- 11st Chair and Department of Cardiology, Medical University of Warsaw, Warsaw, Poland
- 2Research Unit, Maria Sklodowska-Curie Białystok Oncology Center, Białystok, Poland
- 3Institute of Outcomes Research, Maria Sklodowska-Curie Medical Academy in Warsaw, Warsaw, Poland
- 4Research Unit, Polish Society of Disaster Medicine, Warsaw, Poland
- 5Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, Centre of Postgraduate Medical Education, European Health Centre Otwock, Otwock, Poland
- 6Department of Experimental and Clinical Pharmacology, Medical University of Warsaw, Warsaw, Poland
Background: Catheter-directed therapies (CDT) are an alternative to systemic thrombolysis (ST) in pulmonary embolism (PE) patients, but the mortality benefit of CDT is unclear.
Objective: We conducted a systematic review with meta-analysis to compare the efficacy and safety of CDT and ST in intermediate-high and high-risk PE.
Methods: We included (P) participants, adult PE patients; (I) intervention, CDT; (C) comparison, ST; (O) outcomes, mortality, complications, in-hospital treatment, and length of hospital stay; (S) study design, randomized controlled trials (RCTs), or cohort comparing CDT and ST. The primary endpoint was 30-day mortality. Secondary outcomes included treatment-related complications including bleeding, the use of hospital resources, and length of hospital stay.
Results: Eleven studies including 65,589 patients met the inclusion criteria. Thirty-day mortality was lower in the CDT group, compared to ST group [7.3 vs. 13.6%; odds ratio (OR) = 0.51, 95% confidence interval (CI) 0.38–0.69, p < 0.001]. The rates of myocardial injury, cardiac arrest, and stroke were lower in CDT group, compared to ST group (p < 0.001 for all). The rates of any major bleeding, intracranial hemorrhage, hemoptysis, and red blood cell transfusion were lower in patients treated with CDT, compared to ST (p ≤ 0.01 for all). Extracorporeal life support was used more often in patients treated with CDT, compared to ST (0.5 vs. 0.2%, OR = 2.52, 95% CI 1.88–3.39, p < 0.001). The use of hospital resources and length of hospital stay were comparable in both groups.
Conclusion: CDT might decrease mortality in patients with intermediate-high and high-risk PE and were associated with fewer complications, including major bleeding.
Introduction
Acute pulmonary embolism (PE) is the third most frequent cause of cardiovascular morbidity and mortality worldwide (1). Whereas anticoagulation is the mainstay of therapy for low-risk and intermediate-low risk PE patients, the choice of the therapy for patients with intermediate-high and high-risk PE is more complex (2). Systemic thrombolysis (ST) is approved for high-risk PE, but its use for intermediate-risk patients remains uncertain (2, 3). Although ST improves pulmonary perfusion and right ventricle function in patients with PE, it is associated with an overall 10% risk of major bleeding and a 3–5% risk of intracranial hemorrhage (4). In addition, there are numerous patients in whom ST is contraindicated or has failed. Catheter-directed therapies (CDT) are an alternative treatment option for intermediate-high and high-risk PE and for patients with contraindications to ST. CDT are all therapies which are delivered via a catheter placed in the branches of the pulmonary artery directly into the thrombus. They include intrapulmonary administration of low doses of thrombolytic drugs, ultrasound-assisted thrombolysis, mechanical aspiration thrombectomy, and direct clot retrieval systems (5–7). CDT efficiently alleviate right ventricle overload and allow to decrease the dose of thrombolytic drug administered (e.g., local or ultrasound-assisted thrombolysis), or even to entirely avoid thrombolytic drug administration (e.g., thrombectomy-based methods). Preliminary results of registry-based studies and case series suggest the CDT procedural success rate of above 80%, which is defined as improvement in right ventricle function, hemodynamic stabilization, correction of hypoxemia, and survival to hospital discharge. In addition, the rate of major bleeding complications might be reduced in CDT, compared with ST. However, the clear mortality benefit of CDT remains to be demonstrated (8–11). Therefore, we conducted a systematic review with meta-analysis to compare the efficacy and safety of CDT and ST in patients with high and moderate-to-high PE.
Methods
The current systematic review and meta-analysis were conducted according to Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) statement.
Search Strategy
A systematic review was carried out using PubMed, Web of Science, SCOPUS, EMBASE, and Cochrane Collaboration Databases electronic databases for relevant articles published through June 5, 2021, using the following keywords: “pulmonary embolism” OR “PE” OR “embolus” AND “catheter-based therapies” OR “thrombolysis” AND “systemic thrombolysis.” All retrieved articles were reviewed by two authors (ŁS and AG) independently. Any disagreement was resolved through consensus or, if necessary, by discussion with a third author (AP). We restricted articles to published in English language. The reference lists of eligible studies were also manually searched to identify any additional relevant studies. All references were saved in an EndNote (End Note, Inc., Philadelphia, PA, United States) library used to identify the duplicates.
Inclusion and Exclusion Criteria
The PICOS strategy consisting of patient, intervention, comparison, and the outcome was used as a tool to ensure focused clinical questions. The prespecified criteria for studies included in the meta-analysis were as follows: (P) participants, adult patients with PE; (I) intervention, CDT; (C) comparison, ST; (O) outcomes, detailed information for mortality, complications, in-hospital treatment, and length of hospital stay; (S) study design, randomized controlled trials (RCTs), or cohort comparing CDT and ST for their effects on outcomes in patients with PE.
Data Extraction
The titles and abstracts were screened for relevance by two authors (MP and TK) independently; if differences were found, they were discussed until a consensus was reached. The manuscripts of selected titles and abstracts were assessed for inclusion, and the authors were contacted if further information was required. Using the selection criteria enlisted above, the three reviewers (AP, AG, and ŁS) independently identified the papers to be included and excluded, and data from the included papers were extracted using pre-defined extraction flow sheets. The following information was extracted: authors, year of publication, study design, number of patients enrolled, patient characteristics, mortality outcomes, complications, and length of hospital stay.
Quality Assessment
Two investigators (ŁS and AG) independently extracted individual study data and evaluated studies for risk of bias. Any disagreements were discussed and resolved in a consensus meeting with the third reviewer (AP). Data heterogeneity and methodological quality of the included studies were assessed using The Newcastle–Ottawa Scale (12). The scale is divided in three different sections: study selection; comparability and verification of exposure; and outcome investigated. We analyzed questions from each section to receive a star/point. According to the number of points received, cohort studies were categorized as follows: (1) high risk of bias—up to 3 points; (2) moderate risk of bias—4–6 points and; (3) low risk of bias—7–9 points; and cross-sectional studies were categorized as following: (1) high risk of bias—up to 4 points; (2) moderate risk of bias—5–6 points; (3) low risk of bias—7–8 points; and (4) very low risk of bias—9–10 points.
Outcomes
The primary endpoint was 30-day mortality after the index PE episode. Secondary outcomes included treatment-related complications including bleeding, the use of hospital resources [admission to intensive care unit (ICU), invasive mechanical ventilation, use of extracorporeal life support (ECLS), inferior vena cava (IVC) filter insertion], and length of hospital stay.
Statistical Analysis
Statistical analysis was performed with Review Manager (RevMan) software, version 5.4 (Cochrane Collaboration, Oxford, United Kingdom). The Mantel–Haenszel method was used to analyze dichotomous outcomes, and the results were reported as odds ratio (ORs). For continuous measures, we calculated the standardized mean differences (MD). A random-effect model was applied to analyze the data. The results of the meta-analysis are presented as ORs with 95% confidence intervals (CI) for dichotomous measures. When the continuous outcome was reported in a study as median, range, and interquartile range, we estimated means and standard deviations using the formula described by Hozo et al. (13). We quantified heterogeneity in each analysis by the tau-squared and I2 statistics. Heterogeneity was detected with the chi-squared test with n-1 degree of freedom, which was expressed as I2. Values of I2 > 50 and >75% were considered to indicate moderate and significant heterogeneity among studies, respectively. A two-tailed p-value less than 0.05 was considered statistically significant.
Results
Study Selection and Characteristics
The flow diagram showing the stages of database searching and study selection according to the PRISMA guidelines is shown in Figure 1. Of the 765 unduplicated records that were identified during our initial search, the full texts of 52 articles were fully reviewed. Eleven articles met the inclusion criteria and were included in the systematic review. A total of 10 non-randomized controlled trials (RCTs) (13–22) and one RCT (7) published between 2014 and 2021 including 65,589 participants were analyzed. In the included studies, 25,654 patients were treated with CDT and 39,935 were treated with ST. Nine studies were conducted in the United States (13–21), one study in Germany (7), and one study in the South Korea (22). The study characteristics and patient baseline characteristics are shown in Table 1. Detailed inclusion and exclusion criteria, primary outcomes, and findings of individual studies are shown in Supplementary Table 1. According to the Newcastle–Ottawa Scale, the risk of bias was considered very low (7) or low (14–22) (Table 1).
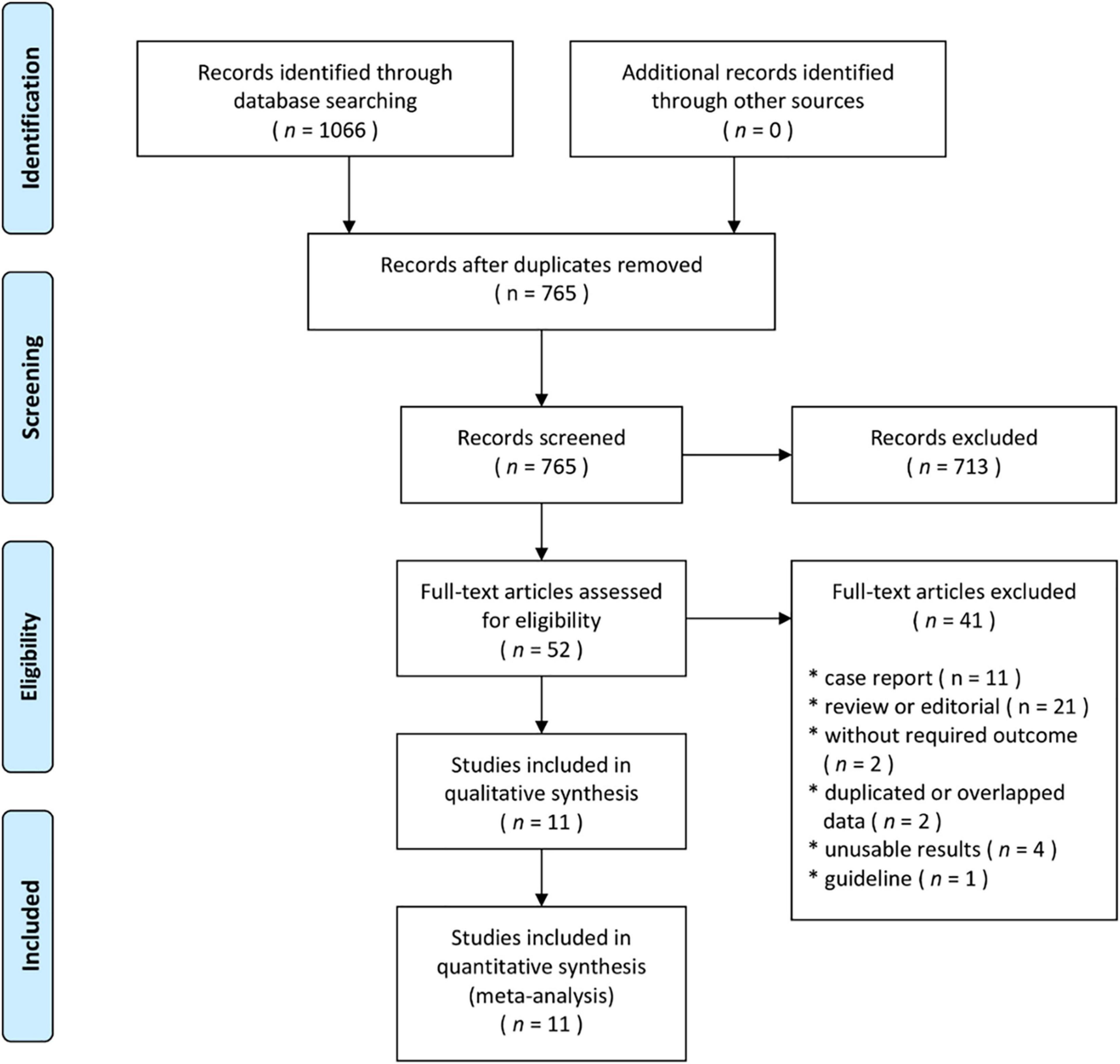
Figure 1. Flow diagram showing the stages of database searching and study selection according to the PRISMA guidelines.
Comparison of patient age, gender, and presentation with massive PE between the CDT and ST groups is shown in Supplementary Figures 1–3. There were no differences regarding the age of patients treated with CDT and ST (MD = -0.84, 95% CI -2.88–1.21, p = 0.42; Supplementary Figure 1) and the gender distribution (OR = 1.01, 95% CI 0.98–1.04, p = 0.52; Supplementary Figure 2). The percentage of patients who presented with massive PE was comparable in both groups (OR = 0.72, 95% CI 0.39–1.31, p = 0.28; Supplementary Figure 3). Comparison of the past medical history of patients treated with CDT and ST is shown in Supplementary Table 2. Diabetes mellitus (DM) was slightly less prevalent, whereas the previous stroke was more prevalent in the CDT group, compared to ST group (21.9 vs. 23.0%, p = 0.02 for DM; 19.2 vs. 15.0%, p = 0.04 for stroke, respectively). In CDT group, less patients presented with shock (8.0 vs. 17.2%, p < 0.001) and cardiac arrest (4.3 vs. 13.3%, p < 0.001), compared to ST group. The rates of other comorbidities were comparable between the groups.
Quality Assessment
A summary of the risk of bias in the included studies is shown in Supplementary Figures 4–7. The one RCT had low overall risk of bias (7). Among non-randomized studies, five had overall low risk of bias (14, 16, 18–20), three moderate risk (13, 21, 22), and two high risk (15, 17). High risk of bias was mostly due to the selection of participants and missing data. Moderate risk of bias was due to the selection of participants, classification of the intervention, and measurement of the reported outcomes. Altogether, the majority of studies included in the meta-analysis had low risk of bias and thus high quality of data.
Primary Outcome
Primary and secondary outcomes are shown in Table 2. In-hospital mortality was reported in seven studies and was lower in the CDT group compared to ST group (6.4 vs. 15.9%, respectively; OR = 0.40, 95% CI 0.30–0.55, I2 = 79%, p < 0.001). Thirty-day mortality (at 28 or 30 days) was reported in four studies and was lower in the CDT group, compared to ST group (7.3 vs. 13.6%; OR = 0.51, 95% CI 0.38–0.69, I2 = 18%, p < 0.001).
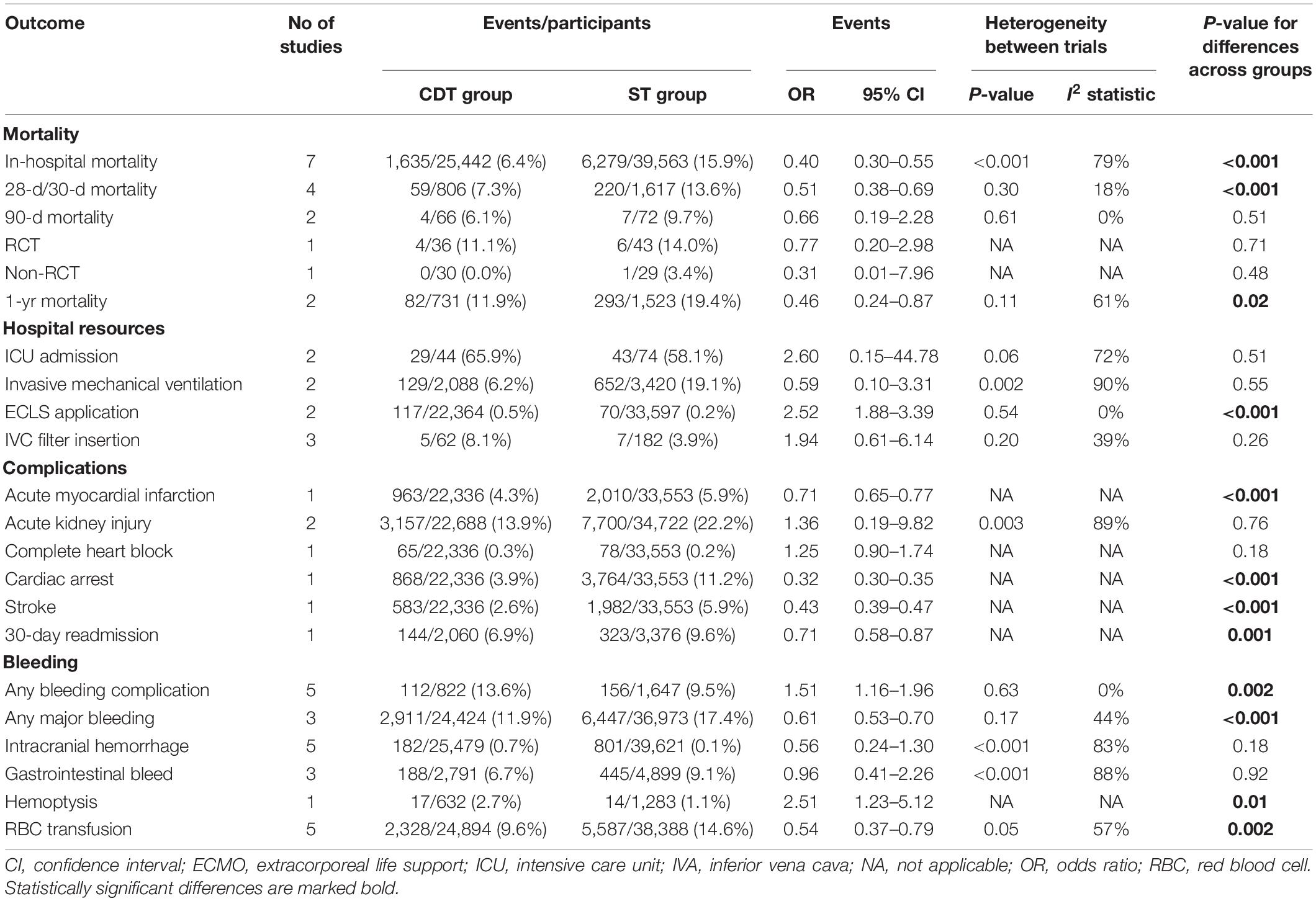
Table 2. Primary and secondary outcomes in patients treated with catheter-directed therapies (CDT) and systemic thrombolysis (ST).
Ninety-day mortality in the CDT group was 6.1% compared to 9.7% for ST group (OR = 0.66; 95% CI: 0.19–2.28, I2 = 0%, p = 0.51). Sub-analysis depending on the study design showed also no statistically significant differences between CDT and ST groups in RCT (11.1 vs. 14.0%, respectively; OR = 0.77, 95% CI: 0.20–2.98, p = 0.71) and in non-RCT (0.0 vs. 3.4%, respectively; OR = 0.31, 95% CI: 0.01–7.96, p = 0.48).
One-year mortality was reported in two studies and was also lower in CDT group, compared to ST group (11.9 vs. 19.4%; OR = 0.46, 95% CI 0.24–0.87, I2 = 61%, p = 0.02).
Secondary Outcomes
Regarding complications, the rates of myocardial injury, cardiac arrest, and stroke were lower in CDT group, compared to ST group (4.3 vs. 5.9% for AMI; 3.9 vs. 11.2% for cardiac arrest; 2.6 vs. 5.9% for stroke; p < 0.001 for all). In addition, patients treated with CDT had lower readmission rate at 30 days, compared to those treated with ST (6.9 vs. 9.6%, p < 0.001). The rates of acute kidney injury and complete heart block were comparable between the groups.
Bleeding complications occurred more often in CDT group, compared to ST (13.6 vs. 9.5%; p = 0.002). However, the rates of any major bleeding, hemoptysis, and red blood cell (RBC) transfusion were lower in patients treated with CDT, compared to ST (11.9 vs. 17.4% for major bleeding, 2.7 vs. 1.1% for hemoptysis, and 9.6 vs. 14.6% for RBC transfusion; p ≤ 0.01 for all). The rate of intracranial hemorrhage and gastrointestinal bleeding was comparable in both groups.
Regarding the use of hospital resources, ECLS was used more often in patients treated with CDT, compared to ST (0.5 vs. 0.2%, OR = 2.52, 95% CI 1.88–3.39, I2 = 0%, p < 0.001). The rates of ICU admission, invasive mechanical ventilation, and IVC filter insertion were comparable in both groups. The length of hospital stay was also comparable in patients treated with CDT and ST (Figure 2).
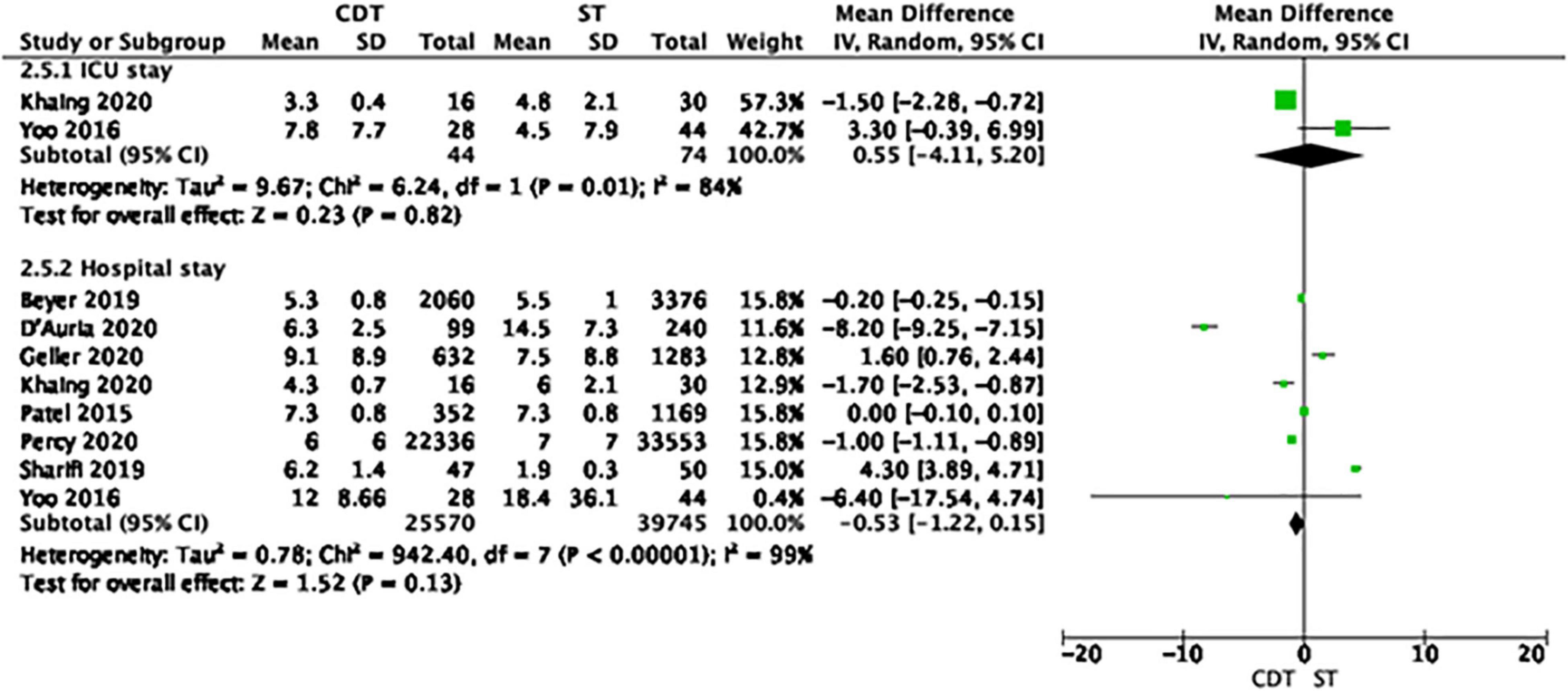
Figure 2. Forest plot of length of hospital stay in the catheter-directed therapies (CDT) group and systemic thrombolysis (ST). Each green rectangle or square represents an individual study, with 95% confidence intervals of the result displayed as black horizontal lines. The diamonds show the result when all the individual studies are combined together and averaged.
Discussion
In this meta-analysis of 11 studies including 65,589 patients with intermediate-high/submassive and high-risk/massive PE, we found patients treated with CDT had lower in-hospital, 30-day and 1-year mortality, less in-hospital complications (AMI, cardiac arrest, stroke, and major bleeding), and lower 30-day readmission rate, compared to those treated with ST. The novelty of our study includes (i) the largest hitherto analyzed a population of 65,589 patients treated with CDT or ST, (ii) the longest follow-up, compared to previous meta-analyses (23, 24), and (iii) inclusion of studies mostly published in the last 2 years, not considered in the previous meta-analyses.
According to the European Society of Cardiology (ESC) guidelines, percutaneous CDT should be considered for patients with high-risk PE, in whom thrombolysis is contraindicated or has failed (class of recommendation IIa, level of evidence C) (2). Although ST remains the mainstay of treatment in intermediate-high and high-risk PE patients, it is associated with relatively high risk of major bleeding (10%) and intracranial bleeding (3–5%) (3, 25). To circumvent bleeding complications, catheter-directed delivery of a low-dose thrombolytic agent has been proposed as safer and more efficient when compared to ST, thus giving rise to the dynamic development of CDT as an alternative to ST in the last decade and shifting paradigms regarding PE management (23). In contrary to the ESC guidelines, CDT were recently suggested not only as an alternative, but also as a first-line treatment strategy in hemodynamically unstable patients with acute PE (26). The feasibility of alternative approaches for CDT has also been suggested as attractive for the treatment of high and intermediate-high PE, such as transcubital venous access (27).
The results of trials comparing ST and catheter-directed thrombolysis are controversial, with some trials suggesting better outcomes (7, 13, 14, 16, 17), and others showing comparable (15, 18, 20–22) or worse outcomes after catheter-directed thrombolysis, compared to ST (19, 28, 29). Recent evidence also showed comparable efficacy and safety of catheter-directed thrombolysis with and without ultrasound, both in intermediate and high-risk groups (8, 30, 31). Since mechanical thrombectomy is performed mostly without the use of thrombolytic agents, it has been hypothesized to reduce major bleeding rates compared with both ST and catheter-directed thrombolysis, especially in intermediate-risk patients. However, the clinical efficacy of mechanical thrombectomy has been demonstrated only in single-arm trials through a surrogate endpoint (reduction of the right ventricle/left ventricle ratio at 48-h follow-up), warranting caution when interpreting the results (32, 33). Hence, the best interventional treatment option in patients with PE remains to be established (34). For this reason, we did not focus on a specific CDT type, but included all CDT in our analysis (Table 1). In contrast to previous recent analyses (35, 36), we compared the techniques which require the involvement of the cath-lab team and specialist equipment against systemic thrombolysis, which can be readily administered in the emergency room. Hence, our analysis was dictated by the practical and organizational considerations.
Compared to a similar meta-analysis published in 2009, the in-hospital mortality decreased over twofold, from 13.6 to 6.4% (23). The decrease in early mortality might be due to two reasons. First, the dynamic development of CDT techniques, along with the increasing experience of the operators, might contribute to better long-term outcomes (37). Second, establishment of pulmonary embolism response teams (PERTs) facilitates the early risk stratification and appropriate patient selection for CDT via multidisciplinary approaches (38). Although the information regarding PERT activity has only been reported in two out of 11 studies, we could not perform any reliable sub-analysis regarding the effect of PERT on patient outcomes. However, preliminary evidence from other trials suggests that PERT implementation improved the time to treatment initiation and decreased both the hospital length of stay and the generated costs (39–41). Thus, the technological, operational, and organizational improvements likely all contribute to better early outcomes in patients with intermediate and high-risk PE.
The mortality at 1 year had not previously been considered in a meta-analysis. However, the 1-year mortality observed in our study (11.9% in the CDT group and 19.4% in the ST group) is comparable with 1-year mortality rates reported in population-based cohort studies (16.8–16.9%) (42, 43). Since data regarding 1-year mortality have only reported in two out of 11 studies included in our analysis, these results should be interpreted with caution and re-evaluated in future trials.
We found a lower rate of in-hospital complications (AMI, cardiac arrest, and stroke) in patients treated with CDT, compared with ST. However, considering that these outcomes were reported only in one study, they should be perceived as hypothesis generating. In our study, CDT was associated with a higher rate of any bleeding (13.6 vs. 9.5%), confirming previous findings (19, 28, 29). Likely, the bleeding events related to CDT are associated with the use of anticoagulants prior to CDT. However, in the absence of individual patient data regarding previous anticoagulation, we could not perform a subgroup analysis to determine the association between anticoagulation before CDT and bleeding risk afterward. The rate of major bleeding and the need for RBC transfusion were lower in CDT than in ST (11.9 vs. 17.4% and 9.6 vs. 14.6%, respectively) and comparable to the major bleeding rate reported in observational studies (13–22.8%) (41, 43), confirming higher safety of CDT relative to ST. Noteworthy, in a network meta-analysis comparing catheter-directed thrombolysis, mechanical thrombectomy, and ST, mechanical thrombectomy was associated with the lowest rate of major bleeding, compared to any other interventional treatment method (24). Further dedicated trials are needed to determine the bleeding risk associated with different CDT.
In our meta-analysis, CDT were associated with comparable use of hospital resources, including ICU admission, invasive mechanical ventilation and IVC filter insertion, comparable length of hospital state, and higher rate of ECLS application. On the contrary, CDT were associated with lower readmission rate, which could theoretically decrease the healthcare costs related to the acute PE episode. However, in the absence of the dedicated economic analysis, we cannot draw any firm conclusion regarding the cost-effectiveness of CDT, compared to ST. Evidence from previous studies showed that CDT might be a more cost-effective treatment strategy compared to ST, despite higher costs of initial hospitalization (16, 21).
Altogether, our analysis suggests that CDT may have the potential to reduce mortality and complications following intermediate and high-risk PE. Our results should be interpreted with caution due to significant heterogeneity between the trials, which is due to differences in study populations (intermediate-high and high-risk PE), CDT (with or without thrombolysis), outcome measured, and duration of follow-up. Future high-quality evidence from randomized controlled trials is required to provide clarity regarding the optimal PE management in specific subgroups of patients, including (i) the efficacy and safety of various CDT therapies among each other and relative to ST, (ii) the best therapy dependent on the patient’s risk of mortality (intermediate vs. high), and (iii) the effect of PERT implementation on clinical outcomes. For example, the Pulmonary Embolism International THrOmbolysis (PEITHO)-3 study (NCT04430569) will investigate whether reduced-dose thrombolysis improves safety while maintaining reperfusion efficacy compared with standard heparin anticoagulation in 650 patients with intermediate-high-risk PE in a randomized, placebo-controlled, double-blind manner (44).
Limitations
Our analysis has several limitations. First and foremost, it combines two essentially different groups of patients with high-risk (massive) PE and intermediate-high-risk (submassive) PE. The risk of death in both groups is substantially different (40 vs. 5–10%). Accordingly, the main treatment goal in patients with high-risk PE is to achieve the prompt reduction in pulmonary artery pressure and resistance, with a concomitant improvement in RV function, whereas the main goal in patients with intermediate-high-risk PE is to prevent the hemodynamic breakdown (2). The combination of different groups of patients and study designs resulted in significant heterogeneity between the trials, requiring very cautious interpretation of the results. Ideally, both groups of patients should be analyzed separately to derive firm conclusion regarding the efficacy and safety of CDT depending on patients’ clinical presentation. However, since the percentage of patients with submassive and massive PE has only been reported in two out of 11 articles included in this analysis (13, 22), such sub-analysis was not feasible. Similarly, a sub-analysis of patients hemodynamically stable or unstable at admission, or those treated with different CDT, could not be done either, since such data were not available in the individual manuscripts. Second, our analysis is mostly based on retrospective studies that differed regarding geographical setting and baseline characteristics, which might have affected the results. Third, we were unable to access individual patient data, such as time from hospital admission to administration of ST or CDT, fibrinolytic agent (alteplase, reteplase, or tenecteplase) and dose (full or half), the use of anticoagulation prior to CDT, detailed PE risk stratification, or co-existent neoplastic disease. Hence, the potential influence of these confounders might potentially have affected the outcomes. Fourth, based on the design of the studies included in this meta-analysis, we could not perform separate comparative analyses of different CDT, including catheter-directed thrombolysis, ultrasound-assisted thrombolysis, and mechanical thrombectomy. Fifth, some of the outcomes such as AMI, cardiac arrest, or stroke have only been reported in one or two studies, which limits the general applicability of the results. Nonetheless, our systematic review with meta-analysis has been performed according to the Cochrane’s methodology and presents data derived from the hitherto largest population of PE patients treated with CDT or ST, with the longest follow-up, compared to previous meta-analyses. Finally, the protocol of this meta-analysis was not pre-registered in PROSPERO due to a significant delay caused by the priority of the research related to COVID-19, according to the information obtained from PROSPERO.
Conclusion
Our meta-analysis showed that CDT might decrease mortality in patients with intermediate-high and high-risk pulmonary embolism and were associated with less complications, including major bleeding, and lower 30-day readmission rate. Our results support further randomized trials to investigate the safety and efficacy use of CDT in patients with PE.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
AP and AG: conceptualization, methodology, and supervision. ŁS, AG, and AP: validation, investigation, and data curation. MP and TK: formal analysis. MG and MK: resources. AP: writing—original draft preparation and project administration. AP, AG, ŁS, and MN: writing—review and editing. SD, AG, and MB: visualization. All authors have been read and approved the manuscript for submission to JTH.
Funding
This manuscript was supported by the Centre of Postgraduate Medical Education, project number 501-1-054-25-22.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.861307/full#supplementary-material
References
1. Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. (2016) 118:1340–7. doi: 10.1161/CIRCRESAHA.115.306841
2. Konstantinides SV, Meyer G, Galié N, Simon R, Gibbs J, Aboyans V, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Respir J. (2019) 54:1901647. doi: 10.1183/13993003.01647-2019
3. Marti C, John G, Konstantinides S, Combescure C, Sanchez O, Lankeit M, et al. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. (2015) 36:605–14. doi: 10.1093/eurheartj/ehu218
4. Daley MJ, Murthy MS, Peterson EJ. Bleeding risk with systemic thrombolytic therapy for pulmonary embolism: scope of the problem. Ther Adv drug Saf. (2015) 6:57–66. doi: 10.1177/2042098615572333
5. Ciampi-Dopazo JJ, Romeu-Prieto JM, Sánchez-Casado M, Romerosa B, Canabal A, Rodríguez-Blanco ML, et al. Aspiration thrombectomy for treatment of acute massive and submassive pulmonary embolism: initial single-center prospective experience. J Vasc Interv Radiol. (2018) 29:101–6. doi: 10.1016/j.jvir.2017.08.010
6. Bhatt A, Al-Hakim R, Benenati JF. Techniques and devices for catheter-directed therapy in pulmonary embolism. Tech Vasc Interv Radiol. (2017) 20:185–92. doi: 10.1053/j.tvir.2017.07.008
7. Kucher N, Boekstegers P, Müller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. (2014) 129:479–86. doi: 10.1161/CIRCULATIONAHA.113.005544
8. Tafur AJ, Shamoun FE, Patel SI, Tafur D, Donna F, Murad MH. Catheter-directed treatment of pulmonary embolism: a systematic review and meta-analysis of modern literature. Clin Appl Thromb. (2017) 23:821–9. doi: 10.1177/1076029616661414
9. Kaymaz C, Akbal OY, Tanboga IH, Hakgor A, Yilmaz F, Ozturk S, et al. Ultrasound-assisted catheter-directed thrombolysis in high-risk and intermediate-high-risk pulmonary embolism: a meta-analysis. Curr Vasc Pharmacol. (2018) 16:179–89. doi: 10.2174/1570161115666170404122535
10. Bajaj NS, Kalra R, Arora P, Ather S, Guichard JL, Lancaster WJ, et al. Catheter-directed treatment for acute pulmonary embolism: systematic review and single-arm meta-analyses. Int J Cardiol. (2016) 225:128–39. doi: 10.1016/j.ijcard.2016.09.036
11. Moriarty JM, Edwards M, Plotnik AN. Intervention in massive pulmonary embolus: catheter thrombectomy/thromboaspiration versus systemic lysis versus surgical thrombectomy. Semin Interv Radiol. (2018) 35:108–15. doi: 10.1055/s-0038-1642039
12. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
13. Hennemeyer C, Khan A, McGregor H, Moffett C, Woodhead G. Outcomes of catheter-directed therapy plus anticoagulation versus anticoagulation alone for submassive and massive pulmonary embolism. Am J Med. (2019) 132:240–6. doi: 10.1016/j.amjmed.2018.10.015
14. Percy ED, Shah R, Hirji S, Tartarini RJ, Yazdchi F, Harloff M, et al. National outcomes of surgical embolectomy for acute pulmonary embolism. Ann Thorac Surg. (2020) 110:441–7. doi: 10.1016/j.athoracsur.2020.02.024
15. Harrison E, Kim JS, Lakhter V, Lio KU, Alashram R, Zhao H, et al. Safety and efficacy of catheter directed thrombolysis (CDT) in elderly with pulmonary embolism (PE). BMJ open Respir Res. (2021) 8:e000894. doi: 10.1136/bmjresp-2021-000894
16. Patel N, Patel NJ, Agnihotri K, Panaich SS, Thakkar B, Patel A, et al. Utilization of catheter-directed thrombolysis in pulmonary embolism and outcome difference between systemic thrombolysis and catheter-directed thrombolysis. Catheter Cardiovasc Interv. (2015) 86:1219–27. doi: 10.1002/ccd.26108
17. D’Auria S, Sezer A, Thoma F, Sharbaugh M, McKibben J, Maholic R, et al. Outcomes of catheter-directed thrombolysis vs. standard medical therapy in patients with acute submassive pulmonary embolism. Pulm Circ. (2020) 10:2045894019898368. doi: 10.1177/2045894019898368
18. Beyer SE, Shanafelt C, Pinto DS, Weinstein JL, Aronow HD, Weinberg I, et al. Utilization and outcomes of thrombolytic therapy for acute pulmonary embolism: a nationwide cohort study. Chest. (2020) 157:645–53. doi: 10.1016/j.chest.2019.10.049
19. Geller BJ, Adusumalli S, Pugliese SC, Khatana SAM, Nathan A, Weinberg I, et al. Outcomes of catheter-directed versus systemic thrombolysis for the treatment of pulmonary embolism: a real-world analysis of national administrative claims. Vasc Med. (2020) 25:334–40. doi: 10.1177/1358863X20903371
20. Khaing P, Paruchuri A, Eisenbrey JR, Merli GJ, Gonsalves CF, West FM, et al. First year experience of a pulmonary embolism response team with comparisons of outcomes between catheter directed therapy versus standard anticoagulation. Hosp Pract. (2020) 48:23–8. doi: 10.1080/21548331.2020.1706315
21. Sharifi M, Awdisho A, Schroeder B, Jiménez J, Iyer P, Bay C. Retrospective comparison of ultrasound facilitated catheter-directed thrombolysis and systemically administered half-dose thrombolysis in treatment of pulmonary embolism. Vasc Med. (2019) 24:103–9. doi: 10.1177/1358863X18824159
22. Yoo J-W, Choi HC, Lee SJ, Cho YJ, Lee JD, Kim HC. Comparison between systemic and catheter thrombolysis in patients with pulmonary embolism. Am J Emerg Med. (2016) 34:985–8. doi: 10.1016/j.ajem.2016.02.037
23. Kuo WT, Gould MK, Louie JD, Rosenberg JK, Sze DY, Hofmann LV. Catheter-directed Therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol. (2009) 20:1431–40. doi: 10.1016/j.jvir.2009.08.002
24. Starodubtsev V, Karpenko A, Lenko E, Rigla J. The better treatment option in acute massive pulmonary embolism. Vasc Dis Manag. (2020) 17:E72.
25. Meyer G, Vicaut E, Konstantinides SV. Fibrinolysis for intermediate-risk pulmonary embolism. N Engl J Med. (2014) 371:581–2.
26. Zuin M, Kuo WT, Rigatelli G, Daggubati R, Vassiliev D, Roncon L. Catheter-directed therapy as a first-line treatment strategy in hemodynamically unstable patients with acute pulmonary embolism: yes or no? Int J Cardiol. (2016) 225:14–5. doi: 10.1016/j.ijcard.2016.09.104
27. Giunio L, Lozo M, Borovac JA, Bradaric A, Zanchi J, Miric D. Feasibility and safety of catheter-directed thrombolysis via superficial cubital vein for the treatment of acute massive and submassive pulmonary embolism. Postepy Kardiol Interwencyjnej. (2021) 17:389–97. doi: 10.5114/aic.2021.112081
28. Avgerinos ED, Saadeddin Z, Abou Ali AN, Fish L, Toma C, Chaer M, et al. A meta-analysis of outcomes of catheter-directed thrombolysis for high-and intermediate-risk pulmonary embolism. J Vasc Surg Venous Lymphat Disord. (2018) 6:530–40. doi: 10.1016/j.jvsv.2018.03.010
29. Pei DT, Liu J, Yaqoob M, Ahmad W, Bandeali SS, Hamzeh IR, et al. Meta-analysis of catheter directed ultrasound-assisted thrombolysis in pulmonary embolism. Am J Cardiol. (2019) 124:1470–7.
30. Rao G, Xu H, Wang JJ, Galmer A, Giri J, Jaff MR, et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute pulmonary embolism: a multicenter comparison of patient-centered outcomes. Vasc Med. (2019) 24:241–7. doi: 10.1177/1358863X19838334
31. Lou BH, Wang LH, Chen Y. A meta-analysis of efficacy and safety of catheter-directed interventions in submassive pulmonary embolism. Eur Rev Med Pharmacol Sci. (2017) 21:184–98.
32. Tu T, Toma C, Tapson VF, Adams C, Jaber WA, Silver M, et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE study. JACC Cardiovasc Interv. (2019) 12:859–69. doi: 10.1016/j.jcin.2018.12.022
33. Sista AK, Horowitz JM, Tapson VF, Rosenberg M, Elder MD, Schiro BJ, et al. Indigo aspiration system for treatment of pulmonary embolism: results of the EXTRACT-PE trial. Cardiovasc Interv. (2021) 14:319–29. doi: 10.1016/j.jcin.2020.09.053
34. Tice C, Seigerman M, Fiorilli P, Pugliese SC, Khandhar S, Giri J, et al. Management of acute pulmonary embolism. Curr Cardiovasc Risk Rep. (2020) 14:24.
35. Pasha AK, Siddiqui MU, Siddiqui MD, Ahmed A, Abdullah A, Riaz I, et al. Catheter directed compared to systemically delivered thrombolysis for pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. (2021) 53:454–66. doi: 10.1007/s11239-021-02556-7
36. Siordia JA, Kaur A. Catheter-directed thrombolysis versus systemic anticoagulation for submassive pulmonary embolism: a meta-analysis. Curr Cardiol Rev. (2021) 18:112–7. doi: 10.2174/1573403X17666210603114116
37. Chopard R, Ecarnot F, Meneveau N. Catheter-directed therapy for acute pulmonary embolism: navigating gaps in the evidence. Eur Hear J Suppl. (2019) 21:I23—-I30. doi: 10.1093/eurheartj/suz224
38. Araszkiewicz A, Kurzyna M, Kopeć G, Roik M, Darocha S, Pietrasik A, et al. Expert opinion on the creating and operating of the regional Pulmonary Embolism Response Teams (PERT). Polish PERT Initiative. Cardiol J. (2019) 26:623–32. doi: 10.5603/CJ.2019.0127
39. Rosovsky R, Chang Y, Rosenfield K, Channick R, Jaff MR, Weinberg I, et al. Changes in treatment and outcomes after creation of a pulmonary embolism response team (PERT), a 10-year analysis. J Thromb Thrombolysis. (2019) 47:31–40. doi: 10.1007/s11239-018-1737-8
40. Liang Y, Shao-Ping NIE, Xiao W, Thomas A, Thompson E, Guan-Qi Z, et al. Role of Pulmonary Embolism Response Team in patients with intermediate-and high-risk pulmonary embolism: a concise review and preliminary experience from China. J Geriatr Cardiol. (2020) 17:510–8. doi: 10.11909/j.issn.1671-5411.2020.08.005
41. Schultz J, Giordano N, Zheng H, Parry BA, Barnes GD, Heresi GA, et al. A multidisciplinary pulmonary embolism response team (PERT)—experience from a national multicenter consortium. Pulm Circ. (2019) 9:2045894018824563. doi: 10.1177/2045894018824563
42. Hoskin S, Brieger D, Chow V, Kritharides L, Ng ACC. Trends in acute pulmonary embolism admission rates and mortality outcomes in Australia, 2002-2003 to 2017-2018: a retrospective cohort study. Thromb Haemost. (2021) 121:1237–45. doi: 10.1055/s-0041-1725932
43. Ebner M, Kresoja K-P, Keller K, Hobohm L, Rogge NIJ, Hasenfuß G, et al. Temporal trends in management and outcome of pulmonary embolism: a single-centre experience. Clin Res Cardiol. (2020) 109:67–77. doi: 10.1007/s00392-019-01489-9
Keywords: pulmonary embolism, catheter-based therapies, meta-analysis, pulmonary embolism response team, PERT
Citation: Pietrasik A, Gąsecka A, Szarpak Ł, Pruc M, Kopiec T, Darocha S, Banaszkiewicz M, Niewada M, Grabowski M and Kurzyna M (2022) Catheter-Based Therapies Decrease Mortality in Patients With Intermediate and High-Risk Pulmonary Embolism: Evidence From Meta-Analysis of 65,589 Patients. Front. Cardiovasc. Med. 9:861307. doi: 10.3389/fcvm.2022.861307
Received: 24 January 2022; Accepted: 25 April 2022;
Published: 16 June 2022.
Edited by:
Jonathan Douxfils, University of Namur, BelgiumReviewed by:
Marco Zuin, University Hospital of Ferrara, ItalyJosip A. Borovac, University of Split, Croatia
Copyright © 2022 Pietrasik, Gąsecka, Szarpak, Pruc, Kopiec, Darocha, Banaszkiewicz, Niewada, Grabowski and Kurzyna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arkadiusz Pietrasik, YXBpZXRyYXNpa0B3dW0uZWR1LnBs
 Arkadiusz Pietrasik1*
Arkadiusz Pietrasik1* Aleksandra Gąsecka
Aleksandra Gąsecka Łukasz Szarpak
Łukasz Szarpak Tomasz Kopiec
Tomasz Kopiec Maciej Niewada
Maciej Niewada Marcin Kurzyna
Marcin Kurzyna