
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 01 April 2022
Sec. Heart Failure and Transplantation
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.856602
This article is part of the Research Topic New Advances in Cardiorenal Syndrome View all 8 articles
 Wenguang Lai1,2,3†
Wenguang Lai1,2,3† Xiaoli Zhao4†
Xiaoli Zhao4† Sijia Yu2,3,5†
Sijia Yu2,3,5† Ziling Mai1,2,3†
Ziling Mai1,2,3† Yang Zhou2,3†
Yang Zhou2,3† Zhidong Huang2,3
Zhidong Huang2,3 Qiang Li2,3
Qiang Li2,3 Haozhang Huang2,3,5
Haozhang Huang2,3,5 Huanqiang Li2,3
Huanqiang Li2,3 Haiyan Wei6
Haiyan Wei6 Dachuan Guo7
Dachuan Guo7 Yun Xie1,2,3
Yun Xie1,2,3 Shanggang Li2,3
Shanggang Li2,3 Hongyu Lu2,3
Hongyu Lu2,3 Jin Liu2,3*
Jin Liu2,3* Shiqun Chen2,3*
Shiqun Chen2,3* Yong Liu1,2,3*
Yong Liu1,2,3*Background: Chronic kidney disease (CKD) is very common in patients who are at a high risk of developing incident heart failure with reduced ejection fraction (HFrEF). However, the harmful effect of CKD on incident HFrEF has not yet been examined among patients with coronary artery disease (CAD) undergoing percutaneous coronary intervention (PCI).
Methods: Patients undergoing PCI with baseline left ventricular ejection fraction (LVEF) ≥ 40% were included from January 2007 to December 2018 (ClinicalTrials.gov NCT04407936). We defined incident HFrEF as a follow-up LVEF of <40% within 3–12 months after discharge. Multivariable logistical regression was performed to examine the association of CKD with incident HFrEF.
Results: Overall, of 2,356 patients (mean age 62.4 ± 10.7 years, 22.2% women), 435 (18.5%) had CKD, and 83 (3.5%) developed incident HFrEF following PCI. The rate of incident HFrEF in the CKD group was higher than that in the non-CKD group (6.9 vs. 2.8%; p < 0.001). Multivariate logistic regression analysis indicated that CKD was an independent risk factor of incident HFrEF [adjusted odds ratio (aOR) = 1.75; 95% CI, 1.03–2.92; p = 0.035] after adjustment for confounders including age, gender, diabetes, hypertension, atrial fibrillation, congestive heart failure (CHF), baseline LVEF, ACEI/ARB, and statins. Furthermore, patients with incident HFrEF have a higher ratio of all-cause mortality compared to those without HFrEF (26.5 vs. 8.1%; p < 0.001).
Conclusions: Our results suggested that CKD was associated with increased risk of incident HFrEF, which was related to higher all-cause mortality in patients with CAD undergoing PCI. On this basis, more aggressive measures should be taken to prevent patients with CKD undergoing PCI from developing HFrEF.
Coronary artery disease (CAD) is the most common cause of heart failure (HF) (1). Even after percutaneous coronary intervention (PCI), patients with CAD still have a high risk of incident heart failure with reduced ejection fraction (HFrEF) (2). Thus, it is necessary to identify risk factors and improve prognosis among patients with CAD undergoing PCI.
Chronic kidney disease (CKD) has been recognized as a leading public health problem worldwide, with a global estimated prevalence of up to 13.4% (3). Previous studies suggested that patients with CKD constitute an increasing proportion of PCI population (4). Meanwhile, patients with CKD are at high risk for adverse cardiovascular events such as recurrent myocardial infarction (MI), HF, and stroke (5–7). Patients with CKD are prone to induced systemic inflammation, volume overload, renin-angiotensin system activation, oxidative stress, and abnormal calcium transport (7–9). These changes also play an essential part in the development of HFrEF (10–13). PCI can improve revascularization, protect the myocardium, and could delay the development of ventricular remodeling and HFrEF in patients with CAD (14). However, whether CKD is independently related to increased risk of incident HFrEF following PCI remains unknown.
Accordingly, we sought to investigate the effect of CKD on incident HFrEF among patients undergoing PCI in a large Chinese population. On this basis, clinicians could be guided to identify early and intervene on incident HFrEF following PCI.
We collected baseline information from the registry of Cardiorenal Improvement (CIN) study (ClinicalTrials.gov NCT04407936) from January 2007 to December 2018 at Guangdong Provincial People's Hospital. PCI was performed on patients according to standard clinical practice guidelines (15–17). A total of 2,356 patients with CAD undergoing PCI were included in this research. Exclusion criteria were as follows: (I) lack of baseline left ventricular ejection fraction (LVEF), (II) baseline LVEF <40%, (III) missing 3–12 month follow-up LVEF after discharge, (IV) lack of preoperative or postoperative creatinine data, (V) patients who died within 1 year after discharge (Figure 1). All the patients were classified into the CKD and non-CKD groups. All personal details were erased to protect data confidentiality of the patients. This clinical study was approved by the Guangdong Provincial People's Hospital ethics committee (no. GDREC2019555H) and complied with the declaration of Helsinki.
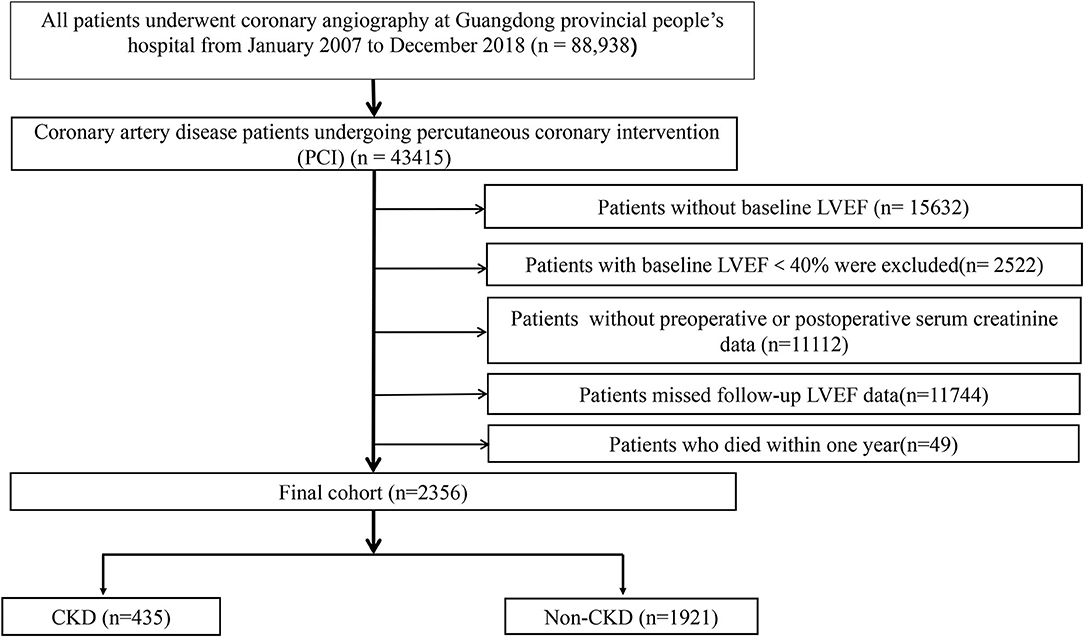
Figure 1. Flow chart of the study population. LVEF, left ventricular ejection fraction; CKD, chronic kidney disease.
All data were collected from the standard clinical electronic medical record system of Guangdong Provincial People's Hospital. All medical records can be accessed to ensure that we have baseline information, such as demographic characteristics, medical history, laboratory examination, and medications on discharge. Two experienced cardiac ultrasound doctors performed echocardiography using the biplane Simpson method from apical 4- and 2-chamber views for the whole cohort during hospitalization. All the cardiac ultrasound doctors have undergone unified training, and senior doctors were responsible for quality control of the ultrasound reports. Follow-up LVEF was calculated by with same method above. Median follow-up echocardiography time was 3.5 months, and median follow-up was 4 years.
The primary outcome was incident HFrEF, defined as a follow-up LVEF of <40% from 3 to 12 months after discharge. HFrEF was defined as LVEF ≤ 40 (18), and CKD was defined as estimated glomerular filtration rate (eGFR) calculated using the Modification of Diet in Renal Disease equation <60 ml/min/1.73 m2 (10). Congestive heart failure (CHF) was defined as New York Heart Association (NYHA) class > 2 or Killip class > 1 (19). Diagnoses of acute myocardial infarction (AMI), diabetes mellitus (DM), and hypertension were identified according to ICD-10.
In this study, the patients were divided into the CKD group and the non-CKD group. Descriptive analysis for continuous variables was shown as means ± standard deviation or medians and interquartile ranges according to distribution. Categorical variables were described as proportions. Kolmogorov-Smirnov test was performed for continuous variables if conforming to normal distribution. Differences in baseline characteristics were compared between the two groups t-test for continuous variables, chi-square test for categorical variables, and Kruskal-Wallis test for abnormal distribution. Time-to-event data were shown in graphs using Kaplan-Meier curves. We calculate sample size according to the rule of thumb of Vittinghoff, Peduzzi, and Harrell (20–22). Namely, a number of events per variable (EPV) of 5 or 10 or greater was applied for a multivariate regression model. Multivariate logistic regression was performed to assess the relationship between CKD and incident HFrEF in patients undergoing PCI by expressing it as odds ratio (OR) with 95% confidence interval (CI). The missing values of all adjusted variables in the regression model were <5%, and we computed a model using multiple imputations with chained equations to fill missing data in the adjusted variables. Three types of models were constructed: unadjusted (model 1), adjusted only for age and gender (model 2), and adjusted for age, gender, DM, hypertension, CHF, atrial fibrillation (AF), baseline LVEF, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARB), and statins (model 3). All statistical analyses were performed using R (ver. 4.0.3). A 2-tailed P value < 0.05 was considered statistically significant.
A total of 2,356 patients with CAD undergoing PCI were enrolled, and included 1,834 males and 522 females (mean age 62.7 ± 10.7 years). Among the patients, 1,421 (60.3%) had hypertension, 730 (31%) had DM, 240 (10.2%) had CHF, 634 (26.9%) had AMI, 123 (5.2%) had valvular heart disease, and 62 (2.6%) had atrial fibrillation (AF). The total population was classified into the CKD group (n = 435) and the non-CKD group (n = 1,921). Patients with CKD were older and more likely to have hypertension, DM, AF, and HF. The CKD group had lower usage of ACEI/ARB, statins, and calcium channel blockers (CCBs). Detailed clinical characteristics of the patients are listed in Table 1.
We found that patients with incident HFrEF were older and more likely to combine with CHF, and they had a lower level of eGFR and baseline LVEF, but had larger left ventricular end-diastolic dimension (LVEDD) and left ventricular end-systolic dimension (LVESD). Detailed information is provided in Supplementary Table 1.
Echocardiographic data from all the patients during the 3- to 12-month follow-up revealed that there were 83 (3.5%) cases of incident HFrEF. The prevalence of incident HFrEF was 6.9% in the CKD group and was 2.5 times higher than that in the non-CKD group. In addition, Kaplan-Meier curves showed higher all-cause mortality in patients with incident HFrEF than in those without HFrEF (log-rank test, P < 0.001) (Figure 2).

Figure 2. Kaplan-Meier curve in terms of all-cause mortality in patients with and without HFrEF. HFrEF, heart failure with reduced ejection fraction; HFrEF = 1, patients with incident HFrEF; HFrEF = 0, patients without incident HFrEF.
Univariate logistic regression analysis revealed that CKD was positively related to incident HFrEF [OR 2.61, 95% CI 1.63–4.11, p < 0.001]. There was also a significant association between CKD and incident HFrEF after adjustment for age and gender [OR 2.38, 95% CI 1.46–3.81, p < 0.001]. This association remained significant even after further adjustment for age, gender, diabetes, CHF, AF, hypertension, ACEI/ARB, statins, and baseline LVEF [OR 1.75, 95% CI 1.03–2.92, p = 0.035] (Figure 3).
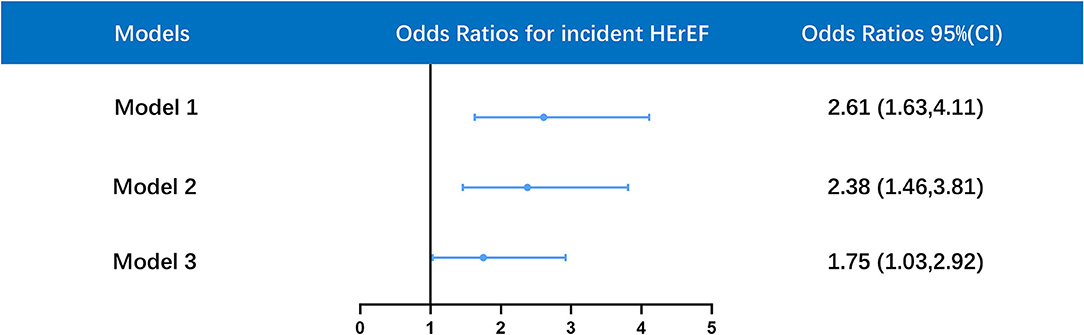
Figure 3. Multivariate logistic regression analysis for association between HFrEF and CKD in different models. Model 1 was unadjusted; model 2 was only adjusted for age and gender; model 3 was adjusted for age, gender, diabetes mellitus (DM), hypertension, atrial fibrillation (AF), congestive heart failure (CHF), baseline left ventricular ejection fraction (LVEF), angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB), statins. OR, odds ratios; CI, confidence interval. (ROC: AUC = 0.844, Hosmer-Lemeshow goodness-of-fit test: P = 0.11).
To our knowledge, this is the first study to analyze the relationship between CKD and incident HFrEF following PCI. In our research, patients with CKD had a higher rate of incident HFrEF. The overall prevalence of incident HFrEF was 3.5%, and the incidence of HFrEF was 2.5-fold higher in the CKD group than in the non-CKD group. CKD was an independent risk factor for incident HFrEF, which was associated with higher all-cause mortality. Our findings pointed to the importance of accounting for CKD in assessing the risk of incident HFrEF. Therefore, targeting CKD should be considered as one focus of prevention efforts for incident HFrEF among patients undergoing PCI.
Previous studies have shown that CKD and CAD play a critical role in the progression of HF. Kottgen et al. reported that the incidence of HF was 3-fold higher in the CKD group than the non-CKD group, and that the incidence of HF in those with CAD was up to five times higher than in those without CAD in a cohort of 14,857 adults in the United States (18). PCI can improve revascularization, protect the myocardium, and could delay the occurrence of HFrEF and ventricular remodeling. Gu et al. found that only 4.6% patients had an occurrence of incident HFrEF following PCI during a median follow-up of 63 months in a cohort of 3,910 patients with CAD (2). However, there is still a paucity of data on the risk of incident HFrEF among patients with CKD undergoing PCI. Our results found that the incidence of HFrEF in patients with CKD undergoing PCI was up to 6.9% within 1 year, which indicated that the risk of incident HFrEF remained high even after timely revascularization.
Furthermore, previous research studies have shown that renal insufficiency has been proposed as a risk factor for HF. Kottgen et al. reported that there was a 95% increased risk of developing HF in patients with CKD compared to patients without CKD (18). Additionally, Domingo et al. also found that renal insufficiency was a strong predictor of HF development among 2,763 women with CAD (23). Similarly, our previous study has shown that acute kidney injury could increase the risk of ventricular remodeling in patients undergoing CAG (24), which indicated that there was a close relationship between renal dysfunction and HF. Patients with CAD still have a risk for incident HFrEF even after PCI and optimal drug therapy (2). Accordingly, based on previous research studies, we explored further the effect of CKD on incident HFrEF among patients undergoing PCI. These two studies showed that renal dysfunction was an independent risk factor for deterioration of cardiac function regardless of transient or chronic impairment. Therefore, clinicians need to pay attention to changes in renal function.
Previous studies have often investigated the effect of CKD on left ventricular function for more than 3 years follow-up (18, 23, 25). Metabolic and hormonal derangements, accompanied with CKD, could impair left ventricular function in a chronic process. In our study, however, CKD could increase the risk of incident HFrEF within 3–12 months even after adjusting for many well-known confounding factors. Some reasons should be noted. The CKD group had more comorbidities like hypertension, DM, CHF, and AF. Pressure and volume overload of the heart caused by high blood pressure and HF may aggravate the deterioration of cardiac function (21). On the other hand, the CKD group was less likely to receive ACEI/ARB. Previous studies have also shown that ACEI/ARB therapy was accompanied with reduced risks of incident HF (2, 22). Moreover, there are some pathophysiologic mechanisms to account for the adverse effect of CKD on incident HFrEF. First, volume overload and renin-angiotensin-aldosterone system activation-mediated cardiac remodeling are known consequences of CKD (23). Moreover, CKD-related abnormalities in calcium transport, fibrinolysis, homocysteine, and systemic inflammation may contribute to the progress of HFrEF as well (26). Besides, the high phosphorus status associated with CKD can promote calcification of cardiac vessels and valves, which further accelerates the process of HFrEF (27).
There are some potential clinical implications from this research. The prevalence of CKD is common in patients undergoing PCI. Therefore, enhancing renal function assessment to screen for CKD is important before PCI, and can remind clinicians of taking measures to protect kidney function, such as formulating hydration measures and reducing the dose of contrast agent (28). In addition, incorporation of CKD in incident HFrEF risk assessment of patients with CAD undergoing PCI can help optimize the selection of high-risk patients who have the potential to obtain the largest advantage of more aggressive cardioprotective prevention treatments (29). Moreover, high-risk patients should be regularly followed up for incident HFrEF in order to detect it early and take renal function protection measures for patients with incident HFrEF such as SGLT2 inhibitors (30). Besides, further studies are needed to explore new strategies to prevent the occurrence of incident HFrEF in patients with CKD undergoing.
Nevertheless, several limitations could be noticed in our study. First, our research was single-center and retrospective, and did not reflect a direct causation. However, our study was large and population-based, and can be a good representative of the population undergoing PCI. Second, there existed a population selection bias when we screened patients, and we excluded patients without follow-up echocardiography. The incidence of HFrEF may be inconsistent among these patients, but we selected a relatively uniform population, and all of them accepted cardiac echocardiography during hospitalization. Third, occurrence of HFrEF is needed to undertake a long-term follow-up, and there was variability in the incidence of HFrEF in the 3–12 months follow-up. However, we mainly evaluated short-term and medium-term incidences, which still could reflect the deterioration of cardiac function. Fourth, the echocardiography data of the patients were collected with a specific time window, but the specific time point was unclear. Finally, our data lacked specific causes of death and information on coronary vessels anatomy, such as number of vessels, entity of coronary stenosis, and target PCI vessels. Therefore, more high-quality studies on the effects of CKD on incident HFrEF following PCI are needed to confirm our findings.
Our results suggested that 6.9% of the patients with CKD undergoing PCI developed HFrEF, and that the prevalence of incident HFrEF was 2.5 times higher in the CKD group than in the non-CKD group. Moreover, CKD was an independent risk factor for incident HFrEF, which was related to higher all-cause mortality in patients with CAD undergoing PCI. This suggests that early identification of high-risk population and aggressive cardioprotective treatment can help further improve the prognosis of these patients.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Guangdong Provincial People's Hospital Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
WL, XZ, and SY: research idea and study design. ZM, YZ, ZH, QL, HH, HuL, HW, YX, DG, and HoL: data acquisition. WL: data analysis/interpretation. SL: statistical analysis. YL, SC, and JL: supervision, mentorship, and writing guidance. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall study by ensuring that questions on the accuracy or integrity of any portion of the study are appropriately investigated and resolved. All the authors read and approved the final version of the manuscript.
This research was funded and supported by Guangdong Provincial science and technology project (2020B1111170011). Guangdong Provincial science and technology project (KJ022021049). The funders had no role in study design, collection and analysis of data, and decision to publish or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.856602/full#supplementary-material
1. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. (2016) 13:368–78. doi: 10.1038/nrcardio.2016.25
2. Gu J, Yin ZF, Xu ZJ, Fan YQ, Wang CQ, Zhang JF. Incident heart failure in patients with coronary artery disease undergoing percutaneous coronary intervention. Front Cardiovasc Med. (2021) 8:727727. doi: 10.3389/fcvm.2021.727727
3. Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. (2019) 1165:3–15. doi: 10.1007/978-981-13-8871-2_1
4. Tsai TT, Messenger JC, Brennan JM, Patel UD, Dai D, Piana RN, et al. Safety and efficacy of drug-eluting stents in older patients with chronic kidney disease: a report from the linked CathPCI Registry-CMS claims database. J Am Coll Cardiol. (2011) 58:1859–69. doi: 10.1016/j.jacc.2011.06.056
5. Ghoshal S, Freedman BI. Mechanisms of stroke in patients with chronic kidney disease. Am J Nephrol. (2019) 50:229–39. doi: 10.1159/000502446
6. Smilowitz NR, Gupta N, Guo Y, Mauricio R, Bangalore S. Management and outcomes of acute myocardial infarction in patients with chronic kidney disease. Int J Cardiol. (2017) 227:1–7. doi: 10.1016/j.ijcard.2016.11.026
7. Tuegel C, Bansal N. Heart failure in patients with kidney disease. Heart. (2017) 103:1848–53. doi: 10.1136/heartjnl-2016-310794
8. House AA. Cardio-renal syndrome type 4: epidemiology, pathophysiology and treatment. Semin Nephrol. (2012) 32:40–8. doi: 10.1016/j.semnephrol.2011.11.006
9. Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. (2016) 12:610–23. doi: 10.1038/nrneph.2016.113
10. Albar Z, Albakri M, Hajjari J, Karnib M, Janus SE, Al-Kindi SG. Inflammatory markers and risk of heart failure with reduced to preserved ejection fraction. Am J Cardiol. (2022) 167:68–75. doi: 10.1016/j.amjcard.2021.11.045
11. Dyadyk OI, Bagriy AE, Yarovaya NF. Disorders of left ventricular structure and function in chronic uremia: how often, why and what to do with it? Eur J Heart Fail. (1999) 1:327–36. doi: 10.1016/S1388-9842(99)00057-4
12. Schwinger RHG. Pathophysiology of heart failure. Cardiovasc Diagn Ther. (2021) 11:263–76. doi: 10.21037/cdt-20-302
13. Wray DW, Amann M, Richardson RS. Peripheral vascular function, oxygen delivery and utilization: the impact of oxidative stress in aging and heart failure with reduced ejection fraction. Heart Fail Rev. (2017) 22:149–66. doi: 10.1007/s10741-016-9573-4
14. Lala A, Desai AS. The role of coronary artery disease in heart failure. Heart Fail Clin. (2014) 10:353–65. doi: 10.1016/j.hfc.2013.10.002
15. Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2013) 61:e179–347. doi: 10.1016/j.jacc.2013.01.014
16. Kushner FG, Hand M, Smith SC Jr, King SB 3rd, Anderson JL, Antman EM, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2009) 54:2205–41. doi: 10.1016/j.jacc.2009.10.015
17. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-Elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-Elevation myocardial infarction. J Am Coll Cardiol. (2016) 67:1235–50.
18. Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. (2007) 18:1307–15. doi: 10.1681/ASN.2006101159
19. Aguiar-Souto P, Ferrante G, del Furia F, Barlis P, Khurana R, di Mario C. Frequency and predictors of contrast-induced nephropathy after angioplasty for chronic total occlusions. Int J Cardiol. (2010) 139:68–74. doi: 10.1016/j.ijcard.2008.10.006
20. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. (1996) 15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4
21. Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. (1995) 48:1503–10. doi: 10.1016/0895-4356(95)00048-8
22. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. (2007) 165:710–8. doi: 10.1093/aje/kwk052
23. Bibbins-Domingo K, Lin F, Vittinghoff E, Barrett-Connor E, Hulley SB, Grady D, et al. Predictors of heart failure among women with coronary disease. Circulation. (2004) 110:1424–30. doi: 10.1161/01.CIR.0000141726.01302.83
24. Li Q, Chen W, Shi S, Huang H, Lai W, Liu L, et al. Acute kidney injury increase risk of left ventricular remodeling: a cohort of 1,573 patients. Front Physiol. (2021) 12:744735. doi: 10.3389/fphys.2021.744735
25. Chae CU, Albert CM, Glynn RJ, Guralnik JM, Curhan GC. Mild renal insufficiency and risk of congestive heart failure in men and women > or =70 years of age. Am J Cardiol. (2003) 92:682–6. doi: 10.1016/S0002-9149(03)00822-1
26. Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. (2007) 116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342
27. Chen J, Budoff MJ, Reilly MP, Yang W, Rosas SE, Rahman M, et al. Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA cardiology. (2017) 2:635–43. doi: 10.1001/jamacardio.2017.0363
28. Liu P, Zhang B, Chen Z, He Y, Du Y, Liu Y, et al. m(6)A-induced lncRNA MALAT1 aggravates renal fibrogenesis in obstructive nephropathy through the miR-145/FAK pathway. Aging. (2020) 12:5280–99. doi: 10.18632/aging.102950
29. Brown E, Wilding JPH, Alam U, Barber TM, Karalliedde J, Cuthbertson DJ. The expanding role of SGLT2 inhibitors beyond glucose-lowering to cardiorenal protection. Ann Med. (2021) 53:2072–89. doi: 10.1080/07853890.2020.1841281
Keywords: chronic kidney disease, percutaneous coronary intervention, coronary artery disease, heart failure with reduced ejection fraction, left ventricular ejection fraction, incidence
Citation: Lai W, Zhao X, Yu S, Mai Z, Zhou Y, Huang Z, Li Q, Huang H, Li H, Wei H, Guo D, Xie Y, Li S, Lu H, Liu J, Chen S and Liu Y (2022) Chronic Kidney Disease Increases Risk of Incident HFrEF Following Percutaneous Coronary Intervention. Front. Cardiovasc. Med. 9:856602. doi: 10.3389/fcvm.2022.856602
Received: 17 January 2022; Accepted: 07 March 2022;
Published: 01 April 2022.
Edited by:
Ernesto Martinez-Martinez, Universidad Complutense de Madrid, SpainReviewed by:
Tokuhisa Uejima, The Cardiovascular Institute Hospital, JapanCopyright © 2022 Lai, Zhao, Yu, Mai, Zhou, Huang, Li, Huang, Li, Wei, Guo, Xie, Li, Lu, Liu, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Liu, bGl1eW9uZ0BnZHBoLm9yZy5jbg==; Shiqun Chen, c2hpcXVuY2hlbkAxMjYuY29t; Jin Liu, bGphdzM5NzAxNzU2OEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.