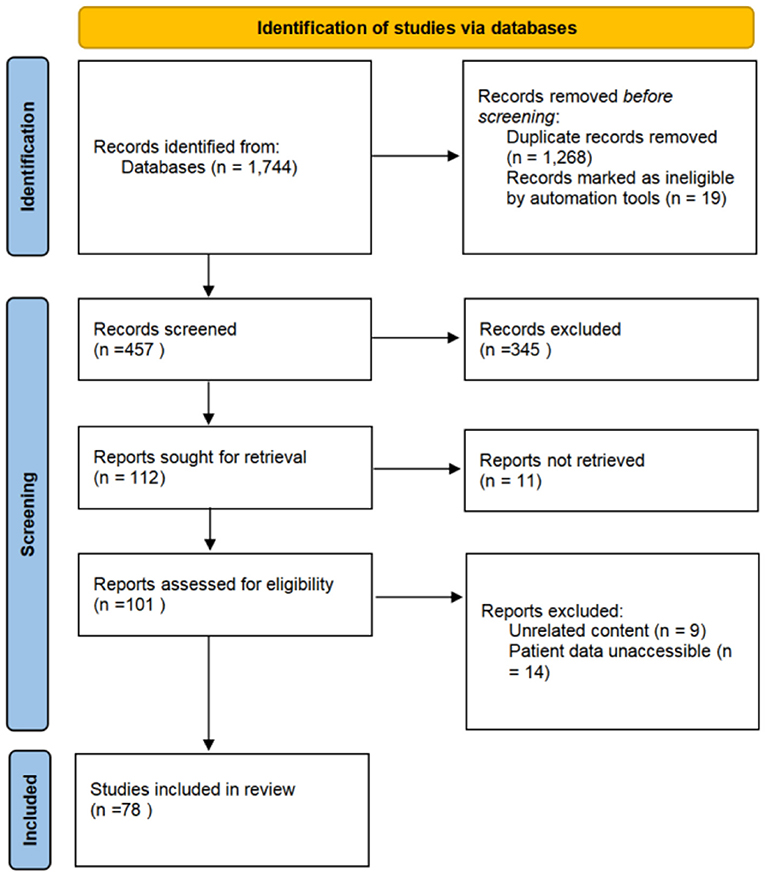
Figure 5. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram of study screening for the literature review. A total of 1,744 studies were identified, from which 78 were included in the review.
Asian patients accounted for the majority of all reported IVL cases in literature. Cardiac involvement is one of the most common characteristics in patients with IVL, which could be found in 80.5% of all cases. Surgery is the primary strategy to remove IVL, both one-stage and staging surgeries could be considered according to patient condition and tumor shape. It should be highlighted that the rate of misdiagnosis on IVLs was high, with 17 cases reported, accounting for 6.5% of total cases (Table 2).
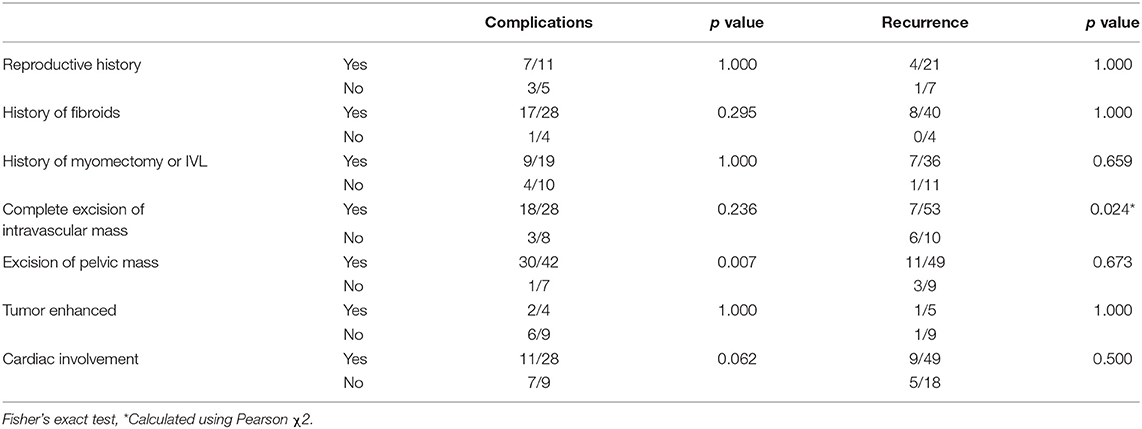
Intravenous leiomyomatosis recurrences are prevalent. However, complete excision of intravascular mass is associated with a reduced recurrence rate (p < 0.05). Nevertheless, the incidence of complications was increased with complete excision, though not significantly compared with non-complete excision (Table 3). In contrast, intrapelvic mass resection might increase the incidence of complications (p < 0.01) but did not significantly reduce IVL recurrence. It is crucial to highlight that these comparisons have relatively little statistical efficacy, and do not reveal which types of patients would have severe complications and die.
Discussion
The cause of AVFs formation in patients with IVL is unknown, and the association between AVF and IVL cannot be confirmed. Spontaneously developed pelvic vascular malformations are rare, and the most common causes include trauma, iatrogenic injury, aneurysm, and malignant tumor-related neovascularization (13, 17, 18). Vascular malformation induced by benign tumors, such as IVL, is seldom reported.
As demonstrated in cases 1 and 4, secondary vascular abnormalities developed around the tumor at the site of IVL invading the vein for unexplained reasons. The contrast agent in the vein appeared in advance under the angiography, indicating that the process of IVL invading the vein may promote local vascular malformation. Sometimes, this vascular malformation can even become the primary diagnosis in patients, masking IVL and leading to missed diagnosis and ignorant of the leiomyomatosis, resulting in tumor development (19).
There are several possible reasons why AVF would form in these patients with IVL. Six patients, including four cases from our center, who had leiomyomatosis complicated by pelvic AVF had received uterine surgery 7–15 years before. Thus, Iatrogenic AVF is to be suspected (15, 16). Furthermore, leiomyomatosis exhibits behaviors resembling malignant tumors; it invades blood vessels and induces angiogenesis. If the tumor invades and destroys both veins and arteries, fistulas could be formed during angiogenesis. AVF may originate from the nutritional artery of the tumor itself, and form AVFs as the tumor invades and grows into the vein. Such a process might be induced by iatrogenic injury during uterine surgery (20). The tumor inside the vein blocks the blood return, increasing local pressure, resulting in sphincter relaxation, and remodeling of the AVF. Some fistulas regressed after pressure relief (21, 22). The observation of pulmonary vascular malformation induced by benign metastasizing leiomyoma (BML) of the lungs increases the possibility of the hypotheses above.
Although pathological research provided evidence for AVF formation in the myometrium, such theories lack the support of long-term imaging surveillance (23, 24). Considering the risk of iatrogenic AVF, gynecologists are advised to avoid injury to arteries and veins during pelvic surgery. Among the reported cases and those reported by us, there were three patients [Case 2~3, and Mizuno et al. (16)] whose AVF and IVL occurred at different sites, which may have other reasons.
Magnetic resonance angiography (MRA) and DSA should be considered for the evaluation in IVL patients with heavy right cardiac load to exclude potential AVF. The advantage of MRA lies in its better ability to differentiate between soft tissue vascular malformations and soft tissue masses (25). Compared with CT, especially in patients with IVL, MRA can reduce the probability of misdiagnosing IVL as venous dissection or other hyper-vascular soft tissue tumors (19). The advantage of angiography is that it can clearly diagnose vascular malformations, which can be treated with coil embolization during the examination (11, 19). If a vascular malformation is found, it should be embolized from the arterial segment before the IVL surgery to reduce the blood flow in the vascular malformation and prevent bleeding during the IVL surgery, which contributes to a safe operation. If the vascular malformation is discovered during the operation, it is often difficult to remove due to tight adhesion and severe bleeding, as in Case 1. At this time, ligation of the artery supplying the AVF should be considered, as in Case 4.
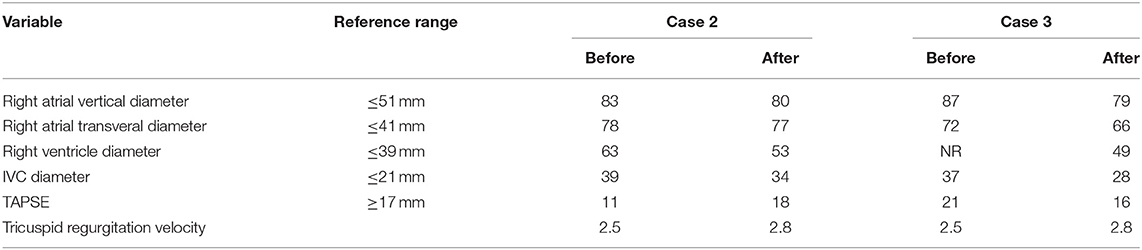
Vascular malformation aggravates the symptoms of IVL and increases the risk of operation. High venous flow caused by vascular malformation increases the right ventricular pressure (Cases 2 and 3). Timely embolization may significantly improve the problem of right ventricular overload, reverse right heart failure, promote the recovery of right ventricular systolic pressure, and improve the blood supply of the lungs (Table 4). The rich blood supply of tumors caused by vascular malformations may promote IVL tumor growth in addition to increasing the risk of surgical bleeding. As in Case 4, the IVL significantly blocks IVC and reaches the heart.
According to statistics, the recurrence rate of intravascular tumor residue was much greater than that of patients with clean intravascular excision (Table 3). This discrepancy could be attributable to the bias introduced by the short sample size. Nevertheless, the ligation of the bilateral internal iliac vein and ovarian vein is helpful to prevent the recurrence or shedding of residual tumor embolus (26). Patients who have a pelvic tumor resection are more likely to experience complications than patients who do not have a pelvic tumor resection (Table 3), which could be owing to the abundance of the pelvic vascular bed, or possibly the creation of tumor-related arteriovenous malformations.
There is currently no consensus on the diagnosis and treatment of IVL, and only a few retrospective cohort studies in big centers with more cases were conducted (10, 27). Ma et al. presented an IVL staging system, categorizing IVLs into stages 1 through 4 based on the extent of intravenous tumor spread (28). Liu et al. categorized IVLs that enter the heart chambers into types 1–5 and discussed surgical plans for each type of IVLs (29). Further classification methods and surgical techniques were discussed by Li et al., although the surgical categorization differs from that suggested by Liu et al. According to the relative diameter between tumor and IVC, Li et al. recommended four types of surgery. Such classification agrees with Ma et al.'s method on stage 3 IVLs, including IVLs reaching and not reaching the right atrium. However, effective imaging tools are still needed to determine whether the tumor in the heart can be retrieved from the IVC in advance according to their reports. Therefore, different institutes should share their surgical experiences and promote diagnostic guidelines to decrease misdiagnosis and missed diagnoses, improve complication management, and decrease significant complications and recurrence.
Limitations and Conclusions
To be clear, our study on IVL complicated by AVF, case series, and literature review has several limitations. First, the cases in the study were mainly from one medical center, some patients may have been overlooked in other places due to the rarity of this condition. The study was retrospective, providing less powerful evidence than prospective studies.
The symptoms of patients with IVL, caused by mass blocking venous reflux, mainly resemble that of right ventricular dysfunction and are easily misdiagnosed due to lack of experience or insufficient imaging examination. Incomplete intravascular IVL resection might contribute to recurrence; therefore, physicians should try to remove the tumor from the vessels completely. Some patients who had IVL complicated by pelvic AVF experienced a higher risk of bleeding in surgery, indicating the importance of dealing with the AVF in advance. Further studies on the above problems are necessary.
We point out that IVL combined with AVF aggravates congestion symptoms of peripheral organs, such as leg edema, ascites, hepatomegaly, jaundice, and intestinal bleeding, which can be seen in our cases and in the literature. Embolization of AVF in advance may reduce the risk of bleeding in IVL surgery. For patients with symptoms of right heart failure after pelvic surgery, imaging with a contrast agent (MRA and DSA) should be recommended to eliminate potential AVFs. In our cases, coil embolization was performed, the right ventricular function improved, and the short-term effect was satisfactory. Moreover, considering the possibility of iatrogenic AVF in patients with IVL, the tumor should be removed carefully, with attention to fine anatomical structure when ligating blood vessels, avoid ligation of arteries and veins together, and reduce secondary arteriovenous malformations.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The study protocol was approved by the Research and Ethics Board of the Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study and for the publication of any potentially identifiable data included in this article.
Author Contributions
HK and YCa analyzed the patient data and were major contributors in writing the manuscript. YZ and YCh made substantial contributions to the study design and gave final approval to the version to be published. All authors read and approved the final manuscript.
Funding
This research was funded by CAMS Innovation Fund for Medical Sciences (CIFMS, numbers 2021-I2M-C&T-A-006 and 2021-I2M-1-016), the Major Research Program of Natural Science Foundation of China (51890892), the National Natural Science Foundation of China (NSFC, numbers 82070492, 81770481, and 82170516), and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2021-JKCS-027).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.878386/full#supplementary-material
References
1. Grella L, Arnold TE, Kvilekval KH, Giron F. Intravenous leiomyomatosis. J Vasc Surg. (1994) 20:987–94. doi: 10.1016/0741-5214(94)90237-2
2. Du J, Zhao X, Guo D, Li H, Sun B. Intravenous leiomyomatosis of the uterus: a clinicopathologic study of 18 cases, with emphasis on early diagnosis and appropriate treatment strategies. Hum Pathol. (2011) 42:1240–6. doi: 10.1016/j.humpath.2010.10.015
3. Price JD, Anagnostopoulos C, Benvenisty A, Kothuru RK, Balaram SK. Intracardiac Extension of Intravenous Leiomyomatosis. Ann Thorac Surg. (2017) 103:e145–7. doi: 10.1016/j.athoracsur.2016.07.037
4. Caldentey G, Flores E, San Antonio R, Caixal G, Sánchez P. A rare cause of intracardiac mass. Int J Cardiol. (2016) 223:91–2. doi: 10.1016/j.ijcard.2016.08.140
5. Su Q, Zhang X, Zhang H, Liu Y, Dong Z, Li G, et al. Intravenous Leiomyomatosis of the Uterus: A Retrospective Single-Center Study in 14 Cases. Biomed Res Int. (2020) 2020:9758302. doi: 10.1155/2020/9758302
6. Deac MO, Sheppard MN, Moat N, Burke SJ, Christmas T, Mohiaddin RH. Images in cardiovascular medicine. From uterus to pulmonary embolus: an uncommon association. Circulation. (2009) 120:e16–19. doi: 10.1161/CIRCULATIONAHA.108.826107
7. Yano M, Katoh T, Nakajima Y, Iwanaga S, Kin R, Kozawa E, et al. Uterine intravenous leiomyomatosis with an isolated large metastasis to the right atrium: a case report. Diagn Pathol. (2020) 15:4. doi: 10.1186/s13000-019-0913-2
8. Gao B, Zhou D, Qian X, Zhang W, Ying L, Wang W. Primary leiomyoma of the inferior vena cava mimicking a cystic neoplasm of the pancreas: a case report. Cardiovasc Pathol. (2020) 46:107097. doi: 10.1016/j.carpath.2018.11.003
9. Gui T, Qian Q, Cao D, Yang J, Peng P, Shen K. Computerized tomography angiography in preoperative assessment of intravenous leiomyomatosis extending to inferior vena cava and heart. BMC Cancer. (2016) 16:73. doi: 10.1186/s12885-016-2112-9
10. Yang C, Fang H, Yang Y, Cai F, Zheng H, Jin B, et al. Diagnosis and surgical management of inferior vena cava leiomyomatosis. J Vasc Surg Venous Lymphat Disord. (2018) 6:636–45. doi: 10.1016/j.jvsv.2018.03.013
11. Fujii M, Sakurai M, Mogi K, Nomura A, Sakata T, Takahara Y. Multiple spontaneous iliac and femoral arteriovenous fistulas. Ann Vasc Surg. (2018) 46:367.e311–367.e313. doi: 10.1016/j.avsg.2017.06.147
12. Wenzl FA, Miljkovic SS, Dabestani PJ, Kessler JJ 2nd, Kotaru TR, Kalamchi LD, et al. A systematic review and individual patient data meta-analysis of heart failure as a rare complication of traumatic arteriovenous fistulas. J Vasc Surg. (2021) 73:1087–94. doi: 10.1016/j.jvs.2020.08.138
13. Yan GW, Li HW, Yang GQ, Bhetuwal A, Liu JP, Li Y, et al. Iatrogenic arteriovenous fistula of the iliac artery after lumbar discectomy surgery: a systematic review of the last 18 years. Quant Imaging Med Surg. (2019) 9:1163–75. doi: 10.21037/qims.2019.05.12
14. Lee VS, Thompson NW, Cho KJ, Goldblum JR. High-output cardiac failure: an unusual manifestation of intravenous leiomyomatosis. Surgery. (1993) 113:466–70.
15. Nishizawa J, Matsumoto M, Sugita T, Matsuyama K, Tokuda Y, Yoshida K, et al. Intravenous leiomyomatosis extending into the right ventricle associated with pulmonary metastasis and extensive arteriovenous fistula. J Am Coll Surg. (2004) 198:842–3. doi: 10.1016/j.jamcollsurg.2003.06.009
16. Mizuno T, Mihara A, Arai H. Intracardiac and intravascular leiomyomatosis associated with a pelvic arterio-venous fistula. Ann Trans Med. (2014) 2:48. doi: 10.3978/j.issn.2305-5839.2014.04.14
17. Brewster DC, Cambria RP, Moncure AC, Darling RC, LaMuraglia GM, Geller SC, et al. Aortocaval and iliac arteriovenous fistulas: recognition and treatment. J Vasc Surg. (1991) 13:253–64; discussion 264-255. doi: 10.1016/0741-5214(91)90218-J
18. Ngan H, Peh WC. Arteriovenous shunting in hepatocellular carcinoma: its prevalence and clinical significance. Clin Radiol. (1997) 52:36–40. doi: 10.1016/S0009-9260(97)80303-0
19. Lee HN, Hyun D, Do YS, Park KB, Bae SH. Soft-Tissue Tumors Mimicking Arteriovenous Malformations. J Vasc Interv Radiol. (2019) 30:1660–4.e1667. doi: 10.1016/j.jvir.2019.07.021
20. Fukuyama A, Yokoyama Y, Futagami M, Shigeto T, Wada R, Mizunuma H, et al. case of uterine leiomyoma with intravenous leiomyomatosis–histological investigation of the pathological condition. Pathol Oncol Res. (2011) 17:171–4. doi: 10.1007/s12253-010-9265-7
21. Tekkök IH, Açikgöz B, Ozgen T, Onol B. Spinal dural arteriovenous fistula adjacent to a spinal neurofibroma–a misleading coexistence. Case report. Paraplegia. (1993) 31:678–83. doi: 10.1038/sc.1993.109
22. Ahn JY, Lee BH, Cho YJ, Joo JY, Lee KS. Dural arteriovenous fistula associated with meningioma: spontaneous disappearance after tumor removal. Neurol Med Chir. (2003) 43:308–11. doi: 10.2176/nmc.43.308
23. Merchant S, Malpica A, Deavers MT, Czapar C, Gershenson D, Silva EG. Vessels within vessels in the myometrium. Am J Surg Pathol. (2002) 26:232–6. doi: 10.1097/00000478-200202000-00010
24. Tang L, Lu B. Intravenous leiomyomatosis of the uterus: a clinicopathologic analysis of 13 cases with an emphasis on histogenesis. Pathol Res Pract. (2018) 214:871–5. doi: 10.1016/j.prp.2018.04.011
25. Flors L, Leiva-Salinas C, Maged IM, Norton PT, Matsumoto AH, Angle JF, et al. MR imaging of soft-tissue vascular malformations: diagnosis, classification, and therapy follow-up. Radiographics. (2011) 31:1321–40; discussion 1340–21. doi: 10.1148/rg.315105213
26. Li H, Xu J, Lin Q, Zhang Y, Zhao Y, Tong H, et al. Surgical treatment strategies for extra-pelvic intravenous leiomyomatosis. Orphanet J Rare Dis. (2020) 15:153. doi: 10.1186/s13023-020-01394-9
27. Zhao Y, Huang ZH, Fu W, Liu TS, Dong R. Clinical analysis of intravenous-cardiac leiomyomatosis. Zhonghua yi xue za zhi. (2020) 100:1741–4. doi: 10.3760/cma.j.cn112137-20191212-02707
28. Ma G, Miao Q, Liu X, Zhang C, Liu J, Zheng Y, et al. Different surgical strategies of patients with intravenous leiomyomatosis. Medicine. (2016) 95:e4902. doi: 10.1097/MD.0000000000004902
Keywords: arteriovenous fistula (AVF), vascular surgery, coil embolization, pelvic mass, intravascular leiomyomatosis (IVL)
Citation: Kan H, Cao Y, Chen Y and Zheng Y (2022) Intravenous Leiomyomatosis Complicated by Arteriovenous Fistula: Case Series and Literature Review. Front. Cardiovasc. Med. 9:878386. doi: 10.3389/fcvm.2022.878386
Received: 18 February 2022; Accepted: 10 May 2022;
Published: 13 June 2022.
Edited by:
Giovanni Battista Levi Sandri, Ospedale di Cassino, ItalyReviewed by:
Mark Christopher Arokiaraj, Pondicherry Institute of Medical Sciences, IndiaLuis Rafael Moscote-Salazar, Latinamerican Council of Neurocritical Care (CLaNi), Colombia
Copyright © 2022 Kan, Cao, Chen and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuehong Zheng, eXVlaG9uZ3poZW5nQHlhaG9vLmNvbQ==; Yuexin Chen, Y3l1ZXhpbjIwMDdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Haoxuan Kan1†
Haoxuan Kan1† Yang Cao
Yang Cao Yuehong Zheng
Yuehong Zheng