
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 20 May 2022
Sec. Coronary Artery Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.870341
 Jing-Lu Jin1,2
Jing-Lu Jin1,2 Hui-Wen Zhang1
Hui-Wen Zhang1 Hui-Hui Liu1
Hui-Hui Liu1 Cheng-Gang Zhu1
Cheng-Gang Zhu1 Yuan-Lin Guo1
Yuan-Lin Guo1 Na-Qiong Wu1
Na-Qiong Wu1 Rui-Xia Xu1
Rui-Xia Xu1 Qian Dong1
Qian Dong1 Jian-Jun Li1*
Jian-Jun Li1*Background: The positive relationship between metabolic healthy obesity (MHO) and cardiovascular risk has been under debate in recent years. Previously, strong evidence supported the causal role of increased plasma lipoprotein(a) [Lp(a)] levels in cardiovascular disease (CVD). The current study aimed to investigate the different associations of Lp(a) and cardiovascular events (CVEs) in patients with coronary artery disease (CAD) and different metabolic phenotypes.
Methods: A total of 5,089 patients who were angiography-proven CAD were consecutively included and followed up for CVEs. Obesity was defined as a body mass index (BMI) ≥25 kg/m2 according to Asia-specific BMI criteria. Patients were divided into four groups according to metabolic phenotypes, namely metabolically healthy/unhealthy non-obese and metabolically healthy/unhealthy obese [metabolically healthy non-obese (MHN), MHO, metabolically unhealthy non-obese (MUN), and metabolically unhealthy obesity (MUO)]. Comparisons of CAD severity and outcomes were performed among four groups. Cox regression analyses and cubic spline models were used to examine the relationship between Lp(a) and CVEs in patients with different metabolic phenotypes.
Results: During a median of 7.5 years’ follow-up, 540 (10.6%) CVEs occurred. MUN and MUO populations had more severe coronary stenosis than MHN ones, while no significant difference in the Gensini score (GS) was observed between MHN and MHO. Patients with MUN and MUO presented a higher risk of CVEs than patients with MHN (hazard ratio [HR]: 1.414, 95% CI: 1.024–1.953–1.556 and HR: 1.747, 95% CI: 1.295–1.363, p < 0.05). In subgroup analysis, restricted cubic spline models showed that there was no association between Lp(a) and CVEs in patients in MHN and MHO, while the MUN and MUO groups presented increasing associations between Lp(a) and CVEs and such association was stronger in the MUO group. In Cox regression analysis, Lp(a) >50 mg/dl was associated with a 2.032- and 2.206-fold higher risk of subsequent CVEs in the MUO and MUN subgroups, respectively.
Conclusion: Among patients with angiography-proven stable CAD, Lp(a) had a more significant prognostic value in both MUO and MUN individuals regardless of obesity, suggesting the importance of screening for cardiovascular risk with Lp(a) in metabolically unhealthy patients.
The prevalence of obesity in Chinese adults has increased significantly, which has become a severe public health issue. The excessive accumulation of body fat in obese individuals was correlated with a surge in rates of several cardiometabolic disorders, such as hypertension, impaired glucose metabolism, hypertriglyceridemia, and low levels of high-density lipoprotein cholesterol (HDL-C). However, some obese individuals have only less than one metabolic disorder and have been categorized as “metabolically healthy obesity” (MHO) (1, 2). As previously reported, MHO is characterized by a proinflammatory phenotype of circulating monocyte subsets (3). In fact, several previous studies have investigated the different relationships between MHO and coronary artery disease (CAD) risk and came to different conclusions. According to previous reports, among metabolic phenotypes, such as MHO, metabolically healthy non-obese (MHN), metabolically unhealthy non-obese (MUN), and metabolically unhealthy obesity (MUO), MHO patients had higher carotid intima-media thickness and a higher risk of heart failure than MHN individuals (4, 5). Moreover, the Atherosclerosis Risk in Communities (ARIC) Study and Evaluation of Chest Pain (PROMISE) study reported controversial results regarding the association between MHO and cardiovascular events (CVEs) (5, 6). Besides, no such study was performed in secondary prevention patients with angiography-proven CAD. Hence, in the current study, approximately 5,000 patients who received coronary angiography were documented. Both coronary severity and outcomes were compared among four metabolic phenotypes.
The full spectrum of risk factors for CAD among patients with different metabolic phenotypes has not been fully determined yet. According to the study by Commodore-Mensah et al. high-sensitivity-cTnT (≥6 ng/L) was associated with a higher risk of cardiovascular disease (CVD) across all metabolic phenotypes (6). Lipid parameters were also crucial in determining the presence and progression of atherosclerosis. Lipoprotein(a) [Lp(a)] is an LDL-like particle that induces proatherogenic and prothrombotic effects via LDL moiety and plasminogen-like apolipoprotein(a) (7, 8). Unlike other lipid parameters, circulating Lp(a) levels are rarely influenced by dietary and environmental factors (9). Thereby, in the setting of different metabolic phenotypes, the levels of L(a) may well reflect the CVE risk in patients with established CAD. Thus, we conducted a prospective, relatively large cohort study to investigate the predictive value of Lp(a) levels for the severity and prognosis of CAD in a Chinese population with different metabolic phenotypes.
Our study complied with the Declaration of Helsinki and was approved by the hospital’s ethical review board (Fu Wai Hospital and National Center for Cardiovascular Diseases, Beijing, China). Informed written consent was obtained from all patients enrolled in this study.
As described in Supplementary Figure 1, before February 2015, 6,505 patients who were scheduled for coronary angiography due to angina-like chest pain were documented. The detailed inclusion criteria were described in the flowchart. Among these patients, 498 were excluded because they did not have angiography-proven CAD (coronary stenosis <50% of at least one coronary artery). Other patients were excluded for acute coronary syndrome, decompensated heart failure, severe liver and/or renal insufficiency, thyroid dysfunction, systematic inflammatory disease, malignant disease, and excessive drinking. Finally, 5,089 of them were enrolled in the study. Patients were followed up at 6 months intervals via telephone or in-person interviews. Trained nurses or physicians who were blinded to the clinical data fulfilled the interview. The primary endpoints (CVEs) were cardiovascular mortality, non-fatal myocardial infarction (MI), and stroke. Non-fatal MI was diagnosed as positive cardiac troponins along with typical chest pain or typical electrocardiogram (ECG) serial changes. Stroke was diagnosed by the presence of typical symptoms and imaging.
Participants were considered metabolically healthy if they met two of the following four criteria: high triglycerides (TGs ≥ 150 mg/dl) or lipid-lowering drugs, increased systolic blood pressure (SBP ≥ 130 mmHg) or diastolic blood pressure (DPB ≥ 85 mmHg) or prescribed anti-hypertensive drugs, high fasting glucose (≥ 100 mg/dl) or medications for diabetes (insulin and oral antidiabetic), and low HDL-C (<40 mg/dl for men and <50 mg/dl for women) (10).
Diabetes mellitus (DM) was diagnosed by fasting plasma glucose (FPG) ≥130 mg/dl or the 2-h plasma glucose of the oral glucose tolerance test ≥200 mg/dl or currently prescribed antidiabetic medications. Hypertension was defined as self-reported hypertension, and currently prescribed antihypertensive drugs. Information on other diseases, family history, and prior therapy of every patient was collected from self-reported medical history.
Blood samples were obtained from each patient from the cubital vein after at least 12-h fasting. In an enzymatic assay, concentrations of total cholesterol (TC), TG, and HDL-C were measured using an automatic biochemistry analyzer (Hitachi 7150, Tokyo, Japan). The calculation of low-density lipoprotein cholesterol (LDL-C) was done using the Friedewald equation: LDL-C = TC-(HDL-C + TG/5). Lp(a) was determined by the immunoturbidimetry method [LASAY Lp(a) auto, SHIMA Laboratories Co., Ltd]. An Lp(a) protein validated standard has been used to calibrate the examination, and the coefficient of variation (CV) value of repetitive measurements was below 10%. The concentrations of glucose were measured by the enzymatic hexokinase method. Hemoglobin A1c (HbA1c) was measured using a Tosoh Automated Glycohemoglobin Analyzer (HLC-723G8, Tokyo, Japan).
Angiographic data were evaluated from catheter laboratory records by three experienced interventional cardiologists as previously reported. The Gensini score (GS) was calculated as the stenosis score by multiplying the location score for all diseased segments. The severity score of each coronary lesion was defined according to the narrowing degree of the coronary artery and its importance (11, 12).
The statistical analyses were performed using SPSS version 21.0 software (SPSS Inc., Chicago, IL, United States) and STATA SE 11.0 software. The values were expressed as the mean ± SD or median (Q1–Q3 quartiles) for the continuous variables and as a number (percentage) for the categorical variables. The Kolmogorov–Smirnov test was used to test the distribution pattern. The differences in clinical characteristics between groups were analyzed using Student’s t-test, Mann–Whitney U-test, χ2-tests, or Fisher’s exact test where appropriate. The event-free survival rates among groups were estimated by the Kaplan–Meier method and compared by the log-rank test. Univariate and multivariate Cox regression analyses were performed to calculate the hazard ratios (HRs). In the multivariate Cox regression models, traditional risk factors, such as age, sex, body mass index (BMI), smoking, and family history of CAD. GS, left ventricular ejection fraction, creatinine, LDL-C, and previous use of statin were used as adjustments. The variables in the definition of the metabolic phenotype were not added in the model. A value of p < 0.05 was considered statistically significant.
Baseline demographic and laboratory characteristics of the study population according to the metabolic phenotype are shown in Table 1. Overall, MHN, MHO, MUN, and MUO phenotypes accounted for 13.5, 12.3, 25.8, and 48.0% of the total population, respectively. Metabolically unhealthy individuals were older, had a higher proportion of men, and had higher levels of FPG, HbA1c, TC, LDL-C, TG, and GS but lower levels of HDL-C and Lp(a). Men and current smokers were most likely to be MHO while the MUO population had the highest levels of FPG, HbA1c, TC, LDL-C, TG and the lowest level of HDL-C.
As shown in Figure 1A, MUN and MUO individuals had more severe coronary stenosis than the MHN ones as assessed using the GS while no significant difference in the GS was observed between the MHN and MHO groups. When Lp(a) was incorporated in the stratification, using Lp(a) <30 mg/dl plus MHN as a reference, Lp(a) <30 mg/dl plus MUN or MUO subgroups had a higher GS, while the most severe coronary stenosis existed in Lp(a) ≥30 mg/dl plus MUN or MUO subgroups (Figure 1B).
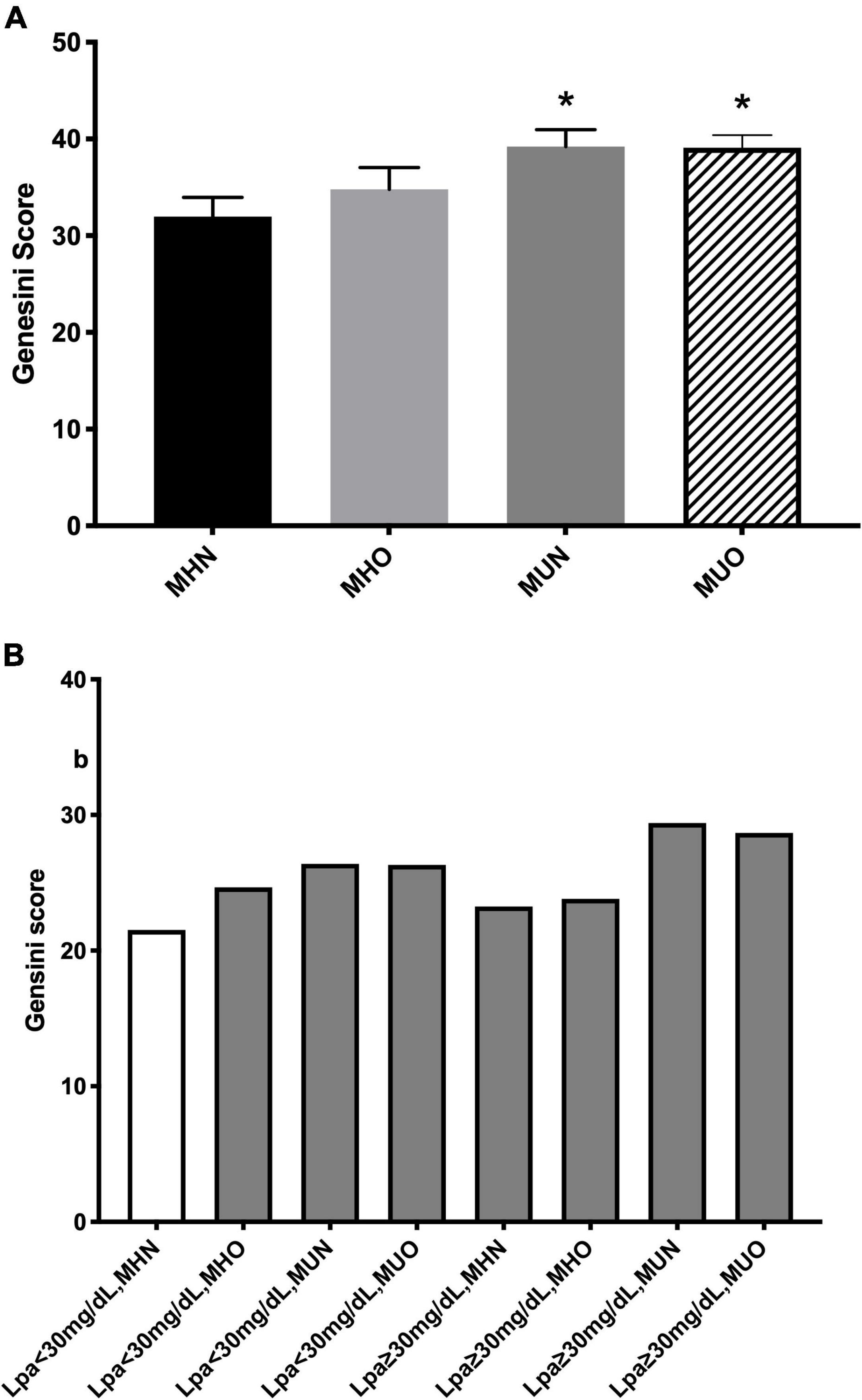
Figure 1. Coronary severity according to (A) metabolic phenotype; and (B) both status of metabolic phenotype and lipoprotein(a) [Lp(a)]. *p < 0.05.
Supplementary Table 1 presents the relative risk of CVEs among four metabolic phenotypes. The MUN and MUO subgroups had a 1.414- and 1.747-fold significant higher risk of CVEs (p < 0.05) while the MHO subgroup did not present a higher cardiovascular risk (p > 0.05).
The Kaplan–Meier analysis (Figure 2A) showed that MUO and MUN subjects had significantly lower event-free survival rates than MHN ones (p < 0.05), while MHO and MHN subgroups presented similar CVE risk. Meanwhile, patients with Lp(a) ≥30 mg/dl showed a higher event rate (Figure 2B). In terms of both metabolic phenotypes and Lp(a) levels, the Lp(a) ≥ 30 mg/dl plus MUN or MUO groups had higher event rates (Figure 2C).
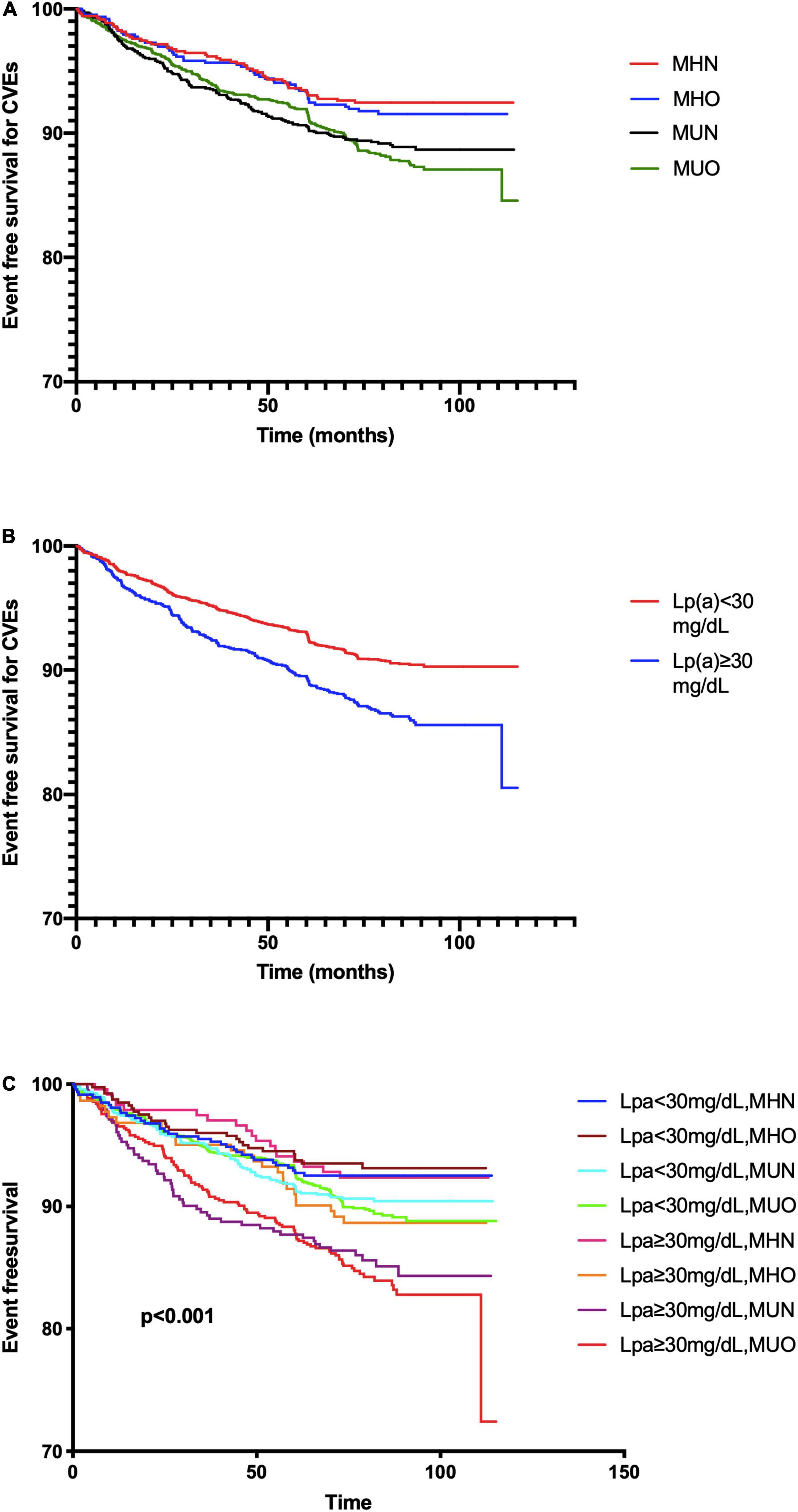
Figure 2. The Kaplan–Meier analysis according to (A) metabolic phenotype; (B) Lp(a) levels; and (C) both status of metabolic phenotype and Lp(a).
Restricted cubic spline models showed that no association between Lp(a) and CVEs existed in the MHN and MHO groups (Figures 3A,B). The MUO and MUN groups had increasing associations between Lp(a) and CVEs, while such association was relatively stronger in the MUO group (Figures 3C,D). Table 2 shows that continuous Lp(a) (per SD increment) was positively associated with CVEs in patients belonging to the MUO and MUN groups (adjusted HR per 1-SD increment: 1.236, 95% CI: 1.063–1.437 and adjusted HR per 1-SD increment: 1.275, 95% CI: 1.151–1.412, respectively, all p < 0.05) but not in those belonging to the MHO and MHN groups (p > 0.05). When Lp(a) was analyzed using more precise cut-off values as 10, 30, and 50 mg/dl, 30 mg/dl ≤ Lp(a) <50 mg/dl and Lp(a) ≥50 mg/dl had 2.016- and 2.032-fold of CVEs risk than Lp(a) <10 mg/dl in the MUN group. In the MUO group, those with 10 mg/dl ≤ Lp(a) <30 mg/dl, 30 mg/dl ≤ Lp(a) <50 mg/dl, and Lp(a) ≥50 mg/dl all had a significantly higher risk of CVEs than the reference group [NGR plus Lp(a) <10 mg/dl].
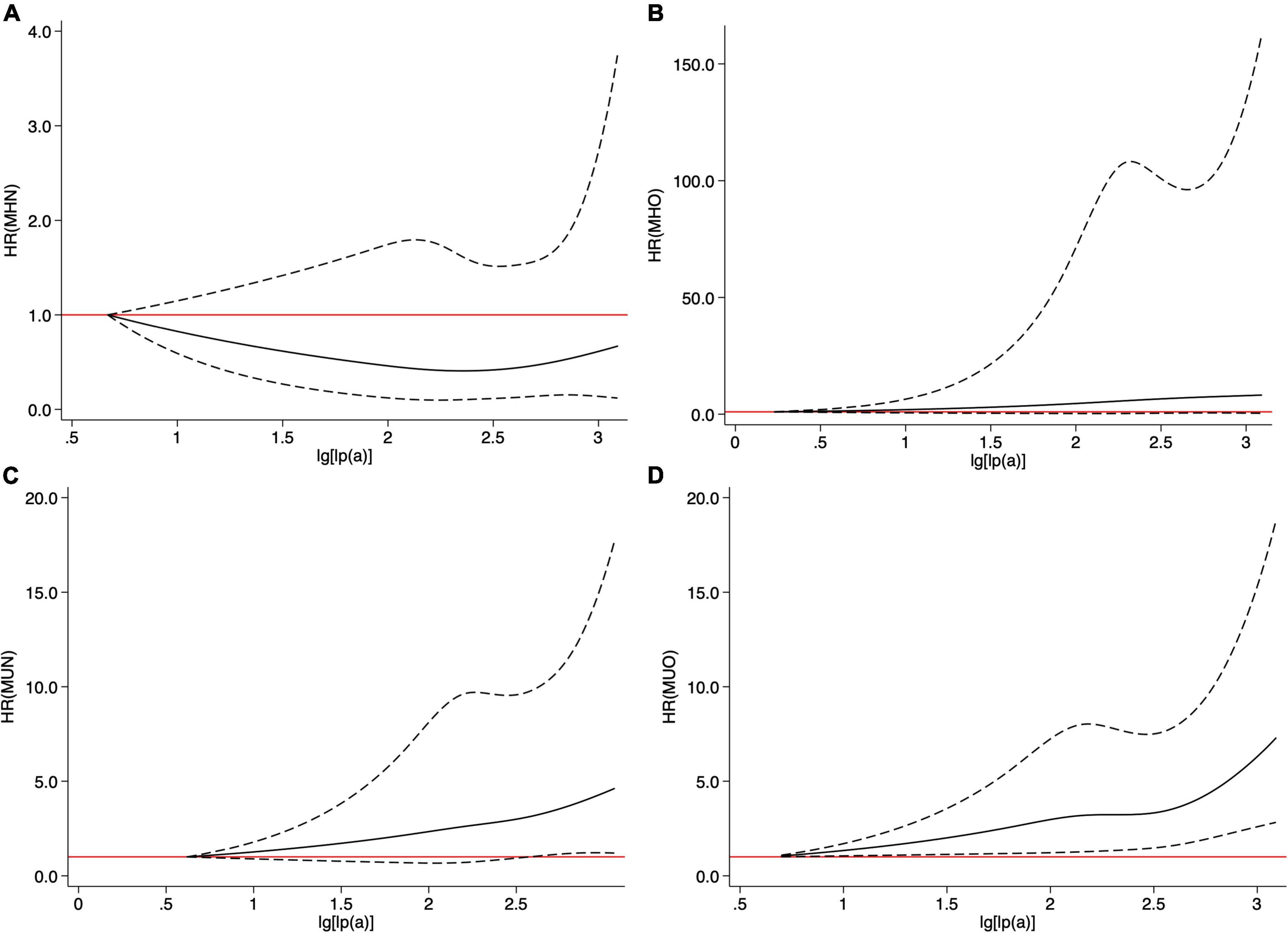
Figure 3. Cubic spline models of Lp(a) in four metabolic phenotypes (A) metabolically healthy non-obese (MHN), (B) metabolically healthy obesity (MHO), (C) metabolically unhealthy non-obese (MUN), and (D) metabolically unhealthy obesity (MUO).
The Lp(a)-associated cardiovascular risk in different metabolic phenotypes is not fully determined. In this prospective cohort study on patients with CAD, we found that obese individuals who were metabolically healthy did not have more severe CAD stenosis or higher cardiovascular risk. However, plasma Lp(a) levels were associated with a higher risk of CVEs in both MUO and MUN individuals regardless of obesity. Furthermore, Lp(a) was more strongly associated with CVEs in MUO than in MUN. These findings suggest that Lp(a) may help to stratify cardiovascular risk in patients with metabolic disorders.
In the past half-century, the prevalence of obesity worldwide has almost tripled (2, 13). Obese individuals may suffer from poor quality of life and have reduced life expectancy due to many comorbidities, such as DM, certain types of cancer, and hypertension (14). It is inexcusable that a subgroup of obese people with relatively fewer cardiometabolic risk factors was described as MHO (15). Whether MHO individuals presented a more severe atherosclerosis burden or had a worse prognosis than MHN ones is currently under debate. Previous studies have elucidated this issue in diverse populations. In the study by the International Childhood Vascular Structure Evaluation Consortium, MHO was associated with carotid intima-media thickness (4). Another study by Lin et al. reported that 46.8% of MHO developed into MUO and presented a higher risk of subclinical atherosclerosis (16). The ARIC study concluded that MHO was associated with a worse prognosis but mostly driven by an excess risk of heart failure. In our study population, 5,089 patients with angiography-proven CAD were followed up for 7 years, and we found that MUN and MUO but not the MHO population had a higher risk of CVEs, suggesting that metabolic health status but not obesity influences the risk of CVEs as well as coronary severity.
Large genetic epidemiological studies have documented an increase in the association of high Lp(a) levels and identified corresponding LPA risk genotypes with a higher risk of CAD. In previous studies, a linear relation between Lp(a) and cardiovascular disease was found in those who reached optimal LDL-C levels (17). In some high-risk patients, recurrent ASCVD events occur despite aggressive LDL-C lowering, which may be attributed to Lp(a)-hyperlipoproteinemia. As an inherited risk factor, Lp(a) is especially significant in younger individuals and proved to be predictive of premature MI (18). However, both dietary intervention and environmental changes had a minor effect on plasma Lp(a) levels. Differences in the Lp(a)-associated risk were identified when patients also had other metabolic risk factors. A similar phenomenon was reported in various literature. When participants were categorized by hypertensive or impaired glucose metabolism status, Lp(a) levels were not related to metabolic control but showed increasing associations with CVEs in patients combined with DM or hypertension (19–21). The International Diabetes Federation’s consensus group had given recommendations to better characterize the underlying diseased conditions beyond those superficial factors of metabolic syndrome (22). In this case, Lp(a) may be a crucial marker that not only represents the lipid disorders but also suggests the proinflammatory and prothrombotic state of individuals with obesity and/or metabolic disorder. To the best of our knowledge, we first reported the different associations between Lp(a) and CVEs in four different metabolic phenotypes.
The American Heart Association (AHA) recently issued a statement confirming that Lp(a) is a genetically determined, causal, and prevalent risk factor for CAD (23). Compelling evidence supported the necessity for the screening of cardiovascular risk by Lp(a) measurement (24). The European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) lipid guidelines also emphasized the importance of Lp(a) measurement in individuals with intermediate risk to further improve the risk stratification (25). In our study population of very high-risk patients with angiography-proven CAD, Lp(a) was reported to play a causal role in the worse prognosis in MUO and MUN individuals. Hence, a reassessment of Lp(a) levels during treatment is greatly necessary for those patients. As the randomized clinical trials to assess the effects of antisense oligonucleotide drugs targeting Lp(a) are in development, this might be a treatment option for very high-risk CAD patients with Lp(a)-hyperlipoproteinemia and metabolic disorders.
The current study had the following limitations. First, the metabolic phenotype is not a permanent state. Transitions from metabolic healthy to unhealthy or obese to non-obese may occur in part of the study population (26). We should also consider that the lack of association between circulating Lp(a) and CVEs in the MHN and MHO groups may be attributed to the lower baseline cardiovascular risk of these individuals. Second, we measured Lp(a) only at baseline, although the parameter was rarely influenced by food and environment, but the follow-up levels of Lp(a) were reported to affect prognosis, which was associated with statin treatment. Third, we did not assess all the metabolic factors such as parameters of insulin resistance due to the features of patients in our study. Hence, more studies are needed to prove our findings.
In summary, our study first reported that metabolic unhealthy Chinese patients with CAD had higher CVEs regardless of obesity. Furthermore, Lp(a) was a valuable marker for worse prognosis in those who were metabolically unhealthy. Finally, stronger associations were found between Lp(a) and CVEs in those individuals with MUO than in individuals with MUN. Thus, Lp(a) measurement may be clinically valuable for assessing CAD risk in metabolically unhealthy individuals.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Fu Wai Hospital and National Center for Cardiovascular Diseases. The patients/participants provided their written informed consent to participate in this study.
J-LJ completed the project, analyzed the data, and wrote the manuscript. J-JL designed the study, interpreted the data, and contributed to critically revising the manuscript. H-WZ contributed to the data collection. N-QW, C-GZ, and Y-LG contributed to the recruitment of patients and the clinical diagnosis of disease and data collection. QD contributed to the collection of clinical data and procedure of laboratory examination. All authors have approved the final manuscript.
This work was partially supported by the Capital Health Development Fund (201614035) and the CAMS Major Collaborative Innovation Project (2016-I2M-1-011) awarded to J-JL. The study sponsors did not participate in the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all the staff and participants of this study for their important contributions.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.870341/full#supplementary-material
1. Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud’homme D, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. (2005) 90:4145–50. doi: 10.1210/jc.2005-0482
2. Bluher M. Metabolically healthy obesity. Endocr Rev. (2020) 41:bnaa004. doi: 10.1210/endrev/bnaa004
3. Christou KA, Christou GA, Karamoutsios A, Vartholomatos G, Gartzonika K, Tsatsoulis A, et al. Metabolically healthy obesity is characterized by a proinflammatory phenotype of circulating monocyte subsets. Metab Syndr Relat Disord. (2019) 17:259–65. doi: 10.1089/met.2018.0132
4. Zhao M, Lopez-Bermejo A, Caserta CA, Medeiros CCM, Kollias A, Bassols J, et al. Metabolically healthy obesity and high carotid intima-media thickness in children and adolescents: international childhood vascular structure evaluation consortium. Diabetes Care. (2019) 42:119–25. doi: 10.2337/dc18-1536
5. Commodore-Mensah Y, Lazo M, Tang O, Echouffo-Tcheugui JB, Ndumele CE, Nambi V, et al. High burden of subclinical and cardiovascular disease risk in adults with metabolically healthy obesity: the atherosclerosis risk in communities (ARIC) study. Diabetes Care. (2021) 44:1657–63. doi: 10.2337/dc20-2227
6. Kammerlander AA, Mayrhofer T, Ferencik M, Pagidipati NJ, Karady J, Ginsburg GS, et al. Association of metabolic phenotypes with coronary artery disease and cardiovascular events in patients with stable chest pain. Diabetes Care. (2021) 44:1038–45. doi: 10.2337/dc20-1760
7. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. (2017) 69:692–711. doi: 10.1016/j.jacc.2016.11.042
8. Tsimikas S, Fazio S, Ferdinand KC, Ginsberg HN, Koschinsky ML, Marcovina SM, et al. NHLBI working group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol. (2018) 71:177–92. doi: 10.1016/j.jacc.2017.11.014
9. Tsimikas S, Hall JL. Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease: a rationale for increased efforts to understand its pathophysiology and develop targeted therapies. J Am Coll Cardiol. (2012) 60:716–21. doi: 10.1016/j.jacc.2012.04.038
10. Hinnouho GM, Czernichow S, Dugravot A, Nabi H, Brunner EJ, Kivimaki M, et al. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. Eur Heart J. (2015) 36:551–9. doi: 10.1093/eurheartj/ehu123
11. Liu HH, Cao YX, Li S, Guo YL, Zhu CG, Wu NQ, et al. Impacts of prediabetes mellitus alone or plus hypertension on the coronary severity and cardiovascular outcomes. Hypertension. (2018) 71:1039–46. doi: 10.1161/HYPERTENSIONAHA.118.11063
12. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. (1983) 51:606. doi: 10.1016/s0002-9149(83)80105-2
13. Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. (2019) 92:6–10. doi: 10.1016/j.metabol.2018.09.005
14. Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. (2010) 363:2211–9. doi: 10.1056/NEJMoa1000367
15. Iacobini C, Pugliese G, Blasetti Fantauzzi C, Federici M, Menini S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism. (2019) 92:51–60. doi: 10.1016/j.metabol.2018.11.009
16. Lin L, Zhang J, Jiang L, Du R, Hu C, Lu J, et al. Transition of metabolic phenotypes and risk of subclinical atherosclerosis according to BMI: a prospective study. Diabetologia. (2020) 63:1312–23. doi: 10.1007/s00125-020-05116-5
17. Albers JJ, Slee A, O’Brien KD, Robinson JG, Kashyap ML, Kwiterovich PO Jr, et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis intervention in metabolic syndrome with low HDL/High triglyceride and impact on global health outcomes). J Am Coll Cardiol. (2013) 62:1575–9. doi: 10.1016/j.jacc.2013.06.051
18. Cesaro A, Schiavo A, Moscarella E, Coletta S, Conte M, Gragnano F, et al. Lipoprotein(a): a genetic marker for cardiovascular disease and target for emerging therapies. J Cardiovasc Med (Hagerstown). (2021) 22:151–61. doi: 10.2459/jcm.0000000000001077
19. Liu HH, Cao YX, Jin JL, Hua Q, Li YF, Guo YL, et al. Lipoprotein (a), hypertension, and cardiovascular outcomes: a prospective study of patients with stable coronary artery disease. Hypertens Res. (2021) 44:1158–67. doi: 10.1038/s41440-021-00668-4
20. Jin JL, Cao YX, Zhang HW, Sun D, Hua Q, Li YF, et al. Lipoprotein(a) and cardiovascular outcomes in patients with coronary artery disease and prediabetes or diabetes. Diabetes Care. (2019) 42:1312–8. doi: 10.2337/dc19-0274
21. Littmann K, Wodaje T, Alvarsson M, Bottai M, Eriksson M, Parini P, et al. The association of lipoprotein(a) plasma levels with prevalence of cardiovascular disease and metabolic control status in patients with type 1 diabetes. Diabetes Care. (2020) 43:1851–8. doi: 10.2337/dc19-1398
22. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome–a new worldwide definition. Lancet. (2005) 366:1059–62. doi: 10.1016/s0140-6736(05)67402-8
23. Reyes-Soffer G, Ginsberg HN, Berglund L, Duell PB, Heffron SP, Kamstrup PR, et al. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement from the American heart association. Arterioscler Thromb Vasc Biol. (2021) 42:e48–60. doi: 10.1161/ATV.0000000000000147
24. Mehta A, Virani SS, Ayers CR, Sun W, Hoogeveen RC, Rohatgi A, et al. Lipoprotein(a) and family history predict cardiovascular disease risk. J Am Coll Cardiol. (2020) 76:781–93. doi: 10.1016/j.jacc.2020.06.040
25. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2020) 41:111–88. doi: 10.1093/eurheartj/ehz455
Keywords: CAD, lipoprotein(a), metabolic phenotypes, coronary severity, outcome
Citation: Jin J-L, Zhang H-W, Liu H-H, Zhu C-G, Guo Y-L, Wu N-Q, Xu R-X, Dong Q and Li J-J (2022) Lipoprotein(a) and Cardiovascular Outcomes in Patients With Coronary Artery Disease and Different Metabolic Phenotypes. Front. Cardiovasc. Med. 9:870341. doi: 10.3389/fcvm.2022.870341
Received: 06 February 2022; Accepted: 20 April 2022;
Published: 20 May 2022.
Edited by:
Paolo Calabrò, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Georgios Christou, Aristotle University, GreeceCopyright © 2022 Jin, Zhang, Liu, Zhu, Guo, Wu, Xu, Dong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Jun Li, bGlqaWFuanVuOTM4QDEyNi5jb20=; MTM5MDEwMTAzNjhAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.