
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 25 April 2022
Sec. Cardiovascular Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.869395
This article is part of the Research Topic Novel and Potential Biomarkers for Prediction of Outcome in Patients with Chronic and Acute Coronary Heart Disease, Volume II View all 9 articles
Background: In this study we investigated the prevalence of undiagnosed impaired glucose tolerance and type-2-diabetes (T2D) among patients with acute myocardial infarction (AMI) and prospectively analyzed whether these patients have a higher long-term mortality.
Methods: The analysis was based on 2,317 AMI patients aged 25–84 years from the population-based Myocardial Infarction Registry Augsburg, recruited between 2009 and 2014 and followed-up until 2019 (median follow-up time 6.5 years [IQR: 4.9–8.1]). AMI patients with a diagnosis of diabetes were divided into a high (>7.0%) and a low HbA1c group (≤7.0%) according to HbA1c values at admission. The remaining patients (without known diabetes) were grouped into normal (<5.7%), elevated (5.7–6.4%), and high (≥6.5%) HbA1c groups. In a multivariable-adjusted COX regression analysis, the association between HbA1c groups and long-term mortality was investigated. Linear regression models were used to identify AMI patients with elevated HbA1c values by means of personal characteristics.
Results: At admission, 29.5% of all patients reported a diagnosis of diabetes. Of all patients without known diabetes, 5.4% had HbA1c values of ≥ 6.5 and 37.9% had HbA1c values between 5.7 and 6.4%. The fully adjusted Cox regression model showed a non-significant trend toward higher long-term mortality for AMI patients with increased HbA1c values (HbA1c 5.7–6.4% HR: 1.05 [0.79–1.38], HbA1c > 6.5% HR: 1.34 [0.77–2.31]). A linear regression model including the variables admission serum glucose, BMI, age, sex and type of infarction (STEMI, NSTEMI) showed only poor prediction of HbA1c values (R2: 11.08%).
Conclusion: A fairly high number of AMI patients without known diabetes have elevated HbA1c values. Though we could not prove a higher risk of premature mortality in these patients, early detection and adequate therapy might lead to reduced diabetes-associated complications and improve long-term outcomes.
The prevalence of diabetes mellitus, and in particular type 2 diabetes, is increasing not only in Europe, but also in most other parts of the world at an alarming rate (1). It is known to be a major risk factor for several diseases, including coronary artery diseases and acute myocardial infarctions (AMI) (2). Diabetes mellitus not only increases the risk of experiencing such an event, but also affects the overall prognosis and outcome. Therefore, it is essential to detect and treat diabetes as early as possible. However, the number of undetected diabetes cases worldwide is as high as the number of diagnosed cases, which presents a major challenge (1). What’s more, prior studies have also suggested a very high prevalence of undiagnosed diabetes in patients with AMI (3–10). Nevertheless, most of these studies were not population-based and thus results might be biased; furthermore, the consequences that “hidden” diabetes in AMI patients may have on long-term mortality has rarely been investigated. Therefore, the aim of this study is to give a reliable estimation of the prevalence of undiagnosed diabetes among AMI patients using population-based data with consecutive enrollment from the Augsburg Myocardial Infarction Registry. In addition, this study examines the association between diabetes/HbA1c values and long-term mortality in AMI patients. Finally, we tried to identify risk factors for elevated HbA1c values in AMI patients.
We used data from the population-based Augsburg Myocardial Infarction Registry (previously the KORA Myocardial Infarction Registry), which was established in 1984 as a part of the MONICA-project (Monitoring Trends and Determinants in Cardiovascular disease) (11). The study area consists of the city of Augsburg, Germany, and the two adjacent counties comprising a total of approximately 680,000 inhabitants. Patients aged between 25 and 84 years who were admitted to one of the eight hospitals in the study area due to AMI were registered. More detailed information on case identification, diagnostic classification of events and quality control of the data can be found in previous publications (11, 12). For the present study, only patients with AMI who were admitted to the University Hospital Augsburg between 2009 and 2014 were included, since blood samples from AMI patients were collected solely in this hospital. All study participants gave written, informed consent. Methods of data collection have been approved by the ethics committee of the Bavarian Medical Association (Bayerische Landesärztekammer), and the study was performed in accordance with the Declaration of Helsinki.
Participants with missing information on diabetes, HbA1c and relevant covariables (n = 501) were excluded. The final study population consisted of 2,317 patients with AMI. For the total of 1,894 patients who survived the first 28 days after the infarction, additional information on long-term survival was available. These patients were followed-up for a median time of 6.5 (IQR: 4.9–8.1) years.
Trained study nurses interviewed the participants during the hospital stay using a standardized questionnaire. In order to confirm the information provided by the patients and to collect additional information, the patients’ medical chart was reviewed. Demographic data and data on cardiovascular risk factors, medical history, comorbidities (including diabetes) and medication before and during hospital stay as well as at discharge was collected for each patient. Furthermore, admission ECG presentation, in-hospital course of the disease and several laboratory parameters including glucose measurement were determined. During the interview, all patients were asked if they suffer from diabetes mellitus. The presence of diabetes mellitus in a patient was additionally extracted from the medical chart. The patient was assigned to the non-diabetes group when there was neither an indication for existing diabetes mellitus (including a previous instance of disturbed glucose tolerance) in the interview nor in the medical chart. All other cases were assigned to the diabetes group. HbA1C values were not used for diabetes grouping. For this study, we did not make a distinction between diabetes mellitus type 1 and type 2.
One variable was generated to register whether it was known that a patient received all four evidence-based medications (EBM) at discharge or not (antiplatelet drug, ACE blockers/ATII antagonist, beta-blockers, statins). Any known cardiological in-hospital complication including cardiogenic shock, left ventricular decompensation, bradycardia, in-hospital reinfarction, ventricular tachycardia and ventricular fibrillation was summarized in one variable (yes/no).
Between 2009 and 2014, plasma samples were collected from AMI cases during the hospital stay and frozen at −80°C.
HbA1c values were measured in stored samples with a reverse-phase cation-exchange high-pressure liquid chromatography (HPLC) method (Analyzer HA 8160; Menarini, Florence, Italy). All other blood parameters were measured in-house during the hospital stay of the patients as part of the regular diagnosis and routine treatment.
The endpoint used in this study was long-term all-cause mortality. Mortality was ascertained by regularly checking the vital status of all registered persons of the MI registry with data from the population registries. Death certificates were obtained from local health departments.
According to HbA1c values at admission, patients with known diabetes were divided into two groups by either high or low HbA1c values (>7.0 vs. ≤7.0% respectively). The remaining patients without known diabetes were grouped into a normal HbA1c group (<5.7%), an elevated group (5.7–6.4%) and a high group (≥6.5%).
For the comparison of categorical variables, Chi-square tests were performed and the results were presented as absolute frequencies with percentages. For normally-distributed continuous variables, Student’s t-test was used. For continuous variables that were not normally distributed, we used non-parametric tests. The results were presented as medians with inter-quartiles ranges.
To further examine the association between diabetes/HbA1c values and long-term mortality, several Cox regression models were calculated. In order to focus on long-term mortality exclusively, we eliminated all patients who died within the first 28 days after infarction. First, a model including only the diabetes/HbA1c group was calculated. A second model included sex and age. According to a literature review, the final model was adjusted for the following confounders: sex, age, typical chest pain symptoms, diabetes, smoking, hyperlipidemia, hypertension, left-ventricular EF ≤ 30%, impaired renal function (according to eGFR), BMI, systolic blood pressure, PCI, bypass surgery, lysis therapy, EBM, and statin prescription at discharge. The proportional hazards assumption was checked by plotting the Schoenfeld residuals against time and searching for any visible correlation. Additionally, a test was performed to check for a significant correlation of the Schoenfeld residuals with time and consequently a violation of the proportional hazard assumption.
In order to identify predictors of elevated HbA1c values among patients without known diabetes, linear regression models were calculated. For each model, the dependent outcome variable was HbA1c. One linear model was calculated for each of the following variables: BMI, age, glucose at admission, sex and type of AMI. Finally, a model including all five variables was calculated to predict HbA1c values. R2 values were used to measure the goodness of adaptation.
All statistical analyses were carried out with the R program version 4.1.0. A p-value of < 0.05 was considered as statistically significant.
A total of 2,317 cases were included in this analysis. The distribution of cases by known diabetes and HbA1c group is displayed in Figure 1. Overall, 70.5% (n = 1,634) of patients had no type of previously known diabetes or disturbed glucose tolerance. Among these patients, 5.4% (n = 89) had HbA1c values of ≥ 6.5%. Another 37.9% (n = 619) of these patients had HbA1c values between 5.7 and 6.4%. A total of 683 patients (29.5%) had pre-known diabetes, of which 34.6% (n = 236) had HbA1c values of > 7%. Among patients in the diabetes group with HbA1c values of ≤ 7%, 4 patients (0.9%) had type 1 diabetes mellitus, 246 patients had type 2 diabetes mellitus (77.4%) and 87 patients had previously known disturbed glucose tolerance (19.5%); for another 10 patients (2.2%) there was no information on diabetes type. Among the patients in the diabetes group with HbA1c values of > 7%, 10 patients (4.2%) had type 1 diabetes mellitus, 220 patients had type 2 diabetes mellitus (93.2%), no patient had previously known disturbed glucose tolerance and for 6 patients (2.5%) there was no information on diabetes type available.
Table 1 displays the baseline characteristics according to diabetes and HbA1c. There were no significant sex-specific differences between the groups. The group with the lowest average age was the group of patients with no diabetes and an HbA1c < 5.7%; the highest average age was seen in the group with diabetes and HbA1c ≤ 7.0%. Diabetic patients were more often diagnosed with hypertension and hyperlipidemia, but were less likely to be current smokers. No significant differences could be observed regarding the occurrence of typical chest pain symptoms. Patients without diabetes were more likely to have a STEMI event. The frequency with which PCI was performed and the prescription of all four EBM at discharge was comparable among the groups. In the Supplementary Material we additionally provide information on statin and anti-diabetic medication use before the event.
Figure 2 shows the (unadjusted) Kaplan Meier curves according to known diabetes and HbA1c groups, and Table 2 displays the results of the COX regression models. Overall, the groups of patients without diabetes had distinctly better long-term survival than the two diabetes groups. Among patients without a diabetes diagnosis, patients with HbA1c values < 5.7% had a noticeable lower long-term mortality compared to the other two groups in the Kaplan Meier curve. This was confirmed by the COX regression models, which predicted a higher long-term mortality for the HbA1c 5.7–6.4%-group and the HbA1C ≥ 6.5%-group in comparison to the group with HbA1c values < 5.7%. However, the differences did not reach statistical significance in the adjusted models. The two diabetes groups, on the other hand, were significantly associated with higher long-term mortality in all COX models. In the fully adjusted model, they had comparable HR values (the group with HbA1C > 7% had marginally higher HR values).
Figure 3 shows the association between certain important characteristics and HbA1c values in patients without known diabetes. Table 3 displays the results of several linear regression models with the dependent variable HbA1c. It shows a positive correlation for glucose at admission, BMI and age with HbA1c. No significant association was found for type of infarction and sex with HbA1c values. A final model, including all mentioned covariables, had an R2 of 11.08%.
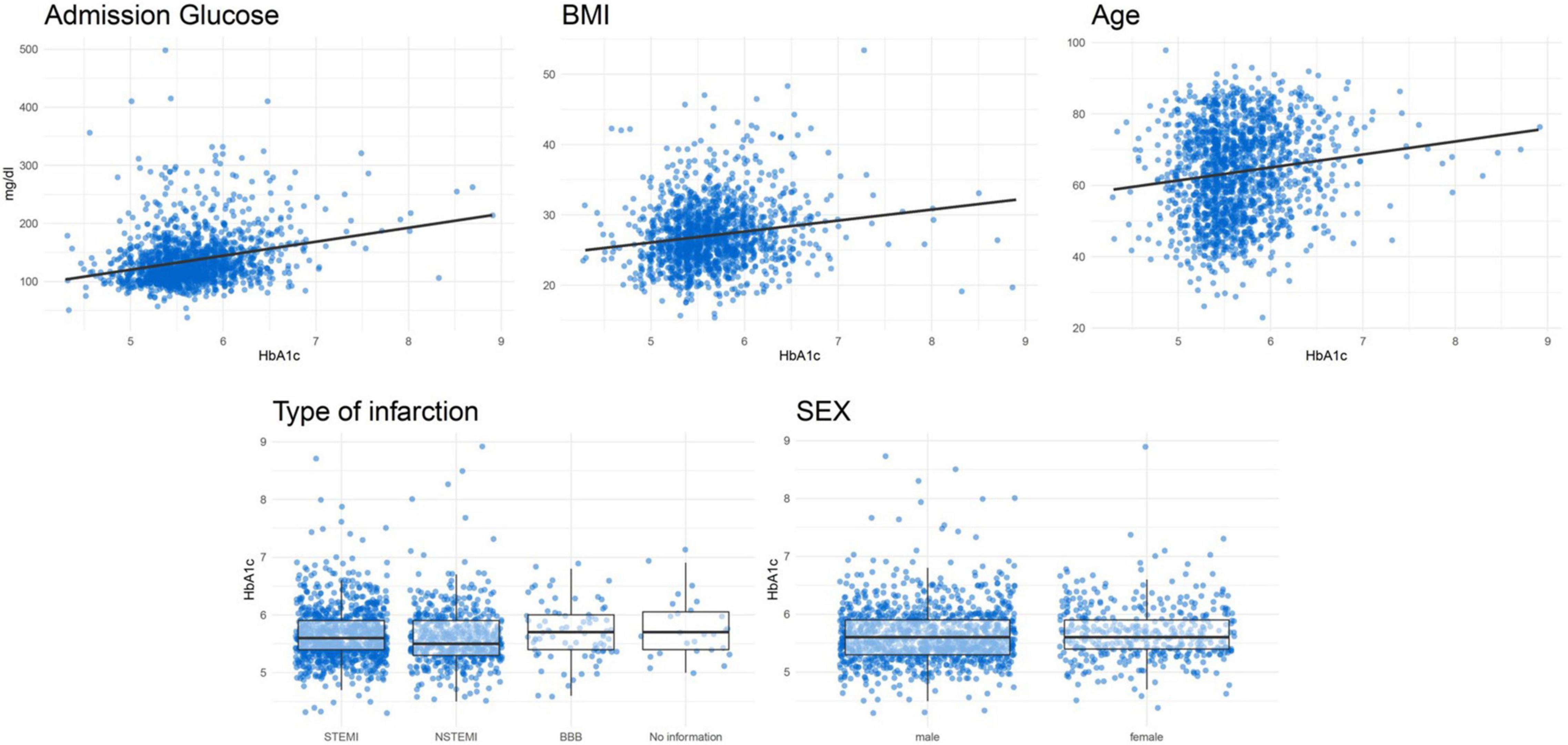
Figure 3. Correlation plots between HbA1c and continuous variables and boxplots for categorical variables for patients without known diabetes.
In the present study, we examined the frequency of elevated HbA1c values among AMI patients with and without known diabetes. HbA1c measurement is an appropriate tool to detect prediabetes and diabetes in general, and in AMI patients in particular, as it reflects average glucose levels over the previous 3 months (13–16). Of all patients without known diabetes, 43.3% had HbA1c values above 5.7%. This threshold is commonly used to distinguish between patients without risk of diabetes and patients with disturbed glucose tolerance or prediabetes (17). Prior studies reported diverging numbers for prevalence of (unknown) prediabetes in the general European population (18). The International Diabetes Federation (IDF), for example, suggested a prediabetes prevalence of about 5% in the European region (19), which is considerably lower than the prevalence we found for AMI patients. This is not surprising, as AMI patients have a higher risk profile with regards to many cardiovascular risk factors, including prediabetes and impaired glucose tolerance. Several prior studies reported a wide range of different results for prevalence of unknown (pre)diabetes among AMI patients (3–10). For the majority of studies, a comparison is difficult, as they were based on different study populations with different inclusion criteria (all AMIs, only STEMI events etc.), as well as different methods (HbA1c, OGTT, fasting glucose) and time points (at admission, at discharge) of determining prediabetes and impaired glucose tolerance. It can be assumed, that the results of the present study are very reliable, as they are based on data from a population-based registry with a high number of consecutive events included. Our results demonstrate that undetected disturbed glucose tolerance and prediabetes are widespread among a representative group of AMI patients.
In a subsequent analysis, we examined the associations between diabetes/HbA1c values and long-term mortality after AMI. Patients with known diabetes had significantly higher long-term mortality compared to the reference group (no known diabetes, normal HbA1c). This is not surprising, as diabetes is a well-known risk factor for AMI and a severe event (20). Nevertheless, among the diabetic patients, the high HbA1c group (> 7.0%) had similar long-term mortality compared to the low HbA1c group.
Among the patients without previously known diabetes, there were no significant differences regarding long-term mortality in the adjusted COX regression models. Nevertheless, a non-significant trend toward higher mortality was observed for patients with HbA1c > 5.7% compared to the reference group. In a prior study, Meier et al. conducted oral glucose tolerance tests in 129 AMI patients and compared long-term mortality in patients with impaired vs. normal glucose tolerance (7). They reported a significantly higher long-term mortality of AMI patients with impaired glucose tolerance compared to AMI patients without impaired glucose tolerance, diverging from the results of the present study, as we did not find a significant difference. In a further study (10), similar long-term mortality as that of the present study was obtained in a STEMI population, comparing subjects with unknown prediabetes (HbA1c 5.7–6.4%) to a normal HbA1c group. In contrast to the present study however, for subjects with unknown diabetes (HbA1c > 6.5%) an analogous 3-year mortality was observed compared to subjects with known diabetes.
One might expect a significantly higher mortality for patients with elevated HbA1c, but that is not what our data suggests. Different explanations for this should be considered. First, a moderately elevated HbA1c might not lead to increased long-term mortality among non-diabetic AMI patients, suggesting that physicians would not need to consider HbA1c levels as a risk factor for long-term mortality. An alternative explanation would be that there is indeed an association which did not reach significance in our study. Even though there was no significant difference in mortality for the first years after the event, it is possible that differences may not develop until a later time point, beyond our median follow-up period of 6.5 years. Most of the patients without known diabetes but with an elevated HbA1c might be in a very early phase of diabetes. As it can take several decades for serious microvascular complications of diabetes to manifest, it is impossible to detect these effects within a limited observational period. Annually, only 5–10% of all patients with prediabetes develop manifest diabetes mellitus, but up to 70% eventually do so within their lifetime (21). This affirms the assumption that the observational period of this study might have been insufficient to detect an increase in the long-term mortality of patients with prediabetes.
Another point to consider is that the outcome variable of this study was long-term mortality. Yet, there are many other adverse outcomes that can be of interest, e.g., myocardial reinfarction, heart failure, secondary mitral wave insufficiency or the progression of coronary artery disease. As those complications usually precede (cardiovascular) mortality, it can be assumed that we would have been able to detect differences in the HbA1c groups with regards to general adverse cardiovascular outcomes and major complications.
For these reasons, it still appears desirable that patients with elevated HbA1 values are identified early. In this way, education of patients (on diet, lifestyle changes), regular follow-up controls and, if necessary, pharmacological interventions (e.g., metformin) can be implemented in an early stage of the disease, which has been proven to reduce disease progression (21). The present study demonstrates that this issue does not affect only a few individual cases, but indeed concerns a considerable proportion of patients with AMI, and is therefore highly relevant.
Since HbA1c may not usually be determined in the context of an AMI, it raises the question of whether it would be useful and practical to routinely determine HbA1c values after AMI for the early detection of prediabetes. A more favorable solution may be to identify factors that predict an increased risk of elevated HbA1c values. In this way, physicians might only need to measure HbA1c values in patients with a high risk of elevated HbA1c levels. We attempted to identify risk factors and characteristics that would potentially predict an increased risk of elevated HbA1c values by calculating linear regression models for the predictor variables glucose at admission, BMI, age, sex and type of infarction. Even though BMI, age and admission glucose were significantly associated with HbA1c levels, their predictive abilities (R2 values) were very limited. Even in a final model including all five predictor variables, the R2 was only 11.08%, which is certainly not sufficient for an adequate risk stratification at the acute event.
The present study is characterized by some particular strengths. First to mention is the large sample size, the consecutive enrollment of patients and long follow-up period with a median time of 6.5 (IQR: 4.9–8.1) years. Standardized data collection was performed by conducting patient interviews during the hospital stay and via patient chart review. Nevertheless, there are also some limitations. Since only patients between 25 and 84 years were included, results cannot be applied to older age-groups, and findings may not be generalized to all ethnic groups. As this is an observational study, causality cannot be proven. Furthermore, it is possible that some confounders, which could have influenced the associations, were not available and could therefore not be included in the analysis. Thus, residual confounding cannot be entirely excluded. Likewise, we might not have considered some valuable predictors of increased HbA1c values in patients without known diabetes.
About 30% of AMI patients have a previously known diagnosis of diabetes, and these patients have a significantly higher risk of earlier death as compared to non-diabetics. In addition, 5.4% of AMI patients without known diabetes have HbA1c values ≥ 6.5%. A further 37.9% have HbA1c values between 5.7 and 6.4%, which demonstrates that undiagnosed (pre)diabetes is a widespread issue among AMI patients. However, in this study we could not identify significant differences between HbA1c groups and long-term mortality in non-diabetic AMI patients. Nonetheless, it remains important to detect derangements of glucose metabolism in these patients at an early stage. Over the course of several years it is quite likely for these patients to develop a manifest diabetes mellitus disease and as a consequence a number of serious long-term complications. By identifying such patients, physicians have a good chance of early intervention (education, lifestyle changes, or medication) and consequent prevention of diabetes-associated complications or even of the manifestation of diabetes mellitus in the first place.
The datasets generated during and/or analyzed during the current study are not publicly available due to data protection aspects but are available in an anonymized form from the corresponding author on reasonable request.
This study involves human participants and was reviewed and approved by Ethics Committee of the Bavarian Medical Association (Bayerische Landesärztekammer). The patients/participants provided their written informed consent to participate in this study.
TS and CM conceived the study. TS performed the statistical analysis and drafted the manuscript. CM supervised data analysis and was responsible for the acquisition of the data. EH, MH, AP, and JL contributed to data acquisition and revised the manuscript. All authors approved the final manuscript.
This work was supported by the Helmholtz Zentrum München, German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education, Science, Research and Technology and by the State of Bavaria and the German Federal Ministry of Health. This research received also support from the Faculty of Medicine, University of Augsburg, and the University Hospital of Augsburg, Germany. Since the year 2000, the collection of MI data has been co-financed by the German Federal Ministry of Health to provide population-based MI morbidity data for the official German Health Report (see http://www.gbe-bund.de).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank all members of the Helmholtz Zentrum München, Institute of Epidemiology, and the Chair of Epidemiology at the University Hospital of Augsburg, who were involved in the planning and conduct of the study. Steering partners of the MI Registry, Augsburg, include the Chair of Epidemiology at the University Hospital of Augsburg and the Department of Internal Medicine I, Cardiology, University Hospital of Augsburg. Many thanks for their support go to the local health departments, the office-based physicians and the clinicians of the hospitals within the study area. Furthermore, we thank DZHZ (Deutsches Zentrum für Herz-Kreislauf-Forschung e.V.) for the cooperation. We also thank Taylor Breuninger for proofreading and language editing. Finally, we express our appreciation to all study participants.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.869395/full#supplementary-material
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
2. Cui J, Liu Y, Li Y, Xu F, Liu Y. Type 2 Diabetes and myocardial infarction: recent clinical evidence and perspective. Front Cardiovasc Med. (2021) 8:644189. doi: 10.3389/fcvm.2021.644189
3. Conaway DG, O’Keefe JH. Frequency of undiagnosed and untreated diabetes mellitus in patients with acute coronary syndromes. Expert Rev Cardiovasc Ther. (2006) 4:503–7. doi: 10.1586/14779072.4.4.503
4. Choi KM, Lee KW, Kim SG, Kim NH, Park CG, Seo HS, et al. Inflammation, insulin resistance, and glucose intolerance in acute myocardial infarction patients without a previous diagnosis of diabetes mellitus. J Clin Endocrinol Metab. (2005) 90:175–80. doi: 10.1210/jc.2004-1795
5. Rathmann W, Icks A, Haastert B, Giani G, Löwel H, Mielck A. Undiagnosed diabetes mellitus among patients with prior myocardial infarction. Z Kardiol. (2002) 91:620–5. doi: 10.1007/s00392-002-0826-y
6. Jessani S, Gangopadhyay K, Patel JV, Lip GY, Millane T. Should oral glucose tolerance testing be mandatory following acute myocardial infarction? Int J Clin Pract. (2007) 61:680–3. doi: 10.1111/j.1742-1241.2007.01301.x
7. Meier JJ, Deifuss S, Gallwitz B, Klamann A, Schmiegel W, Nauck MA. Einfluss einer eingeschränkten Glukosetoleranz auf das Langzeitüberleben nach akutem Myokardinfarkt1 – The LAngendreer Myocardial infarction and Blood glucose in Diabetic patients Assessment (LAMBDA). Dtsch Med Wochenschr. (2002) 127:1123–9. doi: 10.1055/s-2002-31529
8. Meloni L, Montisci R, Sau L, Boi A, Marini A, Ruscazio M. Admission hyperglycemia in acute myocardial infarction: possible role in unveiling patients with previously undiagnosed diabetes mellitus. J Cardiovasc Med (Hagerstown). (2013) 14:821–6. doi: 10.2459/JCM.0b013e32835ec72b
9. Lankisch M, Füth R, Gülker H, Lapp H, Bufe A, Haastert B, et al. Screening for undiagnosed diabetes in patients with acute myocardial infarction. Clin Res Cardiol. (2008) 97:753–9. doi: 10.1007/s00392-008-0674-5
10. Aggarwal B, Shah GK, Randhawa M, Ellis SG, Lincoff AM, Menon V. Utility of glycated hemoglobin for assessment of glucose metabolism in patients With ST-Segment elevation myocardial infarction. Am J Cardiol. (2016) 117:749–53. doi: 10.1016/j.amjcard.2015.11.060
11. Löwel H, Meisinger C, Heier M, Hörmann A. The population-based acute myocardial infarction (AMI) registry of the MONICA/KORA study region of Augsburg. Gesundheitswesen. (2005) 67(Suppl. 1):S31–7. doi: 10.1055/s-2005-858241
12. Kuch B, Heier M, Scheidt W, Kling B, Hoermann A, Meisinger C. 20-year trends in clinical characteristics, therapy and short-term prognosis in acute myocardial infarction according to presenting electrocardiogram: the MONICA/KORA AMI Registry (1985-2004). J Int Med. (2008) 264:254–64. doi: 10.1111/j.1365-2796.2008.01956.x
13. Ishihara M, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, Hata T, et al. Is admission hyperglycaemia in non-diabetic patients with acute myocardial infarction a surrogate for previously undiagnosed abnormal glucose tolerance? Eur Heart J. (2006) 27:2413–9. doi: 10.1093/eurheartj/ehl271
14. Weykamp C. HbA1c: a review of analytical and clinical aspects. Ann Lab Med. (2013) 33:393–400. doi: 10.3343/alm.2013.33.6.393
15. Farmer A. Use of HbA1c in the diagnosis of diabetes. BMJ. (2012) 345:e7293. doi: 10.1136/bmj.e7293
16. Makris K, Spanou L. Is there a relationship between mean blood glucose and glycated hemoglobin? J Diabetes Sci Technol. (2011) 5:1572–83. doi: 10.1177/193229681100500634
17. American Diabetes Association [ADA]. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44:S15–33. doi: 10.2337/dc21-S002
18. Hostalek U. Global epidemiology of prediabetes – present and future perspectives. Clin Diabetes Endocrinol. (2019) 5:5.
19. International Diabetes Federation [IDF].IDF Diabetes Atlas. 8th ed. Brussels: International Diabetes Federation (2017).
20. Norhammar A, Mellbin L, Cosentino F. Diabetes: prevalence, prognosis and management of a potent cardiovascular risk factor. Eur J Prev Cardiol. (2017) 24:52–60. doi: 10.1177/2047487317709554
Keywords: prediabetes, HbA1c, myocardial infarction, long-term mortality, undiagnosed diabetes
Citation: Schmitz T, Harmel E, Heier M, Peters A, Linseisen J and Meisinger C (2022) Undiagnosed Impaired Glucose Tolerance and Type-2 Diabetes in Acute Myocardial Infarction Patients: Fequency, Characteristics and Long-Term Mortality. Front. Cardiovasc. Med. 9:869395. doi: 10.3389/fcvm.2022.869395
Received: 07 February 2022; Accepted: 04 April 2022;
Published: 25 April 2022.
Edited by:
Dennis W. T. Nilsen, Stavanger University Hospital, NorwayReviewed by:
Xuan Li, University of Mississippi Medical Center, United StatesCopyright © 2022 Schmitz, Harmel, Heier, Peters, Linseisen and Meisinger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Timo Schmitz, dGltby5zY2htaXR6QG1lZC51bmktYXVnc2J1cmcuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.