- 1Department of Cardiovascular Surgery, German Heart Center, INSURE (Institute for Translational Cardiac Surgery), TUM, Munich, Germany
- 2German Heart Center Munich, Department of Cardiovascular Surgery, TUM, Munich, Germany
- 3German Heart Center Munich, Department of Cardiology, TUM, Munich, Germany
- 4Centro Medico Nacional Siglo XXI, Hospital de Cardiologia, IMSS, Ciudad de México, Mexico
Background: Bioprosthetic valve fracturing (BVF) results in low gradients following valve-in-valve transcatheter aortic valve replacement (ViV-TAVR). For the commonly used Edwards PERIMOUNT valve data from bench-testing are lacking to provide technical specifications for successful BVF during ViV-TAVR.
Methods: Using four Perimount 19- and 21-mm valves, in-vitro high-pressure balloon valvuloplasty with the True Dilatation Balloon Valvuloplasty Catheter and Atlas Gold PTA Dilatation Catheter was performed to analyze balloon-oversizing and pressure-thresholds to successfully achieve BVF.
Results: High-pressure balloons one millimeter larger than the labeled valve size and pressure rates of 20 atm (for Perimount 19-mm) and > 22 atm (for Perimount 21-mm) were required to achieve BVF. Caliper measurements demonstrated 2.5 mm (Perimount 19-mm) and 1.5 mm (Perimount 21-mm) enlarged inner prosthetic diameters after BVF. The Atlas TM Gold PTA Dilatation Catheter achieved BVF with the Perimount 21-mm, whereas the True TM Dilatation Balloon Valvuloplasty Catheter failed in the Perimount 21-mm either for balloon-rupture or pinhole-defect.
Conclusion: Both 19-mm and 21-mm Perimount P 2900 are amendable to BVF, thereby increasing the inner prosthetic diameter. High-pressure balloons 1 mm larger than the labeled valves are essential for this purpose, and the Atlas Gold PTA Dilatation Catheter alone should ensure success in the 21-mm prosthetics.
Introduction
Valve-in-valve transcatheter aortic valve replacement (ViV-TAVR) is an established therapy for failing surgical bioprostheses in patients with higher operative risks (1, 2). One-year survival after ViV-TAVR is 83%, but mean transprosthetic pressure gradients are determined by the size of bioprosthetics previously implanted (2). In a cohort with small-sized surgical valves (<21 mm inner prosthetic diameter), higher gradients and poor 8-year survival have resulted from ViV-TAVR procedures (1). Bioprosthetic valve fracturing (BVF) is intended to lower transprosthetic gradients in this setting, especially in patients with small surgical valves (3–5). Although technical specifications (ie, balloon types, sizes, and pressure ratings) needed for successful BVF have been documented for various surgical valves through in vitro testing (6–8), there is limited clinical data on BVF utilization frequency or success rates (4, 9). PERIMOUNT surgical valves (Edwards Lifesciences, Irvine, CA, USA), models 2800 and 2900 in particular, have demonstrated inconsistent BVF success (9). Data from in vitro studies of Magna and Magna Ease valves (Edwards Lifesciences) have served to guide clinicians in terms of balloon type and sizing, enabling successful BVF during ViV-TAVR procedures (6). However, the PERIMOUNT model 2700 is known for its resistance to BVF.
Between 2012 and 2018, Edwards PERIMOUNT valves (models 2800 and 2900) have been commonly deployed surgical valves, used in 15,000–20,000 implantations annually throughout Europe and the US (personal communication with Edwards Lifesciences). The present in vitro study was undertaken to better understand the amenability of a model 2900 PERIMOUNT valve to BVF attempts, determining pressure rates and balloon oversizing metrics required for successful BVF implementation.
Methods
Materials
The Edwards PERIMOUNT (model P2900) 19- and 21-mm valves used for study came from institutional stock. The P2900 valve has three pericardial leaflets mounted on a cobalt-chromium-nickel alloy stent frame. Its sewing ring has a silicone rubber core and a polytetrafluoroethylene (PTFE) skirt.
For in vitro BVF testing, two distinctly different high-pressure balloons were engaged: (1) the True Dilatation Balloon Valvuloplasty Catheter (Bard Peripheral Vascular Inc, Temp, AZ, USA), burst-pressure rating of 6 atm, and (2) the Atlas Gold PTA Dilatation Catheter (Bard Peripheral Vascular Inc), burst-pressure rating of 14 atm. The Edwards Inflation Device (Edwards Lifesciences) allows inflation pressures up to 30 atm.
Bioprosthetic Valve Fracturing
Balloons for BVF were sized 1–3 mm beyond true inner diameters of surgical valves, using the Valve in Valve App (UBQO Ltd. [London, UK] and Dr. Vinayak Bapat [Minneapolis, MN, USA]). True inner diameters were obtained before and after BVF by caliper. In the course of BVF, a high-pressure balloon was connected via high-pressure stopcock (Marquis; Merit Medical Systems Inc., South Jordan, UT, USA) to a 50-ml syringe containing dilute contrast and to a high-pressure indeflator (Figure 1). Once the balloon filled with contrast, the stopcock was turned, allowing incremental indeflator pressurization to the point of valve fracture or balloon rupture (Supplementary Video 1). The corresponding pressure level was then recorded.
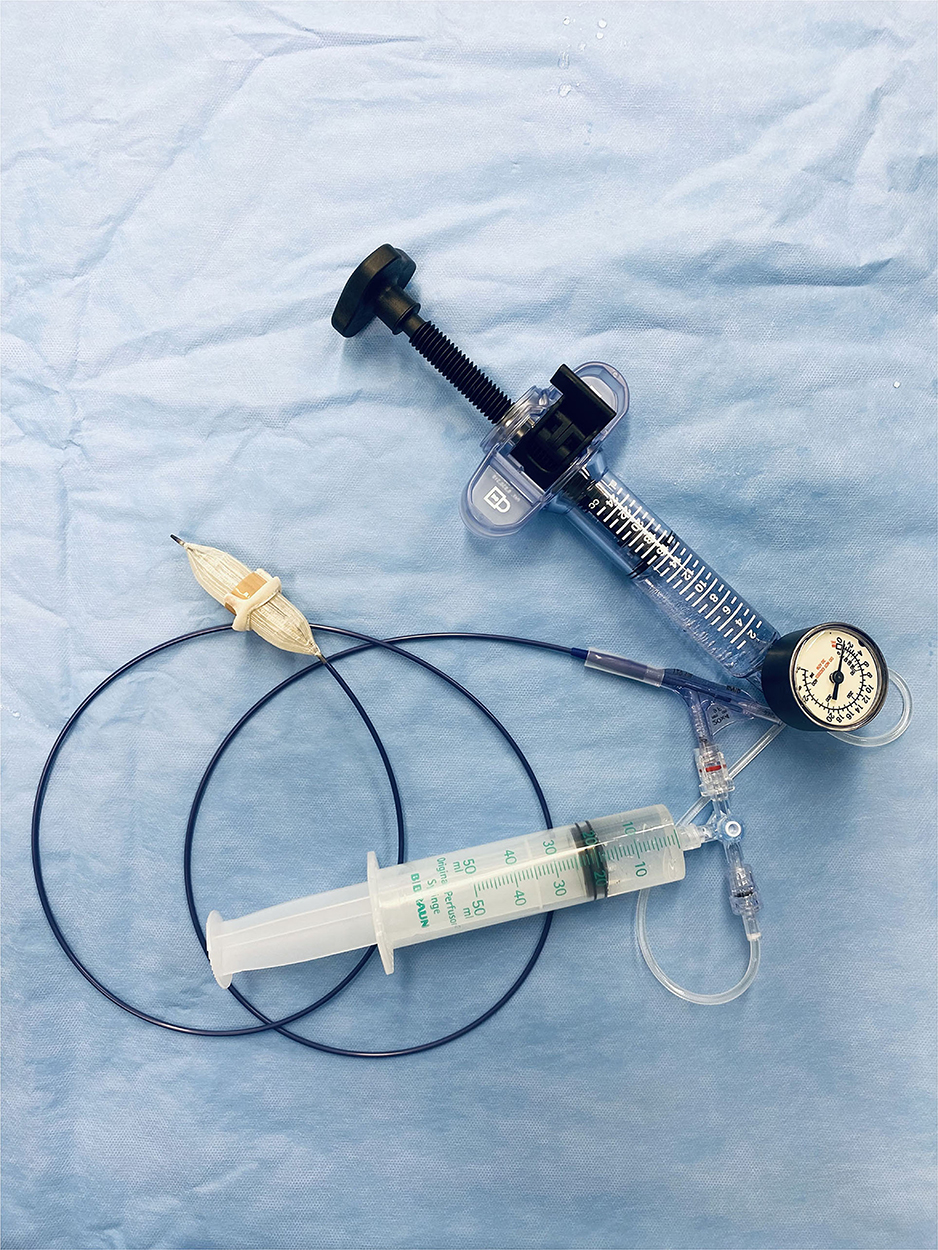
Figure 1. In-vitro test setting with a high-pressure balloon connected via high-pressure stopcock to a 50-ml syringe containing dilute contrast and to a high-pressure indeflator.
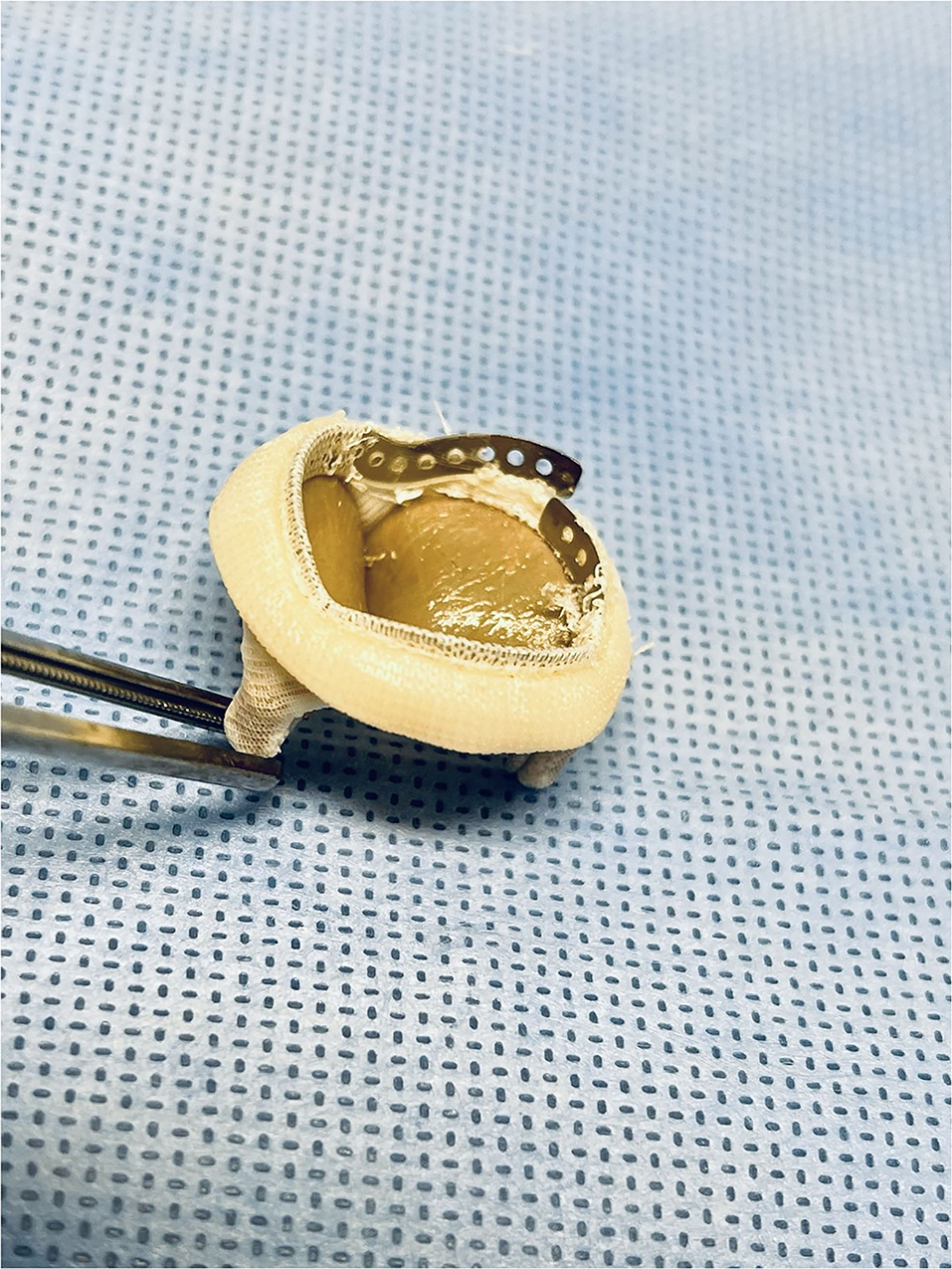
BVF was confirmed under fluoroscopy (Figure 2), with visual inspection after removal of the sewing ring (Figure 3). The ratio of balloon diameter to inner prosthetic diameter was calculated.
Failure of High-Pressure Balloons
Balloon failures were attributed to either ruptures (Supplementary Video 2) or pinhole defects (Supplementary Video 3). In the latter events, inflated balloon volumes remained visibly stable, but further pressure increase failed due to inherent microlesions.
Results
In vitro Bioprosthetic Valve Fracturing
Both the 19- and 21-mm PERIMOUNT P2900 valves were amenable to in vitro BVF, requiring balloons 1 mm larger than labeled valve sizes for procedural success. Applied pressures of 19–20 atm were sufficient to fracture the 19-mm valve, whereas pressures of 22–25 atm were needed for the 21-mm valve (Table 1). Fluoroscopy confirmed frame dehiscence in all valves tested. Caliper measurements also indicated increases in inner diameters after BVF, relative to baseline determinations (19-mm valve: 17.5 mm→ 20 mm; 21-mm valve: 20 mm→ 21.5 mm).
High-Pressure Balloon Performance
In the four 19-mm PERIMOUNT P2900 valves that were tested, BVF was consistently achieved using 20-mm True Dilatation Balloon Valvuloplasty Catheters at pressures of 19–20 atm (Table 1). One pinhole defect surfaced within this test series. An 18-mm Atlas Gold PTA Dilatation Catheter inflated to 30 atm remained intact but failed to achieve BVF.
Four 21-mm PERIMOUNT P2900 valves were similarly tested. BVF was consistently achieved using 22-mm Atlas Gold PTA Dilatation Catheters at pressures of 22–25 atm (Table 1). True Dilatation Balloon Valvuloplasty Catheters at 20-, 21-, and 22-mm sizes failed to achieve BVF due to ruptures or pinhole defects. A 20-mm Atlas Gold PTA Dilatation Catheter inflated to 30 atm remained intact but failed to achieve BVF.
High-Pressure Balloon Defects
During in vitro BVF testing, the high-pressure balloons displayed two modes of failure. There were four ruptures of True Dilatation Balloon Valvuloplasty Catheters at pressures of 18–22 atm (mean, 20 atm), whereas all Atlas Gold PTA Dilatation Catheter remained intact. In the True Dilatation Balloon Valvuloplasty Catheters, pinhole defects undermined balloon pressurization, leading to three failed BVF attempts.
Discussion
During in vitro testing of the Edwards PERIMOUNT P2900 valve (both 19- and 21-mm sizes), fracturing of its bioprosthetic ring was fully achievable. However, a balloon 1 mm larger than the labeled valve size (ie, 3 mm beyond inner prosthetic diameter) was required for success. In the 21-mm valve, higher pressure levels were required to achieve BVF. Only the Atlas Gold PTA Dilatation Catheter was capable of doing so, the True Dilatation Balloon Valvuloplasty Catheter failing entirely. Balloon failures resulted from true ruptures or pinhole defects.
In vitro BVF Studies
Recent in vitro BVF studies have reported technical specifications and feasibility data for various surgical valves other than the PERIMOUNT P2900 (6, 7). Higher pressure levels (ie, 18–24 atm) were required for BVF of surgical bioprostheses with metal rings (e.g., Magna, Magna Ease [Edwards Lifesciences]), as opposed to those with polymer rings (e.g., Epic [Abbott Laboratories, Chicago, IL, USA], Mosaic [Medtronic, Dublin Ireland], Mitroflow [LivaNova, London, UK]) where 8–12 atm sufficed (5–7). Based on bench testing of analogous devices, balloon oversizing of 1 mm beyond stated valve dimension is recommended (6, 7). For the 19- and 21-mm PERIMOUNT Magna valves, the feasibility of BVF using either an Atlas Gold PTA Dilatation Catheter or a True Dilatation Balloon Valvuloplasty Catheter has been proven at high (24-atm) pressure levels (6). Although the PERIMOUNT P2900 and the Magna have similar fluoroscopic appearances, results of our in vitro test series differed. Pressure required (19–20 atm) for the 19-mm PERIMOUNT P2900 was lower than that required (24 atm) for the 19-mm Magna. Also, successful BVF of the 21-mm Magna has been reported at 24 atm, whether by Atlas Gold PTA Dilatation Catheter or True Dilatation Balloon Valvuloplasty Catheter (6). We did not achieve BVF in 21-mm P2900 valves using True Dilatation Balloon Valvuloplasty Catheters. Table 2 summarizes the currently available data on in-vitro BVF studies.
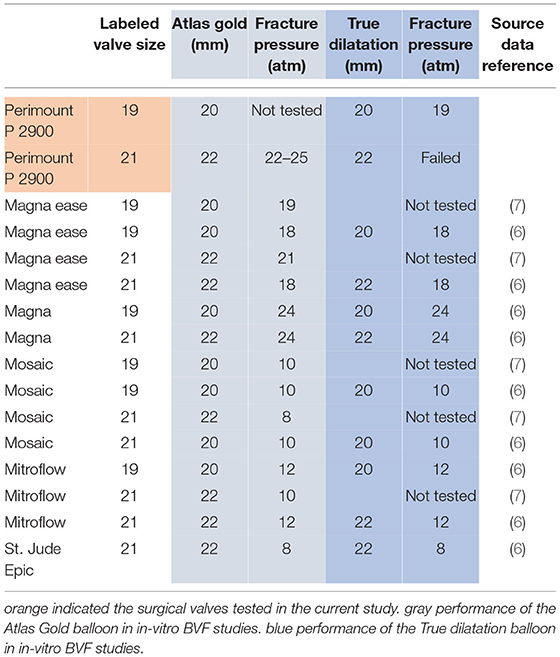
Table 2. Overview of perviously reported data and data acquired within the present study on required pressure rates to achieve in-vitro BVF using the atlas gold PTA dilatation catheter or true dilatation balloon valvuloplasty catheter (6, 7).
Causes of BVF Failure
Existing clinical data on BVF failure rates are sparse (9). Although balloon ruptures during ViV-TAVR procedures are quite evident by fluoroscopy, pinhole balloon defects are more likely signaled indirectly. For instance, manometer readings may indicate pressure loss or stagnation during full fluoroscopic balloon inflation. In such circumstances, balloons should be deflated, and attempts at BVF terminated. Balloon undersizing also precludes successful BVF.
Clinical Data on BVF
Some clinical case series addressing ViV-TAVR have demonstrated lower transvalvular gradients through BVF, compared with its non-use or with postdilatation, respectively (4, 9). As defined by the Valve Academic Research Consortium (VARC), device success is reportedly higher after ViV-TAVR procedures if BVF is performed (93 vs. 68%; p < 0.001) (4); and transvalvular gradients seem to be lower (10). Midterm data on ViV-TAVR with BVF are scant. Immediate postoperative transvalvular gradients in 139 patients treated thusly were low (9.4 ± 5.8 mmHg) but increased significantly (14.6 ± 7.5 mmHg; p < 0.001) at 30 days and remained stable for up to 1-year of follow-up (11). BVF-related complications, such as stroke, annular rupture, and coronary obstruction, have been rare, not exceeding rates cited for ViV-TAVR only (9, 12).
Technical Specifications for BVF Procedure
The present study provides technical specifications for BVF of PERIMOUNT P2900 bioprosthetic valves. Models P2900 and P2800 differ only in their pericardial leaflet treatments. Hence, we presume that the findings herein are applicable to the P2800 model as well.
We do suggest that balloons 1 mm larger than labeled valves be applied in this setting. In vitro BVF of the 21-mm PERIMOUNT P2900 also requires use of an Atlas Gold PTA Dilatation Catheter. Because pinhole defects seemed to account for nearly one-half of our balloon failures, we advise continuous monitoring of manometers during any BVF attempts to clearly identify such defects and abort all non-productive efforts.
Randomized clinical studies of ViV-TAVR procedures conducted alone or in conjunction with BVF are needed to verify the hemodynamic benefit of BVF and to establish protocols for its utilization.
Conclusion
Both 19- and 21-mm PERIMOUNT P2900 valves are amenable to BVF, thereby increasing inner prosthetic diameters. High-pressure balloons 1 mm larger than labeled valve sizes are essential for this purpose, and the Atlas Gold PTA Dilatation Catheter alone should ensure success in 21-mm prosthetics.
Limitations
Despite the limited number of valves tested, results were quite consistent. Still, an in vitro study may not entirely simulate in vivo BVF during transcatheter replacement of a degenerated surgical valve.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
HR is responsible for the study design, data collection, data analysis, data interpretation, and writing the manuscript. HA-C, OD, and ZA contributed in the in-vitro testing and manuscript revision. KV revised the manuscript. RL revised the manuscript and supervised the project. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The manuscript has been copy edited by native English speakers with related biomedical background in BioMed Proofreading® LLC.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.859088/full#supplementary-material
Supplementary Video 1. Successful bioprosthetic valve fracturing.
Supplementary Video 2. High-pressure balloon rupture causing failure of bioprosthetic valve fracturing attempt.
Supplementary Video 3. With pressure increase microlesion with volume loss undermine bioprosthetic valve fracturing attempt.
References
1. Bleiziffer S, Simonato M, Webb JG, Rodés-Cabau J, Pibarot P, Kornowski R, et al. Long-term outcomes after transcatheter aortic valve implantation in failed bioprosthetic valves. Eur Heart J. (2020) 41:2731–42. doi: 10.1093/eurheartj/ehaa544
2. Dvir D, Webb JG, Bleiziffer S, Pasic M, Waksman R, Kodali S, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. (2014) 312:162–70. doi: 10.1001/jama.2014.7246
3. Allen KB, Chhatriwalla AK, Saxon JT, Cohen DJ, Nguyen TC, Webb J, et al. Bioprosthetic valve fracture: technical insights from a multicenter study. J Thorac Cardiovasc Surg. (2019) 158:1317–28.e1. doi: 10.1016/j.jtcvs.2019.01.073
4. Brinkmann C, Abdel-Wahab M, Bedogni F, Bhadra OD, Charbonnier G, Conradi L, et al. Outcomes of valve-in-valve transcatheter aortic valve implantation with and without bioprosthetic valve fracture. Euro Intervention. (2021) 17:848–55. doi: 10.4244/EIJ-D-21-00254
5. Chhatriwalla AK, Allen KB, Saxon JT, Cohen DJ, Aggarwal S, Hart AJ, et al. Bioprosthetic valve fracture improves the hemodynamic results of valve-in-valve transcatheter aortic valve replacement. Circ Cardiovasc Interv. (2017) 10:e005216. doi: 10.1161/CIRCINTERVENTIONS.117.005216
6. Allen KB, Chhatriwalla AK, Cohen DJ, Saxon JT, Aggarwal S, Hart A, et al. Bioprosthetic valve fracture to facilitate transcatheter valve-in-valve implantation. Ann Thorac Surg. (2017) 104:1501–8. doi: 10.1016/j.athoracsur.2017.04.007
7. Johansen P, Engholt H, Tang M, Nybo RF, Rasmussen PD, Nielsen-Kudsk JE. Fracturing mechanics before valve-in-valve therapy of small aortic bioprosthetic heart valves. Euro Intervention. (2017) 13:e1026–31. doi: 10.4244/EIJ-D-17-00245
8. Nielsen-Kudsk JE, Christiansen EH, Terkelsen CJ, Nørgaard BL, Jensen KT, Krusell LR, et al. Fracturing the ring of small mitroflow bioprostheses by high-pressure balloon predilatation in transcatheter aortic valve-in-valve implantation. Circ Cardiovasc Interv. (2015) 8:e002667. doi: 10.1161/CIRCINTERVENTIONS.115.002667
9. Ruge H, Erlebach M, Lange R. Frequency of bioprosthetic valve fracturing utilization in an all-comers valve-in-valve TAVR cohort. Front Cardiovasc Med. (2021) 8:653871. doi: 10.3389/fcvm.2021.653871
10. Sathananthan J, Fraser R, Hatoum H, Barlow AM, Stanová V, Allen KB, et al. bench test study of bioprosthetic valve fracture performed before versus after transcatheter valve-in-valve intervention. Euro Intervention. (2020) 15:1409–16. doi: 10.4244/EIJ-D-19-00939
11. Chhatriwalla AK, Allen KB, Saxon JT, Cohen DJ, Nguyen TC, Loyalka P, et al. 1-Year outcomes following bioprosthetic valve fracture to facilitate valve-in-valve transcatheter aortic valve replacement. Structural Heart. (2021) 5:312–8. doi: 10.1080/24748706.2021.1895456
Keywords: bioprosthetic valve fracturing, valve-in-valve transcatheter aortic valve replacement, in-vitro, balloon rupture, transprosthetic gradient
Citation: Ruge H, Alvarez-Covarrubias HA, Deutsch O, Alalawi Z, Vitanova K and Lange R (2022) Bioprosthetic Valve Fracturing: In vitro Testing of Edwards PERIMOUNT Model P 2900. Front. Cardiovasc. Med. 9:859088. doi: 10.3389/fcvm.2022.859088
Received: 20 January 2022; Accepted: 29 April 2022;
Published: 02 June 2022.
Edited by:
Giuseppe Tarantini, University of Padua, ItalyReviewed by:
Cristina Aurigemma, Agostino Gemelli University Polyclinic (IRCCS), ItalyStylianos Pyxaras, Clinic Fürth, Germany
Copyright © 2022 Ruge, Alvarez-Covarrubias, Deutsch, Alalawi, Vitanova and Lange. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hendrik Ruge, aGVuZHJpa3J1Z2VAYW9sLmNvbQ==; orcid.org/0000-0001-6886-3660
 Hendrik Ruge
Hendrik Ruge Hector A. Alvarez-Covarrubias
Hector A. Alvarez-Covarrubias Oliver Deutsch1,2
Oliver Deutsch1,2 Keti Vitanova
Keti Vitanova