
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 11 July 2022
Sec. Lipids in Cardiovascular Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.872523
This article is part of the Research Topic Cardiovascular Diseases in Autoimmune Diseases: Dyslipidemia and vascular inflammation View all 8 articles
Background: Pulmonary thromboembolism is a common disease frequently encountered in the emergency room and has a high mortality rate. Antiphospholipid syndrome (APS) is a high-risk factor for recurrent pulmonary embolism (PE). It is critical to effectively administer anticoagulants to avoid the recurrence of thrombotic events. This study aims to identify the clinical characteristics of APS patients with PE (APS-PE) and to develop a risk score for determining the presence of APS in PE patients in the emergency situations.
Methods: We retrospectively enrolled 76 PE patients in this study, with 46 patients in the APS-PE group and 30 patients in the non-APS-PE group. We compared differences in demographics, laboratory parameters, and early mortality risk between the two groups. Risk factors for APS-PE were screened using logistic regression analysis. We also developed an early risk score using multivariate analysis weighted points proportional to the β- regression coefficient values and calculated the sensitivity and specificity for APS in PE patients.
Results: In the APS-PE group, we observed a higher proportion of males (43.6 vs. 20%), a higher proportion of low-risk patients (58.7 vs. 10%), lower levels of white blood cells and platelets (PLT), longer activated partial thromboplastin time (APTT), and a slight increase in D-dimer levels. Patients who were triple positive for antiphospholipid antibodies (aPLs) were younger. The APTT gradually increased as the number of positive aPLs increased. The risk factors for APS included male (OR = 5.565, 95% CI 1.176–26.341), decreased PLT (OR = 0.029, 95% CI 0.003–0.330), slightly increased D-dimer (OR = 0.089, 95% CI 0.019–0.426), and prolonged APTT (OR = 4.870, 95% CI 1.189–19.951). The risk score was named MPDA and included male, PLT, D-dimer and APTT, which can predict APS in PE patients with the AUC at 0.888 (95% CI 0.811–0.965).
Conclusion: The risk factors for APS in PE patients are male, low PLT, prolonged APTT and slightly increased D-dimer. The MPDA is a quantitative scoring system which is highly suggestive of APS in PE patients.
Pulmonary embolism (PE) is a group of diseases or clinical syndromes in which various emboli block the pulmonary artery or its branches. In most clinical contexts, emboli refer to blood clots (1). The global incidence, disability rates, and fatality rates of PE are high (1). PE is often accompanied by deep vein thrombosis (DVT) of the lower extremities, and both are referred to as venous thromboembolism (VTE). In addition to anticoagulation and thrombolysis, the causes of PE should be quickly identified and treated to avoid recurrence. Antiphospholipid syndrome (APS) is one of the strongest risk factors for DVT, with an odds ratio (OR ≥ 10) (2).
APS is an autoimmune disease characterized by thrombosis, pregnancy complications, and persistently positive antiphospholipid antibodies (aPLs). APS affects the vessels of tissues and organs, including the arteries, veins, and capillaries. Of the thrombosis, DVT and cerebral artery infarction are the most common complications (3). The incidence of DVT in APS patients could reach 38.9%, while the incidence of PE in APS patients is 14.1% (3). APS can also cause pulmonary hypertension, acute respiratory distress syndrome and intra-alveolar hemorrhage. The incidence of thrombosis in Chinese APS patients was 75.4%, of which 40.1% were DVT, 23.8% were stroke, and 6.7% were PE (4). Any embolism event in these APS patients can be life-threatening. VTE patients with positive aPL have a significantly increased risk of thrombosis recurrence after discontinuation of anticoagulation therapy (5, 6). Different from other PE patients, APS-PE patients require long-term anticoagulation therapy and may need additional immunotherapy (7, 8). Additionally, identifying APS in its early stages, particularly the early recognition of catastrophic antiphospholipid syndrome (CAPS), allows for early treatment, and improve survival.
This study aims to investigate the risk factors of APS from PE and develop a risk score for early recognition of APS.
A total of 192 patients who were diagnosed with their first episode of PE between June 2018 and September 2021 in Peking University People's Hospital were retrospectively enrolled in this study. All demographic characteristics as well as clinical, radiological and laboratory data were collected from their medical records. PE was confirmed in all patients by computed tomographic pulmonary angiography (CTPA). DVT was confirmed by vascular ultrasound of the lower extremities. The laboratory data, including complete blood count and blood coagulation tests, were obtained from samples drawn when the patients were admitted to the Department of Emergency, while aPLs and blood chemistry tests were drawn in the morning of the next day, after the patients had fasted.
APS was diagnosed according to the Sydney criteria (9). We tested for aPLs in PE patients repeatedly at least 12 weeks later. We excluded 96 patients who lacked aPLs results (Figure 1) and 20 PE patients who had other risk factors (e.g., trauma, surgery, immobilization, pregnancy, and oral contraceptive use) within the preceding 12 weeks. In total, 76 patients were included in this study. All patients experienced an initial episode of thrombosis. Forty-six patients were diagnosed with APS, and 30 patients were non-APS. In APS patients, 27 (58.7%) had primary APS (pAPS), 15 (32.6%) had systemic lupus erythematosus, and 1 (2.2%) patient presented with recurrent miscarriages.
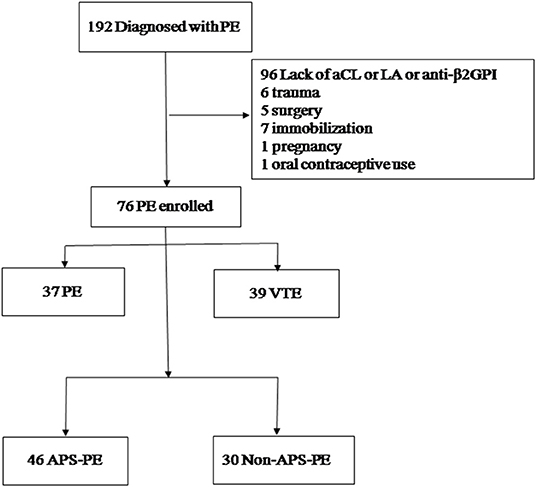
Figure 1. Patient selection flowchart. PE, pulmonary embolism; VTE, venous thromboembolism; DVT, deep vein thrombosis; aCL, anti-cardiolipin antibodies; LA, lupus anti-coagulant; aβ2GPI, anti-β2-glycoprotein I, APS, antiphospholipid syndrome.
The study was approved by the Institutional Review Board (IRB) of Peking University People's Hospital (No. 2022PHB007-001). Given the retrospective nature of this study, the requirement for informed consent was waived.
ELISA was used to identify IgA/IgG/IgM anti-β2-glycoprotein I (aβ2GPI) and IgA/IgG/IgM anti-cardiolipin antibodies (aCL), as previously described (10). Values for aCL > 12 IU/ml and aβ2GPI > 27 RU/ml were identified positive based on local cut-off. The simplified Dilute Russell's Viper Venom Test (dRVVT) was performed using Lupus anticoagulant (LA) 1 Screening reagent and LA2 Confirmatory reagent (STA, USA) according to the manufacturer's instructions. LA activity was considered positive when dRVVT ratios (LA1 screen/LA2 confirmation) were above 1.2 (11).
We calculated the adjusted global APS score (aGAPSS) by scoring the risk factors of each patient, as previously reported (12, 13). Five points were awarded for IgG/IgM aCL, 4 points were awarded for IgG/IgM aβ2GPI antibodies, 4 points for LA, 3 points for hyperlipidemia, and 1 point for arterial hypertension.
The pulmonary embolism severity index (PESI) correlates with the 30-day mortality of acute PE patients (14). Based on the PESI scores and other risk parameters (which accounted for hypertension and shock, right ventricular dysfunction observed during an imaging exam and cardiac laboratory biomarkers), the severity and risk of the early death of acute PE patients were stratified into high, intermediate-high, intermediate-low and low risk, according to the 2019 European Society of Cardiology (ESC) guidelines (15). Considering the complexity of the PESI, we used a simplified PESI (sPESI), which included the following variables: age, cancer, chronic cardiopulmonary disease, heart rate, systolic blood pressure and oxyhemoglobin saturation levels.
The 76 PE patients were divided into two groups based on whether they had APS: the APS-PE group (patients with a PE associated with APS) and the non-APS-PE group.
Seventy-six patients were divided into two groups: the PE group and the VTE group.
Patients were grouped as negative, single positive, double positive, or triple positive according to aPLs. Negative indicated the absence of LA, aCL or aβ2GPI. Single, double and triple positive indicated positivity of any one, two or all three aPLs.
Categorical variables were expressed as ratios and percentages of the total, while continuous variables were expressed as either the median and interquartile range (IQR) or the mean ± SD. The demographics, clinical and laboratory data were compared between PE patients with or without APS and patients with PE or VTE. A t-test or a Mann–Whitney U test was used to test the difference between parametric and non-parametric data between groups. A chi-square test or Fisher's exact test was used to assess categorical variables. One-way ANOVA or a Kruskal-Wallis test was used to compare the different positive antibody groups.
Age, gender and variables with p < 0.1 on univariate analysis were included in binary multivariate logistic regression analysis to identify the risk factors of APS. Risk factors were assigned based on points weighted by multivariate analysis. The β- regression coefficient values were divided by 1.583 (the lowest β- value) and rounded to the nearest integer. A linear transformation of the associated β-regression coefficient was used to score the risk factors (14, 16, 17); we then calculated the risk score for each patient. We also analyzed the areas under the receiver operating characteristic curve (AUC) of various risk factors to identify the sensitivity and specificity of these risk factors and risk scores. Statistical significance was identified using a two-sided p < 0.05; IBM SPSS Statistics version 22.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
Of the 76 subjects, 34.2% were males aged 56 ± 18 years old; 48.7% had PE without DVT, and 51.3% had VTE. Among all the subjects, the proportions of low risk, intermediate-low risk, intermediate-high risk and high risk were 39.5, 39.5, 15.8, and 5.2%, respectively. The positive rates of aCL, LA and aβ2GPI were 31.6, 53.9, and 27.6%, respectively. The positive rates of single, double or triple aPLs were 23.7, 21.1, and 15.8%, respectively. Of all the patients, 15.8% (n = 12) had thrombocytopenia (100 × 109/L), 14.5% of patients belonged to the APS-PE group, and 1.3% belonged to the non-APS-PE group (Tables 1, 2).
In the APS-PE group, there was a higher proportion of males (43.5 vs. 20%) and low-risk subjects (58.7 vs. 10%). The levels of white blood cells (WBC) and platelets (PLT) were lower in the APS-PE group (p < 0.05). Compared to the non-APS-PE group activated partial thromboplastin time (APTT) was significantly longer and the D-dimer level was significantly decreased in the APS-PE group (p < 0.05) (Table 2).
The VTE group had a higher proportion of males (51.3 vs. 16.2%) and APS patients (74.4 vs. 45.9%) (p < 0.05). There were no statistically significant differences between the two groups in terms of age, risk stratification, WBC, hemoglobin, PLT, APTT, D-dimer, the positivity of aPLs, or aGAPSS scores (Table 3).
Patients in the triple-positive group were younger than those in the other three groups. There was also a higher proportion of low-risk patients in the triple-positive group (p < 0.05). As the positive numbers of aPLs increased, the average APTT levels gradually prolonged, and the APTT of the triple positive groups was significantly longer than the other 3 groups. An opposite trend was observed in the D-dimer level (p < 0.05) (Table 4).
The risk factors for APS in PE were male (OR = 5.565, 95% CI 1.176–26.341, p = 0.030), PLT level (OR = 0.029, 95% CI 0.003–0.330, p = 0.004), D-dimer level (OR = 0.089, 95% CI 0.019–0.426, p = 0.002), and APTT level (OR = 4.870, 95% CI 1.189–19.951, p = 0.028). The largest Youden index was used to determine the cut-off value, and the cutoff values for PLT, D-dimer, and APTT were 110 × 109/L, 0.9 mg/L, and 32.2 s, respectively (Tables 5, 6).
To facilitate the clinical application of the predictive model, the β- regression coefficient value (Model I) was rounded to the nearest integer (Model II, named MPDA) and used as the value of each risk factor; this scoring model was displayed in Table 6. Confirmed APS was considered the gold-standard, and the AUC of the two scoring models (Model I and MDPA) were 0.755 (95% CI 0.640–0.870) and 0.888 (95% CI 0.811–0.965). The MPDA had a higher AUC, demonstrating sufficient predictive value. The maximum Youden index (when the score was 2 points) was set as the cutoff value of MPDA, and the sensitivity was 84.6% and the specificity was 75.9% (Figure 2).
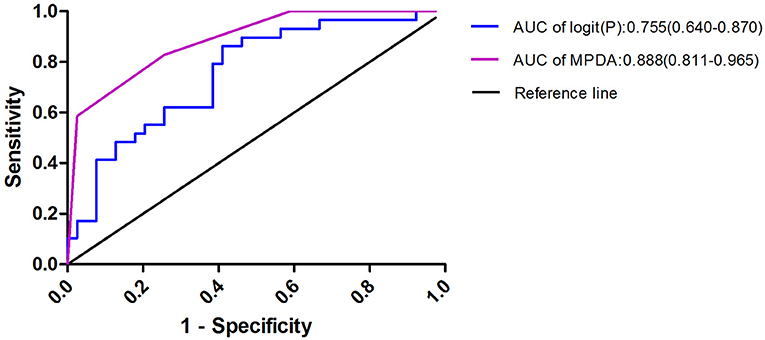
Figure 2. Risk factors were used to produce the receiver operating characteristic curves logit(P) = 1.717 × Male + 3,531 × PLT + 2.421 × D-dimer + 1.583 × APTT; MPDA = 1 × Male + 2 × PLT + 2 × D-dimer + 1 × APTT. When MPDA was more than 2, the predicted sensitivity of APS -PE was 84.6% and the specificity was 75.9%.
Our results demonstrated that there were more males, higher proportions of low-risk patients, lower WBC and PLT levels, prolonged APTT, and lower D-dimer levels in the APS-PE group. Therefore, the MPDA scoring system, based on gender, PLT, D-dimer, and APTT, could help healthcare professionals identify APS patients in emergency situations.
The average age of patients with primary APS who experienced their first thrombotic event was younger than 50 years old (18). The average age of the APS-PE group was 39.7 years, while the average age of the non-APS-PE group was 60.4 years (19), which could be related to the younger age of APS onset (4, 20). Therefore, aPLs should be tested in patients younger than 40 years old who first experienced symptomatic PE or in young patients (<50 years old) with unexplained thrombotic events at an abnormal site or in patients who suffered from pregnancy complications (19, 21, 22).
Compared to patients without thrombosis, there was a higher proportion of males in APS patients with thrombosis and arterial thrombosis complications (23). Our study was consistent with previous studies, which could be related to the sex hormones in young females as a protective factor for vascular endothelium (24). Therefore, APS screening should be considered when PE occurs in young males, especially when accompanied by DVT.
In this study, there were more low-risk patients observed in the APS-PE group, especially in triple positive aPLs patients. This was consistent with previous studies, which found 62.5% of low-risk patients in the APS-PE group and only 29.5% in the non-APS-PE group (19). Therefore, the aPLs test should not be ignored for patients with low-risk pulmonary embolism.
Thrombocytopenia is a common hematological manifestation of APS and occurrs in about 30% of APS patients (3). Among aPL carriers, patients with thrombocytopenia have a high risk of developing thrombosis (25–27). Platelet activation plays a key role in thrombocytopenia and thrombosis. Possible mechanisms are platelet activation and aggregation by aPL, and destruction of platelets by antibodies directed against platelet membrane glycoproteins (28). Platelets play a key role in the pro-thrombotic interaction between aPLs and endothelial cells (29). It has been reported that thrombocytopenia is a predictor of poor APS outcomes and is associated with an increased risk of CAPS and poor long-term survival (30, 31). Therefore, thrombocytopenia in APS patients might indicate more severe APS (including an increased risk of thrombosis) (26). Additionally, thrombocytopenia in APS is not a contraindication for anticoagulation.
Consistent with previous studies (19), prolonged APTT was an indicator of APS in young PE patients. The LA antibody may inhibit the formation of prothrombinase complexes, leading to prolonged APTT (32). Therefore, along with a history of thrombosis or pregnancy morbidity, mild thrombocytopenia and unexplained prolongation of APTT could indicate APS (33).
D-dimer is widely used as a marker for the hypercoagulable state in clinical practice. High D-dimer levels could assist in the diagnosis of venous thrombosis (34, 35). The diagnostic sensitivity of D-dimer to acute PE is high (80–100%), and its negative predictive value could reach 100% (36). The persistent elevation of D-dimer provides further information for predicting the recurrence risk and informing treatment decisions (37). The D-dimer level of the APS-PE group in this study is similar to another study conducted in China (38). Larger sample sizes are required for further research.
It has been proposed that although aPLs persistently present, thrombotic events occur only occasionally. This indicates that environmental factors (infection), inflammatory factors (concomitant autoimmune diseases), or other non-immune procoagulant factors (such as contraceptives containing estrogen, surgery, and immobility) are involved in the thrombosis process, which is known as the “two-hit model” (39). Two thrombosis prediction models, including aPL-S scores and the global APS score (GAPSS) were developed. The aPL-S score includes 3 lupus anticoagulant tests and 6 solid-phase antiphospholipid tests (40), among which a score ≥30 is defined as an independent risk factor for thrombosis (p = 0.006) (40). Among SLE patients, GAPSS ≥10 could be the best risk prediction for assessing the outcome of thrombosis and pregnancy loss (16). Among subjects with primary APS, those with thrombosis had higher GAPSS values than those without. The GAPSS value of patients with recurrence of thrombosis is higher than those without recurrence (20). GAPSS values ≥11 are the most reliable predictor in terms of sensitivity and specificity (20). Both models can be used to assess the risk of thrombosis in APS, but both scores were based on aPLs. Therefore, they cannot be used in the emergency situations. In this study, we proposed an APS prediction model based on conventional clinical indicators to identify APS as a risk factor for primary PE in the emergency room (ER). This score model is sensitive and specific, and the parameters involved are immediately available, which helps ER doctors who must make quick decisions.
The limitations of our study include its design as a single-center retrospective study and the relatively small size of the cohort. Many patients did not test for aPLs, and some patients only checked for one-time aPL. Therefore, several possible APS patients were not included in the study.
Further multicenter prospective studies are needed, and our proposed MPDA score (male, reduced PLT, increased D-dimer, and prolonged APTT) can provide ER physicians with a useful screening tool to manage APS. It can help to quickly identify potential APS-PE patients, assess the need for long-term anticoagulation therapy, and decrease the rate of recurrent thrombosis.
In this study, we explored the independent predictors of APS-PE patients and established an MPDA score for predicting APS in PE patients. In this model, the most sensitive predictor of APS is male, reduced PLT, slightly prolonged D-dimer, and prolonged APTT. The MPDA score, which is based on the above four clinical indicators, provides a basis for quickly identifying these individuals in the ER. PE patients with these characteristics should be tested for aPLs and screened for APS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board (IRB) of Peking University People's Hospital (No. 2022PHB007-001). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
MS, YJ, CL, and WG designed the study, analyzed the data, and drafted the manuscript. CL and JZ helped with statistical analysis, intellectual discussions, and editing. TW, YL, and CL provided critical suggestions for improving the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the Beijing Natural Science Foundation (7192211) and the National Key R&D Program of China (2018YFF0301103).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We sincerely thank all the participants for their contributions, as well as the healthcare workers from the emergency department and rheumatology and immunology department of Peking University of People's Hospital for their enthusiastic help.
1. Essien EO, Rali P, Mathai SC. Pulmonary embolism. Med Clin North Am. (2019) 103:549–64. doi: 10.1016/j.mcna.2018.12.013
2. Mazzolai L, Ageno W, Alatri A, Bauersachs R, Becattini C, Brodmann M, et al. Second consensus document on diagnosis and management of acute deep vein thrombosis: updated document elaborated by the ESC Working Group on aorta and peripheral vascular diseases and the ESC Working Group on pulmonary circulation and right ventricular function. Eur J Prev Cardiol. (2021) 29:zwab088. doi: 10.1093/eurjpc/zwab088
3. Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. (2002) 46:1019–27. doi: 10.1002/art.10187
4. Shi H, Teng J-L, Sun Y, Wu X-Y, Hu Q-Y, Liu H-L, et al. Clinical characteristics and laboratory findings of 252 Chinese patients with antiphospholipid syndrome: comparison with Euro-Phospholipid cohort. Clin Rheumatol. (2017) 36:599–608. doi: 10.1007/s10067-017-3549-1
5. Kearon C, Parpia S, Spencer FA, Baglin T, Stevens SM, Bauer KA, et al. Antiphospholipid antibodies and recurrent thrombosis after a first unprovoked venous thromboembolism. Blood. (2018) 131:2151–60. doi: 10.1182/blood-2017-09-805689
6. Garcia D, Akl EA, Carr R, Kearon C. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood. (2013) 122:817–24. doi: 10.1182/blood-2013-04-496257
7. Farmer-Boatwright MK, Roubey RAS. Venous Thrombosis in the antiphospholipid syndrome. Arterioscler Thromb Vasc Biol. (2009) 29:321–5. doi: 10.1161/ATVBAHA.108.182204
8. Uthman I, Noureldine MHA, Ruiz-Irastorza G, Khamashta M. Management of antiphospholipid syndrome. Ann Rheum Dis. (2019) 78:155–61. doi: 10.1136/annrheumdis-2018-213846
9. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. (2006) 4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x
10. Devreese KMJ, Ortel TL, Pengo V, de Laat B; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibodies. Laboratory criteria for antiphospholipid syndrome: communication from the SSC of the ISTH. J Thromb Haemost. (2018) 16:809–13. doi: 10.1111/jth.13976
11. Devreese KMJ, de Groot PG, de Laat B, Erkan D, Favaloro EJ, Mackie I, et al. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis: update of the guidelines for lupus anticoagulant detection and interpretation. J Thromb Haemost. (2020) 18:2828–39. doi: 10.1111/jth.15047
12. Sciascia S, Radin M, Sanna G, Cecchi I, Roccatello D, Bertolaccini ML. Clinical utility of the global anti-phospholipid syndrome score for risk stratification: a pooled analysis. Rheumatology. (2018) 57:661–5. doi: 10.1093/rheumatology/kex466
13. Radin M, Sciascia S, Erkan D, Pengo V, Tektonidou MG, Ugarte A, et al. The adjusted global antiphospholipid syndrome score (aGAPSS) and the risk of recurrent thrombosis: results from the APS ACTION cohort. Semin Arthritis Rheum. (2019) 49:464–8. doi: 10.1016/j.semarthrit.2019.04.009
14. Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. (2005) 172:1041–6. doi: 10.1164/rccm.200506-862OC
15. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G-J, Harjola V-P, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Respir J. (2019) 54:1901647. doi: 10.1183/13993003.01647-2019
16. Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. GAPSS: the Global Anti-Phospholipid Syndrome Score. Rheumatology. (2013) 52:1397–403. doi: 10.1093/rheumatology/kes388
17. Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. (2000) 83:416–20. doi: 10.1055/s-0037-1613830
18. Piette JC, Cacoub P. Antiphospholipid syndrome in the elderly: caution. Circulation. (1998) 97:2195–6. doi: 10.1161/01.CIR.97.22.2195
19. Na YS, Jang S, Hong S, Oh YM, Lee SD, Lee JS. Clinical phenotype of a first unprovoked acute pulmonary embolism associated with antiphospholipid antibody syndrome. Tuberc Respir Dis. (2019) 82:53–61. doi: 10.4046/trd.2018.0045
20. Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. The global anti-phospholipid syndrome score in primary APS. Rheumatology. (2015) 54:134–8. doi: 10.1093/rheumatology/keu307
21. Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL, Galli M, et al. Update of the guidelines for lupus anticoagulant detection. J Thromb Haemost. (2009) 7:1737–40. doi: 10.1111/j.1538-7836.2009.03555.x
22. Devreese KMJ, Pierangeli SS, de Laat B, Tripodi A, Atsumi T, Ortel TL. Testing for Antiphospholipid antibodies with Solid Phase Assays: guidance from the SSC of the ISTH. J Thromb Haemost. (2014) 12:792–5. doi: 10.1111/jth.12537
23. Matyja-Bednarczyk A, Swadzba J, Iwaniec T, Sanak M, Dziedzina S, Cmiel A, et al. Risk factors for arterial thrombosis in antiphospholipid syndrome. Thromb Res. (2014) 133:173–6. doi: 10.1016/j.thromres.2013.11.012
24. Ross R, Serock M, Khalil R. Experimental benefits of sex hormones on vascular function and the outcome of hormone therapy in cardiovascular disease. Curr Cardiol Rev. (2008) 4:309–22. doi: 10.2174/157340308786349462
25. Hisada R, Kato M, Sugawara E, Fujieda Y, Oku K, Bohgaki T, et al. Thrombotic risk stratification by platelet count in patients with antiphospholipid antibodies: a longitudinal study. J Thromb Haemost. (2017) 15:1782–7. doi: 10.1111/jth.13763
26. Vreede AP, Bockenstedt PL, McCune WJ, Knight JS. Cryptic conspirators: a conversation about thrombocytopenia and antiphospholipid syndrome. Curr Opin Rheumatol. (2019) 31:231–40. doi: 10.1097/BOR.0000000000000595
27. Demetrio Pablo R, Muñoz P, López-Hoyos M, Calvo V, Riancho L, Martínez-Taboada VM. Thrombocytopenia as a thrombotic risk factor in patients with antiphospholipid antibodies without disease criteria. Med Clin. (2017) 148:394–400. doi: 10.1016/j.medcle.2017.04.016
28. Artim-Esen B, Diz-Küçükkaya R, Inanç M. The significance and management of thrombocytopenia in antiphospholipid syndrome. Curr Rheumatol Reps. (2015) 17:14. doi: 10.1007/s11926-014-0494-8
29. Proulle V, Furie RA, Merrill-Skoloff G, Furie BC, Furie B. Platelets are required for enhanced activation of the endothelium and fibrinogen in a mouse thrombosis model of APS. Blood. (2014) 124:611–22. doi: 10.1182/blood-2014-02-554980
30. Pontara E, Banzato A, Bison E, Cattini MG, Baroni G, Denas G, et al. Thrombocytopenia in high-risk patients with antiphospholipid syndrome. J Thromb Haemost. (2018) 16:529–32. doi: 10.1111/jth.13947
31. Pardos-Gea J, Marques-Soares JR, Buján S, Ordi-Ros J, Alijotas-Reig J. Persistent thrombocytopenia predicts poor long-term survival in patients with antiphospholipid syndrome: a 38-year follow-up study. Rheumatology. (2021) 61:1053–61. doi: 10.1093/rheumatology/keab475
32. Abo SM, DeBari VA. Laboratory evaluation of the antiphospholipid syndrome. Ann Clin Lab Sci. (2007) 37:3–14
33. Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. (2018) 378:2010–21. doi: 10.1056/NEJMra1705454
34. Perrier A, Bounameaux H. Cost-effective diagnosis of deep vein thrombosis and pulmonary embolism. Thromb Haemost. (2001) 86:475–87. doi: 10.1055/s-0037-1616245
35. Eichinger S, Hron G, Kollars M, Kyrle PA. Prediction of recurrent venous thromboembolism by endogenous thrombin potential and D-dimer. Clin Chem. (2008) 54:2042–8. doi: 10.1373/clinchem.2008.112243
36. Michiels JJ, Gadisseur A, van der Planken M, Schroyens W, De Maeseneer M, Hermsen JT, et al. Different accuracies of rapid enzyme-linked immunosorbent, turbidimetric, and agglutination D-dimer assays for thrombosis exclusion: impact on diagnostic work-ups of outpatients with suspected deep vein thrombosis and pulmonary embolism. Semin Thromb Hemost. (2006) 32:678–93. doi: 10.1055/s-2006-951296
37. Galioto NJ, Danley DL, Van Maanen RJ. Recurrent venous thromboembolism. Am Fam Physician. (2011) 83:293–300.
38. Bao S, Frempong S, Ruan J. D-Dimer assay may guide LMWH treatment in repeated biochemical pregnancy losses in women with positive antiphospholipid antibody. Clin Lab. (2020) 66. doi: 10.7754/Clin.Lab.2019.190637
39. Schreiber K, Sciascia S, de Groot PG, Devreese K, Jacobsen S, Ruiz-Irastorza G, et al. Antiphospholipid syndrome. Nat Rev Dis Primers. (2018) 4:17103. doi: 10.1038/nrdp.2017.103
Keywords: pulmonary embolism, antiphospholipid syndrome, antiphospholipid antibody, risk factors, score model
Citation: Shi M, Gao W, Jin Y, Zhu J, Liu Y, Wang T and Li C (2022) Antiphospholipid Syndrome-Related Pulmonary Embolism: Clinical Characteristics and Early Recognition. Front. Cardiovasc. Med. 9:872523. doi: 10.3389/fcvm.2022.872523
Received: 09 February 2022; Accepted: 03 June 2022;
Published: 11 July 2022.
Edited by:
Blair Solow, University of Texas Southwestern Medical Center, United StatesReviewed by:
Teresa Iwaniec, Jagiellonian University Medical College, PolandCopyright © 2022 Shi, Gao, Jin, Zhu, Liu, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun Li, MTM4MTExOTAwOThAMTYzLmNvbQ==; Yuansheng Liu, bHlzcGt1QDEyNi5jb20=; Tianbing Wang, d2FuZ3RpYW5iaW5nQHBrdXBoLmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.