- 1Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Cardiology, Beijing AnZhen Hospital, National Clinical Research Centre for Cardiovascular Diseases, Beijing Advanced Innovation Center for Big Data-Based Precision Medicine for Cardiovascular Diseases, Capital Medical University, Beijing, China
- 3Heart Health Research Center (HHRC), Beijing, China
- 4The George Institute for Global Health, The University of New South Wales, Sydney, NSW, Australia
Background: Depression is a prevalent comorbidity in patients with heart failure (HF). However, data regarding the prognostic significance of depression during the early post-discharge period in patients hospitalized with acute HF, regardless of left ventricular ejection fraction (LVEF), were scarce.
Methods and results: The Heart Failure Registry of Patient Outcomes (HERO) study is a prospective, multicenter study of patients hospitalized with acute HF in China. At the first follow-up after discharge (median 4.0, interquartile range [IQR]: 2.4–6.1 weeks), depressive symptoms over the past 2 weeks were assessed using the Patient Health Questionnaire-9 (PHQ-9). Of 3,889 patients, 480 (12.3%) patients had depression (PHQ-9 score ≥ 10). A total of 3,456 patients (11.4% with depression) were included in the prospective analysis. After a median follow-up of 47.1 weeks (IQR: 43.9, 49.3) from the first follow-up, 508 (14.7%) patients died, and 1,479 (42.8%) patients experienced a composite event (death or HF rehospitalization). Cox proportional hazards models were used to assess the association of post-discharge depression with adverse events. After adjustment, post-discharge depression was associated with an increased risk of all-cause mortality (hazard ratio [HR] 2.38 [95% confidence interval (CI): 1.93–2.94]; p < 0.001) and the composite event (HR 1.78 [95% CI: 1.55–2.05]; p < 0.001). A per scale point increase in PHQ-9 score (ranging from 0 to 27 points) was associated with a 7.6% increase in all-cause mortality (HR 1.08 [95% CI: 1.06–1.09]; p < 0.001). In the subgroup analysis, the association between depression and the composite event was significantly stronger in relatively younger patients (< 75 vs. ≥ 75 years; p for interaction = 0.011), and the association between depression and all-cause mortality was significantly stronger in patients with preserved ejection fraction than in those with reduced ejection fraction (p for interaction = 0.036).
Conclusion: Post-discharge depression in patients recently hospitalized with acute HF is associated with an increased risk of adverse events, regardless of LVEF. Screening for depressive symptoms during the early post-discharge period may help to better identify high-risk patients and tailor patient management. Further studies are needed to determine how regular depression screening can help improve patient management and clinical outcomes.
Introduction
Depression is a prevalent comorbidity in patients with heart failure (HF) and is associated with impaired quality of life and increased risk of hospitalization and mortality (1–3). Most previous studies on depression in HF have focused on patients with chronic HF with reduced ejection fraction (HFrEF) from Western and other developed countries (3, 4). Following hospitalization for acute HF, patients are at high risk of readmission and mortality (5, 6). Approximately 30% of patients are re-hospitalized within 2–3 months of discharge, and mortality can reach 15% during this period (7). High rates of events are mainly driven by a subgroup of high-risk patients (8, 9). It is necessary to stratify patients to identify those at high risk of adverse events and potentially improve outcomes. Screening for depressive symptoms during the early post-discharge period can be easily achieved using self-reported questionnaires over the phone in minutes (10). However, the prognostic significance of depression during the early post-discharge period in patients recently hospitalized with acute HF is unknown. In addition, nearly half of patients with HF have a normal left ventricular ejection fraction (LVEF) (11). Nevertheless, data on the associations between depression and HF with preserved ejection fraction (HFpEF) are limited (4). There are substantial differences in the clinical spectrum and pathophysiological mechanisms between HFpEF and HFrEF (11). However, it is unclear whether there is an interaction between LVEF and depression on clinical outcomes.
The prevalence of depression in the general population varies significantly between countries and regions. In the World Mental Health Survey conducted by the World Health Organization, the lifetime prevalence of any mood disorder in the United States was approximately six times higher than in China (21.4 vs. 3.6%, respectively) (12). Similarly, two recent large-scale cross-sectional studies (13, 14) also showed a 5-fold difference in the lifetime prevalence of major depressive disorder between the United States and China (20.6 vs. 3.9%, respectively). A cross-sectional study (15) performed in a Chinese community-dwelling population reported that 12.0% of women and 7.7% of men with chronic HF had depression. To our knowledge, no large-scale prospective studies have been conducted on depression in patients with HF in China. Therefore, we investigated the prevalence, predictors, and prognostic value of post-discharge depression in a prospective cohort of patients hospitalized with acute HF in China.
Methods
Ethical considerations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved centrally by the Ethics Committee on Scientific Research and Clinical Trials at The First Affiliated Hospital of Zhengzhou University (in September 2017; approval number: 2014SY-079), and by the local Health Research Ethics Board of each participating hospital. All patients provided written informed consent.
Study population
The Heart Failure Registry of Patient Outcomes (HERO) study is a prospective, longitudinal, multicenter study aimed at determining the profile, management, and 1-year outcomes of patients hospitalized with acute HF in China (16). Adult (≥18 years) patients admitted to 73 participating hospitals with a primary admission diagnosis of acute HF during the defined period were consecutively recruited. The diagnosis of acute HF was confirmed by trained cardiologists, and patients with an incorrect admission diagnosis were excluded. From 10 November 2017 to 4 November 2018, 5,620 patients hospitalized with acute HF were enrolled. Data on socio-demographic characteristics, medical history, lifestyle, self-reported health status, clinical characteristics at admission, laboratory tests, treatments, and hospital course were collected. Only patients discharged alive who consented to follow-up calls were included. Standardized follow-up calls were made by trained nurses. Follow-up calls were scheduled at 2 weeks and 3, 6, and 12 months after discharge, or until death or withdrawal.
Depressive symptoms evaluation
At the first follow-up after discharge, the Patient Health Questionnaire-9 (PHQ-9) was administered by trained nurses over the phone to assess depressive symptoms. PHQ-9 is a self-reported questionnaire, containing 9 items based on the Diagnostic and Statistical Manual, to determine the presence and severity of depressive symptoms over the past 2 weeks (10). Each depressive symptom score ranges from 0 (not at all) to 3 (nearly every day), with total scores ranging from 0 to 27. PHQ-9 scores of 0–4, 5–9, 10–19, and 20–27 represent minimal, mild, moderate, and severe depression, respectively. A PHQ-9 score ≥ 10 has a sensitivity and specificity of 88% for diagnosing major depressive disorder (10). PHQ-9 is an effective tool for assessing depressive symptoms in patients with HF (17) and in the elderly from China (18). In this study, a PHQ-9 score ≥10 was defined as clinical depression.
Clinical outcomes
The primary outcomes were all-cause mortality and the composite of death or HF rehospitalization (determined from the time of post-discharge depression screening). Vital status and, if applicable, the cause of death of patients who provided consent but were lost to follow-up were determined using administrative data (16).
Statistical analysis
Categorical variables were presented as frequencies and percentages and compared using the chi-square test. Continuous variables were presented as mean ± standard deviation, or median and interquartile range, and compared using the t-test or Mann–Whitney U test, as appropriate. A two-sided p < 0.05 was considered statistically significant. The baseline characteristics of patients with and without depression were compared. Multiple logistic regression analysis was performed using adjusted variables with p < 0.1 in the comparison of baseline characteristics. The outcome of the logistic regression analysis was depression at the first follow-up call. Cox proportional hazards regression analysis was performed to evaluate the associations between depression and adverse events. To evaluate the dose-dependent relationship between depressive symptoms and adverse events, we also included PHQ-9 scores as a continuous variable in the Cox regression analysis. Potential confounding variables were adjusted based on univariate analysis (p < 0.1) and clinical knowledge. The adjusted variables in the multivariate analysis included age; sex; body mass index (BMI); systolic blood pressure; current smoking status; coronary artery disease; diabetes mellitus; chronic obstructive pulmonary disease (COPD); anemia; estimated glomerular filtration rate; serum sodium concentration; in-hospital LVEF (< 40%, 40–49%, ≥ 50%); renin-angiotensin system (RAS) inhibitor, beta-blocker, mineralocorticoid receptor antagonist, and statin use at discharge; and hospital levels. Anemia was defined as hemoglobin < 13 g/dl in men or hemoglobin < 12 g/dl in women (19). Multiple imputations (Markov chain Monte Carlo method) were used to account for missing data. A total of 40 imputed datasets were generated. In-hospital N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels were measured in 1,890 (54.7%) of 3,456 patients. Brain natriuretic peptide levels were available for 880 (25.5%) patients without NT-proBNP measurements. In another sensitivity analysis, brain natriuretic peptide and NT-proBNP levels were log-transformed and standardized, respectively. They were then combined into a new variable, NPs, as described previously (Supplementary Material) (20). In the NPs model, NPs levels (presented as z scores, another indicator of HF severity) were included in the adjusted model to further test the independence of the association between depression and adverse events. Subgroup analysis was performed to explore the interaction between depression and adverse events stratified by age (< 75 vs. ≥75 years), sex, in-hospital LVEF (<40%, 40–49%, and ≥50%), and New York Heart Association (NYHA) class (IV vs. III). Statistical analysis was performed using IBM SPSS Statistics (version 26).
Results
Prevalence and characteristics of patients with depression
Of 5,620 patients hospitalized with acute HF (NYHA class III or IV) in the HERO study, 4,428 patients were discharged alive with consent to be followed up. One hundred and eighty-two patients who died before the first follow-up were excluded. Of 4,246 survivors, 3,889 had valid PHQ-9 sores. In the prospective analysis, 366 patients who were re-hospitalized before the first follow-up and 67 patients who were lost to follow-up after the first follow-up call were excluded. Finally, a total of 3,456 patients were included (Figure 1).
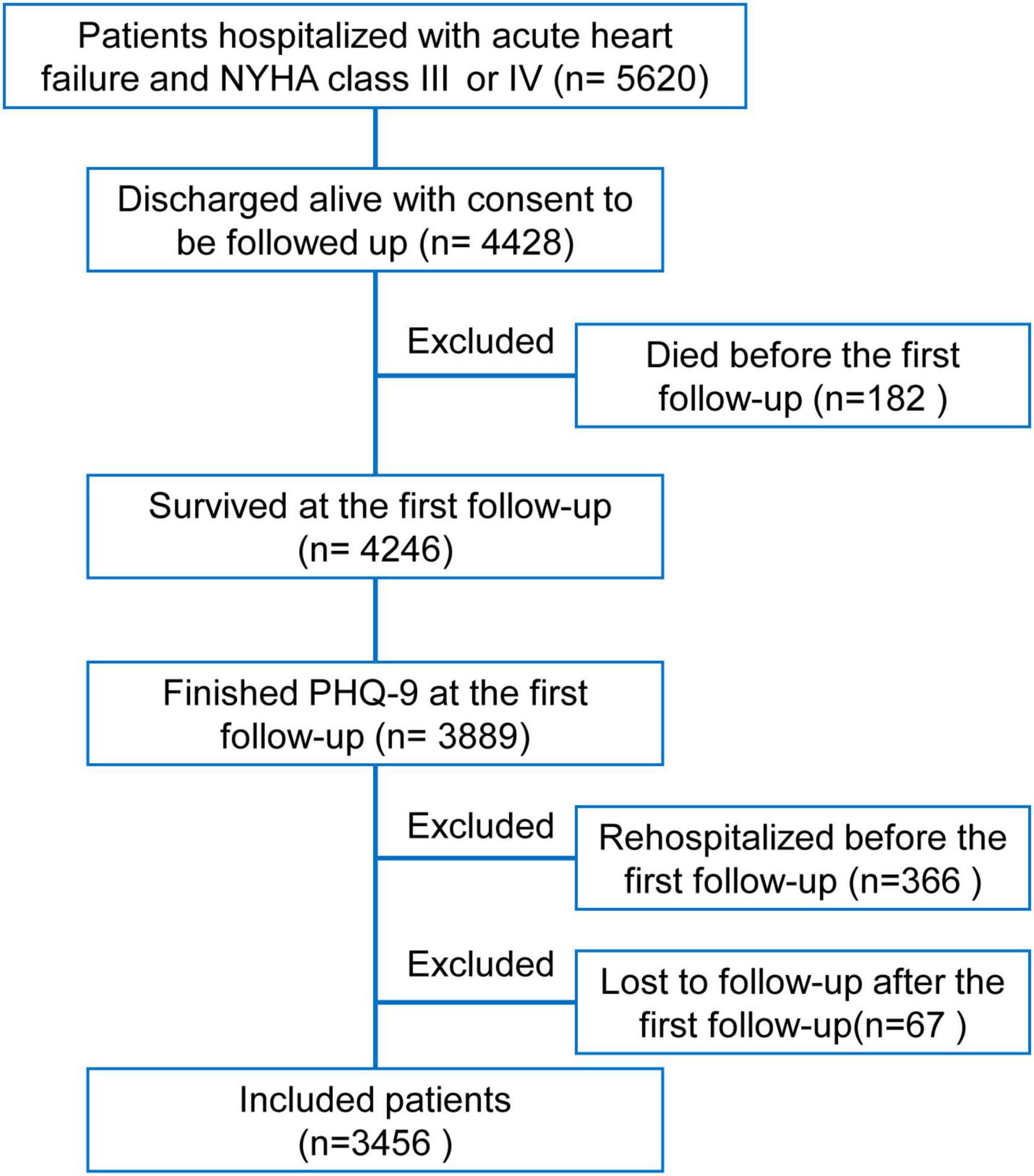
Figure 1. Flowchart of this study. Of 5,620 patients hospitalized with acute heart failure and New York Heart Association class III or IV in the Heart Failure Registry of Patient Outcomes study, 4,428 patients were discharged alive with consent to be followed up. After excluding 182 patients who died before the first follow-up, 3,889 of 4,246 survived patients had valid patient health questionnaire-9 scores. To avoid the effects of repeated hospitalizations in a short period on assessing depressive symptoms, 366 patients who had already been rehospitalized before the first follow-up were excluded. Meanwhile, 67 patients lost to further follow-up calls after the first follow-up were excluded. Finally, a total of 3,456 patients were included in the present analysis.
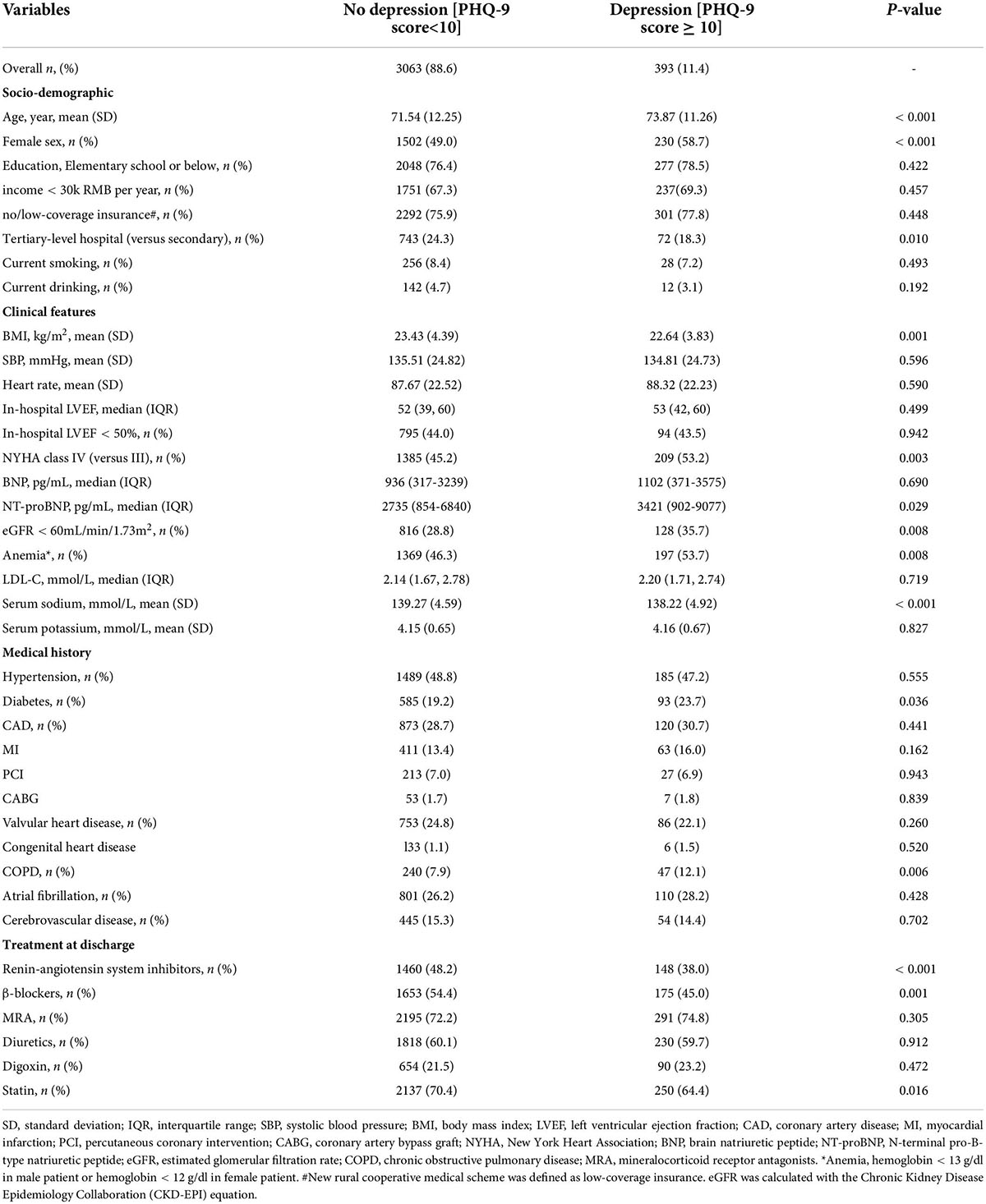
The median first follow-up was 4.0 (interquartile range: 2.4–6.1) weeks after discharge. Of 3,889 patients with available PHQ-9 scores, 480 (12.3%) patients had depression (PHQ-9 score ≥ 10). In the prospective cohort, 393 (11.4%) of 3,456 patients had depression. As shown in Table 1, patients with depression had the following characteristics: female sex, older age, lower BMI, decreased estimated glomerular filtration rate (≤60 ml/min/1.73 m2), higher NYHA class (IV vs. III), higher NT-proBNP levels, lower serum sodium concentration, diabetes mellitus, COPD, and anemia. RAS inhibitor, beta-blocker, and statin use at discharge were lower in patients with depression. Patients with depression were also more likely to be hospitalized in a secondary hospital than in a tertiary hospital. In the adjusted model, female sex, COPD, and diabetes mellitus were independent risk factors for depression; The use of RAS inhibitor and beta-blocker at discharge was independently associated with less depression (Figure 2).
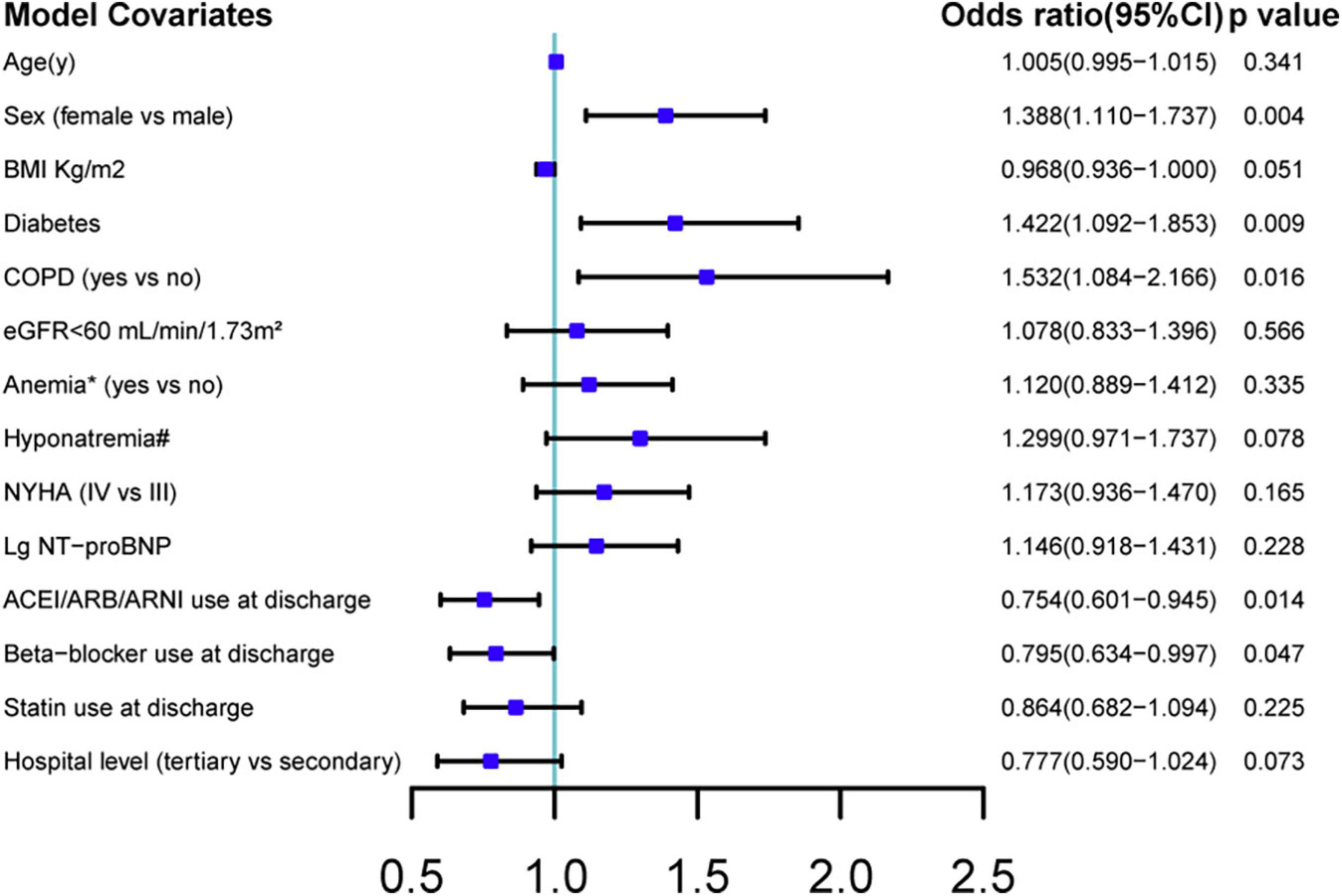
Figure 2. Multiple logistic regression analysis of predictive factors associated with post-discharge depression. BMI, body mass index; NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor-neprilysin inhibitors; Lg NT-proBNP, log-transformed values of N-terminal pro-B-type natriuretic peptide. CI: confidence interval. Factors in baseline characteristics with a p-value < 0.1 were included. Anemia, hemoglobin < 13 g/dl in male patient or hemoglobin < 12 g/dl in female patient. Hyponatremia, serum sodium concentration < 135 mmol/L.
Clinical outcomes in patients with depression
After a median follow-up of 47.1 (interquartile range: 43.9–49.3) weeks from the first follow-up (Figure 3), 508 (14.7%) patients died and 1,479 (42.8%) patients experienced a composite event (death or HF rehospitalization). In the unadjusted, adjusted, and NPs models, post-discharge depression was associated with an increased risk of all-cause mortality and the composite of death or HF rehospitalization (all p < 0.001; Table 2). After adjustment, depression was associated with a 138% increase in all-cause mortality (hazard ratio [HR] 2.38 [95% confidence interval (CI): 1.93–2.94]) and a 78% increase in the composite event rate (HR 1.78 [95% CI: 1.55–2.05]). A per scale point increase in PHQ-9 score was associated with a 7.6% increase in all-cause mortality (HR 1.08 [95% CI: 1.061–1.091]; p < 0.001) and a 4.6% increase in the composite event rate (HR 1.05 [95% CI: 1.036–1.056]; p < 0.001) after adjustment (Supplementary Material). In the sensitivity analysis, the results of the complete case analysis and handling missing data with multiple imputations were consistent (Supplementary Material). In the subgroup analysis, the association between depression and the composite event was significantly stronger in relatively younger patients (<75 vs. ≥ 75 years; p- for interaction = 0.011), and the association between depression and all-cause mortality was significantly stronger in patients with HFpEF than in those with HFrEF (p- for interaction = 0.036; Figure 4).
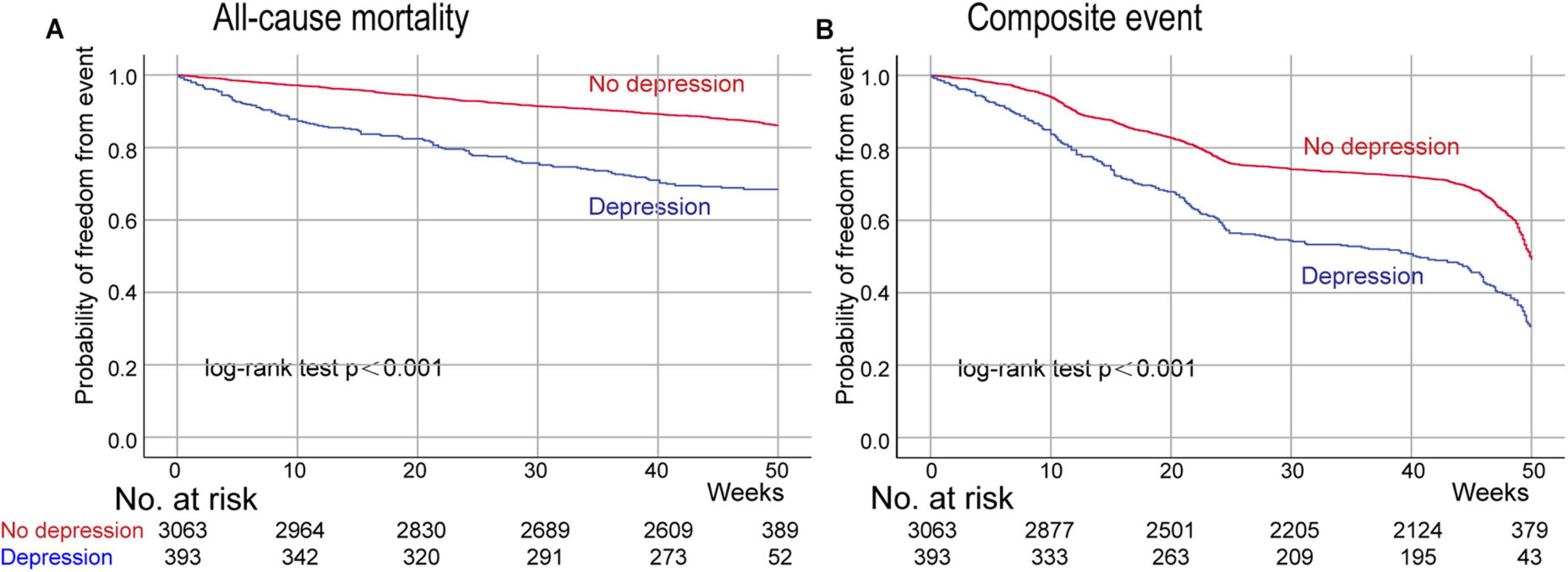
Figure 3. Kaplan–Meier curves for all-cause mortality and the composite event (death or HF rehospitalization) according to post-discharge depression. Day 0 is the time of the first follow-up after discharge. Panel (A), the probability of freedom from all-cause mortality; Panel (B) the probability of freedom from the composite of death or HF rehospitalization.
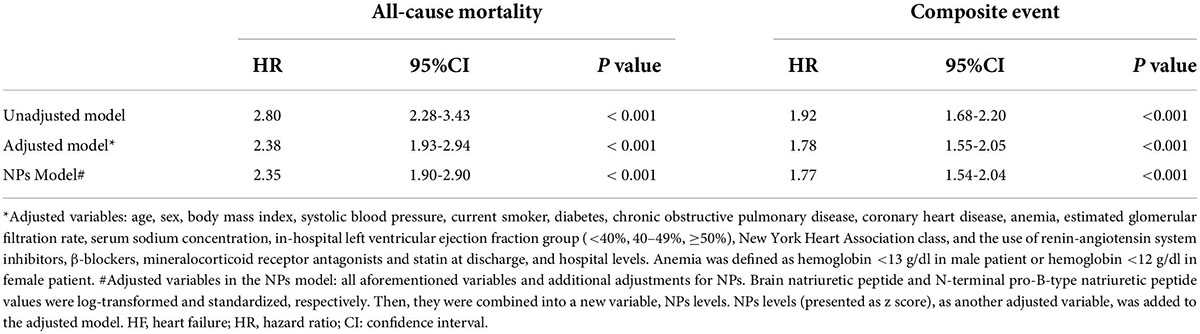
Table 2. The associations of depression with all-cause mortality and the composite event (death or HF rehospitalization).
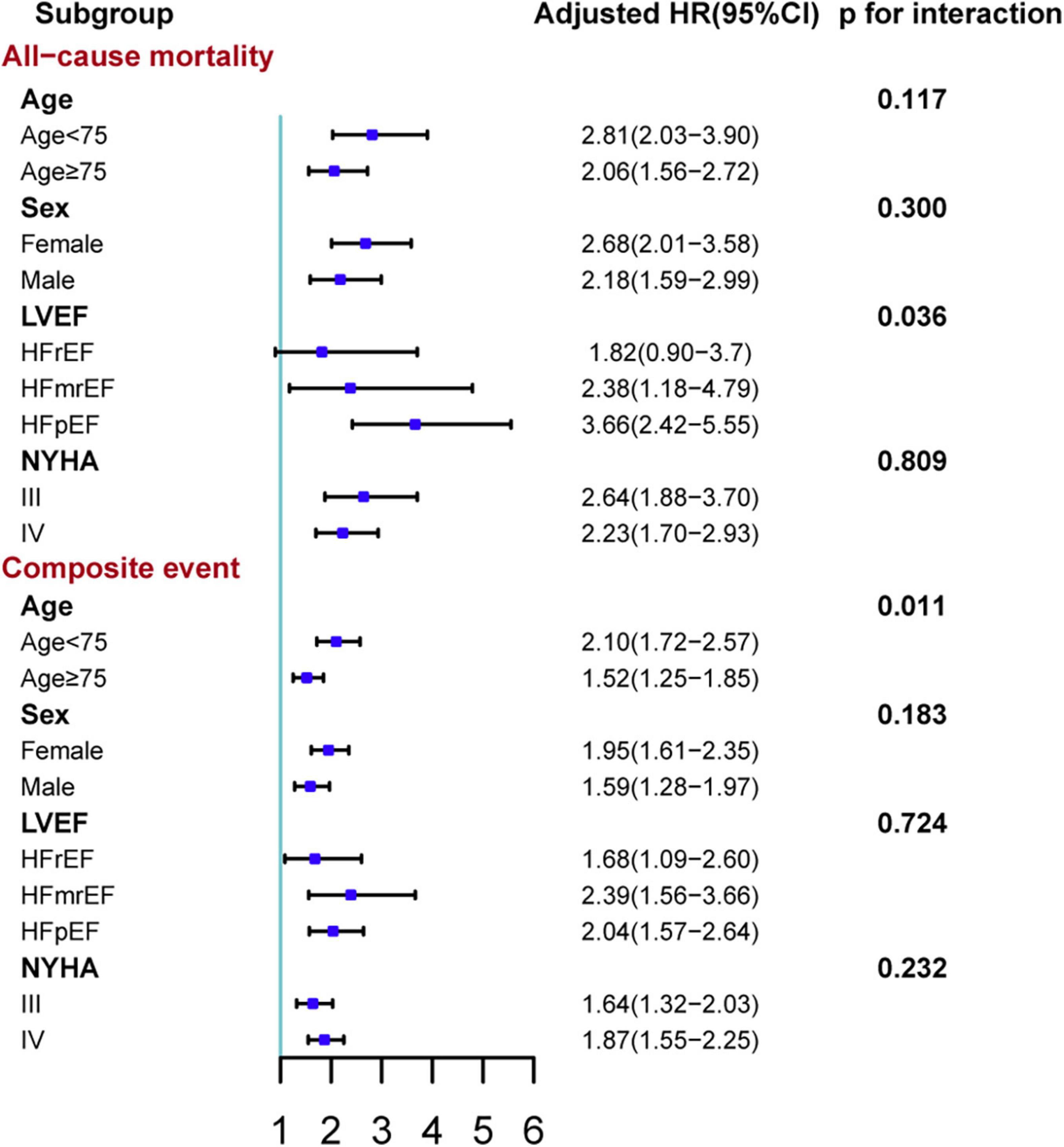
Figure 4. Subgroup analyses of the association between depression and adverse outcomes stratified by age, sex, in-hospital ejection fraction, and NYHA class. The composite event was time to death or HF rehospitalization. Adjusted variables in the Cox proportional hazard model: age, sex, body mass index, systolic blood pressure, left ventricular ejection fraction, current smoker, diabetes, chronic obstructive pulmonary disease, coronary heart disease, anemia, estimated glomerular filtration rate, serum sodium concentration, New York Heart Association class, and the use of renin-angiotensin system inhibitors, β-blockers, mineralocorticoid receptor antagonists and statin at discharge, and hospital levels. Anemia was defined as hemoglobin <13 g/dl in male patient or hemoglobin <12 g/dl in female patient. HFrEF, heart failure with decreased ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction. HR, hazard ratio; CI: confidence interval.
Discussion
In this large-scale prospective study of patients recently hospitalized with acute HF, 12.3% of patients had depression in the early post-discharge period. Most were undiagnosed and untreated. Female sex, older age, lower BMI, lower serum sodium concentration, worse HF severity, more comorbidities, and not using RAS inhibitors, beta-blockers, or statins at discharge were associated with depression. Post-discharge depression in patients recently hospitalized with acute HF was significantly and independently associated with an increased risk of all-cause mortality and the composite of death or HF rehospitalization. The association between post-discharge depression and adverse events was significantly stronger in relatively younger patients and in patients with HFpEF.
Previous studies in Western countries reported that the prevalence of depression in patients with HF was 20–30%, approximately three times the prevalence of major depressive disorder in the general population (2, 14). Depression was also more common in patients with a higher NYHA class, and inpatients were more likely to be depressed than outpatients (2). In this study, we assessed depressive symptoms during the early post-discharge period. This inevitably excluded patients who died before the first follow-up and those lost to the first follow-up. Discharged patients tended to have improved symptoms after in-hospital therapy. The timing of depression assessment and patient selection bias may partly explain the relatively lower prevalence in our study. Of 480 patients with depression, only seven had a history of mental illness, and only four had been treated with antidepressants, indicating an extreme lack of awareness and treatment of depression in patients with HF in China. The under-diagnosis and under-treatment of depression in patients with cardiovascular disease have been reported previously. One study showed that only 11% of patients with depression received appropriate treatment (21). The socioeconomic status (22) and cultural characteristics (e.g., more stigma toward mental illness (23)) of China may have exacerbated this problem. In the China Mental Health Survey, only 0.5% of individuals with major depressive disorder were treated adequately (24). Depression severely decreases the quality of life and increases morbidity and mortality (25). It was estimated that there were 13.7 million patients with HF in China (26), suggesting a large number of patients with undiagnosed depression that warrants attention.
The reasons why depression is prevalent in patients with HF and correlates with poor prognosis are complex and multifactorial (4). Several shared pathological mechanisms between HF and depression have been proposed, including higher levels of inflammatory markers, abnormal activation of the hypothalamic-pituitary-adrenal axis, autonomic dysfunction, and lifestyle factors (4, 27). Consistent with a previous study of chronic HF (4), our study showed that female sex, worse HF severity (higher NYHA class and NT-proBNP levels), and more comorbidities were significant predictors of post-discharge depression in univariate analysis. Depression is both a contributing factor and a consequence of the exacerbation of HF (4). The higher symptom burden, poorer quality of life, and increased activation of shared pathophysiological pathways between depression and HF may account for the higher rate of depression in patients with more severe HF (28). Previous studies reported that higher BMI and younger age were associated with depression in patients with chronic HF (29, 30) and in the general population (14, 31), whereas our findings were the opposite. These differences may partly be explained by the worse HF severity in patients with lower BMI and older age in this study, as they also had a much higher mortality rate during follow-up (Supplementary Material). Theoretically, patients with stable and chronic HF may be more similar to the general population (31). Conversely, in patients recently hospitalized with acute HF, secondary depression due to severe somatic disorders (32) may surpass the effects of a higher BMI and younger age. In this study, COPD and diabetes mellitus were independent predictors of post-discharge depression. These comorbidities may further increase the symptom burden and reduce the quality of life. Patients with COPD (33) and diabetes mellitus (34) tend to have more chronic inflammation, which may be an important mechanism for the increased risk of post-discharge depression. The use of RAS inhibitor and beta-blocker at discharge was independently associated with less post-discharge depression after adjustment. Previous studies have reported that abnormal RAS activation and increased sympathetic tone may contribute to the pathophysiological mechanism of depression in patients with HF (35, 36). Our findings support this hypothesis.
The early post-discharge period after hospitalization for acute HF carries a high risk of adverse events, and post-discharge follow-up during this period is recommended by the current European Society of Cardiology guidelines (7, 11). Our findings highlight the importance of screening for depressive symptoms in this vulnerable phase, and monitoring has significant and independent value for risk stratification. There is currently no clear consensus regarding the treatment of depression in patients with HF (11). Whether depression is a treatable target in HF or serves as a marker of HF severity remains to be determined. Potential interventions for patients with HF and post-discharge depression include treatment for HF and treatment for depression. Depressed patients tend to have lower adherence to HF treatment and poorer self-management and may require more intensive monitoring by clinicians (4). Because of the higher risk of adverse events in patients with HF and depression, a more aggressive treatment strategy for HF may provide a greater benefit. Selective serotonin reuptake inhibitors (SSRI) are widely used as antidepressants (37). However, SSRI failed to improve depression or the prognosis of HF in patients with HFrEF in two randomized controlled trials (38, 39). In the MOOD-HF trial, although escitalopram did not improve depressive symptoms more significantly than placebo, patients in both study arms showed a substantial improvement in depressive symptoms compared with baseline after nurse-coordinated disease management and optimization of HF drug therapy (38). This suggests that optimal treatment of HF may be the key to reducing depressive symptoms. Other studies have shown that exercise training (40), cognitive-behavioral therapy (41), telemedical interventional monitoring (42), and palliative care (43) improve depressive symptoms in patients with HF. These additional interventions may be beneficial for depressive symptoms in post-discharge patients with depression after the optimization of guideline-directed medical therapy for HF. However, the clinical significance of the reduction in depressive symptoms requires further research.
Previous studies on depression in patients with HFpEF are limited. This study is the first to report an interaction between post-discharge depression and in-hospital LVEF on mortality in patients recently hospitalized with acute HF. Our results were inconsistent with those of a recent post hoc analysis of the TOPCAT trial, in which no significant association was found between baseline depression and mortality in patients with chronic HFpEF after a mean follow-up of 3.3 years (44). In the TOPCAT trial, patients with a life expectancy of < 3 years were excluded. Two-thirds of patients had NYHA class I or II symptoms, indicating that the enrolled patients were more likely to have relatively stable HFpEF at baseline (45). Conversely, patients in the HERO study had NYHA class III or IV symptoms at enrolment. Worse HF severity, higher rates of adverse events, shorter time intervals between baseline evaluation of depressive symptoms and the occurrence of events, and more HF-related depression may explain the significant associations in the HERO study. In the TOPCAT trial, worsening depressive symptoms at 12 months were associated with an increased risk of all-cause mortality (HR 1.82 [95% CI: 1.13–2.93) (30), suggesting that depression may be a marker of the deterioration of chronic HFpEF. Our study extended the findings of the TOPCAT trial and indicated that, in patients who have recently experienced acute decompensated HF, depression is a stronger predictor of mortality in HFpEF than in HFrEF.
Limitations
There are several limitations to this study. First, although PHQ-9 is an effective tool with high sensitivity and specificity, it remains a self-reported questionnaire, not diagnostic criteria. PHQ-9 was administered by trained nurses over the phone, not strictly self-administered. Some patients may have been afraid or unwilling to share their poor mental state when answering questions posed by nurses. Second, baseline characteristics were collected during hospitalization in the HERO study, while depressive symptoms were assessed at the first follow-up. The timing discrepancy may have introduced potential confounders and influenced our results. Because depressive symptoms were assessed at the first follow-up, only patients who (i) were discharged, (ii) did not die early after discharge, (iii) had not been rehospitalized before the first follow-up, and (iv) were prepared to answer the PHQ-9 were included. Therefore, selection bias may have influenced the results. Third, the severity of depressive symptoms in patients with HF may have fluctuated. Some studies showed that the improvement or worsening of depression over time was associated with HF outcomes (30, 40). In the HERO study, we only assessed baseline depressive symptoms; therefore, we could not evaluate the associations between dynamic changes in depressive symptoms and clinical outcomes. Finally, the enrolment of patients in the HERO registry was limited to Henan Province, China, with a population of 100 million. Further validation is required in more diverse populations.
Future directions
Post-discharge depression is common in patients recently hospitalized with acute HF and is associated with adverse events. Unfortunately, depression in these patients often goes undiagnosed and untreated. Therefore, clinicians should pay more attention to the post-discharge mental health status of patients with HF. Because traditional antidepressants failed to improve depressive symptoms more significantly than placebo, further studies are needed to determine what can be done to relieve depressive symptoms and improve HF outcomes. Most previous studies on the treatment of depression in HF have focused on patients with HFrEF. Our study suggests that the association between depression and adverse events may be even stronger in patients with HFpEF than in those with HFrEF; therefore, future studies involving the treatment of depression in patients with HFpEF are necessary.
Conclusion
Post-discharge depression in patients recently hospitalized with acute HF is associated with an increased risk of adverse events, regardless of LVEF. Screening for depressive symptoms during the early post-discharge period may help to better identify high-risk patients and tailor patient management. Further studies are needed to determine how regular depression screening can help improve patient management and clinical outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee on Scientific Research and Clinical Trials at the First Affiliated Hospital of Zhengzhou University (in September 2017; approval no. 2014SY-079). Written informed consent to participate in this study was provided by the patients.
Author contributions
JD, XD, and JL contributed to the conception and design of the study. XW, RL, XZ, LinL, and LiL participated in the collection of samples and data. RL and YL performed the statistical analysis and all the results were checked by CJ and LiL. JL wrote the first draft of the manuscript. LinL, YL, XZ, XW, and CJ wrote sections of the manuscript. CM, XD, and JD were critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Thirteenth Five-Year Science and Technology Support Projects by the Ministry of Science and Technology of China (Grant No. 2016YFC1301000) and the National Natural Science Foundation of China (Grant Nos. 82170381 and 81900449). The HERO study was conceived and designed in 2017 and was registered on ChiCTR.org.cn (ChiCTR1800014786).
Acknowledgments
The authors would like to acknowledge the 73 HERO participating hospitals (Supplementary material) and patients who were essential for the HERO study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.858751/full#supplementary-material
References
1. Celano CM, Villegas AC, Albanese AM, Gaggin HK, Huffman JC. Depression and anxiety in heart failure: a review. Harv Rev Psychiatry. (2018) 26:175–84. doi: 10.1097/HRP.0000000000000162
2. Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. (2006) 48:1527–37. doi: 10.1016/j.jacc.2006.06.055
3. Sokoreli I, de Vries JJG, Pauws SC, Steyerberg EW. Depression and anxiety as predictors of mortality among heart failure patients: systematic review and meta-analysis. Heart Fail Rev. (2016) 21:49–63. doi: 10.1007/s10741-015-9517-4
4. Sbolli M, Fiuzat M, Cani D, O’Connor CM. Depression and heart failure: the lonely comorbidity. Eur J Heart Fail. (2020) 22:2007–17. doi: 10.1002/ejhf.1865
5. Cheema B, Ambrosy AP, Kaplan RM, Senni M, Fonarow GC, Chioncel O, et al. Lessons learned in acute heart failure. Eur J Heart Fail. (2018) 20:630–41. doi: 10.1002/ejhf.1042
6. Crespo-Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, et al. European society of cardiology heart failure long-term registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail. (2016) 18:613–25. doi: 10.1002/ejhf.566
7. Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol. (2015) 12:220–9. doi: 10.1038/nrcardio.2015.14
8. Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. (2012) 126:501–6. doi: 10.1161/CIRCULATIONAHA.112.125435
9. Arrigo M, Jessup M, Mullens W, Reza N, Shah AM, Sliwa K, et al. Acute heart failure. Nat Rev Dis Primers. (2020) 6:16. doi: 10.1038/s41572-020-0151-7
10. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
11. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726.
12. Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, et al. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. (2009) 18:23–33. doi: 10.1017/s1121189x00001421
13. Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. (2019) 6:211–24. doi: 10.1016/S2215-0366(18)30511-X
14. Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. (2018) 75:336–46. doi: 10.1001/jamapsychiatry.2017.4602
15. Jia Z, Du X, Du J, Xia S, Guo L, Su X, et al. Prevalence and factors associated with depressive and anxiety symptoms in a Chinese population with and without cardiovascular diseases. J Affect Disord. (2021) 286:241–7. doi: 10.1016/j.jad.2021.02.006
16. Li L, Liu R, Jiang C, Du X, Huffman MD, Lam CSP, et al. Assessing the evidence-practice gap for heart failure in China: the heart failure registry of patient outcomes (HERO) study design and baseline characteristics. Eur J Heart Fail. (2020) 22:646–60. doi: 10.1002/ejhf.1630
17. Hammash MH, Hall LA, Lennie TA, Heo S, Chung ML, Lee KS, et al. Psychometrics of the PHQ-9 as a measure of depressive symptoms in patients with heart failure. Eur J Cardiovasc Nurs. (2013) 12:446–53.
18. Liu ZW, Yu Y, Hu M, Liu HM, Zhou L, Xiao SY. PHQ-9 and PHQ-2 for screening depression in chinese rural elderly. PLoS One. (2016) 11:e0151042. doi: 10.1371/journal.pone.0151042
19. World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Geneva: WHO (2011).
20. Anand IS, Claggett B, Liu J, Shah AM, Rector TS, Shah SJ, et al. Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT trial. JACC Heart Fail. (2017) 5:241–52.
21. Huffman JC, Smith FA, Blais MA, Beiser ME, Januzzi JL, Fricchione GL. Recognition and treatment of depression and anxiety in patients with acute myocardial infarction. Am J Cardiol. (2006) 98:319–24.
22. Kakuma R, Minas H, van Ginneken N, Poz MR, Desiraju K, Morris JE, et al. Human resources for mental health care: current situation and strategies for action. Lancet. (2011) 378:1654–63.
23. Ran M-S, Hall BJ, Su TT, Prawira B, Breth-Petersen M, Li X-H, et al. Stigma of mental illness and cultural factors in Pacific Rim region: a systematic review. BMC Psychiatry. (2021) 21:8. doi: 10.1186/s12888-020-02991-5
24. Lu J, Xu X, Huang Y, Li T, Ma C, Xu G, et al. Prevalence of depressive disorders and treatment in China: a cross-sectional epidemiological study. Lancet Psychiatry. (2021) 8:981–90. doi: 10.1016/S2215-0366(21)00251-0
26. Hao G, Wang X, Chen Z, Zhang L, Zhang Y, Wei B, et al. Prevalence of heart failure and left ventricular dysfunction in China: the China hypertension survey, 2012-2015. Eur J Heart Fail. (2019) 21:1329–37.
27. Mommersteeg PMC, Schoemaker RG, Naudé PJW, Eisel ULM, Garrelds IM, Schalkwijk CG, et al. Depression and markers of inflammation as predictors of all-cause mortality in heart failure. Brain Behav Immun. (2016) 57:144–50. doi: 10.1016/j.bbi.2016.03.012
28. Bekelman DB, Havranek EP, Becker DM, Kutner JS, Peterson PN, Wittstein IS, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail. (2007) 13:643–8.
29. Evangelista LS, Moser DK, Westlake C, Hamilton MA, Fonarow GC, Dracup K. Impact of obesity on quality of life and depression in patients with heart failure. Eur J Heart Fail. (2006) 8:750–5.
30. Chandra A, Alcala MAD, Claggett B, Desai AS, Fang JC, Heitner JF, et al. Associations between depressive symptoms and HFpEF-related outcomes. JACC Heart Fail. (2020) 8:1009–20. doi: 10.1016/j.jchf.2020.06.010
31. Pereira-Miranda E, Costa PRF, Queiroz VAO, Pereira-Santos M, Santana MLP. Overweight and obesity associated with higher depression prevalence in adults: a systematic review and meta-analysis. J Am Coll Nutr. (2017) 36:223–33.
32. Lyra V, Parissis J, Kallergi M, Rizos E, Filippatos G, Kremastinos D, et al. 18 F-FDG PET/CT brain glucose metabolism as a marker of different types of depression comorbidity in chronic heart failure patients with impaired systolic function. Eur J Heart Fail. (2020) 22:2138–46. doi: 10.1002/ejhf.1866
33. Sethi S, Mahler DA, Marcus P, Owen CA, Yawn B, Rennard S. Inflammation in COPD: implications for management. Am J Med. (2012) 125:1162–70.
34. Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diab Rep. (2013) 13:435–44. doi: 10.1016/j.ejphar.2020.173215
35. Vian J, Pereira C, Chavarria V, Köhler C, Stubbs B, Quevedo J, et al. The renin-angiotensin system: a possible new target for depression. BMC Med. (2017) 15:144. doi: 10.1186/s12916-017-0916-3
36. Schnabel RB, Hasenfuß G, Buchmann S, Kahl KG, Aeschbacher S, Osswald S, et al. Heart and brain interactions : pathophysiology and management of cardio-psycho-neurological disorders. Herz. (2021) 46:138–49. doi: 10.1007/s00059-021-05022-5
37. Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA major depressive disorder working group. Mol Psychiatry. (2017) 22:900–9. doi: 10.1038/mp.2016.60
38. Angermann CE, Gelbrich G, Störk S, Gunold H, Edelmann F, Wachter R, et al. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. (2016) 315:2683–93. doi: 10.1001/jama.2016.7635
39. O’Connor CM, Jiang W, Kuchibhatla M, Silva SG, Cuffe MS, Callwood DD, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (sertraline against depression and heart disease in chronic heart failure) trial. J Am Coll Cardiol. (2010) 56:692–9. doi: 10.1016/j.jacc.2010.03.068
40. Blumenthal JA, Babyak MA, O’Connor C, Keteyian S, Landzberg J, Howlett J, et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. JAMA. (2012) 308:465–74. doi: 10.1001/jama.2012.8720
41. Freedland KE, Carney RM, Rich MW, Steinmeyer BC, Rubin EH. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. (2015) 175:1773–82. doi: 10.1001/jamainternmed.2015.5220
42. Koehler J, Stengel A, Hofmann T, Wegscheider K, Koehler K, Sehner S, et al. Telemonitoring in patients with chronic heart failure and moderate depressed symptoms: results of the telemedical interventional monitoring in heart failure (TIM-HF) study. Eur J Heart Fail. (2021) 23:186–94. doi: 10.1002/ejhf.2025
43. Rogers JG, Patel CB, Mentz RJ, Granger BB, Steinhauser KE, Fiuzat M, et al. Palliative care in heart failure: the PAL-HF randomized, controlled clinical trial. J Am Coll Cardiol. (2017) 70:331–41.
44. Liu J, Guo Z, Fan M, Liang W, He X, Wu D, et al. Major depression and clinical outcomes in patients with heart failure with preserved ejection fraction. Eur J Clin Invest. (2021) 51:e13401. doi: 10.1111/eci.13401
Keywords: acute heart failure, depression, heart failure hospitalization, early post-discharge period, heart failure with preserved ejection fraction
Citation: Li J, Jiang C, Liu R, Lai Y, Li L, Zhao X, Wang X, Li L, Du X, Ma C and Dong J (2022) Prognostic value of post-discharge depression in patients recently hospitalized with acute heart failure. Front. Cardiovasc. Med. 9:858751. doi: 10.3389/fcvm.2022.858751
Received: 20 January 2022; Accepted: 05 July 2022;
Published: 02 August 2022.
Edited by:
Leonardo Roever, Federal University of Uberlândia, BrazilReviewed by:
Christiane E. Angermann, Comprehensive Heart Failure Center, University Hospital Würzburg, GermanyH. Lester Kirchner, Geisinger Health System, United States
Copyright © 2022 Li, Jiang, Liu, Lai, Li, Zhao, Wang, Li, Du, Ma and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianzeng Dong, anpkb25nQGNjbXUuZWR1LmNu
 Junlei Li
Junlei Li Chao Jiang
Chao Jiang Rong Liu3
Rong Liu3 Xiaoyan Zhao
Xiaoyan Zhao Xiaofang Wang
Xiaofang Wang Changsheng Ma
Changsheng Ma Jianzeng Dong
Jianzeng Dong